Abstract
Reward-seeking actions can be guided by external cues that signal reward availability. For instance, when confronted with a stimulus that signals sugar, rats will prefer an action that produces sugar over a second action that produces grain pellets. Action selection is also sensitive to changes in the incentive value of potential rewards. Thus, rats that have been prefed a large meal of sucrose will prefer a grain-seeking action to a sucrose-seeking action. The current study investigated the dependence of these different aspects of action selection on cholinergic transmission. Hungry rats were given differential training with two unique stimulus-outcome (S1-O1 and S2-O2) and action-outcome (A1-O1 and A2-O2) contingencies during separate training phases. Rats were then given a series of Pavlovian-to-instrumental transfer tests, an assay of cue-triggered responding. Before each test, rats were injected with scopolamine (0, 0.03, or 0.1 mg/kg, intraperitoneally), a muscarinic receptor antagonist, or mecamylamine (0, 0.75, or 2.25 mg/kg, intraperitoneally), a nicotinic receptor antagonist. Although the reward-paired cues were capable of biasing action selection when rats were tested off-drug, both anticholinergic treatments were effective in disrupting this effect. During a subsequent round of outcome devaluation testing—used to assess the sensitivity of action selection to a change in reward value—we found no effect of either scopolamine or mecamylamine. These results reveal that cholinergic signaling at both muscarinic and nicotinic receptors mediates action selection based on Pavlovian reward expectations, but is not critical for flexibly selecting actions using current reward values.
Keywords: acetylcholine, incentive, goal-directed, Pavlovian, decision making, reward
INTRODUCTION
Reward-paired cues have the ability to provoke and invigorate reward-seeking behavior. For instance, rats tend to increase their performance of a lever-press action that once produced food reward when non-contingently presented with a Pavlovian cue that independently signals food reward (Dickinson et al, 2000; Wassum et al, 2011). Importantly, when rats are trained on distinct pairs of stimulus-outcome (S1-O1 and S2-O2) and action-outcome (A1-O1 and A2-O2) contingencies, these stimuli also acquire the ability to bias action selection at test, such that rats selectively increase their performance of whichever instrumental action was trained with the same outcome as the eliciting stimulus (eg, S1→A1) (Corbit and Balleine, 2005; Kruse et al, 1983).
The influence of reward-paired cues on instrumental action selection is mediated by separate behavioral and neural processes from those underlying the selection of actions based on changes in reward value (Dickinson et al, 2000; Holland, 2004; Ostlund and Balleine, 2007a, 2008b; Wassum et al, 2011). This latter aspect of action selection can be assayed through outcome devaluation testing. Typically, rats are pretrained on two distinct instrumental contingencies involving food outcomes, as described above. They are then allowed to consume freely one of the two foods until they reach a state of sensory-specific satiety. When then given a choice between the two actions, rats will tend to respond in a goal-directed manner, avoiding whichever action was trained with the devalued outcome (Balleine and Dickinson, 1998).
Although considerable progress has been made in identifying the specific neural circuits that support these different aspects of instrumental action selection, much remains unknown about their neurochemistry. The cholinergic system is a particularly interesting target for further study. Acetylcholine acts on two main receptor types in the brain: muscarinic and nicotinic. Both families of receptors are highly expressed in brain areas implicated in instrumental learning and performance, including the striatum, prefrontal cortex, and amygdala (Goldberg et al, 2012; Leslie et al, 2013; Thiele, 2013). Acetylcholine is also known to be involved in regulating dopamine signaling (Cachope et al, 2012; Chapman et al, 1997; Di Giovanni and Shi, 2009; Threlfell et al, 2012), a neuromodulator that has been more directly implicated in motivated behavior and is known to underlie the response-invigorating influence of reward-paired cues on reward-seeking behavior (Dickinson et al, 2000; Lex and Hauber, 2008; Ostlund and Maidment, 2012; Wassum et al, 2011, 2013). While acetylcholine has been implicated in various aspects of reward-motivated behavior (Mendez et al, 2012; Pratt and Kelley, 2004; Ragozzino et al, 2009), much remains unknown about its specific contributions to action selection.
The current study examined this issue by assessing if scopolamine (muscarinic antagonist) or mecamylamine (nicotinic antagonist) pretreatment affects action selection based on either the presentation of a reward-paired cue (using Pavlovian-to-instrumental transfer task) or a reduction in reward value (using outcome devaluation task) (see Figure 1 for schematic of experimental design).
Figure 1.
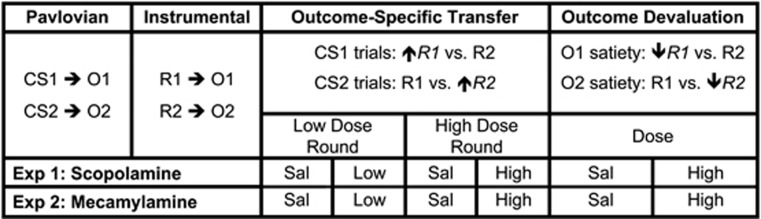
Schematic of experimental design. Hungry rats were given differential Pavlovian and instrumental conditioning before undergoing outcome-specific Pavlovian-to-instrumental transfer testing followed by outcome devaluation testing. Anticipated behavioral effects are indicated by upward and downward arrows, which reflect action-specific increases and decreases in lever press rates, respectively. Experiments 1 and 2 assessed the effects of scopolamine and mecamylamine pretreatment, respectively, in separate sets of rats. Each rat was tested with both low and high drug doses during transfer testing (order counterbalanced), whereas only the high dose was assessed during devaluation testing. Rats were also administered control (saline) tests during each round of testing, as indicated. See Materials and methods for further details. CS, conditioned stimulus; R, response; O, outcome; Sal, saline.
