Abstract
Objective
With the growing number of childhood cancer survivors in the United States, it is important to assess the well-being of these individuals, particularly during the transitional phase of adolescence. Data about adolescent survivors’ overall health and quality of life will help identify survivor subgroups most in need of targeted attention to successfully transition to adulthood.
Participants and Methods
This ancillary study to the Childhood Cancer Survivor Study (CCSS) focused on children 15–19 years of age who had been diagnosed with cancer before the age of 4 years. A cohort of siblings of pediatric cancer survivors of the same ages served as a comparison sample. Adolescent health was assessed using the Child Health and Illness Profile-Adolescent Edition (CHIP-AE) survey.
Results
The teen survey was sent to 444 survivor teens and 189 siblings. Of these 307(69%) survivors and 97 (51%) siblings completed and returned the survey. Overall health profiles of siblings and survivors were similar. Among survivors, females scored significantly below males on Satisfaction, Discomfort, and Disorders domains. Survivors diagnosed with CNS tumors scored less favorably than leukemia survivors in the global domains of Satisfaction and Disorders.
Conclusion
In general, adolescent survivors fare favorably compared to healthy siblings. However, identification of the subset of pediatric cancer survivors who are more vulnerable to medical and psychosocial disorders in adolescence provides the opportunity for design and implementation of intervention strategies that may improve quality of life.
Introduction
Over the past three decades, improvements in the treatment of children with cancer have led to a substantial increase in the number of children surviving into adulthood [1]. It is estimated that there are over 328,000 persons in the U.S. who have survived cancer diagnosed before the age of 20 years, and that one in every 640 young adults is now a survivor of childhood cancer [2]. Because of this expected longevity, maximizing quality of life and minimizing negative late effects are central concerns in the long term care of these patients. Assessment of the well being of adolescents prior to their transition to young adulthood is critical for early identification of the vulnerable subgroup in need of intervention [3, 4].
A major determinant of the quality of life of pediatric cancer survivors is whether or not they have late effects secondary to their cancer treatment. Sixty five to seventy five percent of childhood cancer survivors experience at least one significant late effect; these effects may include neurocognitive impairment, organ system dysfunction, and/or psychological distress [3, 5]. CNS radiation, some neurosurgical complications, and certain chemotherapy regimens are associated with later cognitive deficits in survivors, and with persistent psychological distress [6–10]. Female gender and early age at diagnosis are particular vulnerability factors with more serious deficits [11–13]. While brain development continues throughout childhood and adolescence, brain size increases almost three-fold between birth and two years [14–16]. During these early years of rapid growth, significant sensory, motor, cognitive and emotional development occurs.
Understanding the trajectory of quality of life in adolescent pediatric cancer survivors treated at a young age requires developmentally appropriate assessment of cognitive, motor and emotional maturation within this age cohort. Adolescence involves significant biological (e.g., pubertal), psychological, and contextual changes [17, 18]. Educational expectations increase dramatically as adolescents move from middle to high school when neurocognitive problems can make meeting academic milestones difficult. Visible abnormalities (e.g. visible scars, short stature) can impact self-esteem and psychosocial functioning.
Our study is an ancillary study of the Childhood Cancer Survivor Study (CCSS) [19] and has provided a unique opportunity to study adolescent survivors of childhood cancer with well-characterized cancer diagnoses and treatments. To assess overall health and well-being among adolescents who were treated for cancer at a very young age, their health profiles were compared to health profiles in a cohort of adolescent siblings whose brother/sister had cancer. Not only were survivor/sibling comparisons made, but at-risk survivor subgroups were also identified.
Participants and Methods
Subject Selection and Participation
The parent study (CCSS) is a multi-institutional, longitudinal study of individuals with a diagnosis of leukemia, CNS tumors (all histologies), Hodgkin's disease, non-Hodgkin's lymphoma, kidney tumor, neuroblastoma, soft tissue sarcoma, or bone tumor; diagnosis and initial treatment at one of 26 collaborating institutions; diagnosis date between January 1, 1970 and December 31, 1986; age less than 21 years at diagnosis; and survival five or more years from diagnosis. Details of the study design and descriptions of the cohort have been published previously [19]. The CCSS protocol and contact documents were reviewed and approved by the Human Subjects Committee at each participating institution.
Recruitment for this ancillary study began in 2001. For this study we included childhood cancer survivors who were 15–19 years of age at ancillary study recruitment, had been diagnosed before the age of 4 years, and deemed by their parent as cognitively competent to complete the teen survey independently. Letters were sent to 353 parents of survivors who were younger than 18 years of age, requesting permission to contact their teen; 217 parents gave permission (58%). Demographic and treatment characteristics between teens with and without parental consent differed only by sex, with females comprising 56% of survivor participants. Parents of 208 siblings, ages 15–17 years, were mailed study consents and 120 (57%) gave written consent for their teen to participate. No demographic differences were found between siblings with and without parental permission. Letters and consents were sent directly to 18 and 19 year olds to request permission; 227 (73%) of survivors and 69 (73%) of siblings agreed to receive a survey in the mail.
The Teen Health Survey was subsequently sent to 444 adolescent survivors, and 307 (69%) were completed and returned. The Teen Health Survey was also distributed to 189 siblings, and 97 (51%) of surveys were completed and returned. No differences were found between participant and non-participant groups by age at contact, age at diagnosis, diagnosis, or treatment. However, females were more likely to participate (60% vs. 40% of males).
Outcome Measures
The Child Health and Illness Profile-Adolescent Edition (CHIP-AE) is a survey instrument designed to assess adolescent health-related quality of life, including physical, mental and social experiences [20]. The CHIP-AE describes six health domains from the perspective of the adolescent: 1) Satisfaction with Health - personal perception of overall health and self-esteem; 2) Resilience – activities likely to be protective of future health (such as family involvement, types of food consumed, and physical activity); 3) Discomfort - physical and emotional symptoms, and activity limitations; 4) Risk – risk-taking and disruptive behaviors associated with increased likelihood of poor future health; 5) Disorder - history of and current problems with specific diagnostic conditions; 6) Achievement - social roles appropriate for age (school and work performance). Within these domains, 20 sub-domains can be scored.
The CHIP-AE was selected for this study because it provides the ability to examine the inter-relationship between health profiles including physical and emotional symptoms, limitations of activity, health risk and protective behaviors, and perceptions of health and social role functioning [21–23]. The domains of the CHIP-AE have demonstrated robust reliability with internal consistency correlations above 0.8 on all domains. Validation studies of the CHIP-AE have likewise demonstrated predicted and meaningful differences in health [20–22, 24, 25].
Each domain and subdomain is scored so that a higher score indicates better health. To define outcomes in terms of poor, average and excellent, cut-points for both survivors and siblings were based on the mean score of siblings within each domain, with sibling scores in the lowest 10% coded as poor health, while those in the highest 10% coded as excellent health.
Combinations of poor, average and excellent categories were used to group siblings and survivors into a taxonomy of global health consistent with the work of the CHIP developers [20–23]. Five distinct categories of health, or profile-types, with each profile-type mutually exclusive, were used to assess global health strengths and weaknesses through underlying patterns of physical, mental, and social well-being [21]. These profiles included: 1) Excellent health on three or more of the six domains, no domains of poor health; 2) At least average health on all domains (not to exceed excellent health on more than two domains); 3) Poor health in one domain; 4) Poor health in two domains; 5) Poor health in three or more domains.
