Abstract
Both the chronic development of atherosclerotic lesions and the acute changes in lesion phenotype that lead to clinical cardiovascular events are significantly influenced by the innate and adaptive immune responses to lipoprotein deposition and oxidation in the arterial wall. The rapid pace of discovery of mechanisms of immunologic recognition, effector functions, and regulation has significantly influenced the study of atherosclerosis, and our new knowledge is beginning to affect how we treat this ubiquitous disease. In this review, we discuss recent advances in our understanding of how innate and adaptive immunity contribute to atherosclerosis, as well as therapeutic opportunities that arise from this knowledge.
Keywords: oxidation-specific epitopes, T lymphocytes, B lymphocytes, antibodies, cardiovascular disease
INTRODUCTION
Atherosclerosis is a leading cause of morbidity and mortality worldwide. Despite the widespread use of statins to lower plasma cholesterol, the burden of cardiovascular disease (CVD) that results from atherosclerosis is likely to increase due to the persistence of risk factors such as smoking and sedentary lifestyle, as well as increasing obesity, metabolic syndrome, and diabetes. There have been remarkable advances in our understanding of the cellular and molecular events in the pathogenesis of atherosclerosis that hold the promise of translation into therapies directed at these newly discovered targets. The most significant of these advances is the accumulating knowledge of how the immune system contributes to chronic atherosclerotic lesion development and how immune responses promote destabilization of established arterial lesions resulting in acute catastrophic clinical events.
The concept that the pathogenesis of atherosclerosis involves inflammatory reactions to intimal deposition of lipoprotein is well established and has been thoroughly described in many excellent recent reviews, including one in this journal (1). The relationship between inflammation and immune responses has been clarified by advances in our understanding of innate and adaptive immunology. Innate immune responses are initiated by the body’s recognition of signature molecules that are associated with danger to the organism. These molecules are either of microbial origin [i.e., pathogen-associated molecular patterns (PAMPs)] or self-molecules that should not be expressed or accessible to the immune system unless there is cell injury or death [i.e., damage-associated molecular patterns (DAMPs)]. DAMPs may be molecules that are normally sequestered within cells and released in response to injury, such as heat shock proteins (HSPs) or high-mobility group protein 1, or they may represent newly generated, “altered-self-molecules,” for example, those created by various nonenzymatic chemical modifications, such as the formation of AGEs (advanced glycation end products). Various soluble circulating proteins and cell-surface or intercellular receptors [pattern recognition receptors (PRRs)] recognize DAMPs and PAMPs. Following recognition, these receptors simulate responses that are usually protective, such as killing of microbes or stimulating cleanup and repair of dead tissue. The primary response induced by PRR engagement of PAMPs or DAMPs is inflammation. Often, innate inflammatory responses damage the host, especially when the stimuli are persistent, excessive, or resistant to eradication. Given the nature of the risk factors for atherosclerosis, persistent and sometime excessive stimulation of the innate immune system may be present within the arterial walls of susceptible patients.
Adaptive immune responses can be induced by almost any type of molecular structure (i.e., antigen) when it is recognized, in the right context, by receptors on lymphocytes [membrane immunoglobulins (Igs) on B cells, T cell receptors (TCRs) on T cells]. The somatic DNA recombination mechanisms that form the functional genes encoding Igs in B cells and TCRs generate a tremendous diversity of clonally distributed receptor specificities. In response to antigen recognition, in combination with costimulatory signals induced by innate immune responses, lymphocytes proliferate and differentiate into effector cells that help eradicate infection. Many of these functions, such as effector B cell secretion of antibodies that activate the complement system, are proinflammatory or engage cell-activating Ig Fc receptors on phagocytes. Effector T cells secrete cytokines and express surface proteins that enhance the inflammatory functions of endothelial cells (ECs) and phagocytes. Thus, inflammation—often mediated by the same molecules used in innate responses—is an important way in which the adaptive immune system protects us from infection.
Persistent adaptive responses can also cause tissue damage and disease by promoting inflammation, especially when the inciting antigens are resistant to eradication or there are intrinsic defects in the regulation of lymphocytes. Importantly, unlike the innate immune system, the adaptive immune system has a built-in potential to randomly produce antigen receptors that recognize normal or abnormally modified self-molecules as well as harmless foreign molecules. We rely on various tolerance mechanisms to prevent the survival or activation of the T cells that bear these receptors, but these mechanisms may fail, leading to chronic inflammatory and autoimmune disease.
On the basis of our current understanding, it is reasonable to state that inflammation is always triggered by innate and/or adaptive immune responses to either damaged self- or foreign stimuli. Furthermore, initiation of adaptive immune responses usually requires prior innate responses. Therefore, a thorough understanding of atherosclerosis demands more knowledge about how innate and adaptive immunity operate systemically and locally within the arterial walls of people at risk of atherosclerosis. In this review, we briefly summarize older literature but focus more on recently reported evidence from various basic and clinical research studies that have added to our understanding of immune mechanisms in atherogenesis. The discussion is organized into sections on innate immunity, adaptive immunity, and a synthesis on translational opportunities and challenges. Note that much of the research in this field has relied on low-density lipoprotein receptor-deficient (Ldlr−/−) or apolipoprotein E (ApoE)-deficient (Apoe−/−) mice in which atherosclerotic lesions develop because high plasma cholesterol levels can be sustained. The degree to which data from mouse studies accurately describe human vascular disease is largely unknown, and a major challenge in this field is to acquire more and better human data. Because of space constraints, we are unable to discuss or cite many excellent relevant papers, for which we apologize in advance.
INNATE IMMUNE RESPONSES THAT ARE RELEVANT TO ATHEROSCLEROSIS
A central challenge in atherosclerosis research is the identification of disease-specific PAMPs or DAMPs that provoke innate immune responses in the arterial wall, which in turn promote lesion formation. The traditional view is that the immune system has evolved mainly for protection against infections; therefore, it is plausible that antimicrobial immunity, either toward a specific organism or in aggregate, may contribute to the chronic development of atherosclerosis. However, there is little evidence that infections are the primary inducers of proatherogenic innate responses. This important concept has been the subject of many studies, as recently reviewed elsewhere (2). An attractive alternative hypothesis is that it is recognition of DAMPs that triggers innate immune mechanisms in atherogenesis. In this context, there is considerable evidence that an immune response to bacterial HSPs can subsequently cross-react with HSPs expressed on the surface of ECs injured in the context of atherogenesis, resulting in inflammatory responses (3). Similarly, immune responses to cholesterol crystals in lesions cause inflammatory responses (4). Although these immunological processes may indeed contribute to disease progression, they may be considered secondary, similar to those of other infectious agents, as mentioned above.
Among the many potential endogenous DAMPs that can trigger innate immune responses in atherogenesis, most evidence has focused on products resulting from oxidative reactions, and in particular, lipid peroxidation, as having primary importance. Although elaborate and complex antioxidative mechanisms to reduce oxidized “bystander” molecules to their normal states have evolved, if these mechanisms fail, innate immunity seems to have evolved multiple mechanisms to mediate removal of these oxidatively modified molecules, cells, and debris that, if not sequestered and removed, would be proinflammatory and immunogenic. There is now considerable evidence that a major mechanism by which recognition of oxidation-damaged molecular complexes occurs involves the detection of oxidation-specific epitopes (OSEs), which constitute common motifs of oxidative damage and are ligands for a common set of innate PRRs. Thus, host-derived OSEs are an important class of DAMPs and constitute a major target of innate immunity (reviewed in Reference 5). Because OSEs are created whenever LDL is oxidized (OxLDL) and whenever cells undergo apoptosis and/or death (6)— processes that are central to atherogenesis— they become major disease-specific antigens that engage innate immunity in atherosclerosis. Furthermore, because many OSEs share molecular identity and/or mimicry with epitopes on microbes, there is a link between innate responses to microbial PAMPs and endogenous DAMPs of oxidative origin (5, 7).
OSEs that are prominently expressed on apoptotic cells and cellular debris are proinflammatory and immunogenic if not removed (6). Macrophage PRRs, such as the macrophage scavenger receptors (SRs) CD36 and SR-A [which recognize OSEs on apoptotic cell surfaces such as oxidized phospholipids (OxPLs) and malondialdehyde (MDA)-modified structures, respectively] are intimately involved in the process of apoptotic cell removal (5, 8, 9). Both the IgM natural antibody (NAb) E06 and C-reactive protein (CRP) (10) recognize OxPL and can further participate in such cell removal. Understanding that OSEs form a family of DAMPs recognized by innate PRRs gives rise to a conceptual framework that helps explain why innate immunity is so heavily involved in the pathophysiology of atherosclerosis. Although the usefulness of inhibiting lipid peroxidation in humans remains to be demonstrated, there is widespread acceptance of the importance of oxidation of LDL in atherogenesis (11, 12). Although the original concept that OSEs on OxLDL constituted the ligands leading to enhanced and unregulated uptake by macrophage SRs propelled the oxidation hypothesis to the forefront, it is now apparent that the OSEs are proinflammatory and immunogenic and therefore that, as a class, they are among the most important primary candidate antigens driving immunological responses in atherogenesis. Undoubtedly, there are other major classes of DAMPs that are important innate antigens in atherosclerosis. In the discussion below, we review the roles of various innate PRRs and effectors in atherosclerosis, focusing on OxLDL and OSEs as important DAMPs recognized by these PRRs.
Oxidized Low-Density Lipoprotein and Oxidation-Specific Epitopes
Many lines of evidence suggest that LDL undergoes oxidation in vivo, particularly under conditions of hyperlipidemia and when retained in the vascular wall, as recently reviewed (12). The precise mechanisms that are responsible for formation of OxLDL in vivo are likely to be complex and to consist of both enzymatic and nonenzymatic processes (13). LDL oxidized by exposure to copper in vitro is a widely used model of extensively oxidized LDL and generates various oxidized lipids and oxidized lipid–protein adducts, including OxPL, in which the sn-2 polyunsaturated fatty acid undergoes lipid peroxidation, leading to decomposition products such as MDA. In turn, both OxPL and MDA form covalent adducts with proteins, including ApoB of LDL. ApoB also undergoes direct oxidative attack. Other oxidatively modified lipids, such as oxidized cholesterol esters (OxCEs) also form. Importantly, LDL eluted from plaques in hypercholesterolemic rabbits or humans possess nearly all of the characteristics of in vitro-prepared OxLDL (14).
So-called minimally oxidized LDL (mmLDL) has a lower content (and different types) of OSEs. OxLDL contains abundant truncated aldehydes, ketones, and carboxyl products of advanced PUFA oxidation, whereas mmLDL contains predominantly early lipid peroxidation products of PL and CE formed by 15-lipoxygenase (15-LO), an intracellular enzyme found in all vascular cells under inflammatory conditions (15). Human 15-LO and mouse 12/15-LO have been proposed to play a major role in in vivo LDL oxidation and the development of human and experimental murine atherosclerosis, as reviewed in Reference 16. Accumulation of CE hydroperoxides has been documented in human atherosclerotic lesions and in the lesions of Apoe−/− and Ldlr−/− mice fed a high-fat diet (15, 17). Analyses of the lipids of mmLDL revealed the presence of polyoxygenated OxCEs in which the cholesterol moiety was not oxidized but the arachidonate acyl chain was (15). Such OxCEs have numerous proinflammatory effects on macrophages that are mediated by TLR4 signaling (5), as described below.
Macrophage Scavenger Receptors
Macrophage SRs are so named because they bind and internalize extensively oxidized LDL but not native LDL (18) or mmLDL. SRs are PRRs and include CD36, SR-A1 and SR-A2, SR-BI, MARCO, LOX-1, PSOX, and others (19). Remarkably, although all were originally identified (and so named) for their ability to bind OSEs as found on OxLDL, they also bind a wide variety of different ligands, including many microbial pathogens (PAMPs). CD36 and SR-A have the highest affinity for OxLDL and acetylated LDL, respectively, and are responsible for up to 90% of their uptake by macrophages in vitro, although in vivo other SRs may play important roles as well (reviewed in Reference 19).
The phosphocholine (PC) headgroup of OxPLs in OxLDL is a major ligand mediating binding to CD36 and SR-B1. In contrast, the PC of nonoxidized PL is not recognized. POVPC [1-palmitoyl-2-(5′-oxovaleroyl)-sn-glycero-3-phosphocholine] is an example of an OxPL that binds to CD36. CD36 recognizes the PC on both free POVPC and POVPC covalently linked to ApoB. Furthermore, POVPC linked to bovine serum albumin (BSA) competes with the binding of OxLDL to CD36, as does the IgM NAb E06, which binds specifically to the same PC moiety, as described below. Importantly, cells undergoing apoptosis contain an increased content of such OxPL, and their binding and uptake by macrophages can be inhibited entirely by OxLDL, POVPC-BSA, and E06 (9). Thus, the PC of OxLDL and of apoptotic cells is a DAMP recognized by macrophage SRs.
