Abstract
Purpose/Objectives
Describe frailty and associated factors in breast cancer survivors
Design
Cross-sectional descriptive
Setting
School of nursing
Sample
216 breast cancer survivors (BCS) aged 53–87 not currently participating in exercise
Methods
Performance tests, clinical measures, and self-reported questionnaires provided baseline data analyzed for this study
Main Research Variables
Frailty was defined as meeting 3 of the 5 criteria of the Frailty Phenotype: shrinking, exhaustion, low activity, slowness, and weakness. Data were compared to published data from women in the Cardiovascular Health Study (CHS) and Women’s Health and Aging Study (WHAS).
Findings
18% of BCS aged 70–79 were frail, compared to 11% of CHS and WHAS women aged 70–79. Frailty was more common at a younger age in BCS and more BCS were frail in all age groups compared to CHS women until approximately age 80 when prevalence of frailty was similar in the two groups. 50% of BCS were classified as prefrail because they met 1–2 of the 5 frailty criteria. Higher body mass index increased the odds of frailty and higher physical activity decreased the odds of frailty (OR= 1.12, p=.003 and OR=.99, p=.000 respectively).
Conclusions
Frailty and prefrailty may be common in BCS and may occur at an earlier age than in adults without a history of cancer.
Keywords: breast cancer survivors, frailty, disability
Older women (aged 65+) constitute the largest group of U.S. breast cancer survivors (BCS) (National Cancer Institute). Today, more than 1.6 million U.S. women aged 65+ are BCS (Centers for Disease Control and Prevention, 2011). The long-term physiological symptoms reported by BCS, such as cognitive difficulty, neuropathy, osteoporosis, muscle weakness, weight loss, slow walking speed, and fatigue, may be similar to those of older women without cancer, but may begin at an earlier age (Clough-Gorr, Stuck, Thwin, & Silliman, 2010; Klepin et al., 2010; Maccormick, 2006). A useful approach may be to consider the long-term effects of cancer and cancer treatment as accelerated aging or early-onset frailty (Maccormick, 2006).
Frailty is an overall weakened physiological state, usually associated with advanced age. In 2001, a measureable Frailty Phenotype model was proposed by Fried and colleagues that has been widely adopted in geriatric research and practice. The Frailty Phenotype is a conceptual cycle of inactivity and increasing weakness that cascades into eventual disability and dependence. Fried et al. proposed five criteria to measure frailty and demonstrated that older adults with at least three of the five frailty criteria (unintentional weight loss, exhaustion, weakness, slow walking speed, and low physical activity) were at increased risk of worsening mobility, hospitalization, and death (Fried et al., 2001; Gill, Gahbauer, Han, & Allore, 2010). Frailty, measured by the Frailty Phenotype, has been strongly associated with older age, hospitalization, development of disability, reduced cardiac and pulmonary function, and reduced exercise capacity in older adults without cancer (Avila-Funes et al., 2008; Bandeen-Roche et al., 2006; Boyd, Xue, Simpson, Guralnik, & Fried, 2005; Fernandez-Bolanos et al., 2008; Santos-Eggimann, Cuenoud, Spagnoli, & Junod, 2009; Szanton, Seplaki, Thorpe, Allen, & Fried, 2010; Weiss, Hoenig, Varadhan, Simonsick, & Fried, 2010; Wong et al., 2010; Woo, Chan, Leung, & Wong, 2010).
It is not yet known whether cancer survivors are measurably more frail than other adults of the same age, because frailty has rarely been measured in cancer studies. However, there have been recent calls to include assessment of frailty in research studies and clinical care of cancer patients and survivors (Audisio & van Leeuwen, 2011; Bylow, Mohile, Stadler, & Dale, 2007; Maccormick, 2006; Monfardini & Basso, 2007; Pal, Katheria, & Hurria, 2010; Retornaz et al., 2008). To our knowledge, only one published study has reported frailty in cancer survivors. In that study, 8% of 71 men on androgen deprivation therapy (ADT) were frail, and 56% were prefrail (had one or two criteria for frailty) (Bylow et al., 2011).
The purpose of this study was to measure frailty in 216 older BCS using the five criteria of the Frailty Phenotype. To provide a context for our findings, we present published data on frailty from older women in the Cardiovascular Health Study (CHS) and Women’s Health and Aging Study (WHAS) and a regression model of our BCS data to evaluate characteristics associated with frailty.