Independent variables
Demographics and original cancer diagnosis information was obtained from the treating institution. Cancer treatment information, obtained from medical records, was categorized as chemotherapy (yes/no), radiation therapy (yes/no), and surgery (yes/no).
Data Analysis
Descriptive statistics were used to describe and compare characteristics of the survivors and siblings. Fisher exact tests were used to compare survivors and siblings by sex, race and current age group. Means and standard deviations were calculated for the six domains and 20 subdomains from the CHIP-AE for both adolescent survivors and siblings, and compared by gender within each group using linear models controlling for intra-family correlation [26]. Cohen's d effect sizes were calculated based on the adjusted means and standard deviations (SD). The interaction between diagnosis and sex was evaluated, but not included in final models because it was not significant.
The frequencies and percentages of survivors who scored in the poor category in each of the six domains were calculated and are reported by sex, current age group, age at diagnosis, race/ethnicity, household income, and cancer diagnosis. Predictors of poor outcomes in each domain were evaluated in multivariable logistic regression models and are reported as odds ratios with 95% confidence intervals. The proportions of survivors and siblings in each category of global health were also calculated and were compared both overall and by cancer diagnosis using Fisher’s exact tests.
P-values for this study were set at 0.05. Due to the small sample size (123 male and 184 female survivors), a Bonferroni correction was not applied in order to avoid increasing Type II error (false negatives) which allows us to maximize the statistical power to detect the true difference between male and female survivors in each domain. However, this has the potential to increase our Type I error (false positives) and is therefore a limitation in our analyses [27]. P-values reported should be interpreted in the context of the number of tests that were carried out. All analyses were completed using SAS version 9.2 (Cary, N.C.)
Results
The characteristics of the survivors and siblings who agreed to participate are shown in Table 1. Survivors and siblings differed only by age at study recruitment. When compared to survivors, siblings were more likely to be younger than 18 years of age at the time of the study (60% vs. 42%).
Table 1.
Characteristics of the survivors and siblings
| Survivors (%) | Sibling (%) | P-value | |
|---|---|---|---|
| Sample size | 307 | 97 | |
| Sex | |||
| Female | 184 (60) | 54 (56) | 0.48 |
| Male | 123 (40) | 43 (44) | |
| Race | |||
| White | 272 (89) | 85 (88) | 0.83 |
| Black | 16 (5) | 4 (4) | |
| Hispanic | 4 (1) | 2 (2) | |
| Other race | 15 (5) | 6 (6) | |
| Current age | |||
| <18 | 129 (42) | 58 (60) | 0.002 |
| 18–20 | 178 (58) | 39 (40) | |
| Diagnosis | |||
| Leukemia | 95 (31) | - | - |
| CNS tumor | 40 (13) | - | - |
| Hodgkins disease | 0 (0) | - | - |
| NHL | 4 (1) | - | - |
| Wilms tumor | 56 (18) | - | - |
| Neuroblastoma | 90 (29) | - | - |
| Soft tissue sarcoma | 19 (6) | - | - |
| Bone tumor | 3 (1) | - | - |
| Age at diagnosis | |||
| 0–1 | 198 (65) | - | - |
| 2–3 | 109 (35) | - | - |
| Treatment | |||
| Chemotherapy | 144 (49) | - | - |
| Any Radiation | 11 (4) | - | - |
| Chemo + Any Rad | 99 (33) | - | - |
| Surgery only | 41 (14) | - | - |
Note: Fisher’s Exact tests were used evaluate p-values for the comparison between cases and siblings
Table 2 shows mean scores on the CHIP-AE among siblings and survivors by cancer diagnosis. Overall, compared to siblings, survivors reported more problems with physical activity and disorders, particularly recurrent (e.g. urinary tract infections, asthma), long-term medical (e.g. diabetes) and surgical (e.g. scoliosis) disorders. On the other hand, survivors scored higher (better) than siblings on social problem solving and reported fewer disruptive behaviors, a positive predictor of achievement. In addition, Wilm's tumor survivors reported better functioning in the Emotional Discomfort and Threats to Achievement domains than did siblings. CNS tumor survivors were found to have lower scores than siblings in the global domains of Satisfaction and Disorders, particularly in overall satisfaction with health, self-esteem, physical activity, and long term surgical and psychosocial disorders. Despite these findings, CNS tumor survivors scored higher than siblings in the Risk domain, indicating less involvement in risk taking, fewer threats to achievement, and less association with peers who engaged in negative health behaviors. Additionally, leukemia survivors and those with diagnoses in the “other cancer” category reported more long-term medical disorders than did siblings; both leukemia and neuroblastoma survivors reported more psychosocial disorders than siblings. Neuroblastoma survivors also reported more long-term surgical disorders than did siblings.
Table 2.
Mean (Standard Error) Scores of CHIP-AE domain and subdomains by diagnosis among survivors
| DOMAIN | Sibling | All survivors | Effect Size |
Leukemia | Effect Size |
CNS tumor | Effect Size |
Wilms tumor |
Effect Size |
Neuroblastoma | Effect Size |
Other diagnosis |
Effect Size |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N=97) | (N=307) | (N=95) | (N=40) | (N=56) | (N=90) | (N=26) | |||||||