OxPL also contains other oxidized moieties that are recognized by CD36, including moieties on the sn-2 side chain of both PC and phosphatidylserine-containing PL, that are sufficient ligands to mediate binding of both OxLDL and apoptotic cells to CD36 (20). Binding of both the PC headgroup and the oxidized side-chain moieties of OxPL to different sites on CD36 probably lead to cooperative high-affinity binding of OxLDL and apoptotic cells to CD36. SR-A can bind to both OxLDL and acetylated LDL, but at different molecular sites (21). These data, which illustrate that innate PRRs can bind to multiple OSEs and that some OSEs even have two moieties that serve as DAMPs, suggest that the ability to clear OSEs is evolutionarily important. SR-A also binds MDA epitopes, as occurs on OxLDL, MDA-LDL, and apoptotic cells (reviewed in Reference 5).
The role of individual SRs in atherogenesis in vivo is unclear. CD36 and SR-A mediate high-affinity uptake and appear to account for up to 90% of the uptake of OxLDL in vitro in short experiments in culture; these findings suggest that targeted deletion of these receptors in mice should reduce atherogenesis. Such reduction in atherogenesis has been observed in some experimental studies, but not others, for reasons that are not apparent (reviewed in References 22 and 23). However, note that there are multiple SRs that can mediate uptake of OxLDL, and in vivo genetic targeting of one or more SRs may lead to upregulation of others. Furthermore, even the deletion of a high-affinity receptor mediating OxLDL uptake may not prevent foam cell formation, given that slower rates of OxLDL uptake mediated by less-efficient SRs may still cause the same net cholesterol accumulation (although it may take a longer period of time).
Toll-Like Receptors
Toll-like receptors (TLRs) are an important class of innate PRRs that are widely expressed on many cell types, recognize both PAMPs and DAMPs, and initiate complex signal transduction pathways that mediate strong inflammatory responses. Many published studies have addressed TLRs and their ligands in atherosclerosis (reviewed in Reference 24). TLRs are expressed by ECs, macrophages, dendritic cells (DCs), lymphocytes, and vascular smooth muscle cells, all of which are implicated in atherosclerotic lesion development. Genetic deficiency in the adaptor protein MyD88, which is required for signaling by most TLRs, or a lack-of-function mutation of the adaptor protein TRIF, which is required for TLR3 and TLR4 signaling, protected atherosclerosisprone mice from developing lesions (24, 25).
Studies performed with TLR-deficient atherosclerosis-prone mice, however, have revealed complexities related to the various roles of TLRs in promoting inflammation, protecting against infections, and inducing regulatory mechanisms. Non-bone marrow-restricted deficiency of TLR2 in Ldlr−/− mice reduced atherosclerosis, indicating that there is an endogenous proatherogenic TLR2 ligand in the hypercholesterolemic animals; an exogenous TLR2 ligand that binds to bone marrow-derived cells increased atherosclerosis (24). Deficiency of TLR4 or TLR2 in Apoe−/− mice reduced aortic intimal lipid accumulation and blocked increased expression of interleukin (IL)-1α and monocyte chemotactic protein (MCP)-1 messenger RNA (mRNA) (26). In contrast, TLR4 deficiency leads to markedly enhanced lesion development and increased lesional effector infiltration in Apoe−/− mice infected with the periodontal pathogen Porphyromonas gingivalis (27).
In addition to the possibility that microbial PAMPs may contribute to atherosclerosis by activating TLRs on arterial wall cells, there is evidence that endogenous OSEs found in atherosclerotic lesions can activate both TLR4 and TLR2 in complex ways that often depend on cooperativity between multiple PRRs. For example, OxPL found on OxLDL or on lipoprotein a [Lp(a)] can mediate apoptosis in ER-stressed macrophages through a mechanism requiring both CD36 and TLR2 (28). Other studies suggest that OxLDL activation of CD36 occurs via engagement of the TLR4/TLR6 heterodimer, but not at the cell surface, in endosomes (29). OxPLs also activate TLR2 on macrophages in a signaling pathway mediated by Nrf2, which causes a unique phenotype characterized by decreased phagocytosis and chemotactic activity (30). Furthermore, OxPLs activate TLRs on ECs. For example, OxPLs bind membrane CD14 and an as-yet-unidentified glycosylphosphatidylinositol-anchored receptor, leading to activation of ECs via TLR4 (31). However, OxPLs impair lipopolysaccharide (LPS) activation of TLR4 by binding to soluble CD14 and LPS-binding protein (LBP) and thus compete for LPS recognition (32, 33). To date, most studies have actually utilized mixtures of various PC-containing OxPLs. Future studies with specific OxPLs may help determine the exact interactions between the various OxPLs and TLRs and their coreceptors.
Other OSEs are also ligands for TLRs. mmLDL derived by the action of 15-LO on LDL binds to CD14 and activates macrophages via TLR4/MD-2 (5). A subsequent study showed that OxCE, made by exposure of cholesterol arachidonate to 15-LO, is responsible for many (but not all) TLR4-dependent proinflammatory and proatherogenic macrophage responses to mmLDL, including enhanced macrophage phagocytosis; this process led to enhanced uptake of both OxLDL and native LDL (15). Because the presence of such OxCEs has been demonstrated in both murine and human atherosclerotic lesions, these OSEs, like those of OxPL and MDA, are probably important antigens activating innate responses. Furthermore, activation of TLR4 via OxCE leads to a downstream signaling pathway mediated by Syk, which differs fundamentally from the pathway that is initiated when LPS activates TLR4 signaling. Because of the signaling differences between LPS- and OxCE-induced activation of TLR4, costimulation of macrophages by these two ligands give rise to cooperative effects, leading to greater activation than is achieved by either stimulus alone (34). The cooperative macrophage activation with low levels of both mmLDL (OxCE) and LPS may be relevant to the increased risk of acute CVD events in patients with atherosclerosis that is complicated by chronic infections, obesity, type 2 diabetes, and other conditions associated with subclinical endotoxemia.
Soluble Pattern Recognition Receptors
Many cell-surface PRRs, such as CD14, MD-2, LOX-1, and CD36, also exist in a soluble form. Although their exact functions are unknown, some soluble PRRs can activate their signaling counterparts, but when present at higher concentrations and when bound to different ligands, they are inhibitory, as has been suggested for OxPL bound to soluble CD14 (35).
Other circulating PRRs include LBP, various lectins, pentraxins, and complement proteins. The short acute-phase pentraxin CRP is now widely used as biomarker of inflammation and as an important risk factor for CVD. However, CRP was initially identified as the protein in the C fraction of plasma that bound Streptococcus pneumoniae; it binds the PC moiety on the cell wall polysaccharide of the microbe and mediates enhanced clearance of this and other PC-containing pathogens. CRP similarly binds to the PC moiety of OxPL on OxLDL or on apoptotic cells, but it does not bind to the PC of nonoxidized PLs (10). Furthermore, CRP is colocalized in atherosclerotic lesions with the PC of OxPL, presumably on OxLDL and apoptotic cells (10). Thus, this PRR recognizes the PC of OxPL, as do CD36 and SR-B1 and, as described below, the IgM NAb E06.
In analogy to the role of CRP in binding the PC of OxPL, complement factor H (CFH), a primary inhibitor of the alternative pathway of complement activation, binds the OSE MDA. CFH is the major plasma protein that binds MDA-modified protein (36). A study of its binding properties revealed that the H402 polymorphism, which is strongly associated with age-related macular degeneration (AMD), nearly abolished the ability of CFH to bind MDA. Injection of MDA-modified protein into the eyes of mice was proinflammatory, but the coinjection of CFH blocked the response. This observation suggests that the inability of H402 to bind MDA mediates inflammation in AMD (36). It will be interesting to determine whether CFH plays a similar role in other inflammatory diseases, such as atherosclerosis. Thus, one may consider CFH to be a PRR-recognizing MDA, in analogy to CRP binding to the PC of OxPL.
Natural Antibodies
NAbs can be considered part of the humoral arm of innate immunity. In mice, a primoridial subset of B cells, namely B-1 cells, secrete NAbs, which are predominantly IgM and IgA. NAbs are present at birth or shortly thereafter and are also present in gnotobiotic mice maintained in a completely sterile environment. Most, if not all, IgM in plasma of uninfected mice are of B-1 cell origin, and antigen exposure later in life can cause the expansion of B-1 cell clones, leading to increased IgM levels in plasma. B-1 cells have restricted use of VH/VL genes that are minimally edited, are considered to be of germ-line origin, and are of limited diversity. Because they are conserved by natural selection, NAbs presumably have advantageous properties that maintain homeostasis, such as their crucial role in immediate host defense against PAMPs and DAMPs.
Evidence supporting an atheroprotective role of NAbs is that sIgM−/− Ldlr−/− mice, which cannot secrete IgM, develop significantly greater atherosclerosis than do Ldlr−/− controls (37). Splenectomy enhances atherosclerosis in Apoe−/− mice, and whole B cell transfer into such mice is atheroprotective (38). More recently, investigators showed that B-1 cells, but not B-2 cells, conferred this protection and that transfer of B-1 cells unable to secrete IgM lost the protective capacity; in other words, the IgM were atheroprotective (39). In human populations, OxLDL-specific IgM titers are inversely associated with CVD, at least in univariate analyses (40). All these findings are consistent with an important role for IgM NAbs in limiting atherosclerotic disease.
Consistent with the concept that OSEs are a major target of innate immunity, 20-30% of all IgM in plasma of mice and in newborn human cord blood bind to OSEs (8). The prototypical IgM NAb E06 illustrates well the potential atheroprotective properties of a NAb to an OSE (reviewed in Reference 5). E06, selected for its ability to bind to OxLDL, was cloned from a panel of hybridomas from cholesterol-fed Apoe−/− mice. E06 bound to the PC of OxPL, which is present on OxLDL but also on apoptotic cells. E06 can block the uptake of OxLDL by macrophage SRs CD3 and SR-B1, and it can similarly inhibit the uptake of apoptotic cells; for instance, the PC moiety is a ligand that binds to CD36 and SR-B1. E06 is 100% homologous to the previously cloned, germ-line-encoded NAb T15, an IgA clone that had been studied more than 30 years earlier because it binds to PC (not as part of a PL) that is covalently linked to the cell wall polysaccharide of pathogens and confers optimal protection to mice from lethal infection with S. pneumoniae. The original T15-IgA and E06-IgM NAbs displayed identical binding properties (41). Thus, E06 displays the known ability of a NAb to bind to both an endogenous DAMP and an exogenous PAMP. These seminal studies demonstrate molecular (and immunological) identity between the PC of OxPL present on OxLDL and apoptotic cells, on the one hand, and the PC moiety present on pathogens, on the other hand. As mentioned above, E06 can inhibit OxLDL uptake by macrophages; this observation suggests that E06 is atheroprotective. Indeed, immunization of cholesterol-fed Ldlr−/− mice with heat-killed S. pneumoniae markedly increased E06 titers in plasma, which in turn led to atheroprotection (7).
Approximately 20% to 30% of all IgM derived from B-1 cell clones bind to OSEs (8). Among these, there is a high prevalence of IgM to MDA and the complex structural adducts that are generated when MDA is added to proteins. Remarkably, there is a high prevalence of IgM to MDA-LDL even in wildtype C57BL/6 mice, as well as in healthy adult humans (42). Recently, a distinct population that represents the human B-1 cell equivalent (CD20+CD27+CD43+CD70−) was identified (43). These cells spontaneously secrete IgM, stimulate T cells, express tonic intracellular signaling, and express B cell receptors with typical binding for two specificities, including binding to the classic PC antigen. The prevalence of B-1 cells in cord blood is ~2-5% of all B cells— nearly twice as great as in adults. Furthermore, newborn human umbilical cord blood has a similarly high titer of MDA-specific IgM, which binds apoptotic cells and atherosclerotic tissues (8). Because IgM does not cross the placenta, cord-blood IgM antibodies are thought to represent the human equivalent of innate NAbs in mice. These data suggest that OSEs are an important target of innate NAbs in humans, as they are in mice.
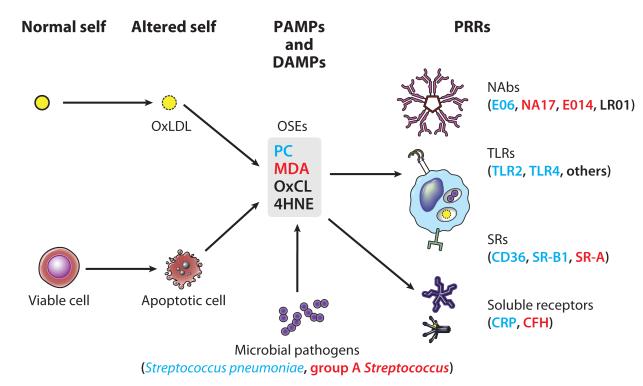
Because human B-1 cells, like B-2 cells, express CD20, they may also be targeted and depleted with anti-CD20 monoclonal antibodies that are currently used to treat autoimmune diseases; this treatment could be unfavorable for CVD. It will be important to monitor IgM titers and CVD parameters in patients who are chronically treated with this therapy. Figure 1 illustrates the concept that OSEs are an important target of multiple innate immune PRRs and uses PC as a representative OSE.