MATERIALS AND METHODS
Subjects
Adult male Sprague–Dawley rats (n=32) were tested in two experiments (n=16 per experiment) using similar experimental procedures, varying only in drug manipulation. Rats were pair housed in a climate-controlled vivarium and tested during the light phase of a 12 : 12 h light : dark cycle. Rats were food restricted (85% free-feeding body weight) for the duration of the experiment, with ad libitum access to water. All procedures were approved by the University of California, Los Angeles Institutional Animal Care and Use Committee, and were performed in accordance with National Research Council's Guide for the Care and Use of Laboratory Animals.
Apparatus
Rats were trained in eight identical Med Associates (East Fairfield, VT) chambers, and housed within light- and sound-attenuating shells. Each chamber contained two retractable levers, located on either side of a recessed food cup, into which 45-mg grain-based food pellets (Bioserv, Frenchtown, NJ) or 20% sucrose solution (0.1 ml) could be delivered. A photo beam sensor detected head entries into the food cup. A house light (24 V, 3 W) provided illumination during all behavioral sessions. White noise (70 dB) and tone (70 dB, 2000 Hz) generators were used to deliver auditory stimuli.
Drugs
Scopolamine hydrochloride (0.03 or 0.1 mg/kg; Experiment 1) or mecamylamine hydrochloride (0.75 or 2.25 mg/kg; Experiment 2) was dissolved in sterile saline and administered by intraperitoneal injection (volume=1 ml/kg) 30 min before testing. Drug doses and injection-to-test intervals are comparable to those used in previous studies (Anagnostaras et al, 1999; Levin et al, 2000; Mendez et al, 2012; Rodgers, 1979) and were selected to minimize nonspecific motor effects.
Pavlovian Conditioning
Rats received eight once-daily sessions of Pavlovian conditioning in which each of two auditory-conditioned stimuli (CSs; tone or noise; 2 min each) was paired with a different food outcome (grain pellets or sucrose solution). The specific Pavlovian stimulus-outcome contingencies were counterbalanced, such that half of the rats were given Tone-Pellet and Noise-Sucrose pairings, and half were given Tone-Sucrose and Noise-Pellet pairings. Each session lasted approximately 40 min and consisted of four Tone and four Noise trials, separated by a variable interval (mean=3.125 min; range=2.25–4 min). During each stimulus, the appropriate outcome was delivered on a random time 30-s schedule, resulting in an average of four outcome deliveries per trial.
Instrumental Conditioning
Rats then received 11 days of instrumental training. Each response (left vs right lever press) was reinforced with a different outcome (pellet or sucrose). Responses were trained in separate sessions, such that rats received two sessions per day, with session order alternating over days. Action-outcome contingencies were counterbalanced with Pavlovian contingencies. Thus, half of the rats in each Pavlovian training condition were trained with Left Press-Pellet and Right Press-Sucrose contingencies, whereas the remaining half were trained with Left Press-Sucrose and Right Press-Pellet contingencies. Each session lasted until 30 rewards were earned or 30 min had elapsed, whichever came first. Over days, the reinforcement schedule was gradually shifted from continuous reinforcement (2 days) to increasingly more effortful random ratio (RR) schedules (3 days each with RR-5, -10, and -20) to establish robust instrumental performance.
Pavlovian-to-Instrumental Transfer Testing
Rats underwent four Pavlovian-to-instrumental transfer tests. On the day before each of the four tests, rats were given a 30-min extinction session during which both levers were extended, but no rewards were delivered. This treatment was used to suppress instrumental performance, rendering it more sensitive to the stimulatory effects of reward-paired cues (Dickinson et al, 2000). Each test lasted 26 min and consisted of four trials (two Tone and two Noise; 2 min each), with the first trial starting 4 min into the session. Trials were presented in alteration (T–N–T–N) and were separated by a fixed 4-min interval. Both levers were continuously available during the tests, but no rewards were delivered. The effects of muscarinic and nicotinic acetylcholine receptor blockade on the expression of transfer performance were assessed by injecting rats with scopolamine (0, 0.03, or 0.1 mg/kg, Experiment 1) or mecamylamine (0, 0.75, or 2.25 mg/kg, Experiment 2), respectively, 30 min before each test. Drug type varied between subjects (and experiments), whereas drug dose varied within subjects. This was accomplished by dividing the four transfer tests into two pairs. For each pair of tests, one test was conducted after saline injection and the other was conducted after drug injection (either low or high dose). The drug dose was changed for the second pair of tests so that each rat was tested in both dose conditions. Order of injections (saline/drug vs drug/saline) was fixed across pairs of tests. This factor was counterbalanced with the order of drug dose across the rounds of testing (low/high vs high/low) as well as with Pavlovian and instrumental training contingencies. Following each transfer test, rats were given 1 day of Pavlovian conditioning using the procedures described above. This was followed by 2–3 days of instrumental training, also using the procedures described above. Lever pressing was reinforced on an RR-10 schedule on the first day of retraining and an RR-20 schedule thereafter. One day of RR-20 training was used for the first two rounds of retraining, whereas 2 days of RR-20 training were provided during the last round of retraining to overcome the repeated extinction testing.