| Satisfaction | 20.34(0.45) | 19.46(0.28) | −0.19 | 19.27(0.46) | −0.24 | 16.14(0.70)** | −0.95 | 21.31(0.60) | 0.22 | 20.19(0.47) | −0.03 | 18.95(0.87) | −0.31 |
| Overall satisfaction | 20.33(0.44) | 19.62(0.27) | −0.16 | 19.31(0.45) | −0.23 | 16.37(0.69)** | −0.91 | 21.33(0.59) | 0.23 | 20.40(0.46) | 0.02 | 19.53(0.86) | −0.18 |
| Self-esteem | 20.28(0.48) | 19.42(0.29) | −0.18 | 19.38(0.48) | −0.19 | 16.71(0.74)** | −0.77 | 21.03(0.63) | 0.16 | 19.94(0.50) | −0.07 | 18.59(0.92) | −0.36 |
| Resilience | 23.04(0.46) | 22.53(0.26) | −0.11 | 21.96(0.47) | −0.24 | 22.08(0.72) | −0.21 | 23.00(0.61) | −0.01 | 23.22(0.49) | 0.04 | 22.01(0.90) | −0.23 |
| Family involvement | 21.30(0.38) | 20.89(0.22) | −0.11 | 20.59(0.39) | −0.19 | 21.13(0.59) | −0.05 | 21.44(0.50) | 0.04 | 20.91(0.40) | −0.10 | 20.39(0.73) | −0.25 |
| Home safety | 24.93(0.41) | 24.74(0.24) | −0.05 | 24.21(0.42) | −0.18 | 26.20(0.64) | 0.32 | 24.71(0.54) | −0.05 | 24.90(0.43) | −0.01 | 23.98(0.80) | −0.24 |
| Social Problem solving | 21.40(0.34) | 22.41(0.20)** | 0.29 | 22.75(0.34)** | 0.40 | 23.60(0.54)** | 0.66 | 21.85(0.45) | 0.13 | 21.97(0.36) | 0.17 | 21.98(0.66) | 0.17 |
| Physical activity | 20.93(0.60) | 19.53(0.35)* | −0.23 | 18.94(0.60)* | −0.34 | 16.61(0.93)** | −0.74 | 20.39(0.80) | −0.09 | 20.97(0.64) | 0.01 | 19.86(1.16) | −0.18 |
| Discomfort | 19.89(0.54) | 19.90(0.30) | 0.003 | 19.60(0.54) | −0.05 | 18.63(0.84) | −0.24 | 20.78(0.71) | 0.17 | 20.12(0.56) | 0.04 | 20.41(1.04) | 0.10 |
| Physical discomfort | 18.28(0.61) | 18.35(0.34) | 0.01 | 17.81(0.61) | −0.08 | 17.53(0.94) | −0.13 | 19.26(0.80) | 0.16 | 18.47(0.64) | 0.03 | 19.30(1.17) | 0.17 |
| Emotional discomfort | 18.05(0.58) | 18.50(0.33) | 0.08 | 18.10(0.58) | 0.01 | 17.72(0.89) | −0.06 | 19.96(0.77)* | 0.34 | 18.55(0.61) | −0.14 | 18.09(1.11) | 0.01 |
| Limitation of activity | 22.10(0.37) | 21.87(0.21) | −0.06 | 21.79(0.37) | −0.09 | 20.44(0.58)* | −0.46 | 22.31(0.50) | 0.06 | 22.18(0.39) | 0.02 | 22.46(0.72) | 0.10 |
| Risks | 19.41(0.54) | 20.58(0.33)* | 0.21 | 19.81(0.55) | 0.08 | 22.94(0.85)** | 0.66 | 20.49(0.72) | 0.20 | 20.68(0.57) | 0.24 | 19.58(1.05) | 0.03 |
| Individual risk-taking | 21.49(0.64) | 22.48(0.37) | 0.15 | 22.08(0.65) | 0.09 | 25.01(1.00)** | 0.56 | 21.82(0.85) | 0.05 | 22.64(0.67) | 0.18 | 20.82(1.24) | −0.11 |
| Threats to achievement | 20.58 0.40) | 21.48(0.23)* | 0.22 | 21.08(0.40) | 0.13 | 22.08(0.62)* | 0.39 | 22.19(0.53)* | 0.41 | 21.47(0.42) | 0.23 | 20.54(0.77) | −0.01 |
| Negative Peer influence | 16.34(0.61) | 17.24(0.36) | 0.15 | 16.19(0.61) | −0.03 | 20.40(0.94)** | 0.69 | 16.77(0.80) | 0.07 | 17.37(0.64) | 0.17 | 16.74(1.19) | 0.07 |
| Disorders | 18.87(0.39) | 17.62(0.24)** | −0.32 | 17.34(0.39)** | −0.40 | 16.73(0.61)** | −0.56 | 18.97(0.52) | 0.03 | 17.70(0.41)* | −0.30 | 16.89(0.76)* | −0.52 |
| Acute minor disorders | 18.54(0.51) | 18.39(0.30) | −0.03 | 17.80(0.52) | −0.15 | 18.87(0.80) | 0.07 | 18.88(0.68) | 0.07 | 18.66(0.54) | 0.02 | 17.92(0.99) | −0.12 |
| Acute major disorders | 19.72(0.51) | 19.99(0.28) | 0.05 | 19.82(0.52) | 0.02 | 20.43(0.79) | 0.14 | 19.95(0.67) | 0.05 | 20.08(0.53) | 0.07 | 19.69(0.99) | −0.01 |
| Recurrent disorders | 19.04(0.49) | 19.18(0.28) | 0.03 | 18.68(0.50) | −0.07 | 19.91(0.77) | 0.18 | 19.98(0.65) | 0.19 | 19.13(0.52) | 0.02 | 18.43(0.95) | −0.13 |
| Long-term medical | 20.34(0.68) | 18.45(0.44)** | −0.27 | 17.53(0.69)** | −0.42 | 18.33(1.05) | −0.30 | 19.92(0.90) | −0.06 | 19.04(0.71) | −0.20 | 16.94(1.31)* | −0.51 |
| Long-term surgical | 16.32(1.08) | 12.85(0.65)** | −0.32 | 14.58(1.09) | −0.17 | 8.65(1.68)** | −0.73 | 14.98(1.42) | −0.13 | 12.15(1.13)** | −0.39 | 10.80(2.08)* | −0.52 |
| Psychosocial disorder | 19.41(0.76) | 16.84(0.47)** | −0.33 | 15.82(0.78)** | −0.48 | 14.43(1.19)** | −0.67 | 19.77(1.02) | 0.05 | 17.21(0.80)* | −0.29 | 17.00(1.48) | −0.32 |
| Achievement | 19.60(0.51) | 19.82(0.29) | 0.05 | 19.11(0.51) | −0.10 | 19.56(0.79) | −0.01 | 20.82(0.67) | 0.25 | 20.33(0.53) | 0.15 | 18.99(0.99) | −0.12 |
| Academic performance | 22.79(0.51) | 22.34(0.31) | −0.09 | 21.47(0.51) | −0.27 | 22.39(0.82) | −0.08 | 23.10(0.68) | 0.06 | 23.20(0.54) | 0.08 | 20.98(0.99) | −0.37 |
| Work performance | 19.34(0.59) | 19.45(0.32) | 0.02 | 18.91(0.57) | −0.09 | 19.87(0.93) | 0.11 | 20.50(0.72) | 0.25 | 19.12(0.58) | −0.05 | 19.65(1.10) | 0.07 |
Note: CHIP-AE = Child Health and Illness Profile-Adolescent Edition
The standard reference population mean on the CHIP-AE is 20, with a standard deviation of 5. Higher score indicates better health
T test was used to compare siblings and diagnosis groups and calculate the p-values:
=p-value < 0.05;
=p-value < 0.01
Cohen's d effect size was calculated comparing survivors to siblings and each diagnostic group with siblings, adjusting for sex and diagnostic group
The mean scores for each domain and subdomain of the CHIP-AE, by gender within survivors, are shown in Table 3. Among survivors, males scored better than females in Satisfaction, Discomfort, and Disorders domains; females reported less overall satisfaction with health, less physical activity, more physical and emotional discomfort, and a greater number of acute minor and recurrent disorders. However, female survivors scored higher than males in the subdomains of problem solving and academic achievement. These sex differences were not seen in the sibling group as shown in the supplemental table: females vs males for Satisfaction (19.98 vs. 20.60, p 0.43), Discomfort (19.50 vs. 20.06, p 0.57), and Disorders (19.00 vs. 18.66, p 0.56).
Table 3.