Methods
Participants and Procedures
For this cross-sectional descriptive study, data of older BCS from two exercise intervention trials were analyzed. Data were collected between 2006 and 2010, in BCS prior to beginning the exercise intervention. Women for both trials were recruited through the Oregon State Cancer Registry, referral by clinical providers, recruitment at breast cancer events, advertisements, and community information sessions. Detailed descriptions of the samples and procedures have been published elsewhere (Loprinzi, Cardinal, Si, Bennett, & Winters-Stone, 2012; Winters-Stone et al., 2011). Both samples consisted of BCS over age 50, interested in participation in an exercise study but not currently participating in resistance exercise (Winters-Stone et al., 2011) or not currently participating in both resistance and aerobic exercise (Loprinzi et al., 2012). BCS in both studies were screened for eligibility and invited to an appointment at study site where they were enrolled and completed a self-report questionnaire and underwent performance tests of physical functioning and other clinical measures.
We combined baseline data because both studies had the same measures of criteria for frailty and other variables and both samples consisted of older BCS. There were some differences between the two samples, as shown in Table 1, but combining the samples was advantageous because it broadened the range of some variables, such as age, physical activity and survival time in the combined sample. The combined sample of 216 BCS were aged 53–87, were generally healthy but inactive, and reported few difficulties in activities of daily living. Most breast cancers were stages I and II with mean completion of chemotherapy or radiation approximately 5–7 years earlier.
Table 1.
Characteristics of two samples of breast cancer survivors from prior studies and combined sample for this study
| Characteristic | Sample 1 (n=105)
|
Sample 2 (n=111)
|
Combined sample (n=216)
|
*p |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | ||
| Age (years) | 62.3 (6.7) | 71.0 (5.1) | 66.7 (7.4) | .00 |
| range:53–83 | range: 64–87 | 53–59: n=44 | ||
| 60–64: n=28 | ||||
| 65–70: n=82 | ||||
| 71–74: n=33 | ||||
| 75–87: n=29 | ||||
| Race (%) | ||||
| Caucasian | 96.2 | 98.2 | 97.2 | |
| African American or Black | 1.0 | 0.0 | 0.5 | |
| Other | 2.9 | 1.8 | 2.3 | |
| Education (%) | ||||
| Less than high school | 0.0 | 0.9 | 0.5 | |
| High school graduate/GED | 26.4 | 29.1 | 27.9 | |
| Associate/technical degree | 8.8 | 18.2 | 13.9 | |
| Bachelor’s degree | 38.5 | 27.3 | 32.3 | |
| Advanced degree | 26.4 | 24.5 | 25.4 | |
| Marital Status (%) | ||||
| Married/Partnered | 56.2 | 59.1 | 57.7 | |
| Divorced/Separated | 26.7 | 21.8 | 24.2 | |
| Widowed | 9.5 | 15.5 | 12.6 | |
| Single | 7.6 | 3.6 | 5.6 | |
| BMI (kg/m2) | 29.5 (5.7) | 29.2 (5.8) | 29.4 (5.8) | |
| Comorbidity (%) | ||||
| 0–2 (not to mildly ill) | 74.3 | 64.5 | 69.3 | |
| 3–4 (moderately ill) | 19.0 | 27.3 | 23.3 | |
| >4 (severely ill) | 6.7 | 8.2 | 7.4 | |
| Difficulty in > 1 ADL (%) | 4.9 | 6.4 | 5.6 | |
| Usual physical activity (kcals/wk) | 1632.3 (1643.3) | 895.3 (1045.0) | 1255.2 (1415.2) | .00 |
| Breast cancer stage (%) | ||||
| Stage 0 | 6.2 | 13.0 | 9.8 | |
| Stage I | 43.3 | 49.1 | 46.3 | |
| Stage II | 45.3 | 29.6 | 37.0 | |
| Stage III | 5.2 | 8.3 | 6.8 | |
| Months since cancer diagnosis | 60.6 (37.9) | 87.1 (45.3) | 74.1 (44.0) | .00 |
| Received chemotherapy (%) | 60.0 | 45.0 | 52.6 | .03 |
| Received radiation therapy (%) | 88.6 | 85.6 | 87.0 | |
| Months since chemotherapy completion | 52.0 (34.1) | 92.8 (46.0) | 70.1 (44.6) | .00 |
| Months since radiation completion | 53.5 (36.1) | 83.6 (42.4) | 68.6 (42.1) | .00 |
| Currently taking AI (%) | 41.7 | 27.5 | 34.4 | |
| Currently taking SERM (%) | 15.5 | 1.8 | 8.5 | |
Note: ADL: Activity of daily living; AI: Aromatase inhibitor; SERM: selective estrogen receptor modulator
p values reported for significant differences between samples 1 and 2: p<.05
Measures of frailty
Frailty was measured using the components of the Frailty Phenotype (Fried et al., 2001), which consists of five criteria: shrinking, exhaustion, low activity, slowness, and weakness. BCS with three or more criteria were classified as frail, those with one or two criteria were classified as prefrail (Fried et al., 2001), and those with no criteria were classified as robust. The criteria were measured as follows:
Shrinking: appendicular lean mass measured dual energy x-ray absorptiometry (DXA, Hologic QDR Discovery Wi, software v.12), adjusted for height and fat mass using linear regression residual ≤ −1.73. Cutpoint was based on the lowest 20th percentile identified in 2984 well-functioning older adults in the Health, Aging and Body Composition Study (HABC) study (Newman et al., 2003).