Outcome Devaluation Testing
Rats were then given 4 days of instrumental retraining (as described above), with the ratio schedule increasing from RR-5 (1 day), to RR-10 (1 day), to RR-20 (2 days). On the next day, they underwent the first of two outcome devaluation tests. Before each test, rats received 1 h ad libitum access to either grain pellets or sucrose solution in their home cages. This sensory-specific satiety procedure is used to reduce temporarily the incentive value of the prefed food, while preserving the value of alternative foods. Thirty minutes into the feeding period, rats were administered scopolamine (0.1 mg/kg, intraperitoneally, Experiment 1), mecamylamine (2.25 mg/kg, intraperitoneally, Experiment 2) or saline before being returned to the home cage for the remaining 30 min of prefeeding, resulting in a 30-min injection-to-test interval, as during transfer testing. They were then placed in the operant chambers for a 5-min choice extinction test in which both levers were available but produced no rewards. Rats underwent instrumental retraining (1 day of RR-5, 1 day of RR-10, and 1 day of RR-20) before undergoing a second devaluation test. Rats were sated on the same outcome before each test (half had pellets devalued and half had sucrose devalued), counterbalancing the identity of the devalued outcome with instrumental training contingencies. Each rat was given one test on saline and a second test on drug, counterbalancing test order with instrumental training contingencies and the identity of the devalued outcome.
Data Analysis
Lever presses and food cup entries were continuously monitored during all behavioral procedures and have been converted to response rates (responses per min) for ease of comparison. The data of primary interest from Experiments 1 and 2 were analyzed separately using repeated-measures ANOVAs. For transfer testing, baseline press rates during 2-min pre-CS periods were averaged across actions and trials. Press rates during CS trials were separated according to whether the action and the CS were trained with the same outcome or different outcomes. Thus, treatment (drug vs saline), dose (low vs high), and CS period (pre-CS, same, different) were included as within-subjects factors in the ANOVA. Analysis of the transfer test data also included planned simple effects analyses to characterize cue-related differences in responding in each drug condition. For outcome devaluation testing, press rates for the two actions were coded according to whether the action was trained with devalued outcome or the non-devalued outcome. Thus, treatment (drug vs saline) and devaluation (devalued vs non-devalued) were included as within-subjects factors in the ANOVA. Statistical significance was set at p=0.05 (two-tailed) for all analyses.
RESULTS
Experiment 1: Effects of Scopolamine
Behavioral training
By the last Pavlovian conditioning session, rats entered the food cup at an average rate of 17.05 times per min (SEM=2.00) during CS periods (between CS onset and the first reward delivery), which was significantly greater than their entry rate during pre-CS periods (7.68 times per min, SEM=0.62) (paired t-test: t15=5.95, p<0.001), indicating that the reward-paired cues had acquired the ability to elicit conditioned approach behavior. Rats also developed robust levels of reward-seeking behavior during instrumental conditioning, such that by the last day of training they averaged 39.04 presses per min (SEM=3.11).
Pavlovian-to-instrumental transfer test
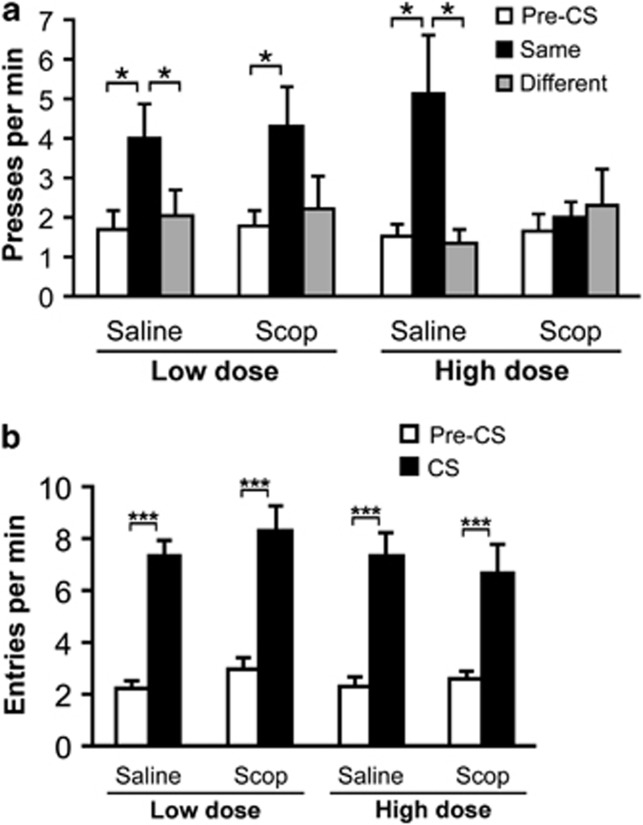
As can be seen in Figure 2a, rats were sensitive to the outcome-selective influence of reward-paired cues, favoring whichever action was trained with the same outcome as the cue being delivered on that trial (main effect of CS period: F1,15=9.86, p<0.001). Importantly, their responsiveness to such cues varied across tests according to whether they were pretreated with scopolamine or saline before testing, as well as the dose they received (treatment × dose × CS period interaction: F2,30=4.17, p=0.025). Planned pairwise comparisons (paired t-test) indicated that press rates were significantly elevated during the CS Same period relative to both the pre-CS period and the CS Different period for both vehicle tests (all p's<0.05). During the low-dose scopolamine test, CS Same press rates were significantly elevated from pre-CS rates (p<0.05), but were only marginally different from CS Different rates (p=0.08). During the high-dose scopolamine test, however, no differences in press rates were detected across CS periods (p's>0.05), indicating that this treatment prevented the cues from altering reward-seeking behavior.