Figure 1.
Summary of oxidation-specific epitopes (OSEs) as major antigens of innate immunity. Oxidation-induced changes in normal self-molecules generate OSEs that are recognized as damage-associated molecular patterns (DAMPs) by the innate immune system. OSEs are also found on apoptotic cells and on microbes (or molecular mimics), where they serve as pathogen-associated molecular patterns (PAMPs). Oxidation of low-density lipoprotein (LDL), which occurs in atherosclerotic lesions, generates several OSEs, such as exposed phosphocholine (PC), malondialdehyde (MDA), oxidized cardiolipin (OxCL), and 4-hydroxynonenal (4HNE). These DAMPs are recognized by various pattern recognition receptors (PRRs) of the innate immune system. For example, PC on OxLDL, apoptotic cells, and Streptococcus pneumoniae is recognized by the innate immunoglobulin M (IgM) natural antibody (NAb) E06, the scavenger receptors (SRs) CD36 and SR-B1, and the pentraxin C-reactive protein (CRP). MDA is recognized by the NAbs E014 and NA17, SR-A, and complement factor H (CFH). An MDA mimic recognized by E014 is found on group A Streptococcus. These OSEs and others are present in atherosclerotic lesions and act as major antigens stimulating innate and adaptive responses that affect the progression of vascular disease.
The NLPR3 Inflammasome
IL-1β, a principal innate immune system cytokine that is most abundantly produced by activated macrophages, is expressed in atherosclerotic lesions and is implicated in lesion inflammation by many in vitro and in vivo studies (reviewed in Reference 44). IL-1β deficiency in Apoe−/− reduces lesion development (45). Recent studies suggest that cholesterol crystals emerge at the earliest time points of diet-induced atherogenesis, concurrently with the appearance of immune cells in the subendothelial space (4). Indeed, lipid peroxidation promotes the formation of crystalline structures of free cholesterol (46). In atherosclerotic lesions, cholesterol crystals are found not only outside cells but inside macrophages, and they activate the intracellular PRR NLRP3 (cryopyrin), the critical component in caspase-1-mediated production of active IL-1 (4, 47). Indeed, cholesterol crystals induced IL-1β secretion by wild-type but not Nlrp3−/− macrophages. Transplantation of Nlrp3−/− or Il-1−/− bone marrow into Ldlr−/− mice significantly reduced diet-induced atherosclerosis in one model (4), but another study found that NLRP3 deficiency did not influence atherogenesis in Apoe−/− mice (48).
VASCULAR ANTIGEN-PRESENTING CELLS: THE BRIDGE BETWEEN INNATE AND ADAPTIVE IMMUNE RESPONSES IN ATHEROSCLEROSIS
Antigen-presenting cells (APCs) are essential to adaptive immune responses because they engulf and process protein antigens and present them in association with MHC complexes on the cell surface for recognition by T cells. DCs are the most important APCs for the activation of näive T cells, whereas DCs, macrophages, and B cells all function as APCs for effector and memory T cells that were previously differentiated from activated näive T cells. DCs constitutively populate most tissues of the body in which—by virtue of their rich repertoire of PRRs, their long dendrite-like cytoplasmic processes, and their robust phagocytic and endocytic activity—they function as efficient sentinels of PAMPs and DAMPs. After innate immune simulation, DCs migrate from tissues into draining lymphoid tissues, where they may interact with recirculating näive T cells. Thus, DCs probably encounter antigens from arterial walls and are involved in activation of T cells specific for these antigens.
There is a well-established and evolving literature on resident vascular APCs in normal and atherosclerotic arteries. The presence of class II MHC-expressing macrophages within the intima of early human atherosclerotic lesions was described in the early 1990s (49). Subsequently, cells with morphology and markers consistent with DCs (CD1a, s100) were described in normal arteries, and these cells were more abundant in early lesions (50, 51). Morphologic and immunohistochemical studies using more specific DC markers have confirmed the existence of human and mouse vascular DCs and have demonstrated their topological proximity with lesional T cells (52, 53). DCs within atherosclerotic lesions take up cholesterol and (presumably) cholesterol-containing lipoproteins, and such lipid-loaded DCs remain capable of antigen presentation (54). Two photon microscopy studies of explanted mouse aortas show that atherosclerotic lesion resident CD11c+ APCs can activate effector T cells (55). To date, there is no definitive evidence that DC migration from arteries to lymphoid tissues is required for initiation of proatherogenic T cell responses; indeed, such artery-to-lymph node migration may not be required to activate näive T cells by antigens such as OxLDL, given that such OSEs can also be generated in extravascular sites.
ADAPTIVE IMMUNE RESPONSES THAT ARE RELEVANT TO ATHEROSCLEROSIS
In humans, the evidence that adaptive immune responses contribute to the development and complications of atherosclerotic lesions is restricted to correlative data from analyses of blood from CVD patients and to examinations of atherosclerotic lesions removed surgically or at autopsy. Specific serum biomarkers of adaptive humoral immune responses that correlate with atherosclerotic disease include levels of antibodies that are specific for HSP60 or HSP65 (reviewed in Reference 56) and for OxLDL (reviewed in References 40 and 57). Many studies have found correlations between various subsets of T cells in the blood of patients with acute coronary syndrome (ACS), discussed below. The strikingly elevated risk of atherosclerotic disease in patients with systemic autoimmune diseases, especially systemic lupus erythematosus and rheumatoid arthritis (58), also supports the hypothesis that adaptive immune responses promote atherosclerosis, although in both of these diseases, systemic inflammation mediated by innate immune cytokines [e.g., tumor necrosis factor (TNF) or type I interferons (IFNs)] may be the downstream culprits in promoting vascular disease.
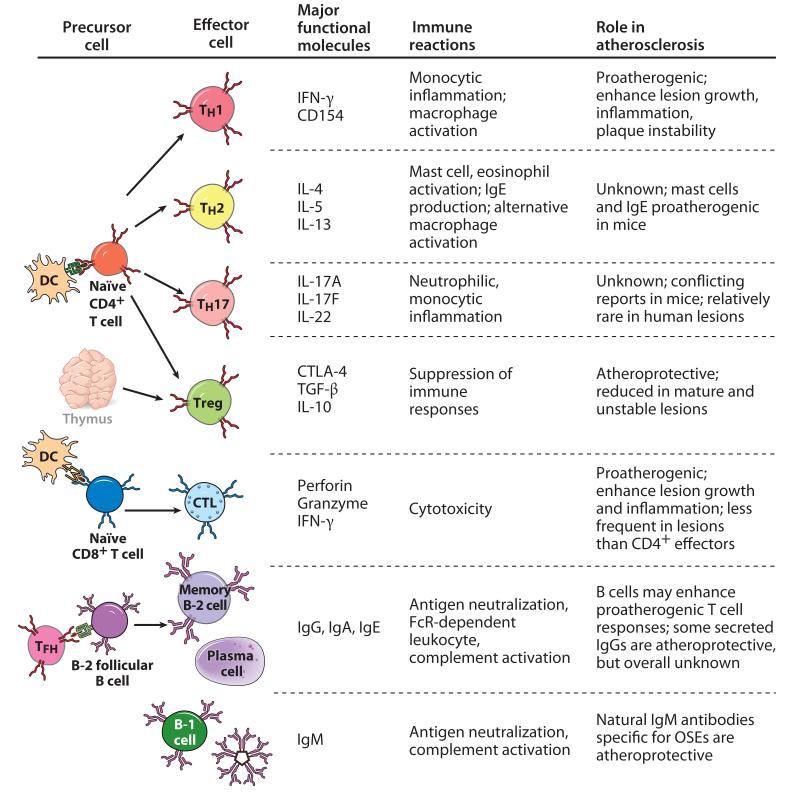
During the past 20 years, genetically manipulated mice have been used to test whether various components of adaptive immunity affect atherosclerotic lesion development and phenotype. The impact of a complete absence of adaptive immunity on atherosclerosis was examined by use of atherosclerosis-prone Ldlr−/− or Apoe−/− mice crossed with Rag1−/−, Rag2−/−, or SCID mice, each of which lacked both T and B lymphocytes. In the presence of very high cholesterol levels, lesion progression was not affected by the absence of lymphocytes, suggesting that adaptive immunity has little impact on atherogenesis in the context of overwhelming hypercholesterolemia, perhaps because macrophages can be activated by OxLDL alone. With more moderate degrees of hypercholesterolemia, atherogenesis was retarded, suggesting that overall, adaptive immunity was proatherogenic (59-61). However, this approach eliminates both proinflammatory and protective effects of T cells, B cells, and antibodies; therefore, more selective genetic manipulations of must be performed to address the contributions of individual components of adaptive immunity to atherosclerosis. The following discussion concentrates more on recent advances and is divided into three major parts focusing on T cells, B cells and the antibodies they produce, and regulatory mechanisms of adaptive immunity. Figure 2 summarizes the influence of different subsets of lymphocytes on atherosclerosis.
Figure 2.
Lymphocyte effector cells and their role in atherosclerosis. CD4+ helper T (TH) cells differentiate from näive CD4+ T cells in response to antigen presentation by dendritic cells (DCs) and include TH1, TH2, and TH17 subsets defined by the cytokines they produce. Likewise, cytotoxic T lymphocytes (CTLs) are derived from antigen-stimulated näive CD8+ T cell precursors. The evidence from mouse models has clearly established that TH1 cells exert proatherogenic effects, but the impact of TH17 or TH2 cells is not clear. CTLs, which kill antigen-bearing target cells and secrete interferon (IFN)-γ, contribute to lesion growth and inflammation. CD4+ regulatory T cells (Tregs), which may be derived from näive T cells in peripheral lymphoid tissues or develop in thymus, have a profound protective effect against lesion growth and inflammation. B-2 cells that recognize proteins or protein-linked antigens receive help from follicular helper T cells (TFH) and differentiate into memory B cells or long-lived plasma cells that secreting high-affinity, isotype-switched antibodies. B-2 cells, but not the antibodies they produce, may promote proatherogenic T cell responses. The overall impact of immunoglobulin G (IgG) secreted by B-2 cells is unknown, although some IgG antibodies to oxidation-specific epitopes (OSEs) provide atheroprotection. In contrast, innate B-1 cells secrete IgM natural antibodies, which overall appear to be atheroprotective. Abbreviations: CTLA, cytotoxic T lymphocyte antigen; IL, interleukin; TGF, transforming growth factor.
T Lymphocytes: General Considerations
Activated CD4+ and CD8+ T cells were first identified in early- and late-stage human atheromata in the 1980s by immunohistochemistry (62, 63). T cells were also identified in early lesions in cholesterol-fed rats (64). These observations stimulated investigators to explore how T cells could influence lesion development and stability. Atherosclerotic lesions in Ldlr−/− and Apoe−/− mice also contain T lymphocytes (65, 66), and targeted gene deletions in these mouse lines have demonstrated that T lymphocytes do have a profound impact on lesion development and phenotype. T cells can theoretically influence atherosclerosis disease in two general ways: (a) by performing effector functions locally within the arterial wall that affect vascular wall cells and other leukocytes and (b) by stimulating T cell-dependent B cell responses within lymphoid organs that produce circulating antibodies, which may promote or inhibit inflammatory events in arterial walls. Many of the putative driving antigens, discussed below, can be present systemically in dyslipidemic individuals; thus, lymphocyte activation may take place within any lymph node or spleen. Studies have found evidence of systemic T cell activation in the spleens of hypercholesterolemic mice (67) and in the blood of patients with ACS or stable angina (68).
T Cell Antigens
It is likely that several different antigens drive T cell responses in atherosclerotic lesions, but definitive identification of any of these antigens remains elusive. The most frequently studied putative T cell antigens in atherosclerosis are those found in OxLDL (69) and native LDL (70). These LDL-associated antigens are most likely to be peptides derived from apolipoprotein B 100 (ApoB-100), the major protein component of LDL particles. Another protein that has been implicated in atherosclerosis is HSP60 (3). In both cases, the relevant MHC-binding peptide epitopes of these proteins that stimulate proatherogenic T cells are not known. T cell responses to native ApoB-100 or HSP60, which correlates with atherosclerotic disease, indicate a loss of tolerance to these self-antigens, perhaps because these same molecules can stimulate innate immune responses that activate DCs (56, 71). ApoB-100 is covalently modified during LDL oxidation in atherosclerotic plaques, which may generate new OSEs to which individuals are not tolerant and/or break tolerance to native peptides of ApoB. Note that other antigens have been implicated in atherosclerosis; they include those made by infectious organisms such as Chlamydia pneumoniae, Helicobacter pylori, Porphyromonas gingivalis, herpes virus, and human immunodeficiency virus (2).