Mean (Standard Deviation (SD)) Scores of CHIP-AE domain and subdomains by gender among survivors
| DOMAIN | SURVIVORS | |||
|---|---|---|---|---|
| Females (N=184) |
Males (N=123) |
P-value | Effect Size |
|
| Satisfaction | 18.71(4.91) | 20.26(4.75) | <0.01 | 0.32 |
| Overall satisfaction | 18.48(4.81) | 20.79(4.59) | <0.001 | 0.49 |
| Self-esteem | 19.22(5.11) | 19.67(4.87) | 0.44 | 0.09 |
| Resilience | 22.26(4.78) | 22.85(4.23) | 0.26 | 0.13 |
| Family involvement | 20.65(4.10) | 21.18(3.40) | 0.22 | 0.14 |
| Home safety | 24.84(4.47) | 24.70(3.76) | 0.76 | −0.03 |
| Problem solving | 23.12(3.22) | 21.71(3.50) | <0.001 | −0.43 |
| Physical activity | 18.61(5.94) | 20.44(6.12) | 0.01 | 0.31 |
| Discomfort | 18.46(5.85) | 21.46(4.61) | <0.001 | 0.56 |
| Physical discomfort | 16.58(6.58) | 20.28(5.24) | <0.001 | 0.61 |
| Emotional discomfort | 16.91(6.29) | 20.20(4.94) | <0.001 | 0.57 |
| Limitation of activity | 21.45(3.97) | 22.35(3.21) | 0.03 | 0.24 |
| Risks | 20.52(5.28) | 20.71(5.89) | 0.76 | 0.04 |
| Individual risk-taking | 22.28(6.17) | 22.71(6.49) | 0.55 | 0.07 |
| Threats to achievement | 21.90(3.59) | 21.13(4.37) | 0.10 | −0.20 |
| Peer influence | 16.63(6.20) | 17.90(6.22) | 0.08 | 0.21 |
| Disorders | 17.32(3.86) | 17.97(4.52) | 0.17 | 0.16 |
| Acute minor disorders | 17.76(5.26) | 19.13(5.02) | 0.02 | 0.27 |
| Acute major disorders | 20.00(4.78) | 20.13(4.80) | 0.81 | 0.03 |
| Recurrent disorders | 18.45(4.81) | 20.01(4.91) | <0.01 | 0.32 |
| Long-term medical | 18.56(6.61) | 18.38(8.30) | 0.84 | −0.02 |
| Long-term surgical | 12.38(11.47) | 13.27(11.31) | 0.50 | 0.08 |
| Psychosocial disorder | 16.96(7.99) | 16.72(8.16) | 0.80 | −0.03 |
| Achievement | 20.22(5.24) | 19.51(4.92) | 0.24 | −0.14 |
| Academic performance | 23.06(5.21) | 21.67(5.26) | 0.03 | −0.27 |
| Work performance | 19.29(4.74) | 19.74 4.49) | 0.48 | 0.10 |
Note: CHIP-AE = Child Health and Illness Profile-Adolescent Edition
Higher score indicates better health
T test was used to compare males and females and calculate the p-values
Cohen's d effect size was calculated comparing males with females
In a multivariable model to evaluate correlates of poor health (mean score < 10% on a given domain) among survivors, several patterns emerged (Table 4). Females were more likely than males to report problems in the Satisfaction, Resilience, and Discomfort domains. Survivors diagnosed between the ages of 2–4 years vs those diagnosed prior to age 2 and teens with lower household income were more likely to report problems with resilience. In addition, non-whites were more likely to report Disorders than were whites. Lastly, when compared with leukemia survivors, CNS survivors were more likely to report problems in Satisfaction and Disorders domains.
Table 4.
Multivariable model of demographic and treatment characteristics associated with poor health among survivors.
| Satisfaction | Resilience | Discomfort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | OR* | 95% CI | P-Value | % | OR* | 95% CI | P-Value | % | OR* | 95% CI | P-Value | |
| Sex | ||||||||||||
| Male | 13.01 | 1.00 | 7.32 | 1.00 | 7.32 | 1.00 | ||||||
| Female | 25.00 | 2.46 | 1.28–4.75 | 0.01 | 13.59 | 2.41 | 1.01–5.78 | 0.05 | 16.85 | 2.71 | 1.22–6.06 | 0.01 |
| Current age group | ||||||||||||
| 15–17 years | 20.16 | 1.00 | 11.63 | 1.00 | 13.18 | 1.00 | ||||||
| 18–20 years | 20.22 | 0.86 | 0.45–1.65 | 0.66 | 10.67 | 0.56 | 0.24–1.30 | 0.18 | 12.92 | 0.97 | 0.46–2.05 | 0.93 |
| Age at diagnosis | ||||||||||||
| < 2 years | 18.18 | 1.00 | 9.09 | 1.00 | 12.63 | 1.00 | ||||||
| 2–4 years | 23.85 | 1.43 | 0.70–2.93 | 0.32 | 14.68 | 2.92 | 1.15–7.42 | 0.02 | 13.76 | 1.24 | 0.53–2.92 | 0.62 |
| Race/ethnicity | ||||||||||||
| White** | 20.45 | 1.00 | 10.41 | 1.00 | 12.64 | 1.00 | ||||||
| Non-white | 18.42 | 1.10 | 0.44–2.77 | 0.84 | 15.79 | 1.96 | 0.71–5.41 | 0.19 | 15.79 | 1.60 | 0.60–4.26 | 0.34 |
| Household income | ||||||||||||
| < 60,000/year | 21.28 | 1.00 | 13.30 | 1.00 | 12.23 | 1.00 | ||||||
| $60,000+/year | 19.27 | 0.77 | 0.41–1.44 | 0.41 | 6.42 | 0.40 | 0.16–0.98 | 0.04 | 14.68 | 1.23 | 0.61–2.51 | 0.56 |
| Diagnosis | ||||||||||||
| Leukemia | 21.05 | 1.00 | 14.74 | 1.00 | 13.68 | 1.00 | ||||||
| CNS Tumor | 40.00 | 2.98 | 1.25–7.07 | 0.01 | 10.00 | 1.01 | 0.28–3.62 | 0.98 | 17.50 | 1.50 | 0.52–4.34 | 0.46 |
| Neuroblastoma | 13.33 | 0.64 | 0.26–1.56 | 0.33 | 10.00 | 1.08 | 0.37–3.16 | 0.89 | 13.33 | 1.08 | 0.40–2.92 | 0.87 |
| Wilms' Tumor | 12.50 | 0.57 | 0.21–1.52 | 0.26 | 7.14 | 0.60 | 0.17–2.10 | 0.43 | 8.93 | 0.68 | 0.22–2.13 | 0.50 |
| Other | 26.92 | 1.43 | 0.51–4.04 | 0.49 | 11.54 | 0.95 | 0.23–3.86 | 0.94 | 11.54 | 0.85 | 0.22–3.36 | 0.82 |
| Risks | Disorders | Achievement | ||||||||||
| % | OR* | 95% CI | P-Value | % | OR* | 95% CI | P-Value | % | OR* | 95% CI | P-Value | |
| Sex | ||||||||||||