Figure 2.
Effects of scopolamine (Scop) pretreatment on outcome-specific Pavlovian-to-instrumental transfer performance. (a) Mean number of lever presses per minute (±SEM) performed during the pre-CS period (collapsed across CSs and actions), during the CS periods, plotted separately for trials with the CS that signaled the same outcome that was earned by the action (Same) and for trials with the alternate CS, which signaled a different outcome (Different). (b) Mean number of food cup approaches (±SEM) during pre-CS and CS periods. Asterisks indicate significant differences (*<0.05, ***<0.001).
As shown in Figure 2b, these reward-paired cues also increased the rate of food cup entries during the transfer test (main effect of cue period: F1,15=118.82, p<0.001). Interestingly, and in contrast to what was observed with instrumental lever pressing, we detected no effect of scopolamine pretreatment on this conditioned approach response (no other main effects or interactions: p's>0.05). Furthermore, a highly significant CS period effect was detected in all test conditions (p's<0.001; paired t-test).
Outcome devaluation test
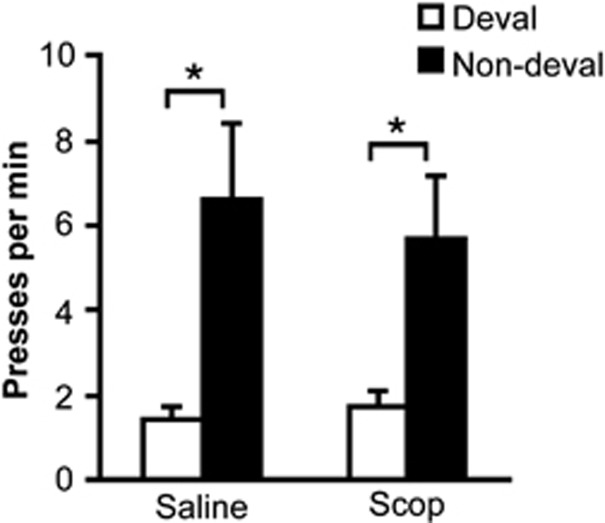
Figure 3 shows that rats exhibited sensitivity to the outcome devaluation procedure, selectively withholding their performance of the action that was previously trained with the devalued outcome (main effect of devaluation: F1,15=7.65, p<0.05). This sensitivity to outcome devaluation was not significantly affected by the high dose of scopolamine (no treatment effect or treatment × devaluation interaction: p's>0.05). Significant devaluation effects were detected for both saline and scopolamine tests (p's<0.05, paired t-test).
Figure 3.
Effect of scopolamine (Scop) pretreatment on outcome devaluation performance. Mean lever presses performed per minute (±SEM), plotted separately for the actions trained with the devalued and non-devalued outcomes. Asterisks indicate significant differences (*<0.05).
Experiment 2: Effects of Mecamylamine
Behavioral training
By the last session of Pavlovian conditioning, rats in this experiment entered the food cup at a higher rate during CS periods (16.00 times per min, SEM=1.49) than during pre-CS periods (9.53 times per min, SEM=0.86) (paired t-test: t15=5.83, p<0.001). They also developed vigorous reward-seeking behavior during instrumental conditioning, averaging 45.33 presses per min (SEM=3.17) during the last day of training.
Pavlovian-to-instrumental transfer test
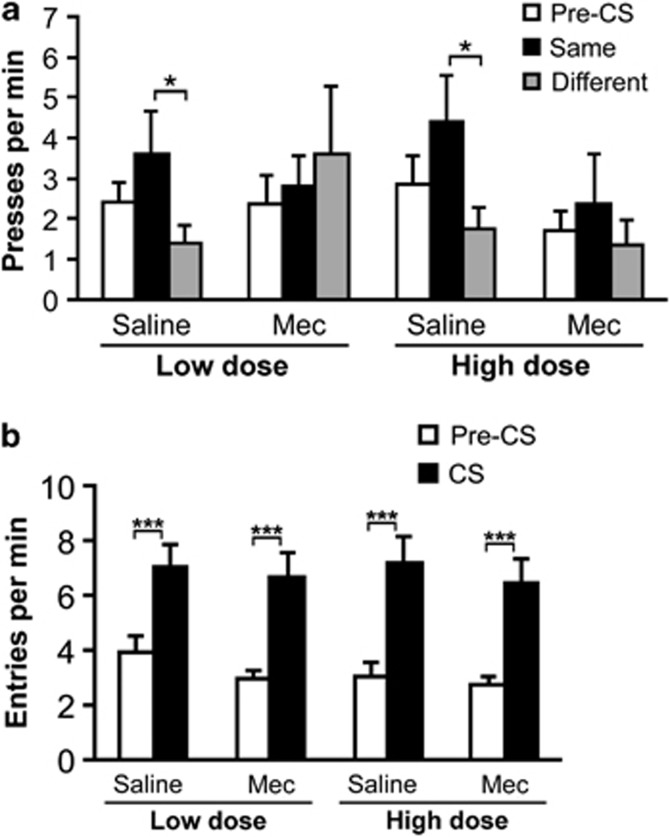
Figure 4a shows that the tendency for reward-paired cues to influence lever pressing varied across treatment conditions (significant treatment × CS period interaction: F2,30=4.03, p=0.028), indicating that mecamylamine was effective in disrupting this aspect of behavior. This disruptive effect did not depend on the dose of mecamylamine used for pretreatment (no effect of dose or any interaction involving dose; p's>0.05). Although the overall main effect of CS period was marginally significant (F1,15=2.97, p=0.066), planned pairwise comparisons (paired t-tests) indicated that press rates were significantly elevated during the CS Same period relative to the CS Different period for both vehicle tests (p's<0.05). However, unlike in Experiment 1, press rates during CS Same did not significantly differ from pre-CS rates during vehicle tests (p's>0.05), perhaps because of the slightly higher pre-CS press rates in the second experiment. Importantly, no evidence of cue-related changes in press rate were detected in either test with mecamylamine (p's>0.05), confirming that nicotinic receptor blockade was also effective in abolishing the influence of Pavlovian cues on reward-seeking behavior.