CD4+ Helper T Cell Subsets
Since the simultaneous presence of CD4+ T cells and IFN-γ gene expression in atherosclerotic lesions was first reported (72, 73), many studies have addressed the effector phenotype of lesional T cells, which is crucial to how the T cells may affect lesion growth and stability. Distinct helper T (TH) cell subsets, defined largely by the cytokines they produce, differentiate from näive CD4+ T cells in response to antigen, costimulators, and cytokines. The three most-studied TH cell subsets are TH1, which secretes IFN-γ; TH2, which secretes IL-4, IL-5, and Il-13; and TH17, which secretes IL-17 (A and F) and IL-22. Although populations of TH cells that arise during an immune response may express different mixtures of cytokines and may not neatly fall into one of these subsets, chronic or repeated antigen exposure often results in the emergence of a dominant polarized TH subset, which may be responsible for manifestations of disease. If one or another TH cell subset has a dominant effect in driving plaque inflammation, either early or late in disease, then a therapeutic opportunity exists to block the cytokines produced by that subset or to block the cytokines or other factors that drive the differentiation of that subset.
Ample evidence indicates that TH1 cells and IFN-γ enhance atherosclerotic lesion development and contribute to lesion rupture. As mentioned above, the identification of IFN-γ protein and mRNA in human lesions (72, 74), as well as the expression of IFN-γ by T cells cloned from human plaques (74), supports the hypothesis that TH1 cells play an important role in atherosclerosis. This concept was further validated by mouse studies in which genetic ablation of IFN-γ or IFN-γ receptor expression significantly reduced lesion development in Ldlr−/− mice (75, 76) but administration of exogenous IFN-γ enhanced lesion development (77). The lineage-defining transcription factor T-bet is essential for TH1 differentiation, and T-bet deficiency in Ldlr−/− mice reduces atherosclerotic lesion formation (78).
IL-12 is the most clearly established cytokine that drives TH1 differentiation from näive T cells, and several studies have implicated this cytokine in the promotion of lesion development and instability. Treatment of Apoe−/− with injections of IL-12 enhanced early lesion development (79). IL-12p40-deficient Apoe−/− mice had significantly reduced aortic lesion development (80), but this finding could also reflect a lack of IL-23, which is required for maximal TH17 cell responses and shares the same p40 subunit found in IL-12. Conversely, a meta-analysis of studies of anti-p40-treated psoriasis patients showed greater risk of major cardiovascular events (81), but again, this observation could reflect changes in either TH1 or TH17 cell differentiation.
IL-18 is an innate immune cytokine that enhances IFN-γ production by T cells and NK cells and also promotes TH1 differentiation. Active IL-18, like IL-1β, is produced by NLRP3 inflammasome-mediated cleavage of an inactive pro-IL-18 precursor; therefore, NLRP3 inflammasome activation by intracellular cholesterol crystals generates IL-18. Most of the available data indicate that IL-18 is proatherogenic. IL-18 and its receptor are expressed by arterial wall cells (82) and in atherosclerotic plaques (83). Several clinical studies have shown that serum IL-18 levels predict risk of CVD (84). IL-18 gene polymorphisms are reported to affect circulating IL-18 levels and clinical manifestations of coronary artery disease (85), although the impact of these polymorphisms on mortality is unknown (86). Most published studies performed on IL-18-deficient or IL-18-treated atherosclerosisprone mice demonstrated that IL-18 promotes lesion development and lesional inflammation and that these effects depend on IFN-γ (87, 88).
IFN-γ exerts various biological effects that are predicted to either promote lesion development or destabilize established lesions. These effects include induction of adhesion molecule and chemokine expression by ECs, stimulation of proinflammatory cytokine and chemokine secretion by macrophages, production of reactive oxygen species and matrix metalloproteinases by macrophages, inhibition of cholesterol efflux from foam cells, and inhibition of collagen synthesis by vascular smooth muscle cells (89).
There are limited and inconsistent data on the influence of TH2 cells or the cytokines they produce (IL-4, IL-5, or IL-13) on both human and mouse atherosclerotic disease. Most reports that showed the presence of IFN-γ and IFN-γ-expressing T cells in atherosclerotic lesions in both mice and humans (discussed above) found no TH2 cytokines. In Ldlr−/− mice that lack the TH1-driving transcription factor T-bet, there are enhanced systemic TH2 responses and decreased lesion development (78). However, some reports suggest that TH2 cells or IL-4 may be associated with advanced atherosclerosis in Apoe −/− mice (73). Furthermore, reduced lesion development was reported in IL-4-deficient Ldlr−/− mice and Apoe−/− mice (80, 90).
TH17 cells were discovered approximately 20 years after TH1 and TH2 cells were first described; this discovery led to a reassessment of the phenotype of TH cells involved in various immune/inflammatory diseases in both mice and humans. TH17 cells and IL-17 play critical roles in protective immunity and immunopathology (reviewed in Reference 91), but their impact on atherosclerosis remains unclear. Although the expression of IL-17A proteins and mRNA in human lesions has been documented (92, 93), correlations between the amount of lesional and blood IL-17 and plaque stability and clinical disease are limited, and no clear message has emerged. Significantly, the ratio of IFN-γ-expressing TH cells to IL-17-expressing T cells extracted from human coronary arteries was approximately 10:1, and many T cells expressing both IL-17 and IFN-γ were present (94). These findings suggest that TH17 cells may be rare in these lesions, although they may be readily detectable only following activation with their cognate antigens.
Several studies using Apoe−/− or Ldlr−/− mice support a proinflammatory and proatherogenic role for TH17 cells and/or IL-17. Blockade of IL-17 and global genetic IL-17A deficiency reduced lesion size, macrophage content, reactive oxygen species, and the expression of various inflammatory cytokines (95-97). In contrast, other studies indicate that IL-17 is atheroprotective. For example, IL-17 administration reduced lesion formation and inflammation (92, 98), whereas IL-17 gene deletion accelerated unstable plaque development (98).
The inconsistencies in the available data concerning the role of TH17 cells probably reflect complex and still poorly understood relationships between TH1 and TH17 responses. An explanation for an atheroprotective effect is that IL-17 blocks differentiation of proatherogenic TH1 cells (92, 99). However, in mouse disease models and in humans, both TH17 and TH1 cells contribute to pathogenesis of the same autoimmune diseases, and it has become evident that these subsets are plastic, that they can redifferentiate from one to the other, and that dual IL-17- and IFN-γ producing T cells are frequently present. An interesting possibility that may explain contradictory data on TH17/IL-17 responses in atherosclerosis relates to the status of microbial flora in the gut. Enteric bacterial flora can profoundly influence the propensity of mice to mount TH17 responses to autoantigens (100); therefore, differences in the gut flora in different animal facilities or patient populations may influence the contribution of TH17 cells to atherosclerosis. Regardless of the differing preclinical data of the impact of IL-17, as anti-IL-17A therapy for psoriasis nears clinical use (101), the opportunity will soon arise to study the impact of IL-17A blockade on clinical manifestations of atherosclerosis.
Cytotoxic T Lymphocytes
CD8+ cytotoxic T lymphocytes (CTLs) are largely αβ TCR-expressing, class I MHC-restricted T cells that recognize peptides derived from cytosolic antigens. CTLs directly kill antigen-expressing target cells via a perforin/granzyme B-dependent mechanism, and they also secrete IFN-γ that activates macrophages. These cells are a mainstay of defense against viruses and intracellular bacteria and fungi, and they contribute to the pathology of many autoimmune diseases and allograft rejection. Although they are usually less abundant than CD4+ cells, CD8+ cells may constitute up to half of the T cells in mature human lesions (102). In most studies of Apoe−/− or Ldlr−/− lesions, very few CD8+ T cells are found. However, when certain immunoregulatory molecules are deficient or blocked, CD8+ T cells are abundant (103-105). CTL deficiency in Apoe−/− mice, which arises because of deletions of Cd8 or Tap1 genes, does not appear to affect lesion development (106, 107). However, a recent report shows that depletion of CD8+ T cells in Apoe−/− mice reduced atherosclerotic lesion development, lesional inflammation, and cell death and that perforin/granzyme B-mediated cytotoxicity was key to the proatherogenic effect of transferred CD8+ T cells (108). Overall, the data on CD8+ T cells in atherosclerosis indicate that this arm of cell-mediated immunity is activated in the context of hypercholesterolemia and within lesions, and although it may not contribute to early lesion development in mice or humans, CTLs can promote plaque inflammation and instability. The nature of the peptide antigens that CTLs recognize in atherosclerotic patients is an open question.
Unusual T Cell Subsets
Invariant natural killer T (iNKT) cells are largely αβ TCR-expressing T cells that have limited diversity because of invariant use of α-chain V and J genes and restricted use of β-chain V genes. These cells recognize glycolipid antigen presented by MHC-like CD1d molecules. iNKT cells behave as innate effectors, producing abundant inflammatory cytokines (including IFN-γ and IL-4) upon activation without the need for clonal expansion and differentiation (109). iNKT cells may also express Fas ligand and perforin and exert cytotoxic effects on other cells. These cells are relatively more abundant in mouse than human immune systems, but they have been implicated as proinflammatory effectors in both human and mouse diseases. Almost all studies inter-rogating the role of iNKT cells in Ldlr−/− or Apoe−/− mice have indicated a proatherogenic role, as reviewed in Reference 110. The relevance of iNKT cells in human atherosclerosis is supported only by limited data, including the finding of these cells (111) and CD1 (112) in human plaques.
CD4+CD28null T cells are cytotoxic and IFN-γ-expressing effector T cells that are infrequently found in healthy young people but increasingly present with age and in the setting of chronic inflammatory diseases (113). These cells express αβ TCRs and killer Ig-like receptors that recognize self MHC molecules. They secrete inflammatory cytokines and have cytotoxic capability. Several studies have correlated increased numbers of these cells in lesions and blood of patients with ACS. A significant fraction of these cells in ACS patients appear to be of a single clone (114), and many recognize HSP60, a putative atherosclerosis-associated autoantigen, in a class II MHC-restricted manner (115).
B Cells and Adaptive Immune Antibody Responses
The humoral arm of adaptive immunity is mediated by B-2 (or follicular B) cells. These cells respond to antigens in a T cell-dependent manner by undergoing isotype switching and affinity maturation, ultimately differentiating into antibody-secreting B cells and long-lived plasma cells that produce high-affinity IgG, IgA, and IgE antibodies. Ample data indicate that B-2 cells respond to atherosclerosis-associated antigens, but assessing their impact on atherogenesis is complicated by the need to assess the impact of the antibodies they secrete versus a potential direct effect of the B cells themselves. With respect to antibodies, OSE-specific IgG molecules are detectable in the blood of healthy individuals and in the blood and lesions of atherosclerosis patients and animal models with atherosclerotic disease (5, 57). In mice, the titers of OxLDL-specific IgG molecules correlate well with lesion progression and regression (116), but such titers in humans are more variable, probably because of greater variability in patient populations and in disease history. Nevertheless, in univariate analyses, there is generally a positive correlation of OxLDL-specific IgG levels with clinical manifestations of CVD (40, 57). Whether or not the titers of such IgG molecules are sufficient to modify CVD, or are only biomarkers of disease, remains unclear.
In animal models, raising the titers of IgG to OSEs by (for example) immunization with homologous MDA-LDL is atheroprotective (117). Additional studies have confirmed that elevations of OSE-specific IgG in atherosclerosis-prone mice, by various methods, reduces lesion formation (5, 118, 119). Even Fab fragments or single-chain variable fragments of anti-OxLDL antibodies, which cannot engage Fc receptors or complement, can protect against atherosclerosis (120). That these OxLDL-specific antibodies inhibit the uptake of OxLDL by macrophages in culture may explain their atheroprotective properties. Overall, these findings suggest that there may be therapeutic value in raising the titers of at least some OSE-specific antibodies. Doing so could be accomplished by passive immunization and/or by active immunization with OSE antigens that raise titers to OSEs of OxLDL.
Other data suggest that B cells are athero-protective through antibody-independent mechanisms. For example, Ldlr−/− bone marrow chimeras with B cell-deficient marrow developed more atherosclerosis than did control chimeras (121) and showed evidence of reduced TH cell activation. A recent study showed that Id3- and CCR6-dependent homing of B-2 cells to aortic adventitia protected Apoe−/− mice against intimal macrophage accumulation in early lesion formation (122). In contrast, other recent mouse studies support a proatherogenic role of B-2 cells. Anti-CD20 antibodies deplete mouse B-2 cells but not peritoneal B-1 cells; these antibodies reduced atherosclerosis in Ldlr−/− and Apoe−/− models, which depended in part on altered DC and T cell activation (99, 123). Apoe−/− or Ldlr−/− mice with B cell-activating factor receptor (BAFF-R) deficiency have a selective reduction in B-2 cells, but not B-1 cells, and reduced IgG titers. These BAFF-R-deficient mice have smaller and less inflamed atherosclerotic lesions than those of control mice (124, 125); again, this finding implicates a proatherogenic role for B-2 cells. Because both anti-CD20 and anti-BAFF antibodies are approved drugs for autoimmune diseases, it will be important to monitor treated patients for the impact of these interventions on CVD status.
