Abstract
BACKGROUND/OBJECTIVES
The objective of this study was to measure and/or estimate the total antioxidant capacity of the Korean diet.
MATERIALS/METHODS
Eighty-one plant foods that were expected to exhibit rather high antioxidant activities were selected from the Korean diet using the Fifth Korean National Health and Nutrition Survey (KNHANES V). These foods were categorized into 11 food groups: cereals, potatoes, legumes, nuts, vegetables, kimchies, mushrooms, fruits, fruit juices, sea weeds, and oils. The foods were mixed in the proportions specified in traditional Korean recipes and analyzed. The measured indicators for antioxidant capacities were total phenolics, 2,2-diphenyl-1-picrylhydrazyl (DPPH), oxygen radical absorbance capacity (ORAC), and Trolox equivalent antioxidant capacity (TEAC).
RESULTS
Total phenolics were high in the fruit juices, nuts, vegetables, and fruits; and the average DPPH, ORAC, and TEAC values were high in the vegetables, fruits, fruit juices, and nuts. The correlation coefficient between the content of total phenolics of each food and the in vitro antioxidant capacity was relatively high at 0.851. The intake of total phenolics per capita per day in the Republic of Korea was estimated to be 127 mg. The total dietary antioxidant capacity (TDAC) values, which were obtained from the total antioxidant capacity of each food, taking into account the intake of each food, were 20,763, 54,335, and 876.4 µmol of Trolox equivalents using the DPPH, ORAC, and TEAC methods, respectively. The food group that contributed the most to the Korean TDAC was cereals at 39.7%, followed by fruits and vegetables at 27.8% and 13.9%, respectively. The contribution of legumes, nuts, fruit juices, and mushrooms was quite minimal at less than 2% each.
CONCLUSIONS
The content of total phenolics and the antioxidant capacity of the Korean diet are significantly correlated and the high contributing food groups are cereals, fruits, and vegetables.
Keywords: Korean diet, total dietary antioxidant capacity, total phenolics intake, dietary antioxidants
INTRODUCTION
Seasonally fresh plant foods provide a wide variety of dietary antioxidants, such as vitamin C, carotenoids, vitamin E, flavonoids, and other phenolic compounds. The additive and synergistic effects of these dietary antioxidant compounds might positively impact human health [1]. It has been widely reported that the high intake of dietary antioxidant may reduce the risk of oxidative stress-related diseases, such as coronary heart disease, cancer, and neurodegenerative diseases by eliminating free radicals and other reactive oxygen and nitrogen species [2,3,4]. Since a typical diet provides more than 25,000 bioactive food constituents [3], understanding the complex role of the diet in such chronic diseases is challenging. Because both the numerous individual functions and the combined additive or synergistic effects of antioxidants in food are crucial for beneficial health effects [2], a food-based or a diet-based research approach is likely to elucidate more health effects than those derived from each individual nutrient or food item.
Total antioxidant capacity takes into account all antioxidants and the synergistic effects among them [5], and describes the cumulative antioxidant capacity of food components scavenging free radicals [6]. The total antioxidant capacity of a diet has been associated with several health benefits in both cross-sectional and randomized intervention studies [5,7,8,9]. In addition, total dietary antioxidant capacity (TDAC) has been shown to be inversely associated with risks of chronic diseases such as heart failure [8], stroke (by inhibition of oxidative stress and inflammation) [5], and gastric cancer [9]. Agudo et al. [10] have shown that the TDAC from fruits and vegetables was inversely related to overall mortality rates in a Spanish cohort of the European Prospective Investigation into Cancer (EPIC).
The influence of different factors on the effectiveness of antioxidants in complex heterogeneous foods and biological systems cannot be evaluated using only a one-assay protocol [4]. Thus, several assays have been used to assess the total antioxidant content of foods, e.g. the oxygen radical absorbance capacity (ORAC) assay [11], the Trolox equivalent antioxidant capacity (TEAC) assay [12], the ferric-reducing ability of plasma (FRAP) assay [13], and the total radical-trapping antioxidant parameter (TRAP) assay [10]. Carson et al. [2] assayed 3,100 food samples for their total antioxidant content using a modified FRAP assay. Using ORAC assay, Rautiainen et al. [8,14] examined how the total antioxidant capacity of diet was associated with the incidence of myocardial infarctions among middle-aged and elderly women. Similarly, a large Italian cohort study [7] suggested that antioxidants may play a role in reducing the risk of cerebral infarction using the total antioxidant capacity value obtained from the TEAC assay. Furthermore, Saura-Calixto and Goni [4] used the mean values of FRAP and 2,2'-azinobis (3-ethylbenzothiazoline-6-sulfunate) (ABTS) assays to estimate the TDAC of the Spanish Mediterranean diet. Moreover, in an EPIC-Spain study [10], data on total antioxidant capacity from plant foods were gathered from published databases that provided the antioxidant capacity measured in foods by four different assays: TRAP, FRAP, TEAC, and ORAC.
TDAC is defined as the antioxidant capacity of all plant foods and beverages (alcoholic and nonalcoholic) consumed daily in a diet [4]. Recently, several studies have evaluated the contribution of food items to the TDAC of a diet. Yang et al. [15] have reported that teas, dietary supplements, and fruits and fruit juices were the major sources of the TDAC for the US population (28, 25, and 17%, respectively), while the largest contributors to the TDAC were beverages (approximately 68% - the mean of FRAP and ABTS values) in the Spanish Mediterranean diet [4].
The Korean diet is a plant-based diet that contains a variety of antioxidants including vitamins, minerals, and phytochemicals [16]. Most diets consumed by Koreans that include fruits and vegetables have been associated with reduced cancer risk [17]. In a meta-analysis of observational studies conducted in Korea and Japan concerning the relationship between fresh and pickled vegetable consumption and gastric cancer, a high intake of fresh vegetables was associated with a decreased risk of gastric cancer [18]. Recent studies have made advances in understanding the relationship between diet and cancer among Korean populations, however, because the history of nutritional cancer epidemiology in Korea is relatively short, the subjects covered and the methodology of the research have been rather limited [17]. Moreover, to the best of our knowledge, there have been no studies on the TDAC of the Korean diet. Thus, it is worthwhile to gain a comprehensive view of this field by examining the antioxidant capacity of the whole Korean diet rather than of single nutrients or foods [4].
The aim of this study was to determine the TDAC of the Korean diet using the Fifth Korean National Health and Examination Survey (KNHANES V) as well as evaluate the contribution of each food group in the Korean diet to the TDAC.