| Male | 13.01 | 1.00 | 17.89 | 1.00 | 11.48 | 1.00 | ||||||
| Female | 8.70 | 0.62 | 0.28–1.36 | 0.23 | 21.20 | 1.43 | 0.77–2.64 | 0.26 | 10.06 | 1.07 | 0.49–2.33 | 0.87 |
| Current age group | ||||||||||||
| 15–17 years | 12.40 | 1.00 | 22.48 | 1.00 | 10.32 | 1.00 | ||||||
| 18–20 years | 8.99 | 0.63 | 0.26–1.54 | 0.31 | 17.98 | 0.75 | 0.40–1.41 | 0.37 | 10.86 | 1.06 | 0.46–2.46 | 0.88 |
| Age at diagnosis | ||||||||||||
| < 2 years | 10.10 | 1.00 | 20.20 | 1.00 | 9.79 | 1.00 | ||||||
| 2–4 years | 11.01 | 0.84 | 0.31–2.27 | 0.73 | 19.27 | 1.29 | 0.62–2.69 | 0.50 | 12.15 | 1.09 | 0.44–2.71 | 0.86 |
| Race/ethnicity | ||||||||||||
| White | 9.29 | 1.00 | 18.22 | 1.00 | 9.51 | 1.00 | ||||||
| Non-White | 18.42 | 1.84 | 0.68–5.01 | 0.23 | 31.58 | 2.38 | 1.08–5.23 | 0.03 | 18.42 | 2.39 | 0.92–6.22 | 0.08 |
| Household income | ||||||||||||
| < 60,000/year | 10.11 | 1.00 | 20.74 | 1.00 | 12.50 | 1.00 | ||||||
| $60,000+/year | 10.09 | 0.95 | 0.42–2.14 | 0.90 | 19.27 | 0.83 | 0.45–1.54 | 0.56 | 7.48 | 0.53 | 0.22–1.25 | 0.15 |
| Diagnosis | ||||||||||||
| Leukemia | 12.63 | 1.00 | 17.89 | 1.00 | 13.83 | 1.00 | ||||||
| CNS Tumor | 2.50 | 0.15 | 0.02–1.27 | 0.08 | 32.50 | 2.63 | 1.06–6.48 | 0.04 | 15.38 | 1.31 | 0.43–4.01 | 0.64 |
| Neuroblastoma | 7.78 | 0.48 | 0.16–1.46 | 0.20 | 22.22 | 1.48 | 0.63–3.45 | 0.37 | 7.87 | 0.47 | 0.15–1.47 | 0.20 |
| Wilms' Tumor | 8.93 | 0.49 | 0.14–1.70 | 0.26 | 12.50 | 0.73 | 0.27–1.97 | 0.53 | 7.41 | 0.53 | 0.15–1.81 | 0.31 |
| Other | 26.92 | 2.19 | 0.71–6.78 | 0.17 | 15.38 | 0.93 | 0.28–3.16 | 0.91 | 8.00 | 0.61 | 0.12–2.98 | 0.54 |
Poor Health – defined for each domain based on 10th percentile cut-point score for sibling sample. See text.
Odds ratio represent ‘worse’ outcome
OR=Standardized risk ratios and 95% confidence intervals for multiple variable models
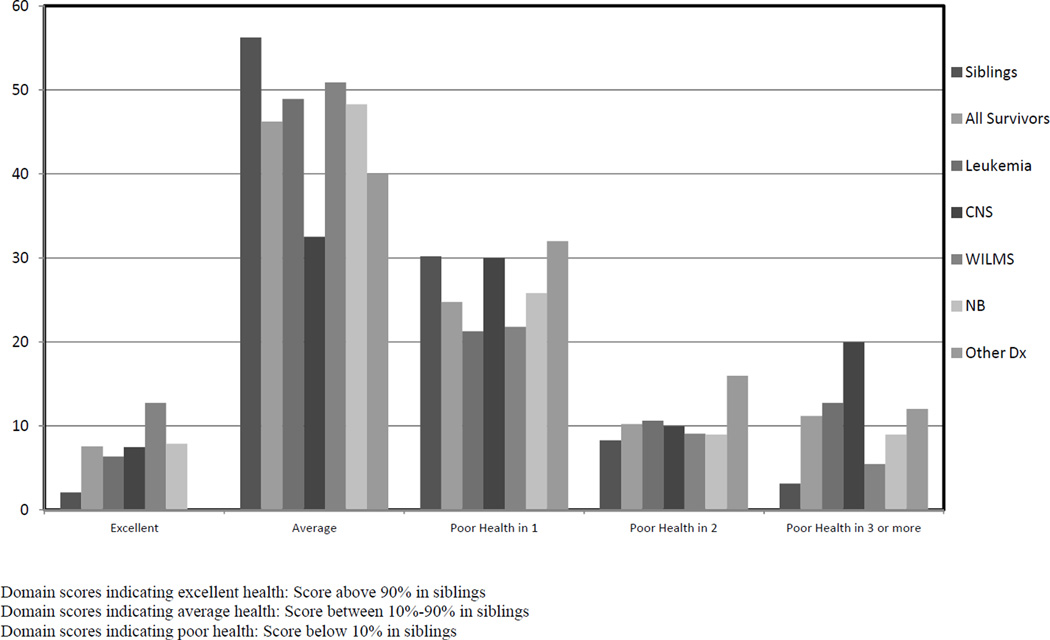
To ascertain a broad view of health in teen survivors, the distribution of overall health was similar among survivors and siblings. However, survivors were more likely to report poor health on three or more domains than were siblings (11% vs. 3%); specifically CNS tumor survivors and those with several other diagnoses, including soft tissue sarcoma, (n=19), bone tumor (n=1) and non-Hodgkin lymphoma (n=1), fared the worst, with 60% reporting poor health in one or more domains compared to 41% in siblings. Wilms’ tumor survivors appeared to do the best, with 13% reporting excellent health.
Discussion
The goal of cancer treatment in children is to cure disease, while using the least toxic strategies to optimize future growth, development, and quality of life. Previous research has indicated that treatment for childhood cancer damages vital organs and increases lifetime risk of chronic disease and less-than-optimal quality of life [28, 29]. Because of the young age and potential longevity among the survivors examined in these analyses, the delayed consequences of therapy may have a significant impact on their abilities to assume life roles as these survivors move from adolescence into adulthood. Therefore, research assessing the overall health of childhood cancer survivors is critical to target interventions to reduce distress in survivors.
Studies from the CCSS document psychosocial and health related problems in adult survivors of childhood cancer [5, 13], and robust and psychologically healthy siblings with only a small subset at risk for developing psychological distress [30]. The good news is that our current analysis indicates that the distribution of health problems of adolescent survivors and siblings is not vastly different. On the other hand, we found that adolescents with significant problems were able to self-report them, similar to other studies which show that many of these problems appear during adolescence [9, 29].
Many studies examining outcomes among childhood cancer survivors have identified young age at diagnosis as a substantial risk factor for poor overall health, in physical, emotional, and social domains [9, 31–35]. Our study further characterizes the period of highest vulnerability to show that survivors diagnosed from ages 2 to 4 years actually had more problems in adolescence than did survivors diagnosed before age 2. This finding deserves further exploration to determine if the differences reflect diagnostic distinctions or potential differences in the ability to conceptualize and remember the illness experience in those above and below age 2. In addition, previous studies have suggested increased vulnerability in female survivors [11–13, 33]. Our study shows similar results, particularly in decreased overall satisfaction, physical activity, and physical and emotional disorders. Despite these self-reported problems, female survivors and siblings demonstrated higher scores in problem solving and academic performance compared to male survivors and siblings.
