Abstract
This paper reviews a number of studies in oral biology and endodontics that deal with the reactivity of the pulpo-dentine complex in response to mechanical and immunological stimuli. It can be hypothesized that these reactions could also apply to changes in dentine sensitivity following periodontal procedures. Some of these changes involve neurogenic inflammation of the pulp under exposed open tubules; this increases the rate of outward fluid flow through the tubules, making the overlying exposed dentine more sensitive. Other changes may be due to inflammation-related nerve sprouting of pulpal nerves, which can lead to innervation ofmore tubules than normal. Changes may also involve upregulation of new, more sensitive ion channels in the membranes of these nerves.
The goal of the paper is to increase awareness of the issues involved in dentine sensitivity, so that future investigators may develop agents or techniques to stimulate mechanisms that mitigate dentine sensitivity or to block mechanisms that aggravate the condition, for therapeutic effect.
Keywords: Dentine sensitivity, pulpal reaction
1. Introduction
According to the hydrodynamic theory of dentine sensitivity, fluid-filled dentinal tubules transmit tactile, osmotic, thermal and evaporative stimuli to the pulpal nerves, which are all interpreted as pain.1,2 A corollary of the theory is that anything which increases dentine fluid shifts in response to hydrodynamic stimuli should increase dentine sensitivity. The term “dentine sensitivity” should be used to describe the sensation experienced by a patient when they become aware that their previously insensitive dentine has become sensitive. The term “hypersensitive dentine” should only be used when the patient describes a significant increase in their dentine sensitivity, ie, hypersensitive dentine is supersensitive with respect to the patient’s previous experience.
Dentinal tubules extend from the pulp to the enamel in coronal dentine or to the cementum in root dentine, and they are filled with a transudate of pulpal fluid. As they are closed at their peripheral ends with enamel or cementum, dentinal fluid is sterile. The only fluid shifts in normal dentine occur in response to extreme hot or cold stimuli.
Although the pulpal tissue pressure is about 15 cm H2O (1.47 kPa) higher than ambient oral pressure,3 the hydraulic resistance offered by enamel or cementum is so high that no outward fluid movement has ever been reported in normal, sealed dentine. However, as soon as the enamel or cementum peripheral seals of dentine are destroyed, some dentinal fluid can slowly seep out of the dentine into the oral cavity. Exposure of root dentine to the oral cavity usually occurs because of gingival recession,4 periodontal disease, improper tooth brushing, overuse of toothpastes5, etc. However, many desensitising toothpastes (and other desensitising therapies) aim to occlude dentinal tubules and reduce dentine permeability.6–10
If curettes are used to remove calculus from root surfaces, it is likely that they will also remove the thin layer of cementum (20–40 μm) and leave smear layer-like debris, combined with blood and/or saliva, covering the exposed dentine. Pashley and Tay have shown that dentinal fluid can slowly seep across smear layers (Fig. 1)11 but smear layers are responsible for 86% of the total resistance to fluid flow across dentine.12 Generally, smear layers prevent patients from experiencing dentine sensitivity because the smear layer debris that is forced down into each dentinal tubule during the creation of smear layers (Fig. 2)13 seals the tubules even better than the resin tags in resin-bonded dentine.14 The presence of smear layers/smear plugs in cervical dentine is thought to be the reason why some patients who have had deep scaling and root surface debridement often have little initial dentine sensitivity even though root dentine is exposed. We now know that those smear layers rapidly become colonised with bacteria, which quickly form biofilms that produce enough organic acids to solubilise the smear layers in 7–10 days (Fig 3).1,13 With the loss of smear layers/smear plugs, dentine tubules become hyperconductive and hence patients develop dentine sensitivity. However, the degree of sensitivity can increase much more, to a level that the patients will classify as “hypersensitive” with respect to their previous degree of dentine sensitivity.
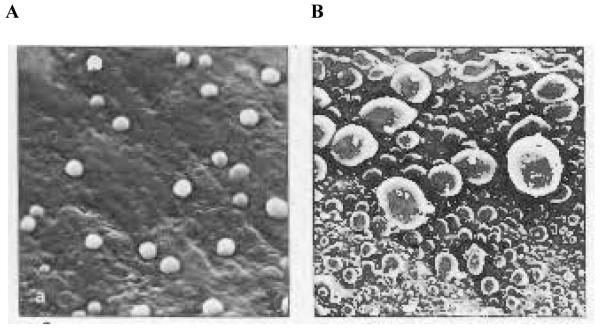
Fig. 1. Scanning electron micrographs of epoxy replicas of vital human dentine surfaces.