Antibodies engage effector and regulatory mechanisms by the binding of their Fc regions to isotype-specific Fc receptors expressed on different cell types or to complement proteins. The impact of some Fc receptors on atherosclerosis has been studied in mice. Deficiency of CD16 (FcγRIII) (126) or the common γ chain of activating Fcγ receptors reduced atherosclerosis (127), whereas deficiency of the inhibitory FcγRIIb receptor had the opposite effect (128, 129). Deficiency of the IgE receptor Fc R1α protected against atherosclerotic lesion growth and inflammation in Apoe−/− mice (130). There is evidence that complement activation occurs in murine and human atherosclerotic lesions, but the significance of antibody-dependent complement activation on atherosclerosis has not been established (reviewed in Reference 131).
REGULATION OF PROATHEROGENIC ADAPTIVE IMMUNE RESPONSES
One can consider atherosclerosis a disease in which there is inadequate regulation of adaptive immune responses to normal or altered components of lipoproteins that accumulate in lymphoid organs and in the arterial intima of humans. In this section, we focus mainly on three interrelated mechanisms of adaptive immune regulation that have been explored in the context of atherosclerosis, namely costimulatory molecules, coinhibitory molecules, and regulatory T cells. These mechanisms are best understood in the context of T cell responses, but they are also relevant to B cell responses.
T Cell Costimulatory and Coinhibitory Pathways
T cell costimulators are membrane proteins expressed on APCs that bind to receptors on T cells concurrently with antigen recognition; they are required for the activation of näive T cells and their differentiation into effectors. Costimulatory blockade is an effective therapy for the treatment of T cell-driven autoimmune disease and, therefore, may be beneficial in inhibiting T cell responses that promote lesion growth or destabilization. The most thoroughly studied costimulatory molecules and receptors are those in the B7/CD28 families. Ligand/receptor pairs of the TNF/TNF receptor superfamilies (TNFSFs/TNFRSFs) also costimulate T cells, and their role is more likelyto enhance activation of effector T cells. The influence of costimulation on atherosclerosis has recently been reviewed (132, 133). A proatherogenic role of the CD80 (B7-1)/CD86 (B7-2) costimulators, which bind CD28 on T cells, is supported by findings of reduced lesion development and reduced hypercholesterolemia-dependent priming of HSP60-reactive T cells in CD80/CD86-deficient Ldlr−/− mice (67). Moreover, lesion formation is reduced in postinjury or homocysteine-mediated mouse models of accelerated atherosclerosis when the mice are treated with the CD80/CD86 blocking agent cytotoxic T lymphocyte antigen 4 (CTLA-4) Ig (134, 135). Gene-deletion and antibody-blocking studies have also demonstrated a proatherogenic influence of the TNFSF costimulatory protein OX40 ligand, which binds the TNFRSF receptor OX40 on T cells (136, 137). CD40 ligand (CD154) is a TN-FSF protein expressed on activated T cells that binds to the TNFRSF protein CD40 on several cell types, including B cells, macrophages, and ECs. CD40 ligand/CD40 interactions are not strictly costimulatory, but they are critical for T cell-dependent B cell responses and for T cell activation of DCs and macrophages, including upregulation of costimulatory molecules. Antibody blockade or genetic deficiencies of the CD40 ligand/CD40 pathway significantly reduce murine atherosclerosis (138-140). The development of therapeutics targeting this pathway has been complicated by the fact that both CD40 and CD40 ligand are expressed on many different cell types, including platelets (reviewed in Reference 141).
T cell activation can be negatively regulated by proteins expressed on APCs that bind to signaling receptors on T cells concurrently with antigen recognition. These so-called coinhibitory pathways are required to maintain tolerance to self-antigens and to physiologically downregulate immune responses to foreign antigens. One well-studied coinhibitory pathway involves PD-1 (PDCD1 or CD279), which is a CD28 family protein expressed on activated T cells that binds to PD-L1 (B7H1, CD274) or PD-L2 (B7-DC, CD273) and engages protein tyrosine phosphatases to counteract kinases activated by antigen recognition and costimulation. Both PD-L1 and PD-L2 are expressed on DCs, but PD-L1 is also expressed on a wide variety of cytokine-activated cells, including ECs, and many tumors. Therapeutic targeting of coinhibitory pathways has been developed to enhance immunity to tumors and viral infections. Genetic deletion of PD-1 or PD-L1 and PD-L2, or antibody blockade of this pathway, enhances atherosclerotic lesion development and plaque T cell numbers in Ldlr−/− mice (104, 105). Furthermore, T cell and DC expression of PD-1 and PD-L1 is decreased in coronary artery disease patients relative to healthy controls (142). These findings highlight the potential CVD risk of blockade of the PD-1/PD-L1 pathway and the therapeutic opportunities of engaging the pathway with agonist reagents.
Regulatory T Cells
Regulatory T cells (Tregs) play an important role in atherogenesis, and their biology has recently been reviewed (143). Tregs include various subsets of T lymphocytes whose major functions are to inhibit immune responses, rather than to provide protective immunity against pathogens. The best-defined subsets of Tregs are αβTCR+CD4+CD25hiCD127lo cells that also express the transcription factor FoxP3. Other molecules expressed by Tregs that have important functional roles are the CD28 family coinhibitory receptor CTLA-4, which binds CD80 and CD86, and the secreted cytokines transforming growth factor (TGF)-β and IL-10. Human and mouse mutations in FoxP3 reveal that Tregs are required to prevent lethal systemic autoimmune disease. FoxP3+ Tregs include thymic Tregs (tTregs), which develop in the thymus and constitute the majority of circulating Tregs in human blood, and peripheral Tregs (pTregs), which arise from näive CD4+ T cells in secondary lymphoid tissues during immune responses. Other subsets of Tregs, including CD8+ Tregs, have been characterized, although their overall contributions to immune regulation are not well understood. The targets of suppression of Tregs include näive and effector CD4+ and CD8+ T cells, DCs, macrophages, and ECs. The mechanisms by which Tregs suppress immune responses include CD25-mediated competitive binding of IL-2 to deprive effector T cells of this growth factor; CTLA-4-mediated competitive binding and perhaps removal of B7-1 and B7-2 on APCs; and suppressive actions of IL-10, IL-35, IL-9, and TGF-β on APCs and T cells.
In humans, an atheroprotective role of Tregs is supported by circumstantial evidence, including the presence of Tregs within human arterial lesions and changes in the numbers of circulating Tregs that correlate with changes in disease activity and/or with effective therapy. Low numbers of CD4+FoxP3+ T cells have been identified in human atherosclerotic plaques at all stages of development (144). Increased numbers of FoxP3+CD3+ T cells were found in carotid plaques from symptomatic patients compared with plaques from asymptomatic patients (145), although the ratio of these putative Tregs to effector T cells was not determined. The enumeration of circulating FoxP3+CD4+ T cells is the low-hanging fruit in studying the status of Tregs in human diseases, but this approach may have limited value in studying the status of Treg function within lymphoid tissues or in atherosclerotic plaques. One study reported decreased numbers of Tregs in patients with ACS (146), whereas another reported that numbers of blood Tregs do not correlate with the severity or extent of carotid or coronary atherosclerosis (147).
Numbers of Tregs in plaques or in the blood of CVD patients appear to decrease in response to various therapeutic interventions, including drug-eluting stent placement (148) and statin therapy (149). Overall, the data suggest that increasingly abundant Tregs may be a feasible goal for pharmacologic immuno modulation of patients who are at risk of complications of unstable plaques. The effects of statins on Treg development and function are particularly interesting, given the enormous number of patients taking these drugs and the well-documented pleiotropic anti-inflammatory effects of statins, independent of their cholesterol-lowering properties (150).
Studies in which Tregs have been depleted, induced, or transferred offer support for an atheroprotective role for Tregs in mouse models of atherosclerosis. Often, these studies show that the changes in numbers of Tregs in aortic lesions are the inverse of changes in numbers of total T cells and macrophages (151). When Tregs are depleted in Apoe−/− mice by anti-CD25 monoclonal antibody treatment, lesion development increases (152). Impaired Treg development or function in bone marrow chimeric Ldlr−/− mice with hematopoietic deficiency of the CD28 or ICOS (inducible T cell costimulator) costimulatory pathways also enhances the development of lesions (152, 153), which can be prevented by Treg transfer (152). Other gene deletions in Apoe−/− or Ldrl−/− mice, such as deletions of the genes encoding the FcRγ chain (154), CCL17 (155), and leptin (156), simultaneously reduce atherosclerosis and increase the number of Tregs. In aggregate, published studies show that the number of Tregs is subject to multiple pathways of regulation and suggest that drugs that target these regulatory pathways can be used to favorably increase the ratio of Tregs to effector T cells in atherosclerotic lesions.
Various strategies have been used to systemically increase Treg in mice and assess the influence on atherosclerosis. Because IL-2 is an essential factor for Treg differentiation and survival, the idea that IL-2 could be used as a drug for autoimmune disease has already been explored. Delivery of IL-2 into lesions in Apoe−/− mice via an IL-2/antifibronectin antibody reversed lesion growth and increased the number of Tregs (157). Furthermore, injection of complexes of IL-2 and anti-IL-2 antibody, a preparation known to enhance IL-2 binding to the IL-2 receptor, into Ldlr−/− and Apoe−/− mice potently expanded the number of Tregs, suppressed early lesion growth, and stabilized established lesions (158, 159). Oral administration of an anti-CD3 antibody to Apoe−/− mice increased systemic and lesional Tregs and reduced the development of atherosclerotic lesions (160).
The mechanisms by which Tregs protect against lesion progression and why they ultimately fail require further study. Decreased Treg-to-T effector ratios in mouse aortas during prolonged hypercholesterolemia correlates with enhanced lesion development and inflammatory content (161), which probably reflects diminishing Treg suppression of T effector responses. The finding that T cell-specific expression of a dominant-negative TGF-β receptor causes enhanced lesion development and inflammation in Apoe−/− mice (162) is consistent with the importance of Treg-derived TGF-β suppression of effector T cells in lesions. Tregs may also suppress innate responses in the plaque, such as EC or macrophage activation by TLR ligands or cytokines. IL-10, which is produced by Tregs and inhibits macrophage activation, has marked atheroprotective functions (163). Interestingly, MyD88 deficiency in DCs causes decreased Treg response and enhanced atherosclerosis in Apoe−/− mice (164). The mechanism for the increased atherosclerosis in this model appears to be reduced Treg/TGF-β suppression of MCP-1 expression, which gives rise to increased inflammatory macrophage accumulation.
IMMUNOTHERAPY FOR ATHEROSCLEROSIS
Approaches to Induce Immune Tolerance to Atherosclerosis Antigens
The holy grail in the fields of autoimmunity and transplant immunology is to achieve longterm specific adaptive immune tolerance to the relevant self- or allogeneic antigens that incite the pathological immune responses, which would avoid nonspecific immunosuppression. This concept has been embraced by investigators studying atherosclerosis. The most frequently reported approaches have included subcutaneous injection of putatively tolerizing preparations of antigens, delivery of antigens via nasal or oral mucosa, and adoptive therapy with antigen-pulsed DCs that have been subjected to tolerizing treatments in vitro. Subcutaneous immunizations that are reported to reduce atherosclerosis in mice include HSP65 in alum adjuvant (165); ApoB-100-derived peptides without adjuvant (166); and ApoB-100 peptide conjugated to various substances, such as cationized BSA (167). In these and other reports, there appeared to be increases in systemic numbers of Tregs in the treated mice.
With the intention of inducing mucosal tolerance to atherosclerosis-relevant antigens, investigators have treated hypercholesterolemic mice with intranasal ApoB-100 fusion protein (169), oral OxLDL (170), or oral HSP60 or HSP60-peptides (170). In each case they found reduced lesion development and evidence for increased numbers of Tregs. Transfer of IL-10-treated ApoB-100-loaded DCs into Apoe−/− mice reduced atherosclerosis (171), and this effect correlated with increased antigen-specific suppressor activity of spleen CD4+ cells in the treated mice. Clearly, better information about the actual antigen specificity of proatherogenic effector T cells and antibodies will be required to refine and optimize tolerance-induction protocols.
Treating Lesion Inflammation
After decades of accumulating scientific evidence, we have entered a period of clinical trials that are designed to directly test the efficacy of anti-inflammatory therapy for the prevention of atherosclerosis-related morbidities and mortality. The JUPITER trial may be considered a seminal example of these trials because it tested whether healthy individuals with elevated high-sensitivity CRP (hsCRP; a systemic biomarker of inflammation) but with normal LDL cholesterol levels (i.e., 107 mg dl−1) would benefit from statin treatment. The results indicated that statin treatment did reduce risk in this population (172), and the most significant decrease was observed in subjects with the greatest reduction in both LDL and hsCRP levels (173). Thus, the findings of the JUPITER trial are consistent with the concept that the beneficial effects of statins are due to both the cholesterol-lowering effect and an anti-inflammatory effect of statins (174). Recall that cholesterol lowering itself is anti-inflammatory (175) and can be considered antigen reduction.
Two currently active clinical trials are designed to directly test whether anti-inflammatory drugs reduce the risk of cardiovascular events without lowering cholesterol. In the CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study), long-term anti-IL-1β antibody therapy is compared with placebo in stable post-myocardial infarction patients with persistently elevated hsCRP (44). In the Cardiovascular Inflammation Reduction Trial (CIRT), low-dose methotrexate treatment is compared with placebo in stable post-myocardial infarction patients with type 2 diabetes (176). In both cases, the end points are recurrent myocardial infarction, stroke, and cardiovascular death.