Figure 4.
Effects of mecamylamine (Mec) pretreatment on outcome-specific Pavlovian-to-instrumental transfer performance. (a) Mean number of lever presses per minute (±SEM) performed during the pre-CS period (collapsed across CSs and actions), during the CS periods, plotted separately for trials with the CS that signaled the same outcome that was earned by the action (Same) and for trials with the alternate CS, which signaled a different outcome (Different). (b) Mean number of food cup approaches (±SEM) during pre-CS and CS periods. Asterisks indicate significant differences (*<0.05, ***<0.001).
Figure 4b shows that mecamylamine did not affect the expression of conditioned food cup approach behavior during the transfer test. Specifically, entry rates were higher during CS periods than during pre-CS periods (main effect CS period: F1,15=41.74, p<0.001), and were generally insensitive to drug pretreatment (no other main effects or interactions, p's>0.05). Furthermore, a highly significant CS period effect was detected in all test conditions (p's<0.001; paired t-test).
Outcome devaluation test
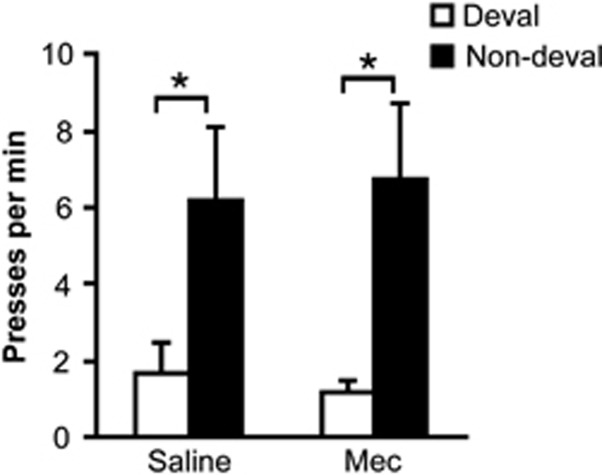
Figure 5 shows that rats were capable of selectively withholding whichever action was previously trained with the devalued outcome (main effect of devaluation: F1,15=7.97, p<0.05), and that this sensitivity to outcome devaluation was not significantly affected by the high dose of mecamylamine (no treatment effect or treatment × devaluation interaction, p>0.05). The devaluation effect was significant for both saline and mecamylamine tests (p's<0.05; paired t-test).
Figure 5.
Effect of mecamylamine (Mec) pretreatment on outcome devaluation performance. Mean lever presses performed per minute (±SEM), plotted separately for the actions trained with the devalued and non-devalued outcomes. Asterisks indicate significant differences (*<0.05).
DISCUSSION
We found that the influence of reward-predictive cues on reward seeking was disrupted when rats were systemically administered muscarinic or nicotinic acetylcholine receptor antagonists. Neither treatment affected baseline rates of instrumental performance nor did they have any detectable effect on conditioned behavior directed toward the food receptacle, indicating that their impact on behavior was specific to the influence of Pavlovian learning on instrumental performance. These treatments also spared rats' ability to choose adaptively between actions in response to a selective reduction in outcome value. Taken together, these findings reveal a novel behavioral function of acetylcholine signaling in the control of reward-seeking actions.
Previous studies have implicated acetylcholine in appetitive learning and behavior. For instance, acetylcholine-depleting striatal lesions have been shown to disrupt appetitive, but spare aversive, learning (Kitabatake et al, 2003). Furthermore, both scopolamine (Sharf et al, 2006; Pratt and Kelley, 2004; Tikhonravov et al, 1997) and mecamylamine (Levin et al, 2000) tend to suppress food-reinforced lever pressing. Although such findings implicate both muscarinic and nicotinic receptors in instrumental performance, they provide little information about the nature of their contributions. It is noteworthy that in each of these studies response-contingent rewards were delivered at test. Indeed, given their observation that intra-accumbens scopolamine infusions suppress both food-reinforced lever pressing and consumption of freely available food, it was suggested that this treatment induces ‘reward devaluation' (Pratt and Kelley, 2004). However, such an interpretation does not distinguish between a possible reduction in the reward's ability to reinforce or maintain performance when delivered in a response-contingent manner vs a change in the incentive value assigned to the reward as a behavioral goal. The latter would affect the ability of the reward to motivate behavior even when performance is evaluated under extinction (ie, without response-contingent feedback about current reward values). In the current study, we found no evidence of response suppression after scopolamine or mecamylamine administration when rats were tested in extinction (at least in the absence of reward-predictive stimuli) nor did we observe any alteration in rats' ability to adjust their performance in response to satiety-induced reward devaluation. Thus, it appears that cholinergic transmission does not have a critical part in retrieving the incentive value of behavioral goals or using previously acquired action-outcome learning to respond in a goal-directed manner.