(A) Smear layer-covered dentine only allowed small amounts of dentinal fluid to permeate through smear layers in the 3–4 min required for the polyvinylsiloxane impression material to set. (B) Much more fluid was observed on the surface of acid-etched dentine free of smear layers.
Reproduced from Pashley and Tay,11 with kind permission from Quintessence Publishing Co.
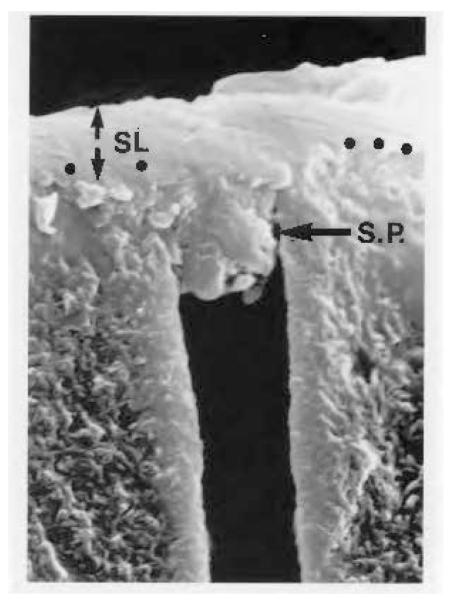
Fig. 2. Smear layer-covered human coronal dentine.
Smear layer (SL) debris slightly penetrates into the lumen of dentinal tubules to form a smear plug (SP).
Reproduced from Pashley,13 with kind permission from the Finnish Dental Society.
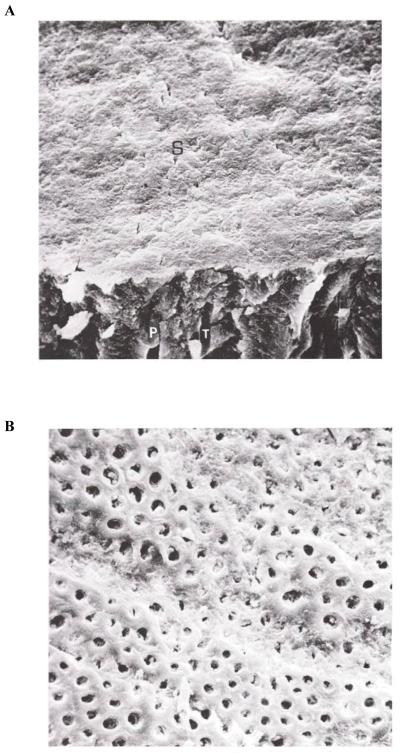
Fig. 3. Smear layer-covered human dentine.
(A) The fractured edge reveals the presence of smear plugs (P) in tubules (T). (B) The smear layer-covered dentine has been exposed to bacterial plaque for 1 week in vivo. Note the loss of the smear layer and the appearance of open dentinal tubules.
Reproduced from Brännström,1 with kind permission from Elsevier.
What mechanisms can cause such supersensitivity? Further increases in dentine permeability caused by further dentine loss could make that dentine even more conductive.15 The nerves responsible for dentine sensation are located in the pulp. If local pulpal inflammation develops, the local tissue pressure can increase. This would increase the outward flow of dentinal fluid and bring pulpal nerves closer to the threshold, thereby making them more sensitive than normal. If the localized pulpal inflammation causes those nerve endings to multiply (ie causes nerve sprouting), then more cervical dentinal tubules would become innervated and the sensitivity of the pulpo-dentine complex would increase even further.16
A recent systematic review of dentine sensitivity17 indicated that tooth sensitivity is high immediately after most periodontal surgical procedures, but that it decreases spontaneously over time.18,19 How can we explain the commonly observed decrease in dentine sensitivity over time in the absence of any additional therapy? Studies of the permeability properties of dentine in vivo have shown that dentine permeability is not constant, but tends to decrease over time in response to the presence of bacteria in oral fluids and/or pulpal inflammation. The evidence for this will be presented below.
