Abstract
Cardiovascular disease represents the most common cause of death in patients with non-alcoholic fatty liver disease (NAFLD). NAFLD patients exhibit an atherogenic dyslipidemia that is characterized by an increased plasma concentration of triglycerides, reduced concentration of high density lipoprotein (HDL) cholesterol, and low density lipoprotein (LDL) particles that are smaller and more dense than normal. The pathogenesis of NAFLD-associated atherogenic dyslipidemia is multifaceted, but many aspects are attributable to manifestations of insulin resistance. Here we review the structure, function and metabolism of lipoproteins, which are macromolecular particles of lipids and proteins that transport otherwise insoluble triglyceride and cholesterol molecules within the plasma. We provide a current explanation of the metabolic perturbations that are observed in the setting of insulin resistance. An improved understanding of the pathophysiology of atherogenic dyslipidemia would be expected to guide therapies aimed at reducing morbidity and mortality in NAFLD patients.
Keywords: Triglycerides, cholesterol, apolipoprotein, insulin resistance, cardiovascular disease
Introduction
Patients with non-alcoholic fatty liver disease (NAFLD) are at increased risk of cardiovascular disease (CVD), particularly when non-alcoholic steatohepatitis (NASH) is present (1–3). Importantly, CVD is the leading cause of death in patients with NAFLD (4). Whereas classical CVD risk factors are frequently prominent in NALFD patients (5), NALFD is a manifestation of insulin resistance and the metabolic syndrome (6), which is not fully captured in estimates of CVD risk (7). This suggests that NAFLD per se can be predictive of CVD risk (8, 9).
Conversely, compared with the general population, patients with CVD risk factors, including dyslipidemia and type 2 diabetes are at a high risk for NAFLD. Indeed, NAFLD is present in 50% of patients with type 2 diabetes and nearly 100% of those with type 2 diabetes plus obesity (10, 11). NASH is also enriched in the obese population up to nearly 90% (10), with 20% of patients being cirrhotic (11). In dyslipidemic patients, 50% exhibited NAFLD, and elevated triglycerides were the most strongly associated with NAFLD (12).
The dyslipidemia typically associated with NAFLD is pro-atherogenic (4, 8). The characteristic findings are increased plasma concentrations of very low density lipoprotein (VLDL) triglycerides and decreased high density lipoprotein (HDL) cholesterol (13). In the setting of insulin resistance, these changes are typically accompanied by increased concentrations of atherogenic small dense low density lipoprotein (LDL) particles, but not necessarily increased LDL cholesterol concentrations (6, 14). These associations in NAFLD were firmly established by the recent Multi-Ethnic Study of Atherosclerosis (MESA) (15). In a population of adults who were free of CVD at the time of enrollment, NAFLD was found to be associated with higher fasting serum triglyceride concentrations and lower concentrations of HDL cholesterol. In the absence of increases in plasma LDL cholesterol concentrations, NAFLD was associated with higher LDL particle concentrations and lower LDL particle size. Even after adjustment for multiple metabolic risk factors, adiposity and clinical measures of insulin resistance, NAFLD remained predictive of the atherogenic dyslipidemia phenotype. This review will focus on our evolving understanding of the relationship between NAFLD and the pathogenesis of atherogenic dyslipidemia.
Structure and composition of plasma lipoproteins
Plasma lipoproteins are macromolecular particles composed of characteristic lipids and proteins that serve to transport otherwise insoluble triglycerides and cholesterol molecules. Lipoproteins consist of a coat composed of a monolayer of amphipathic lipids and apolipoproteins (synonym: apoproteins) that surround a core composed of hydrophobic lipids (16). The coat lipids consist of amphipathic (polar) phospholipids that are admixed with unesterified cholesterol molecules to form a lipid monolayer (17). Ampipathic apolipoproteins associate with and stabilize the monolayer. The hydrophobic core of a lipoprotein particle contains non-polar triglycerides and cholesteryl esters, which are cholesterol molecules covalently esterified to long-chain fatty acids. The amphipathicity of the coat lipids and apoproteins allows these molecules to interact with both the aqueous environment of the plasma and the hydrophobic core lipids.