MATERIALS AND METHODS
Food intake and samples
Estimates of plant food intake in the Korean diet were generated utilizing raw data from the KNHANES V (http://knhanes.cdc.go.kr), which was conducted by the Korean Ministry of Health and Welfare in 2010. The KNHANES was a nationwide representative study that consisted of three sections: a health and behavior interview, a health examination, and a dietary survey. A total of 8,027 persons aged over 1 year had been subjected in the KNHANES. Food items contributed more than 1% of the total dietary intake within each plant food group of the KNHANES were selected and considered to be representative of the plant foods commonly consumed in the Korean diet. Dietary intake per person per day of each food item (81 items) listed in Table 1 represents the average Korean diet. Each individual plant food listed in the KNHANES V (Table 1) was purchased at various local supermarkets.
Table 1.
Intake of plant foods and oils in the Korean diet (KNHANES V, 2010)
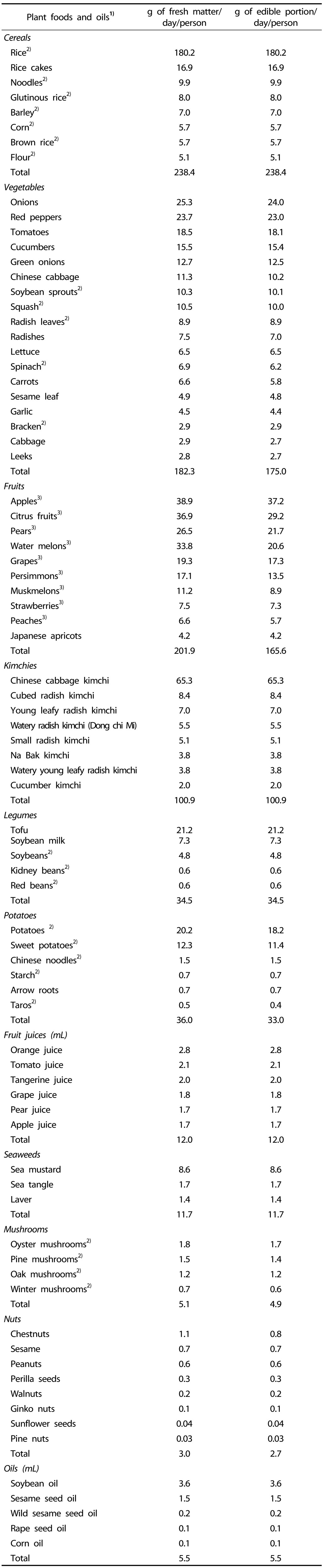
1)Individual items that comprised more than 1% of the total dietary intake by the group selected in this study are representative of the plant foods commonly consumed in the Korean diet.
2)Cooked
3)Peeled
The edible portion in duplicate of the daily amount consumed for each plant food eaten, as specified in Table 1, was weighed and mixed with other food items in the same plant food group of the ten food groups: cereals (total: 238.4 g), vegetables (total: 175.0 g), fruits (total: 165.6 g), kimchies (total: 100.9 g), legumes (total: 34.5 g), potatoes (total: 33.0 g), fruit juices (total: 12.0 mL), seaweeds (total: 11.7 g), mushrooms (total: 4.9 g), and nuts (total: 2.7 g). Each food group becomes a sample for further analysis. These ten samples correspond to the total per capita daily intake of solid plant food in the Korean diet. Each duplicate sample was freeze-dried, ground, and stored prior to analysis. Vegetable oils (total: 5.5 mL) that were individually analyzed included soybean oil, sesame seed oil, wild sesame seed oil, rape seed oil, and corn oil.
Food antioxidant extraction
The extraction of food antioxidants was performed as described by Saura-Calixto and Goni [4], with slight modifications. The ground plant food sample (1 g) was placed in a test tube. Next, 40 mL of methanol/water (50:50, v/v) and 2 N HCl, to obtain a pH of 2.0, were added; and the tube was thoroughly shaken at room temperature for 1 h. The tube was centrifuged at 2,500 g for 10 min, and the supernatant was recovered. To the residue was added 40 mL of acetone/water (70:30, v/v), followed by shaking and centrifugation. Then, the methanol and acetone extracts were combined. The oil mixture (2 g) was mixed with methanol (2 mL), and the mixture was vigorously stirred for 30 min before being centrifuged at 2,500 g. The methanol phase was removed and the extraction was repeated with 2 mL of methanol. Then the methanol extracts were combined. The methanol-acetone extracts of plant foods and the methanol extracts of oils were used as test samples to determine the total phenolics and antioxidant activities (measured in triplicate).
Antioxidant activity assays
Total soluble phenolic content assay
Total soluble phenolic content was determined according to the method of Randhir et al. [19]. The test sample dissolved in distilled water (1 mL) was mixed with 1 mL of ethanol (95%, v/v). To each sample, 0.5 mL of Folin-Ciocalteu reagent (50%, v/v) and 1 mL of 5% Na2CO3 were added. The reaction mixture was left in the dark for 1 h. Its absorbance was then read at 725 nm with 95% ethanol as the blank using a spectrophotometer (Shimadzu Inc. Kyoto, Japan). A standard curve was established using various concentrations of gallic acid in 95% ethanol. Finally, the absorbance values were converted to mg of total soluble phenolics per g of test sample.
ORAC
The ORAC assay was carried out on a Tecan GENios multi-functional plate reader (Tecan Austria, Salzburg, Austria) with fluorescence filters (excitation wavelength: 485 nm and emission filter: 535nm). In the final assay mixture, 40 nM fluorescein was used as the target of free radical attack with either 20 mM 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH) as the peroxyl radical generator in a peroxyl radical scavenging capacity (ORACROO·) assay [20] or H2O2-CuSO4 (0.75% H2O2; 5 µM CuSO4) as the hydroxyl radical generator in a hydroxyl radical scavenging capacity (ORACOH·) assay [21]. Trolox (1 µM) was used as the standard control and prepared fresh daily. The analyzer was programmed to record fluorescence every 2 min after AAPH or H2O2-CuSO4 was added. All fluorescence measurements were expressed relative to the initial reading. The final results were calculated using the difference of the area under the fluorescence decay curve between the blank and the sample. ORACROO· and ORACOH· were expressed as µM of Trolox equivalents (TE). One ORAC unit is equivalent to the net protection area provided by 1 µM Trolox.
DPPH radical-scavenging capacity
Test sample (50 µL) was mixed with 500 µL of 70 µM DPPH in ethanol and centrifuged at 1,000×g for 10 s. The supernatant (200 µL) was transferred to a microplate well and allowed to react for 40 min at 37℃. The absorbance of the resulting solution was measured at 525nm using an ELISA reader (Tecan Austria, Salzburg, Austria). The DPPH scavenging capacity percentage of the extract was calculated as follows:
DPPH radical-scavenging capacity (%) = [(A0-A1)/A0] × 100 where A0 is the absorbance of the control and A1 is the absorbance in the presence of test sample [22].