This paper focuses mainly on responses of the pulpo-dentine complex to immunological or physical irritation and how that may modify patients’ perception of dentinal pain. It ignores many important aspects of pain, especially tooth pain. The highly subjective nature of the pain response is well recognised.20,21
The purpose of this paper is to demonstrate that the reactivity of the pulpo-dentine complex is greater than most clinicians realise and that it can increase or decrease dentine sensitivity. Hopefully, by raising awareness of the issues involved in dentine sensitivity, future investigators can attempt to stimulate or block these responses for therapeutic effect.
2. Background information
Although dentinal tubules have an internal diameter of approximately 1 μm, because they contain collagen fibres, calcium phosphate deposits, etc., hydrodynamically they behave as if their functional diameters are less than 0.1 μm. Pores with such a small diameter are similar to those in the Millipore filters that are used to remove bacteria from solutions.22 Indeed, Michelich and colleagues have shown that it is possible to sterilise bacterial suspensions by filtering them across dentine.23 Thus, although bacteria cannot enter the pulp via fluid-filled dentinal tubules, bacterial products, such as endotoxins and exotoxins, can easily dissolve in dentinal fluid and diffuse to the pulp, even though there is a slow outward fluid flow.24
Saliva contains not only many bacteria, but also large amounts of bacterial by-products that are recognised immunologically in the pulp as foreign, hazardous antigens. When coronal or root dentine is left exposed to the oral cavity, the dentine surfaces are quickly colonised by oral microorganisms that then dissolve the smear layer/smear plugs, thereby increasing the available surface area for bacterial products to diffuse into the pulp through these fluid-filled ‘pathways to the pulp’.
3. In vivo studies
Brännström was among the first to examine pulpal reactions to ground coronal dentine.25 These observations were made in children whose premolars were scheduled for extraction during orthodontic treatment. Within 1 week of exposing normal dentine the smear layers dissolved and the pulps became extremely inflamed (Figs. 4A and 4B).1 The subjects originally reported mild dentine sensitivity to probing or air blasts, but after 1 week were “hypersensitive” compared with their original level of sensitivity. This experiment demonstrated that normal human saliva can cause localised pulpal inflammation within 1 week of dentine exposure.
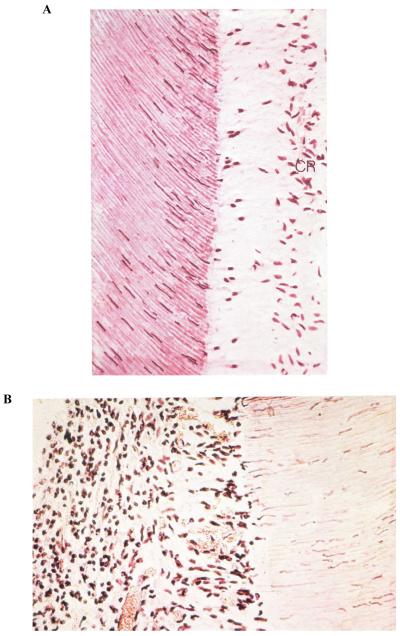
Fig. 4. Histologic appearance of young dental pulp.
(A) After evaporative air blast to exposed dentine surface in vivo. Note the loss of the odontoblastic cell bodies and the appearance of their nuclei in the adjacent dentinal tubules. The cell-free zone is seen just above the cell-rich (CR) zone. There are no inflammatory cells in this pulp. (B) After exposed coronal dentine was left open to oral fluids for 1 week. The pulp is heavily inflamed. This dentine was very hypersensitive. Note the absence of an odontoblast layer and the cell-free and CR zones, and the presence of acute inflammatory cells throughout the pulp. A large dilated venule can be seen in the bottom of the field.
Reproduced from Brännström,1 with kind permission from Elsevier.
This work encouraged Lundy and Stanley to perform similar studies but over longer time periods in adults who had teeth scheduled for extraction for orthodontic, periodontal or prosthetic reasons.26 The investigators prepared class V cavities in the teeth and then measured dentine sensitivity, using dental explorers or air blasts, and tested pulpal sensitivity. The cavities were left unfilled and open to the oral environment. Within 5–12 days, most patients returned to report that those teeth had become very sensitive. Most of the patients regarded the affected teeth as being hypersensitive and the investigators confirmed the hypersensitivity by probing and air blasts. Some teeth were extracted for histological examination, as they had already been scheduled for extraction. Other teeth were not extracted but were followed for weeks to months. Most of the teeth that had been extremely sensitive were shown by histological studies to have severe, acute pulpal inflammation and 5/52 teeth even showed pulpal abscesses. However, those hypersensitive teeth that were not extracted became less sensitive over time. Within 2–4 weeks most of them became much less sensitive. It seemed as if sensitive teeth could spontaneously heal themselves even though the cavities remained open to oral fluids. When these teeth were extracted, the pulps exhibited chronic pulpal inflammation and none of them exhibited any irritation or reparative tertiary dentine formation (a process that usually takes 2–4 months and that can seal off tubules on the pulp side).