Circulating lipoproteins range in size from 5 to >1000 nm, and can be separated according to density (Table 1) (17). High density lipoproteins (HDL) are small, containing the least lipid and the most protein, whereas chylomicrons are large and lipid-rich. Lipoproteins are also distinguished by their apolipoprotein contents (Table 1). Apolipoproteins serve not only to stabilize lipoprotein particles, but also to mediate metabolic functions by acting as receptor ligands or by activating enzymatic activities that promote the metabolism of lipoproteins within the plasma compartment. With the exception of apolipoprotein (apo) B, apolipoproteins, such as apoAI, apoE, and apoCIII, are reversibly associated with lipoprotein particles and can exchange among the particles within the circulation.
TABLE 1.
Characteristics of Plasma Lipoproteinsa
| CM | VLDL | IDL | LDL | HDL | |
|---|---|---|---|---|---|
| Density (g/ml) | <0.95 | 0.95–1.006 | 1.006–1.019 | 1.019–1.063 | 1.063–1.210 |
| Diameter (nm) | 75–1200 | 30–80 | 25–35 | 18–25 | 5–12 |
| Total lipid (% wt) | 98 | 90 | 82 | 75 | 67 |
| Composition, % dry weight | |||||
| Protein | 2 | 10 | 18 | 25 | 33 |
| Triglycerides | 83 | 50 | 31 | 9 | 8 |
| Unesterified cholesterol Cholesteryl esters | 8 | 22 | 29 | 45 | 30 |
| Phospholipids (% wt lipid) | 7 | 18 | 22 | 21 | 29 |
| Major Apolipoproteins | B48,A-I,A-IV, E,CI,CII,CIII | B100, E, CI,CII,CIII | B100,E, CI,CII,CIII | B100 | A-I,A-II,CI, CII,CIII,E |
Abbreviations: CM, chylomicron; VLDL, very low-density lipoprotein; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; HDL, high- density lipoprotein
Adapted with permission from (Jonas, A. (2002) Lipoprotein structure. In: Vance, D.E. & Vance, J.E. (eds.) Biochemistry of Lipids, Lipoproteins and Membranes, 4th edn. Elsevier, Amsterdam, pp. 483–404).
Lipoprotein metabolism and the effects of NAFLD
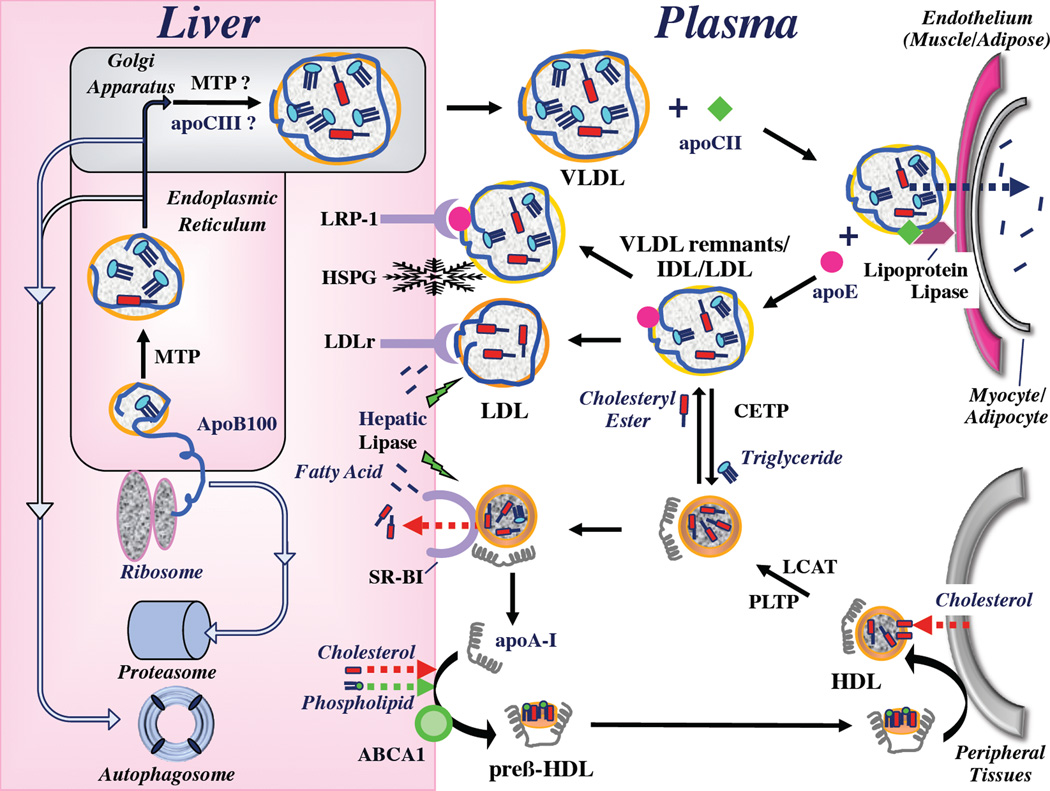
As depicted schematically in Figure 1 and discussed in this review, there are two main functions of lipoproteins: One is the delivery of cholesterol and triglyceride molecules from the liver and intestine to muscle and fat tissue. This is mediated either directly by chylomicron and VLDL particles that contain apoB48 and apoB100, respectively, or, in the case of cholesterol, indirectly by conversion of triglyceride-depleted VLDL particles (“remnants”, sometimes called intermediate density lipoproteins, IDL) to LDL particles. The second is the transport of excess cholesterol from extrahepatic tissues to the liver for elimination via the bile, which is mediated primarily by HDL particles. Both functions are altered in NALFD, and although the MESA study suggests that associations between NALFD and atherogenic dyslipidemia exist even after measures of insulin resistance are taken into account (15), insulin resistance can mechanistically explain many of the key alterations observed in lipoprotein metabolism.