Various anti-inflammatory drugs have been widely used for the treatment of autoimmune diseases that are associated with enhanced risk of atherosclerosis-related comorbidities; these diseases include rheumatoid arthritis, systemic lupus erythematosus, and psoriasis (Table 1). Relatively few studies have addressed whether a particular drug effectively reduces CVD risk in such patients. An example is the treatment of RA patients with methotrexate, which reduces CVD risk (177). This dearth of studies is one of the rationales for CIRT. TNF antagonists have been used for RA and irritable bowel disease for up to 10 years, but these drugs apparently aggravate heart failure (178), and there are no conclusive data that they reduce elevated CVD risk. Biologic drugs targeting IL-17 or the IL-17 receptor are now being tested in clinical trials for the treatment of psoriasis, but no conclusions can yet be made about their effect on CVD risk.
Table 1. Selected potentially beneficial therapeutics for immune or inflammatory diseases in atherosclerosis.
| Drug or method | Current or proposed use | Relevance to immune responses in atherosclerosis |
|---|---|---|
| Statins | Cholesterol lowering to prevent CVD | Reduce the generation of atheroantigens; direct anti-inflammatory effects independent of cholesterol lowering |
| Methotrexate (low dose) | Approved for rheumatologic diseases, IBD, psoriasis; clinical trial ongoing for CVD |
Inhibits purine metabolism, which impairs T cell activation; RA patients have elevated risk of CVD |
| Anti-IL-1β | Clinical trial ongoing for CVD | IL-1β is made in atherosclerotic lesions; cholesterol crystals activate the inflammasome, which leads to IL-1β secretion |
| Anti-IL-17 | Clinical trials for psoriasis | IL-17 may be proatherogenic; psoriasis patients have elevated risk for CVD |
| Anti-p40 (IL-12/IL-23) | Approved for psoriasis | IL-12 is required for TH1 differentiation, and TH1 cells are proatherogenic; IL-23 is required for TH17 differentiation, and IL-17 may be proatherogenic; psoriasis patients have elevated risk of CVD |
| BAFF inhibitor | Approved for SLE | BAFF is required for B-2 B cell responses, and B-2 B cells may be proatherogenic; SLE patients have elevated risk of CVD |
| Anti-CD20 | Approved for RA and B cell lymphomas | Depletes B-2 B but not B-1 B cells in mice, and B-2 B cells may be proatherogenic; B-1 cells are protective, and a newly identified human B-1 B cell subset expresses CD20 |
| CTLA-4 Ig | Approved for RA and renal transplant rejection |
Blocks T cell costimulation by B7-1 and B7-2, and these costimulatory molecules enhance proatherogenic T cell responses |
Abb mations:jBAFF, bJ~cII—activating factor; CTLA, cytotoxic T lymphocyte antigen; CVD, cardiovascular disease; IBD, inflammatory bowel disease; Ig, immunoglobulin; IL, interleukin; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
SUMMARY POINTS.
Inflammation contributes to all stages of atherosclerotic lesion development, ranging from early recruitment of monocytes into the neointima of a fatty streak to the destabilization of mature lesions leading to rupture and arterial thrombosis.
The innate immune system, which has evolved to respond to molecular patterns that are typical of microbial pathogens or damaged tissue, drives inflammatory responses that promote lesion development.
Oxidation-specific epitopes (OSEs) of lipoproteins (and apoptotic cells) are recognized by innate system PRRs and by T and B cells, thereby stimulating both proatherogenic and atheroprotective immune responses.
Adaptive immune responses, which are mediated by T and B lymphocytes, drive inflammatory processes within developing lesions but also generate regulatory cells and antibodies with atheroprotective properties.
CD4+ TH cells with an IFN-γ-producing TH1 phenotype are the most consistently implicated T cell subset in promoting atherosclerotic lesion development and plaque instability. However, the impact of IL-17-producing TH17 cells remains unresolved.
Circulating IgG antibodies to OSEs of LDL and to HSP60 correlate with CVD activity, but their effect on atherosclerosis remains unclear. IgM NAbs specific for OSEs have atheroprotective activity in both in vitro assays and mouse models.
Innate B-1 cells, which produce IgM NAbs that are specific for OSEs, are atheroprotective, whereas some data indicate that B-2 cells exert proatherogenic effects on T cells, independently of Ig secretion.
Regulatory mechanisms restrain proatherogenic immune responses and lesion development, including regulatory T cells, coinhibitory molecules, and anti-inflammatory cytokines.
The strategy of therapeutic targeting of innate and adaptive immune responses to treat atherosclerotic disease has progressed to the clinical trial arena; anti-inflammatory drugs (methotrexate), anticytokine antibodies (anti-IL-1), and cholesterol-lowering drugs (statins) are being used in patients with inflammatory risk factors but normal cholesterol.
The expanding use of immune modulatory drugs, such as antagonists of cytokines or costimulatory molecules, to treat chronic immune and inflammatory diseases provides the opportunity to further interrogate the role of innate and adaptive immune mechanisms in human atherosclerosis.
Therapeutic targeting of antigen-driven proatherogenic adaptive immunity will require progress in identifying the relevant antigens and will benefit from progress in active efforts to treat autoimmune diseases.
ACKNOWLEDGMENTS
We thank the members of our research groups and colleagues, as well as collaborators whose work is related to the topic of review. We apologize to colleagues whose contributions to the field could not be cited or discussed due to space limitations. Our work was supported by the National Institutes of Health.
Footnotes
DISCLOSURE STATEMENT
J.L.W. has patents and patent applications related to the commercial use of oxidation-specific antibodies held by the University of California, San Diego, and is a consultant to Isis Pharmaceutical, Inc., and Regulus Therapeutics. A.H.L. is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Hansson GK, Robertson AK, Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu. Rev. Pathol. Mech. Dis. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 2.Tufano A, Di Capua M, Coppola A, Conca P, Cimino E, et al. The infectious burden in atherothrombosis. Semin. Thromb. Hemost. 2012;38:515–23. doi: 10.1055/s-0032-1315759. [DOI] [PubMed] [Google Scholar]
- 3.Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu. Rev. Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- 4.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011;108:235–48. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, et al. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J. Exp. Med. 2004;200:1359–70. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binder CJ, Hörkkö S, Dewan A, Chang MK, Kieu EP, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 2003;9:736–43. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 8.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Investig. 2009;119:1335–49. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang MK, Bergmark C, Laurila A, Hörkkö S, Han KH, et al. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. USA. 1999;96:6353–58. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang MK, Binder CJ, Torzewski M, Witztum JL. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: phosphorylcholine of oxidized phospholipids. Proc. Natl. Acad. Sci. USA. 2002;99:13043–48. doi: 10.1073/pnas.192399699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Libby P. Counterregulation rules in atherothrombosis. J. Am. Coll. Cardiol. 2012;59:1438–40. doi: 10.1016/j.jacc.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010;30:2311–16. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 13.Miller YI, Choi SH, Fang L, Tsimikas S. Lipoprotein modification and macrophage uptake: role of pathologic cholesterol transport in atherogenesis. Subcell. Biochem. 2010;51:229–51. doi: 10.1007/978-90-481-8622-8_8. [DOI] [PubMed] [Google Scholar]
- 14.Ylä-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J. Clin. Investig. 1989;84:1086–95. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harkewicz R, Hartvigsen K, Almazan F, Dennis EA, Witztum JL, Miller YI. Cholesteryl ester hydroperoxides are biologically active components of minimally oxidized low-density lipoprotein. J. Biol. Chem. 2008;283:10241–51. doi: 10.1074/jbc.M709006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao L, Funk CD. Lipoxygenase pathways in atherogenesis. Trends Cardiovasc. Med. 2004;14:191–95. doi: 10.1016/j.tcm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Leitinger N. Cholesteryl ester oxidation products in atherosclerosis. Mol. Asp. Med. 2003;24:239–50. doi: 10.1016/s0098-2997(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA. 1979;76:333–37. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kzhyshkowska J, Neyen C, Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology. 2012;217:492–502. doi: 10.1016/j.imbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J. Biol. Chem. 2002;277:38503–16. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 21.Freeman M, Ekkel Y, Rohrer L, Penman M, Freedman NJ, et al. Expression of type I and type II bovine scavenger receptors in Chinese hamster ovary cells: lipid droplet accumulation and nonreciprocal cross competition by acetylated and oxidized low-density lipoprotein. Proc. Natl. Acad. Sci. USA. 1991;88:4931–35. doi: 10.1073/pnas.88.11.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witztum JL. You are right too! J. Clin. Investig. 2005;115:2072–75. doi: 10.1172/JCI26130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J. Lipid Res. 2009;50(Suppl.):282–86. doi: 10.1194/jlr.R800066-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtiss LK, Tobias PS. Emerging role of Toll-like receptors in atherosclerosis. J. Lipid Res. 2009;50(Suppl.):340–45. doi: 10.1194/jlr.R800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards MR, Black AS, Bonnet DJ, Barish GD, Woo CW, et al. The LPS2 mutation in TRIF is atheroprotective in hyperlipidemic low density lipoprotein receptor knockout mice. Innate Immun. 2013;19:20–29. doi: 10.1177/1753425912447130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higashimori M, Tatro JB, Moore KJ, Mendelsohn ME, Galper JB, Beasley D. Role of Toll-like receptor 4 in intimal foam cell accumulation in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2011;31:50–57. doi: 10.1161/ATVBAHA.110.210971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi C, Papadopoulos G, Gudino CV, Weinberg EO, Barth KR, et al. Protective role for TLR4 signaling in atherosclerosis progression as revealed by infection with a common oral pathogen. J. Immunol. 2012;189:3681–88. doi: 10.4049/jimmunol.1201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–82. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010;11:155–61. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ. Res. 2010;107:737–46. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walton KA, Hsieh X, Gharavi N, Wang S, Wang G, et al. Receptors involved in the oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine-mediated synthesis of interleukin-8. A role for Toll-like receptor 4 and a glycosylphosphatidylinositol-anchored protein. J. Biol. Chem. 2003;278:29661–66. doi: 10.1074/jbc.M300738200. [DOI] [PubMed] [Google Scholar]
- 32.Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 33.Walton KA, Cole AL, Yeh M, Subbanagounder G, Krutzik SR, et al. Specific phospholipid oxidation products inhibit ligand activation of Toll-like receptors 4 and 2. Arterioscler. Thromb. Vasc. Biol. 2003;23:1197–203. doi: 10.1161/01.ATV.0000079340.80744.B8. [DOI] [PubMed] [Google Scholar]
- 34.Wiesner P, Choi SH, Almazan F, Benner C, Huang W, et al. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor κB and activator protein 1: possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ. Res. 2010;107:56–65. doi: 10.1161/CIRCRESAHA.110.218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Schlieffen E, Oskolkova OV, Schabbauer G, Gruber F, Blüml S, et al. Multi-hit inhibition of circulating and cell-associated components of the Toll-like receptor 4 pathway by oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 2009;29:356–62. doi: 10.1161/ATVBAHA.108.173799. [DOI] [PubMed] [Google Scholar]
- 36.Weismann D, Hartvigsen K, Lauer N, Bennett KL, Scholl HP, et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis MJ. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:417–26. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J. Clin. Investig. 2002;109:745–53. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, et al. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ. Res. 2011;109:830–40. doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- 40.Ravandi A, Boekholdt SM, Mallat Z, Talmud PJ, Kastelein JJ, et al. Relationship of IgG and IgM autoantibodies and immune complexes to oxidized LDL with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk Study. J. Lipid Res. 2011;52:1829–36. doi: 10.1194/jlr.M015776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw PX, Hörkkö S, Chang MK, Curtiss LK, Palinski W, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin.Investig. 2000;105:1731–40. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsimikas S, Willeit P, Willeit J, Santer P, Mayr M, et al. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J. Am. Coll. Cardiol. 2012;60:2218–29. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- 43.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70. J. Exp. Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am. Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, et al. Lack of interleukin-1βdecreases the severity of atherosclerosis in apoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003;23:656–60. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 46.Self-Medlin Y, Byun J, Jacob RF, Mizuno Y, Mason RP. Glucose promotes membrane cholesterol crystalline domain formation by lipid peroxidation. Biochim. Biophys. Acta. 2009;1788:1398–403. doi: 10.1016/j.bbamem.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Rajamäki K, Lappalainen J, Öörnï K, Välimäki E, Matikainen S, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menu P, Pellegrin M, Aubert JF, Bouzourene K, Tardivel A, et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu QB, Oberhuber G, Gruschwitz M, Wick G. Immunology of atherosclerosis: cellular composition and major histocompatibility complex class II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin. Immunol. Immunopathol. 1990;56:344–59. doi: 10.1016/0090-1229(90)90155-j. [DOI] [PubMed] [Google Scholar]