TEAC assay
To the test tube containing 990 µL of 7 mM ABTS solution with 2.45 mM K2S2O8 was added 10 µL of test sample, and the solution was mixed. The ABTS solution was kept in the dark until use. Its absorbance was read at 734 nm using a spectrophotometer (Shimadzu Inc., Kyoto, Japan) for 6 min at 25℃. The absorbance values were converted to mg of Trolox [23].
Total dietary antioxidant capacity
Total dietary antioxidant capacity (TDAC) was defined as the antioxidant capacity of all plant food and beverages consumed daily in a diet [4]. TDAC may represent the amount of antioxidant units (trolox equivalents) present daily in the human gut. The TDAC values were obtained by multiplying the total antioxidant capacity (the average of the DPPH, ORAC, and TEAC assays) and the intake of each food.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS-PC+) software for Windows (version 20.0). Values were expressed as mean values ± standard deviation, unless stated otherwise. Comparison of the means of three measurements using a significance level of P < 0.05 was performed by one-way analysis of variance with a post-hoc comparison using the least significant difference test. Pearson's correlation coefficient test was applied to test the association between the total phenolic content and the antioxidant capacity of the samples analyzed.
RESULTS
The intake of plant foods and vegetable oils in the Korean diet is shown in Table 1. The Korean diet is particularly rich in a wide range of fruits, vegetables, and kimchies. The most widely consumed vegetables, fruits, and kimchies are onions, apples, and Chinese cabbage kimchi, respectively. Rice is the major source of cereals, and soybean oil is the major source of fat.
The antioxidant capacity and total phenolic content of plant foods and vegetable oils are shown in Table 2. All types of plant foods, except mushrooms, vegetable oils, and legumes, presented high antioxidant capacity per gram of edible dry matter by the DPPH radical scavenging assay. In addition, plant foods except legumes (by the ORAC assay) and cereals (by the TEAC assay) showed comparatively high antioxidant capacity per gram of edible dry matter. The greatest values were registered for fruit juices, fruits, and nuts by the DPPH assay; vegetables, fruits, and fruit juices by the ORAC assay; and nuts, fruit juices, and fruits by the TEAC assay. The total phenolic content of every plant food tested showed high values for fruit juices, nuts, and vegetables; while the phenolic content of vegetable oils and cereals was comparatively low.
Table 2.
DPPH radical-scavenging capacity (DPPH), oxygen radical absorbance capacity (ORAC), and trolox equivalent antioxidant capacity (TEAC) of plant foods1),2)
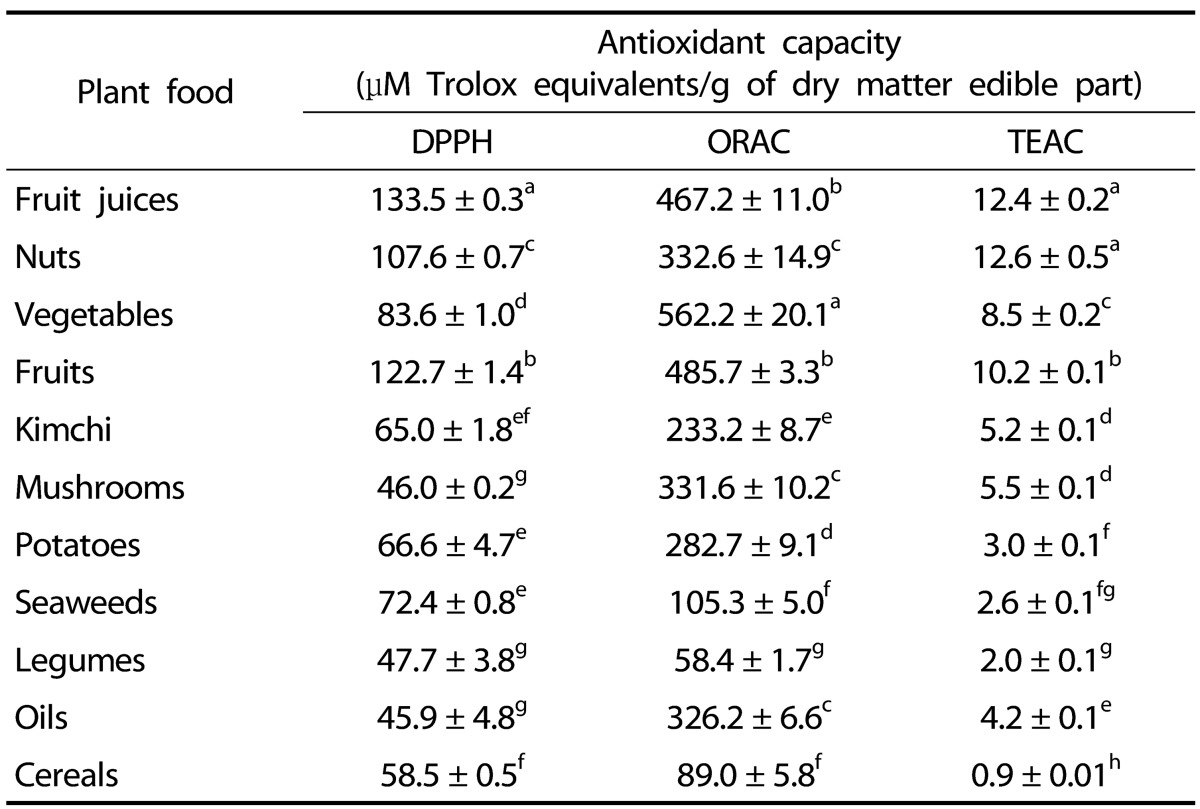
1)Values are the mean ± standard deviation of three replicate experiments of each food.
2)Means with different superscripts in the same column are significantly different by the least significant difference test.
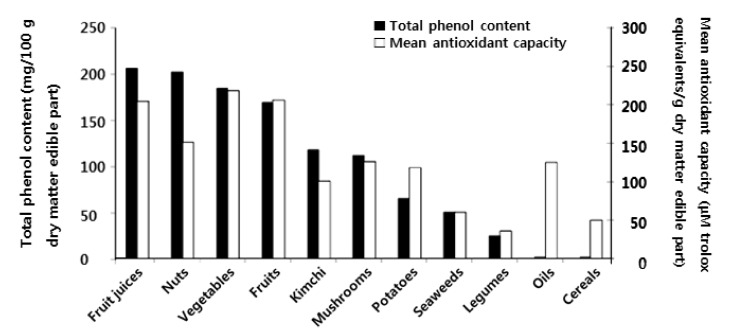
Comparison of total phenolic content and the mean value of the DPPH, ORAC, and TEAC assays of plant foods based on edible dry matter in the Korean diet is shown in Fig. 1. There was a good correlation between the total phenolic content and the mean antioxidant capacity in all types of plant foods except nuts, kimchies, oils, and cereals. The mean antioxidant capacities were relatively low in fruit juices, nuts, and kimchies compared to those of the total phenolics; and the mean antioxidant capacities were relatively high in potatoes, vegetable oils, and cereals. The correlation coefficient (r = 0.851) between the total phenolic content and the mean antioxidant capacity was high.