Figure 1. Simplified schematic summary of hepatic lipoprotein metabolism.
The liver produces VLDL particles, which transport triglycerides through the plasma to muscle and adipose tissue. Assembly begins when apoB100 protein is translated from its mRNA by ribosomes, crossing the membrane of the ER and entering the lumen. ApoB100 is cotranslationally lipidated with triglycerides by the activity of MTP. Cholesteryl esters are incorporated into the lipoprotein core by unclear mechanisms. VLDL particles transit the Golgi apparatus where there is some evidence that they are further lipidated by MTP, as well as apoC-III, though this has not been definitively established (as indicated by “?”). The matured particle is secreted into the plasma. Hepatic VLDL secretion rates are controlled largely by rates of apoB100 degradation, which is mediated by the proteasome or the autophagosome. For clarity, an additional sortilin-mediated pathway of ApoB100 degradation (described in the text) is not displayed. Within the plasma, VLDL particles become fully activated for tissue targeting and lipolysis by the acquisition apoC-II. This permits binding of VLDL to lipoprotein lipase, which is tethered to the surface of endothelial cells. Lipoprotein lipase hydrolyzes triglycerides within the core of the lipoprotein particles, and the fatty acids are taken up into muscle or fat. When this is complete, VLDL particles detach from lipoprotein lipase, apoC-II dissociates and apoE is acquired. The resulting particles are chylomicron (CM) and VLDL remnants and IDL, which are interact for prolonged periods with LPL to become more dense. Remnant lipoproteins (VLDL or IDL) are either taken up into hepatocytes by multiple mechanisms, which include the LDL receptor (LDLr), the LDL-related protein (LRP)-1, a complex formed between LRP-1 and HSPG or HSPG alone. Formation of LDL occurs when IDL particles interact instead with hepatic lipase. Hydrolysis of additional triglycerides renders the particle more dense and enriched with cholesteryl esters. ApoE and apoCII are lost from the particle, leaving apoB100 as the sole apolipoprotein. LDL particles are taken up exclusively by the LDLr.