Consistent with our findings, a recent study found that disrupting cholinergic activity in the dorsomedial striatum had no effect on selecting actions based on the anticipated value of their outcomes, at least under typical conditions (Bradfield et al, 2013). However, such treatment did impair rats' ability to encode changes in action-outcome contingencies and use these remapped associations to guide action selection (Bradfield et al, 2013). This finding fits nicely with a distinct line of research implicating dorsomedial striatal cholinergic signaling in behavioral flexibility (Ragozzino et al, 2002). Taken together with the current results, such findings suggest that acetylcholine's contributions to (uncued) goal-directed behavior may be limited to situations requiring discrimination between new and existing action–outcome associations.
The main finding reported here is that the excitatory influence of Pavlovian reward-paired cues on instrumental reward-seeking behavior requires activation of both muscarinic and nicotinic receptors. This is consistent with numerous reports that tonically active interneurons (TANs) in the striatum, which are believed to be cholinergic, develop a characteristic pause-rebound firing pattern in response to reward-predictive stimuli (Apicella et al, 2011; Matsumoto et al, 2001; Ravel et al, 2003). Interestingly, task-related modulation of TAN firing appears to be more prominent during simple Pavlovian (stimulus-reward) conditioning than during cue-triggered instrumental (stimulus-response-reward) conditioning (Apicella et al, 2011). This is noteworthy because the Pavlovian-to-instrumental transfer task used here was designed to assay the influence of purely Pavlovian cues on reward seeking.
Recent findings indicate that phasic cholingergic activity in the striatum drives local dopamine release via nicotinic receptors on axon terminals (Cachope et al, 2012; Threlfell et al, 2012). Given dopamine's well-established involvement in the response-invigorating influence of reward-paired cues (Dickinson et al, 2000; Lex and Hauber, 2008; Ostlund and Maidment, 2012; Wassum et al, 2011, 2013), such an interaction may be at least partially responsible for mediating the disruptive effect of mecamylamine reported here. However, this would not explain the similar disruption produced by scopolamine, given that it and other muscarinic receptor antagonists tend to facilitate dopamine signaling by blocking acetylcholine autoreceptor activity (Cachope et al, 2012; Chapman et al, 1997; Di Giovanni and Shi, 2009). Furthermore, we recently found that systemic dopamine receptor blockade disrupts the ability of Pavlovian reward-paired cues to invigorate reward seeking (ie, to elevate response rates over pre-cue rates) at doses that spare their ability to bias action selection based on the identity of the anticipated reward (Ostlund and Maidment, 2012). In contrast, this outcome-specific action biasing influence of cues was markedly disrupted by cholinergic receptor blockade in the current study, suggesting that acetylcholine and dopamine make dissociable contributions to this behavioral process.
Instead, acetylcholine's contributions to cue-guided action selection may be mediated, in part, by muscarinic modulation of medium spiny neuron activity (Goldberg et al, 2012), which is ultimately responsible for relaying striatal output to the rest of the brain. Functional inactivation studies have demonstrated that such output—specifically from the dorsal striatum and the nucleus accumbens shell—is required for using Pavlovian reward expectations to select instrumental actions (Corbit and Balleine, 2011; Corbit and Janak, 2007). Another potential locus is the prefrontal cortex, a region that is also known to mediate the influence of reward-paired cues on action selection (Ostlund and Balleine, 2007b). Consistent with this possibility, reward-paired cues have been shown to elicit acetylcholine release in the frontal cortex (Inglis et al, 1994; Parikh et al, 2007). Furthermore, cholinergic input to the prefrontal cortex is implicated in cue detection (Parikh et al, 2007) and other aspects of attention (Dalley et al, 2004) that may contribute to the cue-guided action selection task used here. The amygdala is another prime target for future studies on this topic given its strong cholinergic input and its critical involvement in the influence of Pavlovian cues on instrumental action selection (Corbit and Balleine, 2005; Ostlund and Balleine, 2008a; Prevost et al, 2012). In addition to these possibilities, it should also be noted that our use of systemic anticholinergic treatments leaves open the possibility that these drugs acted peripherally to disrupt cue-evoked reward seeking. Nevertheless, the task- and response-specificity of the impairments produced by these drugs suggests that they were not the product of a gross disturbance in motor control, appetite, or affective state.