Adolescents previously treated for CNS tumors also emerged as a high-risk group, as they had the poorest health satisfaction, the greatest number of health problems related to their diagnosis and treatment, lower self-esteem, lower levels of physical activities, and increased levels of psychosocial disorders. Deficits in social skills have been noted in brain tumor survivors and are associated with lower self-confidence and increased levels of depression [36, 37]. In contrast, those with CNS tumors in this study reported better functioning with respect to social problem solving and lower levels of risky behaviors, suggesting support from their outside environment (i.e. family, school). Perhaps also they remain in a more parental protective and monitored environment, which may inhibit progression to independence as they transition to adulthood to the extent that their cognitive abilities allow. When compared to siblings, these CNS survivors were also more likely to report poor health outcomes on three or more domains, findings that suggest that this is a high risk diagnostic group in which early intervention and preventive measures may be most valuable.
A higher risk for poorer self-reported psychological outcomes in the teen survivors in this study compared to siblings is consistent with previous studies of functioning in adult survivors of childhood cancer, particularly within certain diagnostic groups, such as leukemia, CNS tumors, and neuroblastoma [31, 38]. Survivors diagnosed with leukemia and neuroblastoma also included a significant number who indicated poor health across three or more domains (25% and 22% respectively). Adult survivors with CNS tumors also demonstrated higher rates of impaired physical health [39]. In a parental assessment of young adolescents within the CCSS, survivors between 12–17 years of age were more likely than siblings to have symptoms of anxiety/depression and antisocial behaviors, particularly leukemia and CNS tumor survivors [28]. Results from the MOS SF-36 questionnaire also demonstrated that those diagnosed in the younger age group (characterized as 0–3 years of age) had the poorest outcomes for physical function, physical role, bodily pain, and general health [29].
Data from this study suggest directions for future intervention. Peer support and social acceptance play a formative role in normal adolescent development [40] and the development of peer-based therapeutic programs, social skills training programs and support groups can provide adolescent cancer survivors an opportunity to connect with like-minded peers, decreasing social isolation and fostering a sense of normalcy [41]. Becoming involved in a peer group may help promote a sense of group identity, an important developmental task of adolescence [42]. Also, there is ongoing research to better categorize social functioning deficits in brain tumor patients that will lead to development of interventions aimed at improving peer relationships in CNS survivors [43]. The strength of socio-economic factors influencing health outcomes for children with CNS malignancies has been previously noted [44]; interventions targeting support of low-SES families affected by cancer, with special attention to those who are the parents of very young patients, will be valued additions for pediatric psycho-oncologists.
Certain limitations to this analysis should be considered when interpreting these results. First, there were some parents who would not give permission for their adolescent to receive this survey. However, a comparison of respondents and non-respondents showed no cancer and demographic differences, except for females being more likely than males to participate. Secondly, the CCSS has a relatively small percentage of non-white participants, limiting the generalizability of the results. Lastly, these data were collected on a sample of young children diagnosed in 1970–1986, when both treatments and supportive care were different than they are currently, a time factor limiting the generalizability of these findings.
Conclusion
Results from this study show that despite the known long-term effects of cancer treatments in early life, many adolescent survivors in this large and diverse sample have average to good health related quality of life in many domains. Subgroups of pediatric cancer survivors are more vulnerable to medical and psychosocial disorders in adolescence. Early identification and intervention to counter potential health risks and support beneficial behaviors in adolescents who have had cancer when very young are critical to reduce morbidity and enhance the likelihood that these survivors will enjoy a productive, satisfying adult life. Further research is needed to identify the mechanisms that mediate between physical effects, psychosocial well-being and functioning, allowing us to develop interventions that take into account many of the components conducive to successful transition from adolescence into young adulthood.
Supplementary Material
Figure 1.
Percent of teens within each distinct health profile-types
Acknowledgments
This work was supported by NCI grant CA 55727 and ACS Grant RSG-01-021-01-CCE.
Abbreviations
- CCSS
Childhood Cancer Survivor Study
Appendix
The Childhood Cancer Survivor Study (CCSS) is a collaborative, multi-institutional project, funded as a resource by the National Cancer Institute, of individuals who survived five or more years after diagnosis of childhood cancer. CCSS is a retrospectively ascertained cohort of 20,346 childhood cancer survivors diagnosed before age 21 between 1970 and 1986 and approximately 4,000 siblings of survivors, who serve as a control group. The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and Canada. The study is currently funded by a U24 resource grant (NCI grant # U24 CA55727) awarded to St. Jude Children’s Research Hospital. Currently, we are in the process of expanding the cohort to include an additional 14,000 childhood cancer survivors diagnosed before age 21 between 1987 and 1999. For information on how to access and utilize the CCSS resource, visit www.stjude.org/ccss
CSS Institutions and Investigators
St. Jude Children’s Research Hospital, Memphis, TN
Ann & Robert H. Lurie Children’s Hospital of Chicago, IL
Children's Healthcare of Atlanta/Emory University, Atlanta, GA
Children's Hospitals and Clinics of Minnesota Minneapolis, St. Paul, MN
Seattle Children’s Hospital, Seattle, WA
Children’s Hospital Colorado, Aurora, CO
Children’s Hospital, Los Angeles, CA
Children’s Hospital, Oklahoma City, OK
Children’s Hospital of Orange County, Orange, CA
Children’s Hospital of Philadelphia, Philadelphia, PA
Children’s Hospital of Pittsburgh, Pittsburgh, PA
Children’s National Medical Center, Washington, DC
Cincinnati Children’s Hospital Medical Center, Cincinnati, OH
City of Hope Medical Center, Los Angeles, CA