- 50.Bobryshev YV, Lord RS. S-100-positive cells in human arterial intima and in atherosclerotic lesions. Cardiovasc. Res. 1995;29:689–96. [PubMed] [Google Scholar]
- 51.Waltner-Romen M, Falkensammer G, Rabl W, Wick G. A previously unrecognized site of local accumulation of mononuclear cells. The vascular-associated lymphoid tissue. J. Histochem. Cytochem. 1998;46:1347–50. doi: 10.1177/002215549804601202. [DOI] [PubMed] [Google Scholar]
- 52.Bobryshev YV. Dendritic cells and their role in atherogenesis. Lab. Investig. 2010;90:970–84. doi: 10.1038/labinvest.2010.94. [DOI] [PubMed] [Google Scholar]
- 53.Paulson KE, Zhu S-N, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ. Res. 2010;106:383–90. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 54.Packard RR, Maganto-García E, Gotsman I, Tabas I, Libby P, Lichtman AH. CD11c+ dendritic cells maintain antigen processing, presentation capabilities, and CD4+ T cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ. Res. 2008;103:965–73. doi: 10.1161/CIRCRESAHA.108.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, et al. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J. Clin. Investig. 2012;122:3114–26. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grundtman C, Kreutmayer SB, Almanzar G, Wick MC, Wick G. Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011;31:960–68. doi: 10.1161/ATVBAHA.110.217877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gounopoulos P, Merki E, Hansen LF, Choi SH, Tsimikas S. Antibodies to oxidized low density lipoprotein: epidemiological studies and potential clinical applications in cardiovascular disease. Minerva Cardioangiol. 2007;55:821–37. [PubMed] [Google Scholar]
- 58.Kahlenberg JM, Kaplan MJ. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu. Rev. Med. 2013;64:249–63. doi: 10.1146/annurev-med-060911-090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daugherty A. The effects of total lymphocyte deficiency on the extent of atherosclerosis in apolipoprotein E−/− mice. J. Clin. Investig. 1997;100:1575–80. doi: 10.1172/JCI119681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4+ T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–22. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 61.Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J. Clin. Investig. 2001;108:251–59. doi: 10.1172/JCI11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–38. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 63.Emeson EE, Robertson AL., Jr. T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am. J. Pathol. 1988;130:369–76. [PMC free article] [PubMed] [Google Scholar]
- 64.Haraoka S, Shimokama T, Watanabe T. Participation of T lymphocytes in atherogenesis: sequential and quantitative observation of aortic lesions of rats with diet-induced hypercholesterolaemia using en face double immunostaining. Virchows Arch. 1995;426:307–15. doi: 10.1007/BF00191369. [DOI] [PubMed] [Google Scholar]
- 65.Roselaar SE, Kakkanathu PX, Daugherty A. Lymphocyte populations in atherosclerotic lesions of apoE−/− and LDL receptor−/− mice. Decreasing density with disease progression. Arterioscler. Thromb. Vasc. Biol. 1996;16:1013–18. doi: 10.1161/01.atv.16.8.1013. [DOI] [PubMed] [Google Scholar]
- 66.Zhou X, Stemme S, Hansson GK. Evidence for a local immune response in atherosclerosis. CD4+ T cells infiltrate lesions of apolipoprotein E-deficient mice. Am. J. Pathol. 1996;149:359–66. [PMC free article] [PubMed] [Google Scholar]
- 67.Buono C, Pang H, Uchida Y, Libby P, Sharpe AH, Lichtman AH. B7-1/B7-2 costimulation regulates plaque antigen-specific T cell responses and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation. 2004;109:2009–15. doi: 10.1161/01.CIR.0000127121.16815.F1. [DOI] [PubMed] [Google Scholar]
- 68.Bergström I, Backteman K, Lundberg A, Ernerudh J, Jonasson L. Persistent accumulation of interferon-γ-producing CD8+CD56+ T cells in blood from patients with coronary artery disease. Atherosclerosis. 2012;224:515–20. doi: 10.1016/j.atherosclerosis.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 69.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA. 1995;92:3893–97. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, et al. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J. Exp. Med. 2010;207:1081–93. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ketelhuth DF, Rios FJ, Wang Y, Liu H, Johansson ME, et al. Identification of a danger-associated peptide from apolipoprotein B100 (ApoBDS-1) that triggers innate proatherogenic responses. Circulation. 2011;124:2433–43. doi: 10.1161/CIRCULATIONAHA.111.051599. [DOI] [PubMed] [Google Scholar]
- 72.Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 73.Zhou X, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J. Clin. Investig. 1998;101:1717–25. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stemme S. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl Acad. Sci. USA. 1995;92:3893–97. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-γon the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 2003;23:454–60. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 76.Gupta S. IFN-γ potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Investig. 1997;99:2752–61. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-γenhances atherosclerosis in apolipoprotein E−/− mice. Am. J. Pathol. 2000;157:1819–24. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buono C. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl Acad. Sci. USA. 2005;102:1596–601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee TS, Yen H-C, Pan C-C,, Chau LW. The role of interleukin 12 in the development of atherosclerosis in apoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 1999;19:734–42. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- 80.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 2003;163:1117–25. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tzellos T, Kyrgidis A, Zouboulis CC. Re-evaluation of the risk for major adverse cardiovascular events in patients treated with anti-IL-12/23 biological agents for chronic plaque psoriasis: a meta-analysis of randomized controlled trials. J. Eur. Acad. Dermatol. Venereol. 2012;27:622–27. doi: 10.1111/j.1468-3083.2012.04500.x. [DOI] [PubMed] [Google Scholar]
- 82.Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schönbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J. Exp. Med. 2002;195:245–57. doi: 10.1084/jem.20011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mallat Z. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104:1598–603. doi: 10.1161/hc3901.096721. [DOI] [PubMed] [Google Scholar]
- 84.Jefferis BJ, Papacosta O, Owen CG, Wannamethee SG, Humphries SE, et al. Interleukin-18 and coronary heart disease: prospective study and systematic review. Atherosclerosis. 2011;217:227–33. doi: 10.1016/j.atherosclerosis.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tiret L, Godefroy T, Lubos E, Nicaud V, Tregouet DA, et al. Genetic analysis of the interleukin-18 system highlights the role of the interleukin-18 gene in cardiovascular disease. Circulation. 2005;112:643–50. doi: 10.1161/CIRCULATIONAHA.104.519702. [DOI] [PubMed] [Google Scholar]
- 86.Hernesniemi JA, Heikkilä A, Raitakari OT, Kahönen M, Juonala M, et al. Interleukin-18 gene polymorphism and markers of subclinical atherosclerosis. The Cardiovascular Risk in Young Finns Study. Ann. Med. 2010;42:223–30. doi: 10.3109/07853891003769940. [DOI] [PubMed] [Google Scholar]
- 87.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E−/− mice through release of interferon-γ. Circ. Res. 2002;90:E34–38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 88.Elhage R, Jawien J, Rudling M, Ljunggren H-G, Takeda K, et al. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc. Res. 2003;59:234–40. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 89.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011;31:969–79. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 90.King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler. Thromb. Vasc. Biol. 2002;22:456–61. doi: 10.1161/hq0302.104905. [DOI] [PubMed] [Google Scholar]
- 91.Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu. Rev. Pathol. Mech. Dis. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 2009;206:2067–77. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, Idu MM, et al. Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J. Pathol. 2010;220:499–508. doi: 10.1002/path.2667. [DOI] [PubMed] [Google Scholar]
- 94.Eid RE. Interleukin-17 and interferon-γ are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–32. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Erbel C. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J. Immunol. 2009;183:8167–75. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 96.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, et al. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–55. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ. Res. 2012;110:675–87. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Danzaki K, Matsui Y, Ikesue M, Ohta D, Ito K, et al. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2012;32:273–80. doi: 10.1161/ATVBAHA.111.229997. [DOI] [PubMed] [Google Scholar]
- 99.Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, et al. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 2010;207:1579–87. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–27. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Waisman A. To be 17 again—anti-interleukin-17 treatment for psoriasis. N. Engl. J. Med. 2012;366:1251–52. doi: 10.1056/NEJMe1201071. [DOI] [PubMed] [Google Scholar]
- 102.van der Wal AC, Das PK, Bentz van de Berg D, van der Loos CM, Becker AE. Atherosclerotic lesions in humans: in situ immunophenotypic analysis suggesting an immune mediated response. Lab. Investig. 1989;61:166–70. [PubMed] [Google Scholar]
- 103.Olofsson PS, Söderström LA, Wågsäter D, Sheikine Y, Ocaya P, et al. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation. 2008;117:1292–301. doi: 10.1161/CIRCULATIONAHA.107.699173. [DOI] [PubMed] [Google Scholar]
- 104.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J. Clin. Investig. 2007;117:2974–82. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bu DX, Tarrio M, Maganto-García E, Stavrakis G, Tajima G, et al. Impairment of the programmed cell death 1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31:1100–7. doi: 10.1161/ATVBAHA.111.224709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elhage R, Gourdy P, Brouchet L, Jawien J, Fouque MJ, et al. Deleting TCR αβ+ or CD4+ T lymphocytes leads to opposite effects on site-specific atherosclerosis in female apolipoprotein E-deficient mice. Am. J. Pathol. 2004;165:2013–18. doi: 10.1016/s0002-9440(10)63252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kolbus D, Ljungcrantz I, Söderberg I, Alm R, Björkbacka H, et al. TAP1-deficiency does not alter atherosclerosis development in apoE−/− mice. PLoS ONE. 2012;7:e33932. doi: 10.1371/journal.pone.0033932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kyaw T, Winship A, Tay C, Kanellakis P, Hosseini H, et al. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE−/− mice. Circulation. 2013;127:1028–39. doi: 10.1161/CIRCULATIONAHA.112.001347. [DOI] [PubMed] [Google Scholar]
- 109.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013;13:101–17. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 110.Getz GS, Vanderlaan PA, Reardon CA. Natural killer T cells in lipoprotein metabolism and atherosclerosis. Thromb. Haemost. 2011;106:814–19. doi: 10.1160/TH11-05-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bobryshev YV, Lord RS. Co-accumulation of dendritic cells and natural killer T cells within rupture-prone regions in human atherosclerotic plaques. J. Histochem. Cytochem. 2005;53:781–85. doi: 10.1369/jhc.4B6570.2005. [DOI] [PubMed] [Google Scholar]
- 112.Melian A, Geng YJ, Sukhova GK, Libby P, Porcelli SA. CD1 expression in human atherosclerosis. A potential mechanism for T cell activation by foam cells. Am. J. Pathol. 1999;155:775–86. doi: 10.1016/S0002-9440(10)65176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dumitriu IE, Araguas ET, Baboonian C, Kaski JC. CD4+CD28 null T cells in coronary artery disease: when helpers become killers. Cardiovasc. Res. 2009;81:11–19. doi: 10.1093/cvr/cvn248. [DOI] [PubMed] [Google Scholar]
- 114.Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, et al. Monoclonal T cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–88. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 115.Zal B, Kaski JC, Arno G, Akiyu JP, Xu Q, et al. Heat-shock protein 60-reactive CD4+CD28null T cells in patients with acute coronary syndromes. Circulation. 2004;109:1230–35. doi: 10.1161/01.CIR.0000118476.29352.2A. [DOI] [PubMed] [Google Scholar]
- 116.Tsimikas S, Palinski W, Witztum JL. Circulating autoantibodies to oxidized LDL correlate with arterial accumulation and depletion of oxidized LDL in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001;21:95–100. doi: 10.1161/01.atv.21.1.95. [DOI] [PubMed] [Google Scholar]