Fig. 1.
Comparison of total phenol content and mean antioxidant capacity (mean of DPPH, ORAC and TEAC values) of plant foods based on the dry matter edible part in the Korean diet.
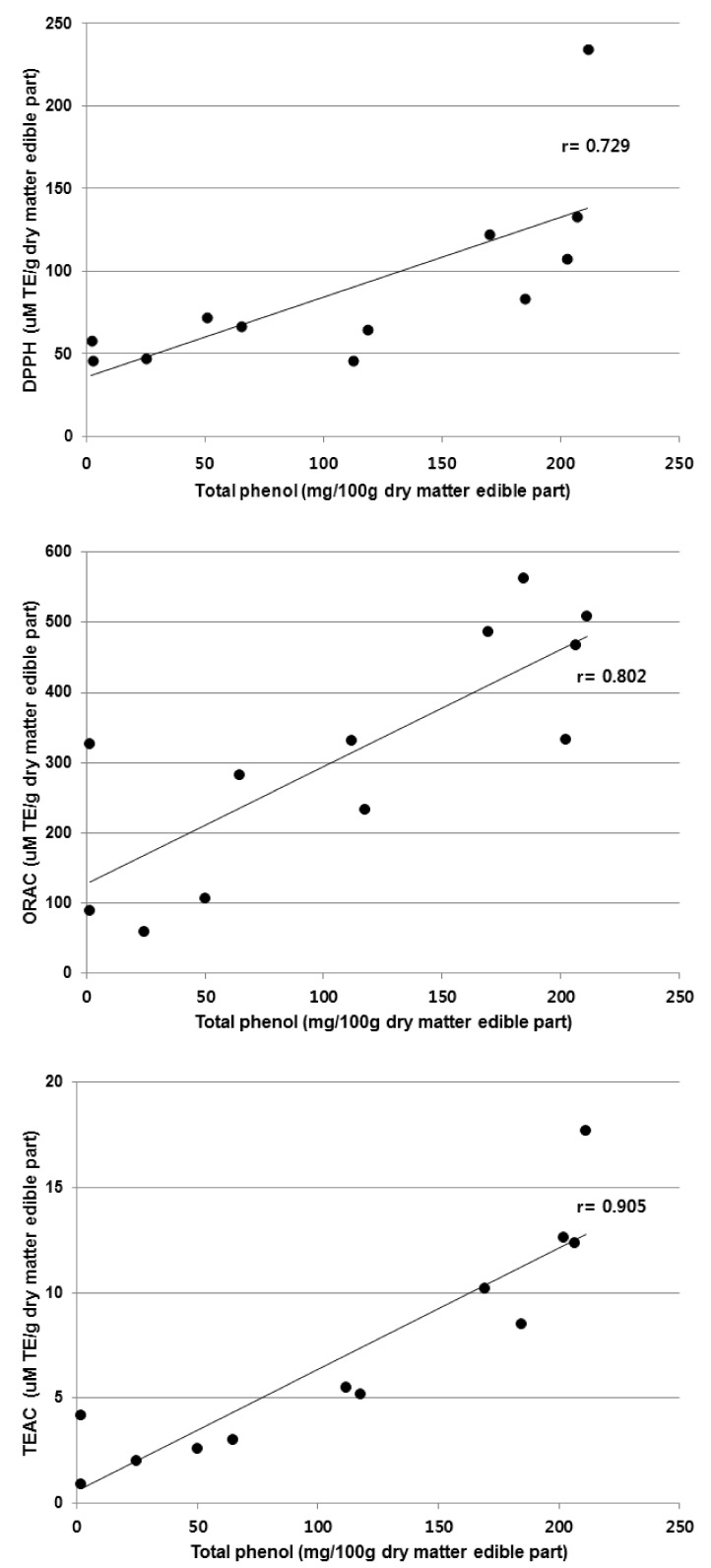
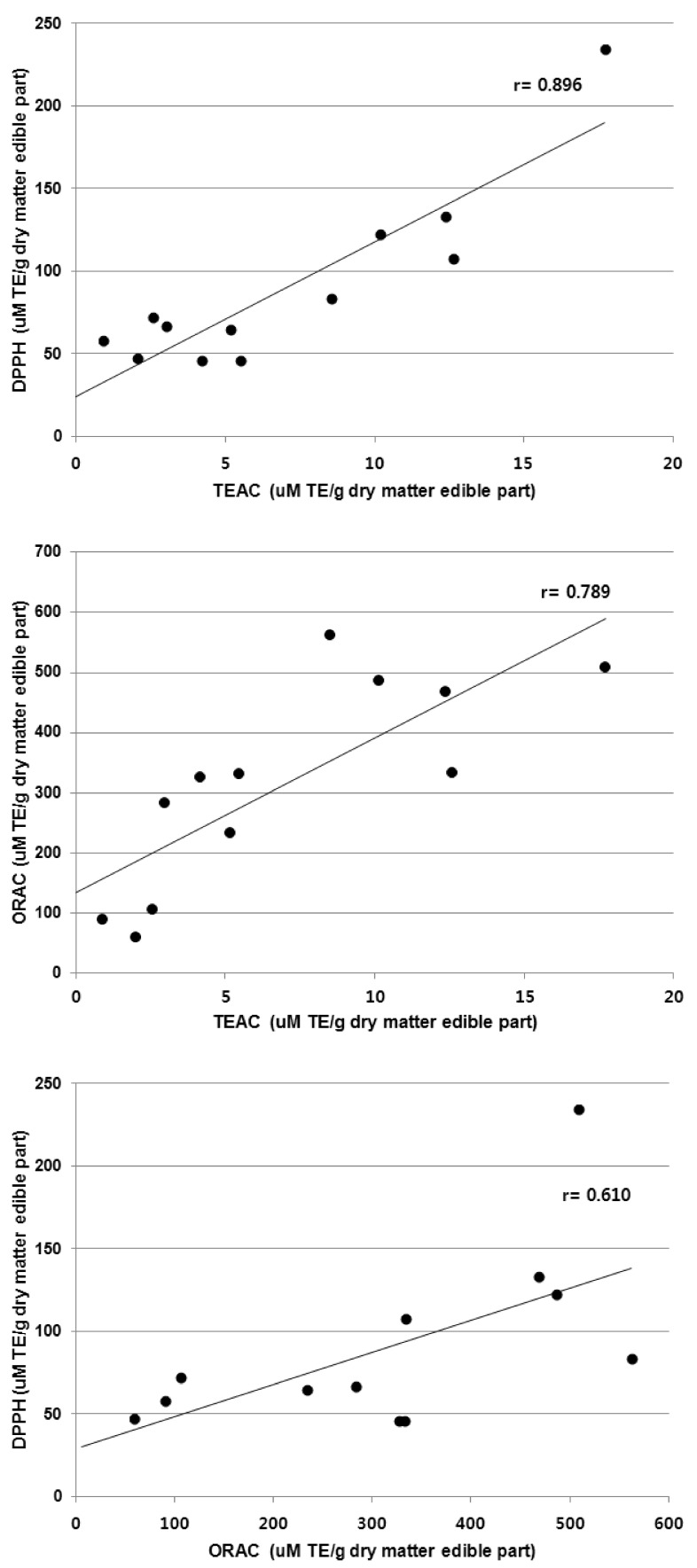
The relationship between the total phenolic content and the three assays (DPPH, ORAC, and TEAC) is presented in Fig. 2 and Fig. 3. The correlation coefficient between the total phenolic content and the TEAC assay was the greatest (r = 0.905), followed by the ORAC assay (r = 0.802) and the DPPH assay (r = 0.729) (Fig. 2). There were high correlations between the TEAC and DPPH assays (r = 0.896) as well as the TEAC and ORAC assays (r = 0.789); while the ORAC assay showed slightly less correlation with the DPPH assay (r = 0.610) (Fig. 3).