Exhaustion: self-reported score on SF-36 Vitality Scale (Ware, Kosinski, & Gandek, 2000) less than 45.85 (normed) for BCS aged 50–74 years or less than 42.73 (normed) for BCS aged 75+ years. Cutpoints were lowest quartile of scale in general U.S. population (Ware, 2005).
Low activity: kcals per week in moderate-vigorous intensity activity measured by the Community Healthy Activities Model Program for Seniors (CHAMPS) physical activity questionnaire (Stewart et al., 2001). Less than 270 kcal/week was selected as the cutpoint to conform to cutpoint used by Fried et al. for the Phenotype of Frailty (Fried et al., 2001).
Slowness: walking four meters at < 1 meter/second using fastest time recorded from two attempts. Cutpoint chosen based on findings in the HABC study of older adults that walking speed <1m/sec predicted mortality (Cesari et al., 2005).
Weakness: grip strength measured by handgrip dynamometry (Tanaka Instr., Tokyo Japan) ≤ 17 kg for BCS with BMI ≤ 23, ≤ 17.3 kg for BCS with BMI 23.1–26, ≤ 18 kg for BCS with BMI 26.1–29, or ≤ 21 kg for BCS with BMI > 29. Cutpoints were selected to conform to cutpoints used by Fried et al. and Bandeen-Roche et al. (Bandeen-Roche et al., 2006; Fried et al., 2001).
Measures of participant characteristics
Participant characteristics (age, race, education, and marital status) were measured by written answers to items on the study questionnaire. Cancer type, dates, stage, treatments, and current use of an aromatase inhibitor or a selective estrogen receptor modulator were also self-reported. Other variables were measured by study staff using clinical measures, performance tests, and DXA. Comorbidity was measured by self-reported items on the Charlson Comorbidity Index (Charlson, Pompei, Ales, & MacKenzie, 1987). Cancer was included in the comorbidity measure only if it was treated less than five years ago or had metastasized. Comorbidity was categorized according to the Charlson weighted scores as score 0–2 not or mildly ill, 3–4 moderately ill, or >4 severely ill. Disability was measured by difficulty in activities of daily living measured by a sum of “yes” answers to difficulty in six items on the Personal Role Domain scale, part of the disability component of the Late-Life Function and Disability Instrument (Jette et al., 2002). The items asked about ability to take care of house, finances, own health, personal care, local errands, and meal preparation. Physical activity was measured by the self-report CHAMPS questionnaire (Stewart et al., 2001) of frequency and duration of various activities and answers were transposed to kcal per week using CHAMPS protocols.
Comparison to published data from CHS and WHAS studies
Participants in the CHS study were a large sample (N= 5317) of community-dwelling older adults whose data, collected in 1989–90 were used to validate the original Frailty Phenotype (Fried et al., 2001). Subsequently, a subsample of 1741 older women aged 70–79 from CHS was assessed for frailty criteria and compared with a sample of 786 older women aged 70–79 from WHAS (Bandeen-Roche et al., 2006). These two samples of older community-living women had a broad range of socioeconomic, functional, and health status, and thus provide a reasonable comparison for frailty status with older BCS. CHS and WHAS data were previously published (Bandeen-Roche et al., 2006; Fried et al., 2001) and permission was obtained to reproduce a comparison table from a prior study (Bandeen-Roche et al., 2006).
Statistical analysis
Descriptive statistics were used to characterize the sample and compare BCS with CHS and WHAS data. Robust, prefrail, and frail groups in the BCS sample were compared using Analysis of Variance (ANOVA) with least significant difference post-hoc analysis between pairs. Logistic regression models using SPSS (v. 20) were constructed to evaluate associations among sample characteristics and frailty status. Independent variables were entered in one block (age, BMI, physical activity, disability, comorbidity, prior chemotherapy, prior radiation treatment, current AI therapy, and current SERM therapy) in a logistic regression model with frailty as the condition of interest, compared to robust and prefrail combined. Physical activity was included as an independent variable in logistic regression models in its full continuous variation, because it was not collinear with the dependent variable frailty (r = −.25) even though a low cutpoint of physical activity was one of the five criteria of frailty.