HDL particles are formed when newly secreted ApoA-I interacts with ATP binding cassette protein AI (ABCA1) on the surface of hepatocytes. This promotes the incorporation of phospholipid and cholesterol from hepatocyte plasma membranes into a discoidal-shaped pre-β-HDL particle, which can accept excess cellular cholesterol through ABCA1. Subsequently, lecithin cholesterol:acyltransferase (LCAT) promotes the formation of spherical HDL, which are much better configured to receive cholesterol from cell membranes of peripheral tissues through ABCG1. The combined activities of LCAT and phospholipid transfer protein (PLTP allow for enlargement of the HDL particle and enable it to take on even more cholesterol. Cholesteryl ester transfer protein (CETP) exchanges cholesteryl esters in the core of HDL for triglycerides from the core of VLDL, VLDL remnants, IDL, and even LDL particles. At the liver, HDL particles bind to scavenger receptor, class B type I (SR-BI), which promotes hepatic uptake of only the cholesteryl esters. Hepatic lipase hydrolyzes triglycerides from the core, which remodels the particle and allows for optimal activity of SR-BI. ApoA-I dissociates from the particle and participates in the formation of new HDL.
Triglyceride transport
ApoB-containing lipoproteins transport triglycerides through the plasma to muscle, which utilizes the fatty acids for energy production, and to adipose tissue, which stores fatty acids for future energy consumption. Within the intestinal epithelium, dietary triglycerides are ultimately packaged into apoB48 containing chylomicrons that are secreted into the lymphatics (18, 19). In the liver, endogenous triglycerides are packaged into apoB100-containing VLDL particles that are secreted directly into the plasma. The intravascular metabolism of these particles facilitates the delivery of triglycerides by a lipolytic process that generates remnant particles that are removed from the circulation mainly by the liver. The cellular mechanisms by which chylomicrons and VLDL are assembled and metabolized are quite similar, and this review will focus on VLDL metabolism, which is altered in the setting of NAFLD and contributes directly to the pathogenesis of both hepatic steatosis and atherogenic dyslipidemia.
Assembly of VLDL particles in the liver
Under physiological conditions, the secretion rate of VLDL particles is well matched to supply hepatic triglycerides for the metabolic demands of oxidative tissues, with excess triglycerides directed towards adipose tissue for storage. As will be reviewed below, the initial molecular assembly of VLDL depends primarily upon the supply of hepatic triglycerides and the activity of microsomal triglyceride transfer protein (MTP). If these are deficient, apoB100 will undergo degradation and little VLDL will be assembled. The triglycerides that are incorporated into VLDL particles are preferentially synthesized within liver from plasma fatty acids, which either originate from adipose tissue or the diet, or can be synthesized de novo by hepatocytes. In response to fasting, fatty acids are released from white adipose tissue and transported to the liver. In the absence of dietary triglycerides, their incorporation into VLDL triglycerides ensures a continuous supply of “concentrated” fatty acids for delivery to muscle. Cholesterol molecules that are derived largely from intestinal sources are incorporated into the core of the VLDL particles (18) following esterification by the activity of the microsomal enzyme acyl-coenzyme A:cholesterol acyltransferase (ACAT) 2 (20, 21).
During translation from its mRNA, apoB100 translocates into the lumen of the endoplasmic reticulum (ER) (21–23). Within the ER, the activity of MTP (23) incorporates triglyceride molecules into apoB100, which promotes its proper folding. Proper folding of apoB100 also requires the participation of a number of chaperone proteins located in both the cytosol and ER lumen (21). Once formed, the nascent VLDL particles (each containing one apoB100 molecule) are further lipidated in the Golgi apparatus (22), in an incompletely defined process, which may involve a pool of MTP in this organelle, or fusion with lipid droplets or another VLDL particle (24). This maturation process of nascent VLDL to a more highly lipidated particle also appears to engage the intracellular activity of apoCIII, which mobilizes triglycerides within the ER for fusion to the newly formed VLDL particles (25).