Fig. 2.
Correlation of total phenolic content and antioxidant capacities obtained from DPPH, ORAC and TEAC assays.
Fig. 3.
Correlation of antioxidant capacities obtained from TEAC assay and those obtained from ORAC or DPPH assay.
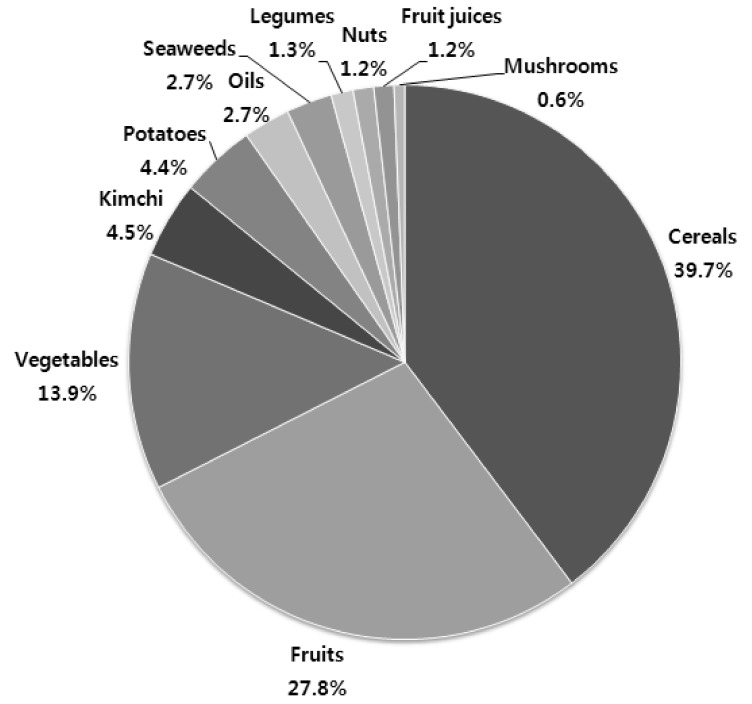
Total phenol intake was estimated as 127 mg/100 g of edible dry matter/person/day by the Folin-Ciocalteu method (Table 3). The total antioxidant capacity in the Korean diet was estimated to be 20,763, 54,335, and 876 µM Trolox equivalent by the DPPH, ORAC, and TEAC assays, respectively. The contribution of each specific plant food to the TDAC was dependent on both food intake and food antioxidant capacity. Thus, the contribution of total antioxidant capacity obtained from the mean value of the DPPH, ORAC, and TEAC assays and food intake to the TDAC is shown in Fig. 4. The largest contributor to the TDAC was cereals (39.7%), followed by fruits (27.8%) and vegetables (13.9%), while kimchies and potatoes accounted for nearly 5% of the TDAC; in addition, the contribution of legumes, nuts, fruit juice, and mushrooms was very low.
Table 3.
Total antioxidant capacity and polyphenol per capita daily intake in the Korean diet
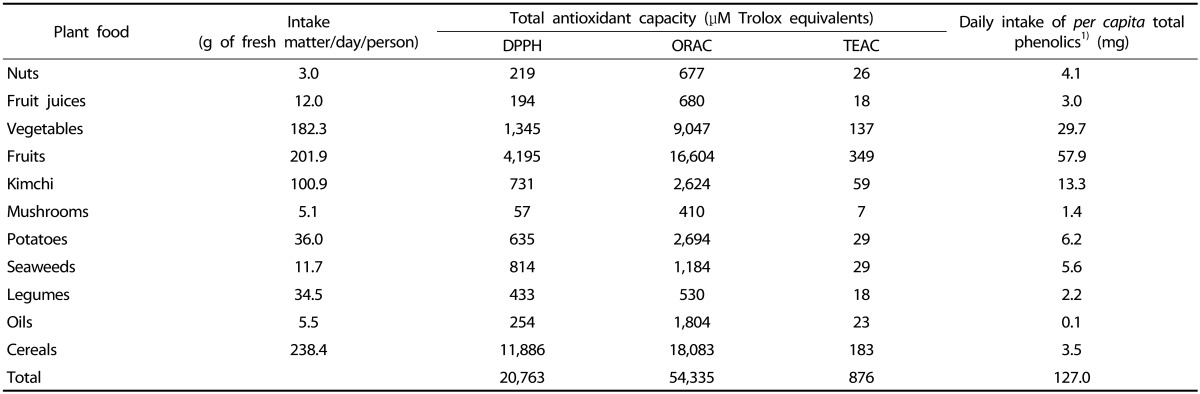
1)Daily intake of per capita total phenolics of the food groups were obtained by multiplying the total phenol content per g of fresh matter of edible part and the dietary intake of each food.
Fig. 4.
Contribution of total antioxidant capacities (mean of DPPH, ORAC and TEAC) from all plant foods as a percentage of total dietary antioxidant capacities (TDAC) from per capita daily intake in the Korean diet. The TDAC values were obtained by multiplying the total antioxidant capacity of each food group and the intake of each food.
DISCUSSION
The Korean diet is largely composed of various vegetables, fruits, cereals, and fermented foods, with plant foods as the major component. It is presumed that several antioxidants are significant parts of the diet, and synergistic effects of the antioxidants are expected to maximize the health benefit of the foods. Therefore, it is worthwhile to evaluate the antioxidant capacity of the Korean diet at the level of total antioxidant capacity rather than at the single food or nutrient level.