Results
Prevalence of frailty in BCS and comparison of characteristics of robust, prefrail, and frail groups of BCS
The criteria for frailty were met by 12.5% (n=27) and the criteria for prefrailty were met by 50% (n=108) of 216 BCS. Table 2 shows personal characteristics and cancer variables in BCS who were robust (no frailty criteria), prefrail, and frail. The prefrail and frail BCS were similar in most characteristics, though frail BCS had significantly higher BMI and lower levels of usual activity compared to both prefrail and robust BCS. Younger age of robust BCS was significantly different from ages of both prefrail and frail BCS. Categories of comorbidity were in expected direction, with least illness in robust BCS, followed by prefrail BCS and frail BCS.
Table 2.
Baseline characteristics of breast cancer survivors (BCS) in three frailty groups
| Characteristic | Robust (n=81)
|
Prefrail (n=108)
|
Frail (n=27)
|
*p |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | ||
| Age (yrs) | 66.7 (7.1) | 67.6 (7.6) | 69.3 (6.1) |
a: <.01 b: <.01 |
| BMI (kg/m2) | 27.4 (4.8) | 29.6 (5.2) | 34.5 (7.4) |
a: <.01 b: <.01 c: <.01 |
| Comorbidity (Charlson Comorbidity Index) (%) | ||||
| 0–2 (not to mildly ill) | 84.0 | 63.9 | 46.2 |
a: <.01 b: <.01 |
| 3–4 (moderately ill) | 9.9 | 28.7 | 42.3 | |
| >4 (severely ill) | 6.2 | 7.4 | 11.5 | |
| Difficulty in > 1 ADL (%) | 1.2 | 9.4 | 3.8 | |
| Usual physical activity (kcals/wk) | 2033.0 (1357.5) | 901.9 (1316.4) | 299.7 (631.7) |
a: <.01 b: <.01 c: .03 |
| Breast cancer stage (%) | ||||
| Stage 0 | 5.1 | 12.9 | 12.0 | |
| Stage I | 53.2 | 44.6 | 32.0 | |
| Stage II | 39.2 | 34.7 | 40.0 | |
| Stage III | 2.6 | 7.9 | 16.0 | |
| Months since cancer diagnosis | 80.2 (48.8) | 68.9 (38.0) | 76.8 (48.6) | |
| Received chemotherapy (%) | 61.2 | 47.2 | 48.1 | |
| Received radiation therapy (%) | 84.0 | 89.8 | 85.2 | |
| Months since chemotherapy completion | 73.2 (47.4) | 65.9 (42.1) | 74.6 (45.0) | |
| Months since radiation completion | 73.8 (46.0) | 64.3 (37.1) | 71.9 (49.5) | |
| Currently taking AI (%) | 35.0 | 36.4 | 24.0 | |
| Currently taking SERM (%) | 13.8 | 6.5 | 0 |
Note: ADL: Activities of daily living; AI: Aromatase inhibitor; SERM: selective estrogen receptor modulator
Signifies p-value of significant difference between Robust and Prefrail
Signifies p-value of significant difference between Robust and Frail
Signifies p-value of significant difference between Prefrail and Frail
Frailty in BCS compared to older women in CHS and WHAS
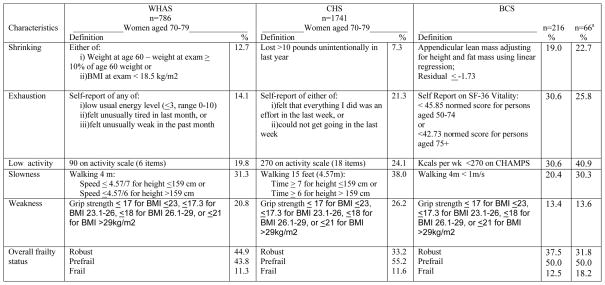
The measures of each frailty criterion in the three groups (BCS, women in CHS, and women in WHAS) are shown in Figure 1, along with the proportion of women in each group who met each criterion. The BCS were younger (65% were younger than 70 years) than CHS and WHAS women (aged 70–79), yet the proportion of BCS who were frail (12.5%) is similar to the proportions in CHS and WHAS (11.6% and 11.3% respectively). In 66 BCS who were same age as CHS and WHAS women (70–79 years), frailty was higher in BCS (18.2%) than in CHS women (11.6%) and WHAS women (11.3%). Prefrail status in women aged 70–79 years was similar in the three groups, lowest in WHAS women (43.8%) followed by BCS (50.0%) and CHS women (55.2%).
Figure 1.