Lundy and Stanley concluded that somehow the permeability of dentine had decreased spontaneously.26 In 1969, there no mechanisms were known to decrease dentine permeability. Indeed, studies of dentine permeability did not start in earnest until 1976. It is important to remember that the pulpal tissue pressure in inflamed pulps increases from 15 cm H2O (1.47 kPa) to 40–50 cm H2O (3.92–4.90 kPa).27 This increases the outward dentinal fluid flow, making pulpal nerves more likely to fire in response to hydrodynamic stimuli.
4. In vitro studies
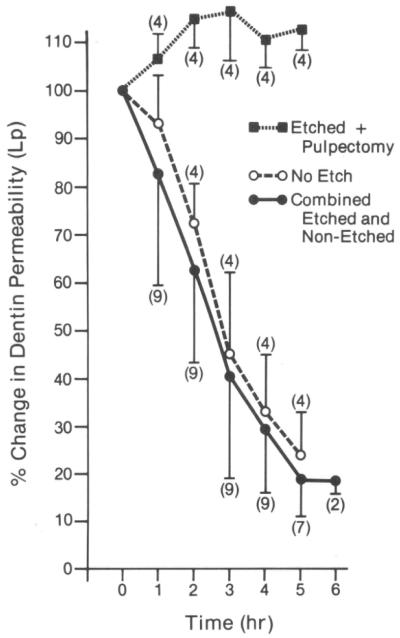
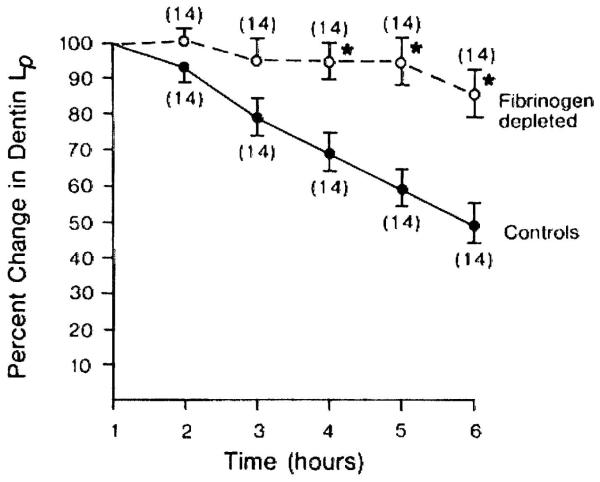
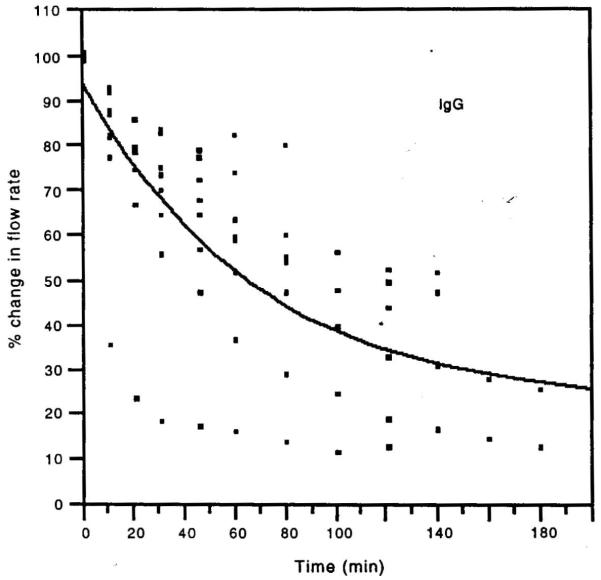
In 1983–1984, Pashley and colleagues reported that when cavities were prepared in dog teeth and the permeability evaluated every hour for 8 hours, the permeability of dentine fell by 15% per hour in vital teeth, but not in teeth that had undergone pulpotomy (Fig. 5).28 This occurred in the absence of bacteria or saliva. However, histology showed that the pulps of the vital teeth were inflamed. It was thought that cavity preparation plus repeated fluid infiltration across dentine into the pulp probably induced sufficient mechanical irritation to cause pulpal inflammation. The investigators suspected that plasma proteins were leaking from pulpal blood vessels into dentinal fluid and then diffusing into the tubules, where they reduced the functional diameter of dentinal tubules, resulting in decreases in dentine permeability. As fibrinogen is one of the largest plasma proteins (>300 KDa), the investigators treated an experimental group with a snake venom preparation that specifically destroys plasma fibrinogen. After multiple injections of this venom, the dogs’ plasma fibrinogen levels were shown to fall to near zero levels. When cavities were prepared in the teeth of these dogs, dentine permeability did not decrease significantly for 5 hours (Fig. 6), while the control dogs’ dentine permeability fell significantly.29 The investigators concluded that pulpal inflammation allows large plasma proteins to escape from pulpal blood vessels and diffuse into dentinal tubules, where they decrease dentine permeability. Others have shown that immunoglobulins (Igs) such as IgG can produce progressively lower dentine permeability over time (Fig. 7).30
Fig. 5. Changes in dentine permeability of dog dentine in vivo.