Known antioxidants in the diet are vitamin C, vitamin E, polyphenolics, and carotenoids, and the content of total phenolics in plants are known to show various physiological activities, such as antioxidant and anticancer activities [4]. In the Korean diet, the phenolics in fruit juices and nuts were the highest, followed by those in vegetables and fruits (Table 2). In a preliminary study in Korea, the phenolic content in Korean nuts was higher than that in vegetables, which might have been be due to the antioxidant capacity of the chestnut, which is the most popular nut in Korea [24,25]. Zamora-Ros et al. [26] also have reported that the phenolic content in the nuts in the European diet is greater than the phenolics in oranges and other fruits.
When the total antioxidant capacity, the average of the DPPH, ORAC, and TEAC assays, for each food group was compared, the values from the vegetables, fruits, fruit juices, and nuts were showing very high antioxidant capacities. The antioxidant capacity of the European diet, which is known to be similar to the Korean diet, has been studied using the FRAP and ABTS assays as indicators [4], and the antioxidant activity is the greatest in nuts, followed by fruits and vegetables, showing a similar trend to the one shown in this study.
The total phenolic content and antioxidant capacity of the plant foods in the Korean diet showed a very high correlation (DPPH, r = 0.723; ORAC, r = 0.802; TEAC, r = 0.905). This result indicates a good agreement with the reports of other researchers [4,27] and implies that phenolics are the major contributing factor for the antioxidant activity of the Korean diet. The study by Saura-Calixto and Goni [4] has shown that the correlation coefficients between the phenolic content and the antioxidant capacity of plant foods were 0.9924 and 0.9446 with FRAP and ABTS, respectively. Kratchanova et al. [27] also have shown that the total polyphenol content and the ORAC value of water extracts from 25 Bulgarian medicinal plants have a high correlation coefficient (r = 0.950).
The simple comparison of the antioxidant capacity and the phenolic content per unit weight of plant food has limited applicability in evaluating the impact of the food component on human physiology. Therefore, food intake should be taken into account. It was estimated in this study that the total daily intake of total phenolics was 127 mg/person/day (Table 3), which is greater than the sum of the amount of vitamin C (94 mg) [28], beta-carotene (4.7 mg), and vitamin E (14.3 mg) [29] consumed by Koreans. It has been reported that the antioxidant capacity of phenolics is greater than that of vitamins and carotenoids measured in vitro or ex vivo [30,31]. In addition, Vinson et al. [32] have reported that the major antioxidants of fruits are phenolics rather than vitamin C or carotenoids. Moreover, the intake of total phenolics in the Spanish Mediterranean diet [4] was estimated to be greater than that in the Korean diet. This result might be attributed to the consumption of certain beverages, mainly wine and coffee.
Studies on individual food items, not at the level of the whole diet, might over-estimate their potential antioxidant effects within a whole diet [4]. Since the contribution of each specific food items to the TDAC is dependent on both food intake and food antioxidant capacity, the contribution to the TDAC of any given food can be minimal with low food intake in spite of an individual food having a high antioxidant capacity. This phenomenon is evident in this study; the contribution of nuts and fruit juices, which showed a high antioxidant capacity per unit weight of the food, was less than 1% of the TDAC in the Korean diet. In contrast, the contribution of cereals, despite a low antioxidant capacity per unit weight, was 39.7% of the TDAC, which is a significant portion of the value due to the high consumption rate of cereals in Korea.
Recent cohort studies in Denmark have revealed that the intake of cereal [33] and dietary fiber from cereal [34] decreased the incidence of rectal cancer; and a Spanish cohort study [35] has shown that dietary fiber from cereals decreases hypertension. Thus, the results of the antioxidant capacity of cereals having a health-benefit effect as the greatest contributor to the Korean TDAC agree with the significant contribution of cereals to the antioxidant capacity of the Korean diet.
As shown in Fig. 4, the contributions of fruits and vegetables, which have a rather high antioxidant capacity and intake rate, to the Korean TDAC were 27.8% and 13.9%, respectively. In the Spanish Mediterranean diet [4], in contrast to this study, beverages such as coffee and wine contributed 68% of the TDAC, and were followed by fruits and vegetables contributing 20%. In the U.S.A., tea contributed the highest at 28% of the total antioxidant capacity, followed by dietary supplements and fruits and fruit juice at 25% and 17%, respectively. The contribution of vegetables was less than 2% [15]. In terms of contribution to the TDAC, the contribution of fruits and vegetables in the Korean diet was at 41.7%, and this level is significantly greater than that in the Spanish Mediterranean diet or the US diet at 20% or 19%, respectively. A series of studies on the antioxidant capacity of vegetables and fruits has been conducted [2,36,37], and an association between the consumption of a diet high in fruits and vegetables and a decreased risk of chronic degenerative diseases is well documented [38,39]. In the Spanish Mediterranean diet studies, the intake of fruits and vegetables and the incidence of myocardial infarction showed a reverse correlation [14,39,40]. Meanwhile, in a recent cohort study of the male population of various ethnic groups, the intake of fruits and vegetables was inversely correlated with the incidence of colorectal cancer [40]. In addition, in a study of Chinese females, the intake of fruits decreased the incidence of heart disease [41]; and a meta-analysis of various cohort studies revealed that the intake of fruits and vegetables decreases the incidence of stroke [7]. Furthermore, in a comparative study of normal and gastric cancer patients in Korea, the intake of fresh vegetables and fruits decreased the incidence of gastric cancer [42]. In a similar study with normal and breast cancer patients in Korea, the intake of vegetables decreased the incidence of the breast cancer [43]. Both in Korea and Japan, the intake of fresh vegetables decreased the incidence of gastric cancer, but the intake of pickled vegetables increased the incidence of gastric cancer [44]; unfortunately the TDAC was not utilized in that study. Therefore, it is highly recommended that any nutritional epidemiological studies on the Korean diet and oxidative stress-related disease should utilize the TDAC for obtaining meaningful results. The results of this study on the contribution of various food groups to the TDAC could be utilized for the development of policy regarding the improvement of the antioxidant capacity of the Korean diet. In technical terms, the consumption of various kinds of cereals could be recommended as a policy in order to increase the antioxidant capacity of the cereals consumed; even though the total antioxidant capacity per unit weight is small, the consumption rate is high. As a second option, the consumption of nuts could be recommended because of their high antioxidant capacity. The influence of different factors on the effectiveness of antioxidants in complex heterogeneous foods and biological systems cannot be evaluated using a single assay method [4]. Although these in vitro methods are widely used to evaluate antioxidant capacities in foods and biological systems, further studies on ex vivo using specific human cells or human in vivo studies about effectiveness of antioxidants of foods in human are needed in the future.