Comparison of prefrailty and frailty among BCS, women in WHAS and women in CHS.
aBCS aged 70–79 years
Notes: BCS = Breast cancer survivors; WHAS = Women’s Health and Aging Study; CHS = Cardiovascular Health Study; BMI = body mass index
WHAS and CHS portion of table previously published and used with permission from Oxford University Press. Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P et al. Phenotype of Frailty: Characterization in the Women’s Health and Aging Studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–6.
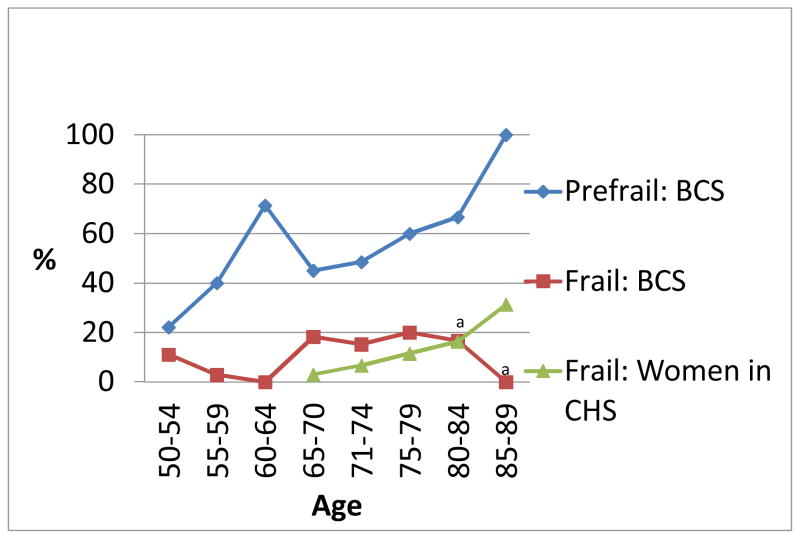
Age may be an important factor in many aspects of cancer survivorship including onset of frailty (Lichtman, 2012). To describe the data more fully in terms of age, prefrail and frail status are shown in five-year age ranges in Figure 2, for BCS (age range 53–87) compared to women in CHS (age range 65–90) (Fried et al., 2001). Data were not available in five year increments for WHAS women. Frailty was more common at a younger age (18% of BCS aged 65–70 were frail, compared to 3% of CHS women), while the percentage of frail CHS women began to increase at an older age and increased gradually with increasing age. BCS had higher proportions of frailty in all age groups until age 80–84 when proportion of frailty in BCS and CHS were approximately equal. The BCS sample had only 9 women aged above 80 years, so comparisons at older ages are not likely to be accurate. Prefrailty was high in BCS as shown in Figure 2, but comparison data on prefrailty in CHS were not available in five-year increments.
Figure 2.
Proportion of frailty and prefrailty by age in breast cancer survivors and women in the Cardiovascular Health Study
Note: Data in five-year increments was not available for prefrail women in CHS or for frail or prefrail women in Women’s Health and Aging Study (WHAS)
Abbreviations: BCS: Breast cancer survivors; CHS: Cardiovascular Health Study
Prefrail: meets 1–2 of 5 frailty criteria; Frail: meets 3 of 5 frailty criteria
an=6 BCS aged 80–84, n=3 BCS aged 85–89
Characteristics associated with frailty in BCS
In a logistic regression model to predict odds of being frail/prefrail compared to robust, BCS with higher BMI (OR= 1.12, p=.003, 95% CI: 1.04, 1.19) and lower physical activity levels (OR=.99, p=.000, 95% CI: .99,1.00) were more likely to be frail or prefrail. All other variables in the model (age, disability, comorbidity, chemotherapy, radiation therapy, current SERM [selective estrogen receptor modulator], current AI [aromatase inhibitor]) were not significantly associated with likelihood to be frail or prefrail.
Discussion
Using criteria for a phenotype of frailty developed and validated in older adults (Fried et al., 2001), our findings suggest that BCS may become frail at an earlier age than older women without cancer. In this study, a greater proportion of younger BCS were frail than similarly aged women without cancer (18% of BCS aged 65–70 were frail, compared to 3% of CHS women) and frailty affected more BCS between ages 70–79 (18% compared to 11–12% of CHS and WHAS women). This finding is remarkable in the context of this particular sample of BCS, a healthy group who signed up to begin a 12-month exercise studies and who reported low levels of comorbidity and disability in ADL. Frailty may be even more prevalent in BCS who are less healthy.