Dentine permeability increased in teeth that had coronal pulp tissue removed just before preparing class V cavities on the buccal surface (dotted line, square symbols). Dentine permeability decreased rapidly in vital teeth over time (solid line with round filled symbols). Numbers in parentheses are numbers of individual teeth studied.
Reproduced from Pashley et al.,28 with kind permission from Elsevier.
Fig. 6. Changes in dentine permeability in dog teeth with and without fibrinogen.
Teeth from fibrinogen-depleted dogs (treated with snake venom 2 weeks before the permeability assessment) showed higher, more constant dentine permeability over 6 hours than did teeth from dogs with normal fibrinogen plasma levels.
Lp, hydraulic conductivity
Reproduced from Pashley et al.,29 with kind permission from Elsevier.
Fig. 7. Permeability of dentine treated with immunoglobulin G (IgG) in vitro.
Dentinal permeability decreased during passage of 160 kDa IgG from the pulpal side of the dentine toward the occlusal side under a simulated normal pulpal pressure of 20 cm H2O.
Reproduced from Hahn and Overton,30 with kind permission from Elsevier.
5. Discussion
Does this endogenous mechanism operate in all patients who develop dentine hypersensitivity? One would think so from the evidence presented thus far. However, there are multiple changes that take place in inflamed pulps that make them so sensitive that they respond in a hypersensitive manner even though the permeability of dentine may be reduced by 50–75%. Trowbridge and Kim reported that inflammatory changes in the pulp decreased pain thresholds.31,32
The presence of bacterial antigens in the pulp causes the release of a host of mediators of inflammation (e.g. histamines, bradykinin, prostaglandins, neuropeptides, etc.). These mediators cause pulpal fibroblasts to begin dividing rapidly in an effort to repair the collagen in the pulpal connective tissue that is being destroyed by polymorphonuclear cell-derived matrix metalloproteinases. These fibroblasts express nerve growth factor, which stimulates nearby pulpal nerves to begin sprouting new branches (Fig 8).33–35 The nerves multiply, making the region more richly innervated than normal. In addition, receptors in the cell membranes of those sprouting nerves recognise bacterial antigens and inflammatory signals, leading to stimulation of the synthesis of additional, more sensitive sodium-channel proteins.36–38 These can be immune stained and visualised in pulpal tissue by confocal microscopy (Fig. 9).34,35
Fig. 8. Schematic diagram illustration the consequences of pulpal irritation.
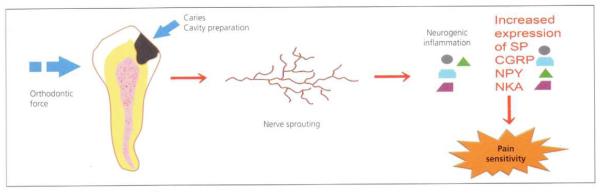
Regardless of source, pulpal irritation induces pulpal inflammation that causes nerve sprouting. Increased numbers of pulpal nerves release increased amounts of substance P (SP), calcitonin-gene-related peptide (CGRP), neuropeptide Y (NPY) and neurokinin A (NKA). These neuropeptides sustain neurogenic inflammation that contributes to maintaining sensitive dentine in a hypersensitive state.
Reproduced from Caviedes-Bucheli et al.,33 with kind permission from Elsevier.