In summary, all plant food groups in the Korean diet showed that the content of total phenolics is positively correlated with in vitro antioxidant indices: the DPPH, ORAC, and TEAC values. The intake of cereals, fruits, and vegetables in the Korean diet is the major contributor to the TDAC, an index of the total antioxidant capacity of a diet. It is hoped that the biofunctional effects, including the antioxidant capacity, of the Korean diet are evaluated by incorporating the concept of food intake in addition to the efficacy per unit weight of the individual food in order to describe the contribution of health-benefit effects to the whole diet. In this regard, it was also confirmed in this study that the TDAC could be a useful marker in future investigations concerning the relationship between the Korean diet and diseases associated with oxidative stress. In practice, when one would like to study the relationship of oxidative stress-related disease and dietary patterns using epidemiology to evaluate the superiority of the Korean diet, the use of the TDAC index should be considered first, and correlations between Korean dietary patterns and the incidence of diseases need to be revealed.
Footnotes
This research was supported by the Globalization of Korean Foods R&D program funded by the Ministry of Agriculture, Food and Rural Affairs, Republic of Korea and the Research Funds from Hannam University in 2013.
References
- 1.Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78:517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 2.Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, Willey C, Senoo H, Umezono Y, Sanada C, Barikmo I, Berhe N, Willett WC, Phillips KM, Jacobs DR, Jr, Blomhoff R. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. 2010;9:3. doi: 10.1186/1475-2891-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Cancer Research Fund; American Institute for Cancer Research (US) Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington D.C.: American Institute of Cancer Research; 2007. [Google Scholar]
- 4.Saura-Calixto F, Goñi I. Antioxidant capacity of the Spanish Mediterranean diet. Food Chem. 2006;94:442–447. [Google Scholar]
- 5.Rautiainen S, Larsson S, Virtamo J, Wolk A. Total antioxidant capacity of diet and risk of stroke: a population-based prospective cohort of women. Stroke. 2012;43:335–340. doi: 10.1161/STROKEAHA.111.635557. [DOI] [PubMed] [Google Scholar]
- 6.Pellegrini N, Serafini M, Colombi B, Del Rio D, Salvatore S, Bianchi M, Brighenti F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J Nutr. 2003;133:2812–2819. doi: 10.1093/jn/133.9.2812. [DOI] [PubMed] [Google Scholar]
- 7.Del Rio D, Agnoli C, Pellegrini N, Krogh V, Brighenti F, Mazzeo T, Masala G, Bendinelli B, Berrino F, Sieri S, Tumino R, Rollo PC, Gallo V, Sacerdote C, Mattiello A, Chiodini P, Panico S. Total antioxidant capacity of the diet is associated with lower risk of ischemic stroke in a large Italian cohort. J Nutr. 2011;141:118–123. doi: 10.3945/jn.110.125120. [DOI] [PubMed] [Google Scholar]
- 8.Rautiainen S, Levitan EB, Mittleman MA, Wolk A. Total antioxidant capacity of diet and risk of heart failure: a population-based prospective cohort of women. Am J Med. 2013;126:494–500. doi: 10.1016/j.amjmed.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Serafini M, Jakszyn P, Luján-Barroso L, Agudo A, Bas Bueno-de-Mesquita H, van Duijnhoven FJ, Jenab M, Navarro C, Palli D, Boeing H, Wallström P, Regnér S, Numans ME, Carneiro F, Boutron-Ruault MC, Clavel-Chapelon F, Morois S, Grioni S, Panico S, Tumino R, Sacerdote C, Ramon Quirós J, Molina-Montes E, Huerta Castaño JM, Barricarte A, Amiano P, Khaw KT, Wareham N, Allen NE, Key TJ, Jeurnink SM, Peeters PH, Bamia C, Valanou E, Trichopoulou A, Kaaks R, Lukanova A, Bergmann MM, Lindkvist B, Stenling R, Johansson I, Dahm CC, Overvad K, Jensen M, Olsen A, Tjonneland A, Lund E, Rinaldi S, Michaud D, Mouw T, Riboli E, González CA. Dietary total antioxidant capacity and gastric cancer risk in the European prospective investigation into cancer and nutrition study. Int J Cancer. 2012;131:E544–E554. doi: 10.1002/ijc.27347. [DOI] [PubMed] [Google Scholar]
- 10.Agudo A, Cabrera L, Amiano P, Ardanaz E, Barricarte A, Berenguer T, Chirlaque MD, Dorronsoro M, Jakszyn P, Larrañaga N, Martínez C, Navarro C, Quirós JR, Sánchez MJ, Tormo MJ, González CA. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain) Am J Clin Nutr. 2007;85:1634–1642. doi: 10.1093/ajcn/85.6.1634. [DOI] [PubMed] [Google Scholar]
- 11.DeLange RJ, Glazer AN. Phycoerythrin fluorescence-based assay for peroxy radicals: a screen for biologically relevant protective agents. Anal Biochem. 1989;177:300–306. doi: 10.1016/0003-2697(89)90056-0. [DOI] [PubMed] [Google Scholar]
- 12.Miller NJ, Rice-Evans CA. Spectrophotometric determination of antioxidant activity. Redox Rep. 1996;2:161–171. doi: 10.1080/13510002.1996.11747044. [DOI] [PubMed] [Google Scholar]
- 13.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 14.Rautiainen S, Levitan EB, Orsini N, Åkesson A, Morgenstern R, Mittleman MA, Wolk A. Total antioxidant capacity from diet and risk of myocardial infarction: a prospective cohort of women. Am J Med. 2012;125:974–980. doi: 10.1016/j.amjmed.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Yang M, Chung SJ, Chung CE, Kim DO, Song WO, Koo SI, Chun OK. Estimation of total antioxidant capacity from diet and supplements in US adults. Br J Nutr. 2011;106:254–263. doi: 10.1017/S0007114511000109. [DOI] [PubMed] [Google Scholar]
- 16.Kwon OR. Functionality of Korean food: science and practice. Food Ind Nutr. 2011;16:11–14. [Google Scholar]
- 17.Woo HD, Kim J. Nutritional epidemiology of cancer in Korea: recent accomplishments and future directions. Asian Pac J Cancer Prev. 2011;12:2377–2383. [PubMed] [Google Scholar]
- 18.Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer. 2011;11:135–140. doi: 10.5230/jgc.2011.11.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randhir R, Shetty P, Shetty K. L-DOPA and total phenolic stimulation in dark germinated fava bean in response to peptide and phytochemical elicitors. Process Biochem. 2002;37:1247–1256. [Google Scholar]