Cook Children’s Medical Center, Ft. Worth, TX
Dana-Farber Cancer Institute/Children’s Hospital, Boston, MA
Fred Hutchinson Cancer Research Center, Seattle, WA
Hospital for Sick Children, Toronto, ON
International Epidemiology Institute, Rockville, MD
Mayo Clinic, Rochester, MN
Memorial Sloan-Kettering Cancer Center, New York, NY
Miller Children’s Hospital, Long Beach, CA
National Cancer Institute, Bethesda, MD
Nationwide Children's Hospital, Columbus, Ohio
Riley Hospital for Children, Indianapolis, IN
Roswell Park Cancer Institute, Buffalo, NY
St. Louis Children’s Hospital, St. Louis, MO
Stanford University School of Medicine, Stanford, CA
Texas Children’s Hospital, Houston, TX
University of Alabama, Birmingham, AL
University of Alberta, Edmonton, AB
University of California-Los Angeles, CA
University of California-San Francisco, CA
University of Chicago, Chicago, IL
University of Michigan, Ann Arbor, MI
University of Minnesota, Minneapolis, MN
University of Southern California, Los Angeles, CA
UT-Southwestern Medical Center, Dallas, TX
U.T.M.D. Anderson Cancer Center, Houston, TX
Leslie L. Robison, PhD#+, Melissa Hudson, MD*++
Greg T. Armstrong, MD, MSCE +, Daniel M. Green, MD +
Kevin R. Krull, PhD+, Kiri Ness, PhD+
Kimberley Dilley, MD, MPH*
Lillian Meacham, MD*, Ann Mertens, PhD+
Joanna Perkins, MD, MS*
Scott Baker, MD*, Eric Chow, MD, MPH +
Brian Greffe, MD*
Kathy Ruccione, RN, MPH*
John Mulvihill, MD+
Leonard Sender, MD*
Jill Ginsberg, MD*, Anna Meadows, MD +
Jean Tersak, MD *
Sadhna Shankar, MD*, Roger Packer, MD+
Paul Bowman, MD, MPH*
Mark Greenberg, MBChB*, Paul C. Nathan, MD, MSc*+
John Boice, ScD+
Vilmarie Rodriguez, MD*
Charles Sklar, MD*+, Kevin Oeffinger, MD+
Jerry Finklestein, MD*
Roy Wu, PhD+, Nita Seibel, MD+, Peter Inskip, ScD+
Julia Rowland, PhD+
Laura Martin, MD*, Sue Hammond, MD+
Terry A. Vik, MD*
Denise Rokitka, MD, MPH*
Robert Hayashi, MD*
Neyssa Marina, MD, MS*, Sarah S. Donaldson, MD +
Zoann Dreyer, MD*
Kimberly Whelan, MD, MSPH*
Jacqueline Casillas, MD, MSHS*, Lonnie Zeltzer, MD+
Robert Goldsby, MD*
Tara Henderson, MD, MPH*
Raymond Hutchinson, MD*
Daniel C. Bowers, MD*
Louise Strong, MD*+, Marilyn Stovall, MPH, PhD+
*Institutional Principal Investigator
+ Project Co-Principal Investigator
‡ Member CCSS Steering Committee
#Project Principal Investigator (U24 CA55727)
References
- 1.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review 1975–2009 (Vintage 2009 Populations) Bethesda, MD: National Cancer Institute; 2012. [Google Scholar]
- 2.Mariotto AB, Rowland JH, Yabroff KR, Scoppa S, Hachey M, Ries L, Feuer EJ. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 3.Ellenberg L, Liu Q, Gioia G, Yasui Y, Packer RJ, Mertens A, Donaldson SS, Stovall M, Kadan-Lottick N, Armstrong G, Robison LL, Zeltzer LK. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23(6):705–717. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadan-Lottick NS, Zeltzer LK, Liu Q, Yasui Y, Ellenberg L, Gioia G, Robison LL, Krull KR. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102(12):881–893. doi: 10.1093/jnci/djq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS, Schwartz CL, Leisenring W, Robison LL. Chronic Health Conditions in Adult Survivors of Childhood Cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 6.Ris MD, Beebe DW. Neurodevelopmental outcomes of children with low-grade gliomas. Dev Disabil Res Rev. 2008;14:196–202. doi: 10.1002/ddrr.27. [DOI] [PubMed] [Google Scholar]
- 7.Stuber ML, Kazak AE, Meeske K, Barakat L, Guthrie D, Garnier H, Pynoos R, Meadows A. Predictors of Posttraumatic Stress Symptoms in Childhood Cancer Survivors. Pediatrics. 1997;100:958–964. doi: 10.1542/peds.100.6.958. [DOI] [PubMed] [Google Scholar]
- 8.Stuber ML, Meeske KA, Krull KA, Leisenring W, Stratton K, Huber M, Zebrack B, Uijtdehaage SH, Mertens AC, Robison LL, Zeltzer LK. Prevalence and Predictors of Posttraumatic Stress Disorder in Adult Survivors of Childhood Cancer: a report from the Childhood Cancer Survivor Study. Pediatrics. 2010;125(5):e1124–e1134. doi: 10.1542/peds.2009-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maurice-Stam H, Oort FJ, Last BF, Brons PPT, Caron HN, Grootenhuis MA. Longitudinal assessment of health-related quality of life in preschool children with non-CNS cancer after the end of successful treatment. Pediatric Blood and Cancer. 2008;50:1047–1051. doi: 10.1002/pbc.21374. [DOI] [PubMed] [Google Scholar]
- 10.von Essen L, Enskar K, Kreuger A, Larsson B, Sjoden PO. Self-esteem, depression and anxiety among Swedish children and adolescents on and off cancer treatment. Acta Paediatrica. 2000;89:229–236. doi: 10.1080/080352500750028889. [DOI] [PubMed] [Google Scholar]
- 11.Waber DP, Carpentieri SC, Klar N, Silverman LB, Schwenn M, Hurwitz CA, Mullenix PJ, Tarbell NE, Sallan SE. Cognitive sequelae in children treated for acute lymphoblastic leukemia with dexamethasone or prednisone. J Pediatar Hematol Oncol. 2000;22:206–213. doi: 10.1097/00043426-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Gragert MN, Ris MD. Neuropsychological late effects and rehabilitation following pediatric brain tumor. J Pediatr Rehabil Med. 2011;4(1):47–58. doi: 10.3233/PRM-2011-0153. [DOI] [PubMed] [Google Scholar]
- 13.Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Casillas J, Tsao JC, Lu Q, Krull KR. Psychological status in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2396–2404. doi: 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsujimoto S. The prefrontal cortex: functional neural development during early childhood. Neuroscientist. 2008;14(4):345–358. doi: 10.1177/1073858408316002. [DOI] [PubMed] [Google Scholar]
- 15.Pihko E, Nevalainen P, Stephen J, Okada Y, Lauronen L. Maturation of somatosensory cortical processing from birth to adulthood revealed by magnetoencephalography. Clin Neurophysiol. 2009;120(8):1552–1561. doi: 10.1016/j.clinph.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 16.García-Molina A, Enseñat-Cantallops A, Tirapu-Ustárroz J, Roig-Rovira T. Maturation of the prefrontal cortex and development of the executive functions during the first five years of life. Rev Neurol. 2009;48(8):435–440. [PubMed] [Google Scholar]
- 17.Turner CD, Rey-Casserly C, Liptak C, Chordas C. Late effects of therapy for pediatric brain tumor survivors. J Child Neurology. 2009;24(11):1455–1463. doi: 10.1177/0883073809341709. [DOI] [PubMed] [Google Scholar]
- 18.Masten AS. Regulatory Processes, Risk, and Resilience in Adolescent Development. Annals of the New York Academy of Sciences. 2004;1021:310–319. doi: 10.1196/annals.1308.036. [DOI] [PubMed] [Google Scholar]