Fig. 9. Confocal microscopy of pulpal nerves.
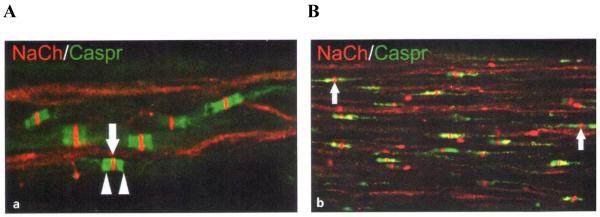
Sodium channels (NaCh; red) and Caspr (green) immunoreactions within pulpal nerves in (A) a normal pulp and (B) a painful pulp. Caspr identifies nodes of Ranvier in myelinated nerves. There are more unmyelinated nerves in painful (i.e. inflamed) than normal pulps.
Reproduced from Byers et al.,35 with kind permission from Quintessence Publishing Co.
Thus, bacterial substances from saliva and plaque – which can diffuse into the pulp and produce pulpal inflammation, elevated pulpal pressures, nerve sprouting, and more sensitive nerves, etc. – are responsible for continued dentine hypersensitivity in a subset of the population of patients who initially develop dentine sensitivity. Perhaps that dentine hypersensitivity is sustained because these patients have had multiple episodes of local pulpitis that healed by scar formation in the pulp. Scar tissue is avascular and would not leak as many large plasma proteins into dentinal fluid as normal pulp. Perhaps not all patient pulpal tissues react to bacterial products with the same degree of inflammation. There are many possibilities.
6. Concluding remarks
What are some solutions to this problem? Patients who have routine dentine sensitivity that is non-plaque related should continue to perform their usual oral hygiene. Patients who have truly supersensitive dentine should try to remove plaque from their teeth every day because mechanical and chemical plaque control seems to reduce dentine sensitivity in many patients. There is a need to be able to reduce dentine permeability from the outside of the tooth as well as from the inside. Advances in the development of effective dentinal tubule occlusion technologies should lead to sufficient occlusion of open, sensitive dentinal tubules, which will reduce the permeability of that dentine to the extent that bacterial antigens can no longer diffuse into the pulp. That should allow pulpal inflammation to minimise and eventually resolve. The nerves that had sprouted should lose those excess branches, and the number of nerves in the pulp should return to normal. Possibly the extra-sensitive sodium channels in the nerves can be replaced by normal, less sensitive sodium channels.
Although some desensitising formulations also contain anti-inflammatory substances (essential oils, triclosan, cetylpyridinium chloride, etc.), future desensitising treatments might include anti-inflammatory agents in addition to tubule-occluding agents. Future studies should examine the efficacy of topically applied hydrocortisone or other anti-inflammatory agents for the treatment of dentine sensitivity.
Acknowledgement
Editorial assistance was provided by Dr Julie Ponting of Anthemis Consulting Ltd, funded by Johnson & Johnson.
Footnotes
Conflict of interest statement Preparation of this article was sponsored by Johnson & Johnson Consumer & Personal Products Worldwide.
References
- 1.Brännström M. Dentin and pulp in restorative dentistry. Wolfe Medical Publications Ltd; London: 1982. [Google Scholar]
- 2.Brännström M. Etiology of dentin hypersensitivity. Proceedings of the Finnish Dental Society. 1992;88(Suppl 1):7–14. [PubMed] [Google Scholar]
- 3.Ciucchi B, Bouillaguet S, Holz J, Pashley DH. Dentinal fluid dynamics in human teeth, in vivo. Journal of Endodontics. 1995;21:191–4. doi: 10.1016/S0099-2399(06)80564-9. [DOI] [PubMed] [Google Scholar]
- 4.Pashley DH, Tay FR, Haywood VB, Collins MA, Drisko CL. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. Compendium of Continuing Education in Dentistry. 2008;8(Special Issue):1–37. [Google Scholar]
- 5.Adams D, Addy M, Absi EG. Abrasive and chemical effects of dentifrices. In: Embery G, Rolla G, editors. Clinical and biological aspects of dentifrices. Oxford University Press; Oxford: 1992. pp. 345–55. [Google Scholar]