- 20.Kurihara H, Fukami H, Asami S, Toyoda Y, Nakai M, Shibata H, Yao XS. Effects of oolong tea on plasma antioxidative capacity in mice loaded with restraint stress assessed using the oxygen radical absorbance capacity (ORAC) assay. Biol Pharm Bull. 2004;27:1093–1098. doi: 10.1248/bpb.27.1093. [DOI] [PubMed] [Google Scholar]
- 21.Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med. 1993;14:303–311. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- 22.Chen HM, Muramoto K, Yamauchi F, Fujimoto K, Nokihara K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J Agric Food Chem. 1998;46:49–53. doi: 10.1021/jf970649w. [DOI] [PubMed] [Google Scholar]
- 23.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 24.Lee HJ, Chung MJ, Cho JY, Ham SS, Choe M. Antioxidative and macrophage phagocytic activities and functional component analyses of selected Korean chestnut (Castanea crenata S. et Z.) cultivars. J Korean Soc Food Sci Nutr. 2008;37:1095–1100. [Google Scholar]
- 25.Barreira JC, Ferreira IC, Oliveira MB, Pereira JA. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008;107:1106–1113. [Google Scholar]
- 26.Zamora-Ros R, Rothwell JA, Scalbert A, Knaze V, Romieu I, Slimani N, Fagherazzi G, Perquier F, Touillaud M, Molina-Montes E, Huerta JM, Barricarte A, Amiano P, Menéndez V, Tumino R, de Magistris MS, Palli D, Ricceri F, Sieri S, Crowe FL, Khaw KT, Wareham NJ, Grote V, Li K, Boeing H, Förster J, Trichopoulou A, Benetou V, Tsiotas K, Bueno-de-Mesquita HB, Ros M, Peeters PH, Tjønneland A, Halkjær J, Overvad K, Ericson U, Wallström P, Johansson I, Landberg R, Weiderpass E, Engeset D, Skeie G, Wark P, Riboli E, González CA. Dietary intakes and food sources of phenolic acids in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr. 2013;110:1500–1511. doi: 10.1017/S0007114513000688. [DOI] [PubMed] [Google Scholar]
- 27.Kratchanova M, Denev P, Ciz M, Lojek A, Mihailov A. Evaluation of antioxidant activity of medicinal plants containing polyphenol compounds. Comparison of two extraction systems. Acta Biochim Pol. 2010;57:229–234. [PubMed] [Google Scholar]
- 28.Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Korea Health Statistics 2010: Korea National Health and Nutrition Examination Survey (KNHANES V-1) Cheonwon: Korea Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 29.Na YJ, Lee SH. Development and validation of a quantitative food frequency questionnaire to assess nutritional status in Korean adults. Nutr Res Pract. 2012;6:444–450. doi: 10.4162/nrp.2012.6.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48:3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez-Moreno C, Jiménez-Escrig A, Saura-Calixto F. Study of low-density lipoprotein oxidizability indexes to measure the antioxidant activity of dietary polyphenols. Nutr Res. 2000;20:941–953. [Google Scholar]
- 32.Vinson JA, Su X, Zubik L, Bose P. Phenol antioxidant quantity and quality in foods: fruits. J Agric Food Chem. 2001;49:5315–5321. doi: 10.1021/jf0009293. [DOI] [PubMed] [Google Scholar]
- 33.Kyrø C, Skeie G, Loft S, Landberg R, Christensen J, Lund E, Nilsson LM, Palmqvist R, Tjønneland A, Olsen A. Intake of whole grains from different cereal and food sources and incidence of colorectal cancer in the Scandinavian HELGA cohort. Cancer Causes Control. 2013;24:1363–1374. doi: 10.1007/s10552-013-0215-z. [DOI] [PubMed] [Google Scholar]
- 34.Hansen L, Skeie G, Landberg R, Lund E, Palmqvist R, Johansson I, Dragsted LO, Egeberg R, Johnsen NF, Christensen J, Overvad K, Tjønneland A, Olsen A. Intake of dietary fiber, especially from cereal foods, is associated with lower incidence of colon cancer in the HELGA cohort. Int J Cancer. 2012;131:469–478. doi: 10.1002/ijc.26381. [DOI] [PubMed] [Google Scholar]
- 35.Alonso A, Beunza JJ, Bes-Rastrollo M, Pajares RM, Martínez-González MA. Vegetable protein and fiber from cereal are inversely associated with the risk of hypertension in a Spanish cohort. Arch Med Res. 2006;37:778–786. doi: 10.1016/j.arcmed.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Song W, Derito CM, Liu MK, He X, Dong M, Liu RH. Cellular antioxidant activity of common vegetables. J Agric Food Chem. 2010;58:6621–6629. doi: 10.1021/jf9035832. [DOI] [PubMed] [Google Scholar]
- 37.Yao H, Chen Y, Shi P, Hu J, Li S, Huang L, Lin J, Lin X. Screening and quantitative analysis of antioxidants in the fruits of Livistona chinensis R. Br using HPLC-DAD-ESI/MS coupled with pre-column DPPH assay. Food Chem. 2012;135:2802–2807. doi: 10.1016/j.foodchem.2012.07.076. [DOI] [PubMed] [Google Scholar]
- 38.Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv Nutr. 2012;3:506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martínez-González MA, Fernández-Jarne E, Martínez-Losa E, Prado-Santamaría M, Brugarolas-Brufau C, Serrano-Martinez M. Role of fibre and fruit in the Mediterranean diet to protect against myocardial infarction: a case-control study in Spain. Eur J Clin Nutr. 2002;56:715–722. doi: 10.1038/sj.ejcn.1601382. [DOI] [PubMed] [Google Scholar]
- 40.Nomura AM, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Pike MC, Kolonel LN. Association of vegetable, fruit, and grain intakes with colorectal cancer: the Multiethnic Cohort Study. Am J Clin Nutr. 2008;88:730–737. doi: 10.1093/ajcn/88.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu D, Zhang X, Gao YT, Li H, Yang G, Huang J, Zheng W, Xiang YB, Shu XO. Fruit and vegetable intake and risk of CHD: results from prospective cohort studies of Chinese adults in Shanghai. Br J Nutr. 2014;111:353–362. doi: 10.1017/S0007114513002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HJ, Chang WK, Kim MK, Lee SS, Choi BY. Dietary factors and gastric cancer in Korea: a case-control study. Int J Cancer. 2002;97:531–535. doi: 10.1002/ijc.10111. [DOI] [PubMed] [Google Scholar]
- 43.Lee SA, Kang D, Nishio H, Lee MJ, Kim DH, Han W, Yoo KY, Ahn SH, Choe KJ, Hirvonen A, Noh DY. Methylenetetrahydrofolate reductase polymorphism, diet, and breast cancer in Korean women. Exp Mol Med. 2004;36:116–121. doi: 10.1038/emm.2004.17. [DOI] [PubMed] [Google Scholar]
- 44.Kim HJ, Lim SY, Lee JS, Park S, Shin A, Choi BY, Shimazu T, Inoue M, Tsugane S, Kim J. Fresh and pickled vegetable consumption and gastric cancer in Japanese and Korean populations: a meta-analysis of observational studies. Cancer Sci. 2010;101:508–516. doi: 10.1111/j.1349-7006.2009.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]