The findings reported here reveal a new role for cholinergic transmission in the expression of cue-triggered reward-seeking behavior. While the Pavlovian-to-instrumental transfer task—shown here to be acetylcholine-dependent—was designed to study a fundamental aspect of motivated behavior under normal conditions, it may also be very useful for modeling compulsive behavior (Ostlund and Balleine, 2008b, 2008c). Notably, it has been shown that devaluing the reward that a Pavlovian cue signals does not disrupt that cue's ability to provoke reward seeking (Holland, 2004; Rescorla, 1994), suggesting that such cues trigger actions through an automatic process akin to (but distinct from) that controlling the performance of stimulus-response-guided habits, rather than through more goal-directed processes (Balleine and Ostlund, 2007). This may be particularly relevant to the study of addiction, given the role of drug-related cues in precipitating drug craving and relapse. Indeed, a recent transfer study found that Pavlovian cues associated with passive intravenous cocaine delivery have the ability to provoke cocaine seeking in rats (LeBlanc et al, 2012). Furthermore, studies using a food-motivated version of the transfer task have shown that rats given repeated exposure to psychostimulants exhibit heightened levels of cue-triggered food seeking (Leblanc et al, 2013a; LeBlanc et al, 2013b; Saddoris et al, 2011; Wyvell and Berridge, 2001), suggesting that these drugs cause long-lasting alterations in the neural substrates of Pavlovian incentive motivation. The current findings indicate that this behavioral influence of Pavlovian learning critically depends on cholinergic signaling through muscarinic and nicotinic receptors, suggesting that drug-induced adaptations in the cholinergic system may contribute to compulsive drug seeking by augmenting the influence of Pavlovian cues.
FUNDING AND DISCLOSURE
This work was supported by grants from the National Institute on Drug Abuse to SBO (DA029035) and NTM (DA09359 and DA05010). The authors are solely responsible for the views expressed here. The authors declare no competing financial interests.
Acknowledgments
We thank Drs Kate Wassum, Ian Mendez, and Shahrdad Lotfipour for providing constructive comments on this work, and Yoel Sitbon for providing technical assistance.
References
- Anagnostaras SG, Maren S, Sage JR, Goodrich S, Fanselow MS. Scopolamine and Pavlovian fear conditioning in rats: dose-effect analysis. Neuropsychopharmacology. 1999;21:731–744. doi: 10.1016/S0893-133X(99)00083-4. [DOI] [PubMed] [Google Scholar]
- Apicella P, Ravel S, Deffains M, Legallet E. The role of striatal tonically active neurons in reward prediction error signaling during instrumental task performance. J Neurosci. 2011;31:1507–1515. doi: 10.1523/JNEUROSCI.4880-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Ostlund SB. Still at the choice-point: action selection and initiation in instrumental conditioning. Ann N Y Acad Sci. 2007;1104:147–171. doi: 10.1196/annals.1390.006. [DOI] [PubMed] [Google Scholar]
- Bradfield LA, Bertran-Gonzalez J, Chieng B, Balleine BW. The thalamostriatal pathway and cholinergic control of goal-directed action: interlacing new with existing learning in the striatum. Neuron. 2013;79:153–166. doi: 10.1016/j.neuron.2013.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CA, Yeomans JS, Blaha CD, Blackburn JR. Increased striatal dopamine efflux follows scopolamine administered systemically or to the tegmental pedunculopontine nucleus. Neuroscience. 1997;76:177–186. doi: 10.1016/s0306-4522(96)00358-2. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. J Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J Neurosci. 2011;31:11786–11794. doi: 10.1523/JNEUROSCI.2711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Inactivation of the lateral but not medial dorsal striatum eliminates the excitatory impact of Pavlovian stimuli on instrumental responding. J Neurosci. 2007;27:13977–13981. doi: 10.1523/JNEUROSCI.4097-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Shi WX. Effects of scopolamine on dopamine neurons in the substantia nigra: role of the pedunculopontine tegmental nucleus. Synapse. 2009;63:673–680. doi: 10.1002/syn.20650. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Smith J, Mirenowicz J. Dissociation of Pavlovian and instrumental incentive learning under dopamine antagonists. Behav Neurosci. 2000;114:468–483. doi: 10.1037//0735-7044.114.3.468. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Ding JB, Surmeier DJ. Muscarinic modulation of striatal function and circuitry. Handb Exp Pharmacol. 2012;208:223–241. doi: 10.1007/978-3-642-23274-9_10. [DOI] [PubMed] [Google Scholar]
- Holland PC. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. J Exp Psychol Anim Behav Process. 2004;30:104–117. doi: 10.1037/0097-7403.30.2.104. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Day JC, Fibiger HC. Enhanced acetylcholine release in hippocampus and cortex during the anticipation and consumption of a palatable meal. Neuroscience. 1994;62:1049–1056. doi: 10.1016/0306-4522(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Kitabatake Y, Hikida T, Watanabe D, Pastan I, Nakanishi S. Impairment of reward-related learning by cholinergic cell ablation in the striatum. Proc Natl Acad Sci USA. 2003;100:7965–7970. doi: 10.1073/pnas.1032899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse JM, Overmier JB, Konz WA, Rokke E. Pavlovian conditioned-stimulus effects upon instrumental choice behavior are reinforcer specific. Learn Motiv. 1983;14:165–181. [Google Scholar]
- Leblanc KH, Maidment NT, Ostlund SB.2013Impact of repeated intravenous cocaine administration on incentive motivation depends on mode of drug delivery Addict Bioldoi: 10.1111/adb.12063 [DOI] [PMC free article] [PubMed]