- 117.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc. Natl. Acad. Sci. USA. 1995;92:821–25. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hansson GK, Nilsson J. Vaccination against atherosclerosis? Induction of atheroprotective immunity. Semin. Immunopathol. 2009;31:95–101. doi: 10.1007/s00281-009-0151-x. [DOI] [PubMed] [Google Scholar]
- 119.Nilsson J, Björkbacka H, Fredrikson GN. Apolipoprotein B100 autoimmunity and atherosclerosis—disease mechanisms and therapeutic potential. Curr. Opin. Lipidol. 2012;23:422–28. doi: 10.1097/MOL.0b013e328356ec7c. [DOI] [PubMed] [Google Scholar]
- 120.Tsimikas S, Miyanohara A, Hartvigsen K, Merki E, Shaw PX, et al. Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. J. Am. Coll. Cardiol. 2011;58:1715–27. doi: 10.1016/j.jacc.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Major AS, Fazio S, Linton MF. B lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 2002;22:1892–98. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 122.Doran AC, Lipinski MJ, Oldham SN, Garmey JC, Campbell KA, et al. B cell aortic homing and atheroprotection depend on Id3. Circ. Res. 2012;110:e1–12. doi: 10.1161/CIRCRESAHA.111.256438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J. Immunol. 2010;185:4410–19. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- 124.Kyaw T, Tay C, Hosseini H, Kanellakis P, Gadowski T, et al. Depletion of B2 but not B1α B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PLoS ONE. 2012;7:e29371. doi: 10.1371/journal.pone.0029371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sage AP, Tsiantoulas D, Baker L, Harrison J, Masters L, et al. BAFF receptor deficiency reduces the development of atherosclerosis in mice—brief report. Arterioscler. Thromb. Vasc. Biol. 2012;32:1573–76. doi: 10.1161/ATVBAHA.111.244731. [DOI] [PubMed] [Google Scholar]
- 126.Kelly JA, Griffin ME, Fava RA, Wood SG, Bessette KA, et al. Inhibition of arterial lesion progression in CD16-deficient mice: evidence for altered immunity and the role of IL-10. Cardiovasc. Res. 2010;85:224–31. doi: 10.1093/cvr/cvp300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hernández-Vargas P, Ortiz-Muñoz G, Lopez-Franco O, Suzuki Y, Gallego-Delgado J, et al. Fcγ receptor deficiency confers protection against atherosclerosis in apolipoprotein E knockout mice. Circ. Res. 2006;99:1188–96. doi: 10.1161/01.RES.0000250556.07796.6c. [DOI] [PubMed] [Google Scholar]
- 128.Zhao M, Wigren M, Dunér P, Kolbus D, Olofsson KE, et al. FcγRIIB inhibits the development of atherosclerosis in low-density lipoprotein receptor-deficient mice. J. Immunol. 2010;184:2253–60. doi: 10.4049/jimmunol.0902654. [DOI] [PubMed] [Google Scholar]
- 129.Mendez-Fernandez YV, Stevenson BG, Diehl CJ, Braun NA, Wade NS, et al. The inhibitory FcγRIIb modulates the inflammatory response and influences atherosclerosis in male apoE−/− mice. Atherosclerosis. 2011;214:73–80. doi: 10.1016/j.atherosclerosis.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang J, Cheng X, Xiang MX, Alanne-Kinnunen M, Wang JA, et al. IgE stimulates human and mouse arterial cell apoptosis and cytokine expression and promotes atherogenesis in apoE−/− mice. J. Clin. Investig. 2011;121:3564–77. doi: 10.1172/JCI46028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Speidl WS, Kastl SP, Huber K, Wojta J. Complement in atherosclerosis: friend or foe? J. Thromb. Haemost. 2011;9:428–40. doi: 10.1111/j.1538-7836.2010.04172.x. [DOI] [PubMed] [Google Scholar]
- 132.Lichtman AH. T cell costimulatory and coinhibitory pathways in vascular inflammatory diseases. Front. Physiol. 2012;3:18. doi: 10.3389/fphys.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J. Clin. Investig. 2013;123:27–36. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ma K, Lv S, Liu B, Liu Z, Luo Y, et al. CTLA4-IgG ameliorates homocysteine-accelerated atherosclerosis by inhibiting T cell overactivation in apoE−/− mice. Cardiovasc. Res. 2013;97:349–59. doi: 10.1093/cvr/cvs330. [DOI] [PubMed] [Google Scholar]
- 135.Ewing MM, Karper JC, Abdul S, de Jong RC, Peters HA, et al. T cell co-stimulation by CD28-CD80/86 and its negative regulator CTLA-4 strongly influence accelerated atherosclerosis development. Int. J. Cardiol. 2013 doi: 10.1016/j.ijcard.2012.12.085. In press. [DOI] [PubMed] [Google Scholar]
- 136.Wang X, Ria M, Kelmenson PM, Eriksson P, Higgins DC, et al. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nat. Genet. 2005;37:365–72. doi: 10.1038/ng1524. [DOI] [PubMed] [Google Scholar]
- 137.van Wanrooij EJ, van Puijvelde GH, de Vos P, Yagita H, van Berkel TJ, Kuiper J. Interruption of the Tnfrsf4/Tnfsf4 (OX40/OX40L) pathway attenuates atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2007;27:204–10. doi: 10.1161/01.ATV.0000251007.07648.81. [DOI] [PubMed] [Google Scholar]
- 138.Schönbeck U, Sukhova GK, Shimizu K, Mach F, Libby P. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc. Natl. Acad. Sci. USA. 2000;97:7458–63. doi: 10.1073/pnas.97.13.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mach F, Schönbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–3. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 140.Lutgens E, Gorelik L, Daemen MJ, de Muinck ED, Grewal IS, et al. Requirement for CD154 in the progression of atherosclerosis. Nat. Med. 1999;5:1313–16. doi: 10.1038/15271. [DOI] [PubMed] [Google Scholar]
- 141.Lievens D, Eijgelaar WJ, Biessen EA, Daemen MJ, Lutgens E. The multi-functionality of CD40L and its receptor CD40 in atherosclerosis. Thromb. Haemost. 2009;102:206–14. doi: 10.1160/TH09-01-0029. [DOI] [PubMed] [Google Scholar]
- 142.Lee J, Zhuang Y, Wei X, Shang F, Wang J, et al. Contributions of PD-1/PD-L1 pathway to interactions of myeloid DCs with T cells in atherosclerosis. J. Mol. Cell Cardiol. 2009;46:169–76. doi: 10.1016/j.yjmcc.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 143.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, van der Wal AC. Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS ONE. 2007;2:e779. doi: 10.1371/journal.pone.0000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patel S, Chung SH, White G, Bao S, Celermajer DS. The “atheroprotective” mediators apolipoproteinA-I and Foxp3 are over-abundant in unstable carotid plaques. Int. J. Cardiol. 2010;145:183–87. doi: 10.1016/j.ijcard.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 146.Zhang WC, Wang J, Shu YW, Tang TT, Zhu ZF, et al. Impaired thymic export and increased apoptosis account for regulatory T cell defects in patients with non-ST segment elevation acute coronary syndrome. J. Biol. Chem. 2012;287:34157–66. doi: 10.1074/jbc.M112.382978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ammirati E, Cianflone D, Banfi M, Vecchio V, Palini A, et al. Circulating CD4+CD25hiCD127lo regulatory T cell levels do not reflect the extent or severity of carotid and coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010;30:1832–41. doi: 10.1161/ATVBAHA.110.206813. [DOI] [PubMed] [Google Scholar]
- 148.Potekhina AV, Provatorov SI, Sokolov VO, Pylaeva EA, Masenko VP, et al. CD4+CD25highCD127low regulatory T cells in patients with stable angina and their dynamics after intracoronary sirolimus-eluting stent implantation. Hum. Immunol. 2011;72:553–57. doi: 10.1016/j.humimm.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 149.Zhang D, Wang S, Guan Y, Wang L, Xie W, et al. Effect of oral atorvastatin on CD4+CD25+ regulatory T cells, FoxP3 expression, and prognosis in patients with ST-segment elevated myocardial infarction before primary percutaneous coronary intervention. J. Cardiovasc. Pharmacol. 2011;57:536–41. doi: 10.1097/FJC.0b013e318211d016. [DOI] [PubMed] [Google Scholar]
- 150.Bu DX, Griffin G, Lichtman AH. Mechanisms for the anti-inflammatory effects of statins. Curr. Opin. Lipidol. 2011;22:165–70. doi: 10.1097/MOL.0b013e3283453e41. [DOI] [PubMed] [Google Scholar]
- 151.Entin-Meer M, Afek A, George J. Regulatory T cells, FoxP3 and atherosclerosis. Adv. Exp. Med. Biol. 2009;665:106–14. doi: 10.1007/978-1-4419-1599-3_8. [DOI] [PubMed] [Google Scholar]
- 152.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 2006;12:178–80. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 153.Gotsman I, Gupta R, Lichtman AH. Impaired regulatory T cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–55. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 154.Ng HP, Burris RL, Nagarajan S. Attenuated atherosclerotic lesions in apoE-Fcγ-chain-deficient hyperlipidemic mouse model is associated with inhibition of Th17 cells and promotion of regulatory T cells. J. Immunol. 2011;187:6082–93. doi: 10.4049/jimmunol.1004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Weber C, Meiler S, Döring Y, Koch M, Drechsler M, et al. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J. Clin. Investig. 2011;121:2898–910. doi: 10.1172/JCI44925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, et al. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007;27:2691–98. doi: 10.1161/ATVBAHA.107.149567. [DOI] [PubMed] [Google Scholar]
- 157.Dietrich T, Hucko T, Schneemann C, Neumann M, Menrad A, et al. Local delivery of IL-2 reduces atherosclerosis via expansion of regulatory T cells. Atherosclerosis. 2012;220:329–36. doi: 10.1016/j.atherosclerosis.2011.09.050. [DOI] [PubMed] [Google Scholar]
- 158.Foks AC, Frodermann V, ter Borg M, Habets KLL, Bot I, et al. Differential effects of regulatory T cells on the initiation and regression of atherosclerosis. Atherosclerosis. 2011;218:53–60. doi: 10.1016/j.atherosclerosis.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 159.Dinh TN, Kyaw TS, Kanellakis P, To K, Tipping P, et al. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ regulatory T cells and attenuates development and progression of atherosclerosis: clinical perspective. Circulation. 2012;126:1256–66. doi: 10.1161/CIRCULATIONAHA.112.099044. [DOI] [PubMed] [Google Scholar]
- 160.Sasaki N, Yamashita T, Takeda M, Shinohara M, Nakajima K, et al. Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation. 2009;120:1996–2005. doi: 10.1161/CIRCULATIONAHA.109.863431. [DOI] [PubMed] [Google Scholar]
- 161.Maganto-García E, Tarrio ML, Grabie N, Bu D, Lichtman AH. Dynamic changes in aortic regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 2011;24:185–95. doi: 10.1161/CIRCULATIONAHA.110.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Robertson AK. Disruption of TGF-β signaling in T cells accelerates atherosclerosis. J. Clin. Investig. 2003;112:1342–50. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Potteaux S, Esposito B, van Oostrom O, Brun V, Ardouin P, et al. Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 2004;24:1474–78. doi: 10.1161/01.ATV.0000134378.86443.cd. [DOI] [PubMed] [Google Scholar]
- 164.Subramanian M, Thorp E, Hansson GK, Tabas I. Treg-mediated suppression of atherosclerosis requires MyD88 signaling in DCs. J. Clin. Investig. 2013;123:179–88. doi: 10.1172/JCI64617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Klingenberg R, Ketelhuth DFJ, Strodthoff D, Gregori S, Hansson GK. Subcutaneous immunization with heat shock protein-65 reduces atherosclerosis in apoE−/− mice. Immunobiology. 2012;217:540–47. doi: 10.1016/j.imbio.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 166.Herbin O, Ait-Oufella H, Yu W, Fredrikson GN, Aubier B, et al. Regulatory T cell response to apolipoprotein B100-derived peptides reduces the development and progression of atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2012;32:605–12. doi: 10.1161/ATVBAHA.111.242800. [DOI] [PubMed] [Google Scholar]
- 167.Wigren M, Kolbus D, Dunér P, Ljungcrantz I, Söderberg I, et al. Evidence for a role of regulatory T cells in mediating the atheroprotective effect of apolipoprotein B peptide vaccine. J. Int. Med. 2011;269:546–56. doi: 10.1111/j.1365-2796.2010.02311.x. [DOI] [PubMed] [Google Scholar]
- 168.Dunér P, To F, Beckmann K, Björkbacka H, Fredrikson GN, et al. Immunization of apoE−/− mice with aldehyde-modified fibronectin inhibits the development of atherosclerosis. Cardiovasc. Res. 2011;91:528–36. doi: 10.1093/cvr/cvr101. [DOI] [PubMed] [Google Scholar]
- 169.Klingenberg R. Intranasal immunization with an apolipoprotein B-100 fusion protein induces antigen-specific regulatory T cells and reduces atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010;30:946–52. doi: 10.1161/ATVBAHA.109.202671. [DOI] [PubMed] [Google Scholar]
- 170.van Puijvelde GH. Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation. 2006;114:1968–76. doi: 10.1161/CIRCULATIONAHA.106.615609. [DOI] [PubMed] [Google Scholar]
- 171.Hermansson A, Johansson DK, Ketelhuth DF, Andersson J, Zhou X, Hansson GK. Immunotherapy with tolerogenic apolipoprotein B100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation. 2011;123:1083–91. doi: 10.1161/CIRCULATIONAHA.110.973222. [DOI] [PubMed] [Google Scholar]
- 172.Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 173.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–82. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 174.Ridker P. Moving beyond JUPITER: Will inhibiting inflammation reduce vascular event rates? Curr. Atheroscler. Rep. 2012;15:1–6. doi: 10.1007/s11883-012-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Aikawa M, Sugiyama S, Hill CC, Voglic SJ, Rabkin E, et al. Lipid lowering reduces oxidative stress and endothelial cell activation in rabbit atheroma. Circulation. 2002;106:1390–96. doi: 10.1161/01.cir.0000028465.52694.9b. [DOI] [PubMed] [Google Scholar]
- 176.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J. Thromb. Haemost. 2009;7(Suppl. 1):332–39. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 177.Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernan MA, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am. J. Cardiol. 2011;108:1362–70. doi: 10.1016/j.amjcard.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Listing J, Strangfeld A, Kekow J, Schneider M, Kapelle A, et al. Does tumor necrosis factor α inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum. 2008;58:667–77. doi: 10.1002/art.23281. [DOI] [PubMed] [Google Scholar]