- 19.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, Li FP, Meadows AT, Mulvihill JJ, Neglia JP, Nesbit ME, Packer RJ, Potter JD, Sklar CA, Smith MA, Stovall M, Strong LC, Yasui Y, Zeltzer LK. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 20.Starfield B, Riley AW, Green BF, Ensminger ME, Ryan SA, Kelleher K, Kim-Harris S, Johnston D, Vogel K. The Adolescent Child Health and Illness Profile: A Population-Based Measure of Health. Med Care. 1995;33:553–566. doi: 10.1097/00005650-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Riley AW, Green B, Forrest CB, Starfield B, Kang M, Ensminger M. A taxonomy of adolescent health: Development of the adolescent health profile types. Medical Care. 1998;36:1228–1236. doi: 10.1097/00005650-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Riley AW, Forrest CB, Starfield B, Green B, Kang M, Ensminger M. Reliability and validity of the adolescent health profile-types. Medical Care. 1998;36(8):1237–1248. doi: 10.1097/00005650-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Starfield B, Ensminger ME, Green BF, Riley AW, Ryan S, Kim-Harris S, Crawford KV, Johnston D. Manual for The Child Health and Illness Profile: Adolescent EditionTM (CHIP-AETM) Baltimore, MD: Johns Hopkins University; 1995. 1999. [Google Scholar]
- 24.Hack M, Cartar L, Schluchter M, Klein N, Forrest CB. Self-perceived health, functioning and well-being of very low birth weight infants at age 20 years. Journal of Pediatrics. 2007;151:635–641. 641.e1–641.e2. doi: 10.1016/j.jpeds.2007.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerson AC, Riley A, Fivush BA, Pham N, Fiorenza J, Robertson J, Chandra M, Trachtman H, Weiss R, Furth SL. Council on Pediatric Nephrology and Urology of New York/New Jersey; Kidney and Urology Foundation of America. Assessing health status and health care utilization in adolescents with chronic kidney disease. Journal of the American Society of Nephrology. 2005;16(5):1427–1432. doi: 10.1681/ASN.2004040258. [DOI] [PubMed] [Google Scholar]
- 26.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: Wiley Series in Probability and Statistics; 2004. pp. 187–234. [Google Scholar]
- 27.Thomas V Perneger. What’s wrong with Bonferroni adjustments. Br Med J. 1998;316(7139):1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultz KAP, Ness KK, Whitton J, Recklitis C, Zebrack B, Robison LL, Zeltzer L, Mertens AC. Behavioral and Social Outcomes in Adolescent Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. J Clin Oncol. 2007;25:3649–3656. doi: 10.1200/JCO.2006.09.2486. [DOI] [PubMed] [Google Scholar]
- 29.Zeltzer LK, Lu Q, Leisenring L, Tsao J, Recklitis C, Armstrong G, Mertens AC, Robison LL, Ness KK. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: A report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2008;17(2):435–446. doi: 10.1158/1055-9965.EPI-07-2541. [DOI] [PubMed] [Google Scholar]
- 30.Buchbinder D, Casillas J, Krull KR, Goodman P, Leisenring W, Recklitis C, Alderfer MA, Robison LL, Armstrong GT, Kunin-Batson A, Stuber M, Zeltzer LK. Psychological outcomes of siblings of cancer survivors: A Report from the Childhood Cancer Survivor Study. Psychooncology. 2011 Dec;20(12):1259–1268. doi: 10.1002/pon.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrera M, Schulte F, Spiegler BJ. Factors influencing depressive symptoms of children treated for a brain tumor. J Psychosoc Onc. 2008;26:1–16. doi: 10.1300/j077v26n01_01. [DOI] [PubMed] [Google Scholar]
- 32.Nathan PC, Ness KK, Greenberg ML, Hudson M, Wolden S, Davidoff A, Laverdiere C, Mertens A, Whitton J, Robison LL, Zeltzer L, Gurney JG. Health-related quality of life in adult survivors of childhood Wilms’ tumor or neuroblastoma. A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2007;49:704–715. doi: 10.1002/pbc.20949. [DOI] [PubMed] [Google Scholar]
- 33.Ness KK, Gurney JG, Zeltzer LK, Leisenring W, Mulrooney DA, Nathan PC, Robison LL, Mertens AC. The impact of limitations in physical, executive and emotional function on health-related quality of life among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Arch Phys Med Rehabil. 2008;89:128–136. doi: 10.1016/j.apmr.2007.08.123. [DOI] [PubMed] [Google Scholar]
- 34.Pogany L, Barr RD, Shaw A, Speechley KN, Barrera M, Maunsell E. Health status in survivors of cancer in childhood and adolescence. Qual Life Res. 2006;5(1):159–160. doi: 10.1007/s11136-005-0198-7. [DOI] [PubMed] [Google Scholar]
- 35.Ness KK, Morris EB, Nolan VG, Howell CR, Gilchrist LS, Stovall M, Cox CL, Klosky JL, Gajjar A, Neglia JP. Physical performance limitations among adult survivors of childhood brain tumors. Cancer. 2010;116(12):3034–3044. doi: 10.1002/cncr.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurney JG, Krull KR, Kadan Lottick N, Nicholson HS, Nathat PC, Zebrack B, Tersak JM, Ness KK. Social Outcomes in the Childhood Cancer Study Cohort. J Cin Oncol. 2009;27:2390–2395. doi: 10.1200/JCO.2008.21.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulte F, Barrera M. Social competence in childhood brain tumor survivors: a comprehensive review. Support Care Cancer. 2010;18(12):1499–1513. doi: 10.1007/s00520-010-0963-1. [DOI] [PubMed] [Google Scholar]
- 38.Holmqvist AS, Wiebe T, Hjorth L, Lindgren A, Ora I, Moell C. Young age at diagnosis is a risk factor for negative late socio-economic effects after acute lymphoblastic leukemia in childhood. Pediatr Blood Cancer. 2010;55(4):698–707. doi: 10.1002/pbc.22670. [DOI] [PubMed] [Google Scholar]
- 39.Perkins JL, Kunin-Batson AS, Youngren NM, Ness KK, Ulrich KJ, Hansen MJ, Petryk A, Steinberger J, Anderson FS, Baker KS. Long-term follow-up of children who underwent hematopoeitic cell transplant (HCT) for AML or ALL at less than 3 years of age. Pediatr Blood Cancer. 2007;49(7):958–963. doi: 10.1002/pbc.21207. [DOI] [PubMed] [Google Scholar]
- 40.Treadgold CL, Kuperberg A. Been there, done that, wrote the blog: the choices and challenges of supporting adolescents and young adults with cancer. J Clin Onco. 2010;28(32):4842–4849. doi: 10.1200/JCO.2009.23.0516. [DOI] [PubMed] [Google Scholar]
- 41.Barakat LP, Hetzke JD, Foley B, Carey ME, Gyato K, Phillips PC. Evaluation of a social-skills training group intervention with children treated for brain tumors: a pilot study. J Pediatr Psychol. 2003;28(5):299–307. doi: 10.1093/jpepsy/jsg019. [DOI] [PubMed] [Google Scholar]
- 42.Zebrack B, Bleyer A, Albritton K, Medearis S, Tang J. Assessing the health care needs of adolescent and young adult cancer patients and survivors. Cancer. 2006;107(12):2915–2923. doi: 10.1002/cncr.22338. [DOI] [PubMed] [Google Scholar]
- 43.Ach E, Gerhardt CA, Barrera M, Kupst MJ, Meyer EA, Patenaude AF, Vannatta K. Psycho-Oncology. 2013;22(8):1731–1737. doi: 10.1002/pon.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Butler RW, Fairclough DL, Katz EB, Kazak AE, Noll RB, Thompson RD, Sahler OJ. Intellectual functioning and multi-dimensional attentional processes in long-term survivors of a central nervous system related pediatric malignancy. Life Science. 2013 doi: 10.1016/j.lfs.2013.05.017. pii 0024-3205 (13), 00294-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.