- LeBlanc KH, Maidment NT, Ostlund SB. Repeated cocaine exposure facilitates the expression of incentive motivation and induces habitual control in rats. PLoS One. 2013;8:e61355. doi: 10.1371/journal.pone.0061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc KH, Ostlund SB, Maidment NT. Pavlovian-to-instrumental transfer in cocaine seeking rats. Behav Neurosci. 2012;126:681–689. doi: 10.1037/a0029534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie FM, Mojica CY, Reynaga DD. Nicotinic receptors in addiction pathways. Mol Pharmacol. 2013;83:753–758. doi: 10.1124/mol.112.083659. [DOI] [PubMed] [Google Scholar]
- Levin ED, Mead T, Rezvani AH, Rose JE, Gallivan C, Gross R. The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs food self-administration in rats. Physiol Behav. 2000;71:565–570. doi: 10.1016/s0031-9384(00)00382-6. [DOI] [PubMed] [Google Scholar]
- Lex A, Hauber W. Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learn Mem. 2008;15:483–491. doi: 10.1101/lm.978708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N, Minamimoto T, Graybiel AM, Kimura M. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J Neurophysiol. 2001;85:960–976. doi: 10.1152/jn.2001.85.2.960. [DOI] [PubMed] [Google Scholar]
- Mendez IA, Gilbert RJ, Bizon JL, Setlow B. Effects of acute administration of nicotinic and muscarinic cholinergic agonists and antagonists on performance in different cost–benefit decision making tasks in rats. Psychopharmacology (Berl) 2012;224:489–499. doi: 10.1007/s00213-012-2777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. The contribution of orbitofrontal cortex to action selection. Ann N Y Acad Sci. 2007;1121:174–192. doi: 10.1196/annals.1401.033. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J Neurosci. 2008;28:4398–4405. doi: 10.1523/JNEUROSCI.5472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. The disunity of Pavlovian and instrumental values. Behav Brain Sci. 2008;31:456–457. [Google Scholar]
- Ostlund SB, Balleine BW. On habits and addiction: an associative analysis of compulsive drug seeking. Drug Discov Today Dis Models. 2008;5:235–245. doi: 10.1016/j.ddmod.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Maidment NT. Dopamine receptor blockade attenuates the general incentive motivational effects of noncontingently delivered rewards and reward-paired cues without affecting their ability to bias action selection. Neuropsychopharmacology. 2012;37:508–519. doi: 10.1038/npp.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WE, Kelley AE. Nucleus accumbens acetylcholine regulates appetitive learning and motivation for food via activation of muscarinic receptors. Behav Neurosci. 2004;118:730–739. doi: 10.1037/0735-7044.118.4.730. [DOI] [PubMed] [Google Scholar]
- Prevost C, Liljeholm M, Tyszka JM, O'Doherty JP. Neural correlates of specific and general Pavlovian-to-instrumental transfer within human amygdalar subregions: a high-resolution fMRI study. J Neurosci. 2012;32:8383–8390. doi: 10.1523/JNEUROSCI.6237-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Jih J, Tzavos A. Involvement of the dorsomedial striatum in behavioral flexibility: role of muscarinic cholinergic receptors. Brain Res. 2002;953:205–214. doi: 10.1016/s0006-8993(02)03287-0. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Mohler EG, Prior M, Palencia CA, Rozman S. Acetylcholine activity in selective striatal regions supports behavioral flexibility. Neurobiol Learn Mem. 2009;91:13–22. doi: 10.1016/j.nlm.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel S, Legallet E, Apicella P. Responses of tonically active neurons in the monkey striatum discriminate between motivationally opposing stimuli. J Neurosci. 2003;23:8489–8497. doi: 10.1523/JNEUROSCI.23-24-08489.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Transfer of instrumental control mediated by a devalued outcome. Anim Learn Behav. 1994;22:27–33. [Google Scholar]
- Rodgers RJ. Effects of nicotine, mecamylamine, and hexamethonium on shock-induced fighting, pain reactivity, and locomotor behaviour in rats. Psychopharmacology (Berl) 1979;66:93–98. doi: 10.1007/BF00431996. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Stamatakis A, Carelli RM. Neural correlates of Pavlovian-to-instrumental transfer in the nucleus accumbens shell are selectively potentiated following cocaine self-administration. Eur J Neurosci. 2011;33:2274–2287. doi: 10.1111/j.1460-9568.2011.07683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, McKelvey J, Ranaldi R. Blockade of muscarinic acetylcholine receptors in the ventral tegmental area prevents acquisition of food-rewarded operant responding in rats. Psychopharmacology (Berl) 2006;186:113–121. doi: 10.1007/s00213-006-0352-0. [DOI] [PubMed] [Google Scholar]
- Thiele A. Muscarinic signaling in the brain. Annu Rev Neurosci. 2013;36:271–294. doi: 10.1146/annurev-neuro-062012-170433. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Tikhonravov DL, Shapovalova KB, Dyubkacheva TA. Effects of microinjection of scopolamine into the neostriatum of rats on performance of a food conditioned reflex at different levels of fixation. Neurosci Behav Physiol. 1997;27:312–318. doi: 10.1007/BF02462901. [DOI] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Balleine BW, Maidment NT. Differential dependence of Pavlovian incentive motivation and instrumental incentive learning processes on dopamine signaling. Learn Mem. 2011;18:475–483. doi: 10.1101/lm.2229311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Loewinger GC, Maidment NT. Phasic mesolimbic dopamine release tracks reward seeking during expression of Pavlovian-to-instrumental transfer. Biol Psychiatry. 2013;73:747–755. doi: 10.1016/j.biopsych.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered ‘wanting' for sucrose reward. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]