An important finding was the high level of prefrailty across the age ranges of BCS, especially the continuous increase in prevalence of prefrailty after age 70. Prefrailty, measured as having one or two frailty criteria, may be an early sign of impending frailty (Bylow et al., 2007). We do not know if the age of onset of prefrailty or the steep increase in prevalence in older BCS is unusual because age data on prefrailty in WHAS and CHS have not been published. However, 20% prefrailty in BCS aged 50–54 increasing to 45% prefrailty in BCS aged 71–74 warrants further investigation. Longitudinal data on frailty and age would be clinically useful; if prefrailty predicts later frailty and occurs at an earlier age in cancer survivors, reasonably simple performance tests, such as walking speed, may predict early onset frailty that could be prevented.
Frailty describes a seriously debilitated physical state, or syndrome, that has been shown to increase with age in older adults without cancer (Rockwood, 2005). Prior studies have shown a strong association of frailty, albeit measured in different ways, with adverse outcomes for older adults without cancer, including falls, fractures, hospitalization, disability, nursing home admission, reduced mobility, and shorter time until death (Bandeen-Roche et al., 2006; Boyd et al., 2005; Ensrud et al., 2008; Fried, Ferrucci, Darer, Williamson, & Anderson, 2004; Fried et al., 2001; Rockwood et al., 2004). If frailty occurs sooner or more frequently in BCS, it is likely that the same adverse effects also may occur more frequently or at a younger age. This study provides the first data that frailty and prefrailty may indeed be common in BCS and may occur at an earlier age than in adults without a history of cancer.
Exhaustion, low activity, and shrinking were the most frequent criteria of frailty found in BCS in this study, while slowness and weakness were the most frequent criteria of frailty in women in WHAS or CHS. It is possible that the differences in these components are related to symptoms of cancer treatment. For example, fatigue is often reported as a long-term symptom by BCS, and may be the reason for the exhaustion component being more common in BCS than in women without cancer. However, our data did not show an association between cancer treatments and frailty in regression models. Future studies of frailty in cancer survivors could measure other potential factors associated with early frailty, such as persistent neuropathies, reduced voluntary activity due to habits formed during illness, fear of lymphedema, or other factors.
Our finding that 19% of BCS had evidence of shrinking may be due to actual reduced muscle mass (sarcopenia) in BCS, or may be due to more accurate measurement of sarcopenia in this study than in WHAS or CHS. In this study, sarcopenia was measured by lean mass adjusted for height and fat mass, a more accurate measure than a simple self-report of lost weight as in the WHAS and CHS studies. Adjusted lean mass was shown to be more accurate in detecting sarcopenia, especially in obese individuals, in the HABC study (Newman et al., 2003). The accuracy of adjusted lean mass to measure sarcopenia may be especially important in cancer survivors. In a study of 71 prostate cancer survivors on ADT, self-reported weight loss did not indicate frailty, but when BMI >30 was substituted for shrinking, 9% of survivors were “obese frail” and 56% were “obese prefrail”(Bylow et al., 2011). Our finding that frail BCS had significantly higher mean BMI (mean 34.5) than prefrail or robust BCS indicates that obesity, as well as shrinking or weight loss, may be a risk factor for frailty in cancer survivors (Bylow et al., 2011). The logistic regression model in this study showed that each one unit increase in BMI increased the odds of being frail by 12%. Sarcopenic obesity, which develops when fat mass is disproportionate to muscle mass, has been associated with chemotherapy, chemotherapy-induced ovarian failure, and tamoxifen therapy in women cancer survivors, including premenopausal women (Costa, Varella, & del Giglio, 2002; Demark-Wahnefried et al., 2001; Demark-Wahnefried, Rimer, & Winer, 1997; Demark-Wahnefried, Winer, & Rimer, 1993; Hoskin, Ashley, & Yarnold, 1992). Consideration of both shrinking and obesity in the context of sarcopenia may be important in future studies of frailty and functioning among cancer survivors.
The finding that increased physical activity reduced the odds of frailty is in the expected direction. A one unit increase in caloric expenditure per week is very small, with a correspondingly small (less than 1%) decrease in odds of frailty. The significant odds ratio should be interpreted with caution because self-report of physical activity is only generally accurate. Thus, the small association shown by the odds ratio may not be valid. Instead, the general finding that physical activity reduced the risk for frailty when other variables were controlled may provide a rationale for future studies focused on this potential protective factor.
This study was limited by the characteristics of the samples from our prior studies. In general, BCS in this study were healthy and willing to undertake a lengthy exercise study. It is possible that frailty may be even more common in BCS who are not as healthy and well-functioning. On the other hand, frailty may be less common in BCS who exercise regularly. Some have proposed that cancer treatments and cancer symptoms may cause early-onset frailty in survivors of cancer (Bylow et al., 2007; Maccormick, 2006), but perhaps the long-term survivors in this study were too far beyond treatment to show an association with particular risk factors, such as type of treatment. The associations between frailty, obesity, and low physical activity may be especially relevant to cancer survivors and warrant further study. Though our data only describe and do not establish cause, this study is among the first to measure criteria for frailty in cancer survivors. The findings provide preliminary evidence that early-onset frailty as a result of cancer treatment, hypothesized by Maccormick in 2006 (Maccormick, 2006), may be a reality for many BCS.