- 6.Yilmaz HG, Kurtulmus-Yilmaz S, Cengiz E, Bayindir H, Aykac Y. Clinical evaluation of Er,Cr:YSGG and GaAlAs laser therapy for treating dentine hypersensitivity: A randomized controlled clinical trial. Journal of Dentistry. 2011;39:249–54. doi: 10.1016/j.jdent.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 7.West NX, Hooper SM, O’Sullivan D, Hughes N, North M, Macdonald EL, et al. In situ randomised trial investigating abrasive effects of two desensitising toothpastes on dentine with acidic challenge prior to brushing. Journal of Dentistry. 2012;40:77–85. doi: 10.1016/j.jdent.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Olley RC, Pilecki P, Hughes N, Jeffery P, Austin RS, Moazzez R, Bartlett D. An in situ study investigating dentine tubule occlusion of dentifrices following acid challenge. Journal of Dentistry. 2012;40:585–93. doi: 10.1016/j.jdent.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Bakry AS, Tamura Y, Otsuki M, Kasugai S, Ohya K, Tagami J. Cytotoxicity of 45S5 bioglass paste used for dentine hypersensitivity treatment. Journal of Dentistry. 2011;39:599–603. doi: 10.1016/j.jdent.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Tschoppe P, Zandim DL, Martus P, Kielbassa AM. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. Journal of Dentistry. 2011;39:430–7. doi: 10.1016/j.jdent.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Pashley DH, Tay FR. Pulpodentin complex. In: Hargreaves KM, Goodis HE, Tay FR, editors. Seltzer and Bender’s dental pulp. 2nd ed Quintessence Publishing Co; Chicago: 2012. pp. 47–66. [Google Scholar]
- 12.Pashley DH, Livingston MJ, Greenhill JD. Regional resistances to fluid flow in human dentine in vitro. Archives of Oral Biology. 1978;23:807–10. doi: 10.1016/0003-9969(78)90159-0. [DOI] [PubMed] [Google Scholar]
- 13.Pashley DH. Smear layer: overview of structure and function. Proceedings of the Finnish Dental Society. 1992;88(Supplement 1):215–24. [PubMed] [Google Scholar]
- 14.Carrilho MR, Tay FR, Sword J, Donnelly A, Agee KA, Nishitani Y, et al. Dentine sealing provided by smear layer/smear plugs vs. adhesive resins/resin tags. European Journal of Oral Sciences. 2007;115:321–9. doi: 10.1111/j.1600-0722.2007.00465.x. [DOI] [PubMed] [Google Scholar]
- 15.Pashley DH. Dynamics of the pulpo-dentine complex. Critical Reviews in Oral Biology and Medicine. 1996;7:104–33. doi: 10.1177/10454411960070020101. [DOI] [PubMed] [Google Scholar]
- 16.Kerns DG, Scheidt MJ, Pashley DH, Homer JA, Strong SL, Van Dyke TE. Dentinal tubule occlusion and root hypersensitivity. Journal of Periodontology. 1991;62:421–8. doi: 10.1902/jop.1991.62.7.421. [DOI] [PubMed] [Google Scholar]
- 17.Draenert ME, Jakob M, Kunzelmann KH, Hickel R. The prevalence of tooth hypersensitivity following periodontal therapy with special reference to root scaling. A systematic review of the literature. American Journal of Dentistry. 2013;26:21–7. [PubMed] [Google Scholar]
- 18.Clayton DR, McCarthy D, Gillam DG. A study of the prevalence and distribution of dentine sensitivity in a population of 17-58-year-old serving personnel on an RAF base in the Midlands. Journal of Oral Rehabilitation. 2002;29:1–23. doi: 10.1046/j.1365-2842.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- 19.Tammaro S, Wennström JL, Bergenholtz G. Root-dentin sensitivity following non-surgical periodontal treatment. Journal of Clinical Periodontology. 2000;27:690–7. doi: 10.1034/j.1600-051x.2000.027009690.x. [DOI] [PubMed] [Google Scholar]
- 20.McGrath PA. Psychological aspects of pain perception. Archives of Oral Biology. 1994;39(Supplement):55S–62S. doi: 10.1016/0003-9969(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 21.Alexander JI. Biochemical, physiological and psychological aspects of pain and pain management. In: Addy M, Embery G, Edgar WM, Orchardson R, editors. Tooth wear and sensitivity. Martin-Dunitz; London: 2000. pp. 268–81. [Google Scholar]