Implications for Nursing
Gerontological nurses are familiar with the multi-component syndrome of frailty and usually assess for signs of frailty in older women without cancer, especially those over age 80. However, oncology nurses may not be thinking about frailty when evaluating the well-being of BCS, especially those who are younger than 80. Our findings suggest that nurses should consider the possibility of frailty, and especially prefrailty, at a younger-than-expected age in BCS. Recognition of prefrailty may be especially important because awareness and early intervention may delay or prevent frailty. More knowledge about the prevalence and causes of frailty in BCS, and perhaps in survivors of other cancers, could be gained if nurse scientists include measures of the components of the frailty phenotype in future research among cancer survivors, even if frailty is not the focus of the study.
Implications for Nursing.
Nurses should be alert to prefrailty or frailty at a younger age in BCS. Awareness and early intervention may delay or prevent frailty.
Knowledge Translation.
There is potential for BCS to be frail even when they are not yet “elderly”
Prefrailty in BCS is important to recognize because it suggests impending frailty that could lead to reduced physical functioning or poor health
Prefrailty and frailty could be assessed in BCS over age 50 in a clinical setting using a few questions about weight, fatigue, and activity levels plus, if warranted, simple tests of walking speed and grip strength
Acknowledgments
Funding support from the Susan G. Komen For the Cure Foundation and the National Cancer Institute (1R01 CA120123)
References
- Audisio RA, van Leeuwen B. When reporting on older patients with cancer, frailty information is needed. Ann Surg Oncol. 2011;18(1):4–5. doi: 10.1245/s10434-010-1327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Funes JA, Helmer C, Amieva H, Barberger-Gateau P, Le Goff M, Ritchie K, Dartigues JF. Frailty among community-dwelling elderly people in France: the three-city study. J Gerontol A Biol Sci Med Sci. 2008;63(10):1089–1096. doi: 10.1093/gerona/63.10.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Fried LP. Phenotype of Frailty: Characterization in the Women’s Health and Aging Studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- Boyd CM, Xue QL, Simpson CF, Guralnik JM, Fried LP. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005;118(11):1225–1231. doi: 10.1016/j.amjmed.2005.01.062. [DOI] [PubMed] [Google Scholar]
- Bylow K, Hemmerich J, Mohile SG, Stadler WM, Sajid S, Dale W. Obese frailty, physical performance deficits, and falls in older men with biochemical recurrence of prostate cancer on androgen deprivation therapy: a case-control study. Urology. 2011;77(4):934–940. doi: 10.1016/j.urology.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylow K, Mohile SG, Stadler WM, Dale W. Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer?: A conceptual review. Cancer. 2007;110(12):2604–13. doi: 10.1002/cncr.23084. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report/Cancer Survivors-United States 2007. 2011 [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, Pahor M. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53(10):1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Clough-Gorr KM, Stuck AE, Thwin SS, Silliman RA. Older breast cancer survivors: Geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up. J Clin Oncol. 2010;28(3):380–386. doi: 10.1200/JCO.2009.23.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LJ, Varella PC, del Giglio A. Weight changes during chemotherapy for breast cancer. Sao Paulo Med J. 2002;120(4):113–117. doi: 10.1590/S1516-31802002000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, Rimer BK. Changes in Weight, Body Composition, and Factors Influencing Energy Balance Among Premenopausal Breast Cancer Patients Receiving Adjuvant Chemotherapy. J Clin Oncol. 2001;19(9):2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Rimer BK, Winer EP. Weight gain in women diagnosed with breast cancer. J Am Diet Assoc. 1997;97(5):519–526. doi: 10.1016/s0002-8223(97)00133-8. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Winer EP, Rimer BK. Why women gain weight with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1993;11(7):1418–1429. doi: 10.1200/JCO.1993.11.7.1418. [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Cummings SR. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168(4):382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- Fernandez-Bolanos M, Otero A, Zunzunegui MV, Beland F, Alarcon T, de Hoyos C, Castell MV. Sex differences in the prevalence of frailty in a population aged 75 and older in Spain. J Am Geriatr Soc. 2008;56(12):2370–2371. doi: 10.1111/j.1532-5415.2008.02032.x. [DOI] [PubMed] [Google Scholar]
- Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of Disability in the Last Year of Life. N Engl J Med. 2010;362(13):1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin PJ, Ashley S, Yarnold JR. Weight gain after primary surgery for breast cancer--effect of tamoxifen. Breast Cancer Res Treat. 1992;22(2):129–132. doi: 10.1007/BF01833342. [DOI] [PubMed] [Google Scholar]
- Jette AM, Haley SM, Coster WJ, Kooyoomjian JT, Levenson S, Heeren T, Ashba J. Late life function and disability instrument: I. Development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci. 2002;57(4):M209–216. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- Klepin HD, Geiger AM, Tooze JA, Newman AB, Colbert LH, Bauer DC, Kritchevsky SB. Physical performance and subsequent disability and survival in older adults with malignancy: Results from the Health, Aging and Body Composition study. J Am Geriatr Soc. 2010;58(1):76–82. doi: 10.1111/j.1532-5415.2009.02620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman SM. Call for changes in clinical trial reporting of older patients with cancer. J Clin Oncol. 2012;30(8):893–894. doi: 10.1200/JCO.2011.41.0696. [DOI] [PubMed] [Google Scholar]
- Loprinzi PD, Cardinal BJ, Si Q, Bennett JA, Winters-Stone KM. Theory-based predictors of follow-up exercise behavior after a supervised exercise intervention in older breast cancer survivors. Support Care Cancer. 2012 doi: 10.1007/s00520-011-1360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: A cause of early onset frailty? Med Hypotheses. 2006;67(2):212–215. doi: 10.1016/j.mehy.2006.01.045. [DOI] [PubMed] [Google Scholar]
- Monfardini S, Basso U. Oncological causes of frailty in older cancer patients. Eur J Cancer. 2007;43(8):1230–1231. doi: 10.1016/j.ejca.2007.02.004. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. SEER: Surveillance, Epidemiology, and End Results. Retrieved March 1, 2011, from National Cancer Institute http://seer.cancer.gov/statfacts/html/breast.html.
- Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Harris TB. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51(11):1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60(2):120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- Retornaz F, Monette J, Batist G, Monette M, Sourial N, Small D, Bergman H. Usefulness of frailty markers in the assessment of the health and functional status of older cancer patients referred for chemotherapy: a pilot study. J Gerontol A Biol Sci Med Sci. 2008;63(5):518–522. doi: 10.1093/gerona/63.5.518. [DOI] [PubMed] [Google Scholar]
- Rockwood K. Frailty and its definition: A worthy challenge. J Am Geriatr Soc. 2005;53(6):1069–1070. doi: 10.1111/j.1532-5415.2005.53312.x. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Howlett SE, MacKnight C, Beattie BL, Bergman H, Hebert R, McDowell I. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian Study of Health and Aging. J Gerontol A Biol Sci Med Sci. 2004;59(12):1310–1317. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64(6):675–681. doi: 10.1093/gerona/glp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS Physical Activity Questionnaire for Older Adults: Outcome for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Szanton SL, Seplaki CL, Thorpe RJ, Jr, Allen JK, Fried LP. Socioeconomic status is associated with frailty: the Women’s Health and Aging Studies. J Epidemiol Community Health. 2010;64(1):63–67. doi: 10.1136/jech.2008.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE. How to Score the Version 2 of the SF-36 Health Survey. Vol. 1998. Boston, MA: The Health Institute, New England Medical Center; 2005. [Google Scholar]
- Ware JE, Kosinski M, Gandek B. SF-36 Health Survey: Manual & Interpretation Guide. Lincoln, RI: Qualitymetric; 2000. [Google Scholar]
- Weiss CO, Hoenig HH, Varadhan R, Simonsick EM, Fried LP. Relationships of cardiac, pulmonary, and muscle reserves and frailty to exercise capacity in older women. J Gerontol A Biol Sci Med Sci. 2010;65(3):287–294. doi: 10.1093/gerona/glp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters-Stone KM, Dobek J, Nail L, Bennett JA, Leo MC, Naik A, Schwartz A. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res Treat. 2011;127(2):447–456. doi: 10.1007/s10549-011-1444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Weiss D, Sourial N, Karunananthan S, Quail JM, Wolfson C, Bergman H. Frailty and its association with disability and comorbidity in a community-dwelling sample of seniors in Montreal: a cross-sectional study. Aging Clin Exp Res. 2010;22(1):54–62. doi: 10.3275/6675. [DOI] [PubMed] [Google Scholar]
- Woo J, Chan R, Leung J, Wong M. Relative contributions of geographic, socioeconomic, and lifestyle factors to quality of life, frailty, and mortality in elderly. PLoS One. 2010;5(1):e8775. doi: 10.1371/journal.pone.0008775. [DOI] [PMC free article] [PubMed] [Google Scholar]