- 22.Michelich V, Pashley DH, Whitford GM. Dentin permeability: A comparison of functional versus anatomical tubular radii. Journal of Dental Research. 1978;57:1019–24. doi: 10.1177/00220345780570110301. [DOI] [PubMed] [Google Scholar]
- 23.Michelich V, Schuster GS, Pashley DH. Bacterial penetration of human dentin in vitro. Journal of Dental Research. 1980;59:1398–1403. doi: 10.1177/00220345800590080701. [DOI] [PubMed] [Google Scholar]
- 24.Pashley DH, Matthews WG. The effects of outward forced convective flow on inward diffusion in human dentin in vitro. Archives of Oral Biology. 1993;38:577–82. doi: 10.1016/0003-9969(93)90122-3. [DOI] [PubMed] [Google Scholar]
- 25.Brännström M. The elicitation of pain in human dentine and pulp by chemical stimuli. Archives of Oral Biology. 1962;7:59–62. doi: 10.1016/0003-9969(62)90048-1. [DOI] [PubMed] [Google Scholar]
- 26.Lundy T, Stanley HR. Correlation of pulpal histopathology and clinical symptoms in human teeth subjected to experimental irritation. Oral Surgery, Oral Medicine, and Oral Pathology. 1969;27:187–201. doi: 10.1016/0030-4220(69)90172-8. [DOI] [PubMed] [Google Scholar]
- 27.Stenvik A, Iversen J, Mjör IA. Tissue pressure and histology of normal and inflamed tooth pulps in macaque monkeys. Archives of Oral Biology. 1972;17:1501–11. doi: 10.1016/0003-9969(72)90037-4. [DOI] [PubMed] [Google Scholar]
- 28.Pashley DH, Kepler EE, Williams EC, Okabe A. Progressive decrease in dentine permeability following cavity preparation. Archives of Oral Biology. 1983;28:853–8. doi: 10.1016/0003-9969(83)90043-2. [DOI] [PubMed] [Google Scholar]
- 29.Pashley DH, Galloway SE, Stewart FP. Effects of fibrinogen in vivo on dentin permeability in the dog. Archives of Oral Biology. 1984;29:725–8. doi: 10.1016/0003-9969(84)90179-1. [DOI] [PubMed] [Google Scholar]
- 30.Hahn CL, Overton B. The effects of immunoglobulins on the convective permeability of human dentine in vitro. Archives of Oral Biology. 1997;42:835–43. doi: 10.1016/s0003-9969(97)00080-0. [DOI] [PubMed] [Google Scholar]
- 31.Kim S, Trowbridge H. Pulpal reaction to caries and dental procedures. In: Cohen S, Burns RC, editors. Pathways of the Pulp. Mosby; St. Louis: 1998. pp. 414–33. [Google Scholar]
- 32.Trowbridge HO. Pulp biology: progress during the past 25 years. Australian Endodontic Journal. 2003;29:5–12. doi: 10.1111/j.1747-4477.2003.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 33.Caviedes-Bucheli J, Munoz HR, Azuero-Holguin MM, Ulate E. Neuropeptides in dental pulp: the silent protagonists. Journal of Endodontics. 2008;34:773–88. doi: 10.1016/j.joen.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Byers M, Närhi MVO. Dental injury models: Experimental tools for understanding neuroinflammatory nociceptor functions. Critical Reviews in Oral Biology and Medicine. 1999;10:4–39. doi: 10.1177/10454411990100010101. [DOI] [PubMed] [Google Scholar]
- 35.Byers M, Henry MA, Närhi MVO. Dental innervation and its response to tooth injury. In: Hargreaves KM, Goodis HE, Tay FR, editors. Seltzer and Bender’s dental pulp. 2nd ed Quintessence Publishing Co; Chicago: 2012. pp. 133–57. [Google Scholar]
- 36.Renton T, Yiangou Y, Plumpton C, Tate S, Bountra C, Anand P. Sodium channel Nav1.8 immunoreactivity in painful human dental pulp. BMC Oral Health. 2005;5:5. doi: 10.1186/1472-6831-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo S, Perry GM, Levinson SR, Henry MA. Nav1.7 expression is increased in painful human dental pulp. Molecular Pain. 2008;4:16. doi: 10.1186/1744-8069-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henry MA, Luo S, Foley BD, Rzasa RS, Johnson LR, Levinson SR. Sodium channel expression and localization at demyelinated sites in painful human dental pulp. Journal of Pain. 2009;10:750–8. doi: 10.1016/j.jpain.2009.01.264. [DOI] [PMC free article] [PubMed] [Google Scholar]