The critical role of MTP in VLDL production is evidenced by the hepatic steatosis that occurs as a result of its genetic absence (26, 27) or pharmacologic inhibition (28). Lipid droplet-associated proteins, including the perilipins and fat-inducing transmembrane (FIT) protein 2, also play important supporting roles in the assembly of VLDL particles by controlling the availability of triglycerides for lipidating apoB100 (29). A coding region polymorphism (rs738409[G], encoding I148M) in patatin-like phospholipase domain-containing 3 (PNPLA3, synonym adiponutrin) is strongly associated with NAFLD (30–32), and recent functional studies have revealed that PNPLA3 modulates lipid droplet metabolism (33–35). In humans, I148M PNPLA3 reduces hepatic VLDL secretion, possibly by reducing the mobilization of triglycerides stored in lipid droplets (35).
The translation of apoB100 in the liver is constitutive, which facilitates the rapid production VLDL particles in response to increased rates of triglyceride synthesis by providing an ever-ready pool of protein for lipoprotein assembly. As observed for MTP, hepatic steatosis follows the genetic absence of apoB100, as well as mutations that truncate apoB (36) and antisense lipid-lowering pharmacotherapy that targets apoB100 mRNA for degradation (37). As alluded to above, the hepatic secretion of VLDL is further regulated by intracellular degradation of apoB100 (38–40). If the initial lipidation step during apoB100 translocation across the ER membrane fails, apoB100 in the liver is directed to the proteasome-mediated ER-associated degradation (ERAD) pathway. If this step is successful, but subsequent VLDL maturation is interrupted, then in another quality control pathway, apoB100 is degraded by a post-ER, pre-secretory proteolytic process (“PERPP”) that depends upon autophagy and is augmented by insulin, by fish oil fatty acids and by ER stress (40). An additional example of degradation is mediated by sortilin, which binds apoB100 within the Golgi apparatus and traffics it to the lysosome, in what may be a variant of the autophagic pathway (41, 42).
Insulin suppresses VLDL particle secretion rates by several mechanisms (25, 42). In an exception to constitutive translation of apoB100 mRNA, it can reduce the availability of apoB100 mRNA for this purpose. Insulin can also decrease transcription of the genes encoding both MTP and apoCIII by inhibiting the activity of the transcription factor FoxO1 and ultimately increase degradation of apoB100. Insulin also enhances the interaction between sortilin and apoB100 trafficking in cell culture, and this correlates with repression of apoB100 secretion (24).
In the setting of insulin resistance, multiple metabolic abnormalities conspire to increase the secretion of VLDL particles. Insulin resistance leads to increased lipolysis within white adipose tissue, with increased delivery of free fatty acids to the liver and increased expression of hepatic fatty acid transport proteins (43). At the same time, hyperinsulinemia leads to increased sterol regulatory element binding protein (SREBP) 1c-mediated lipogenesis by both liver X receptor (LXR)-dependent and independent mechanisms (6, 43, 44) and hyperglycemia leads to lipogenesis by the activation of ChREBP (44, 45). Thus, the net effects of these changes are to increase the supply of hepatic triglycerides due to greater uptake of plasma free fatty acids and to higher rates of de novo fatty acid synthesis (6, 43). There is also loss of suppression of MTP and apoCIII transcription, leading to increased efficiency of VLDL particle assembly (25, 46). On the other hand, increased lipid accumulation within liver can also counteract these effects of insulin (6). The accumulation of fatty acids within the liver also promotes chronic ER stress, which enhances the degradation of apoB-100 (6, 40), while at the same time leading to further stimulation of lipogenesis (6, 47, 48). Notwithstanding these effects, although the VLDL particles can become triglyceride-enriched, the number of particles (dictated by apoB100 availability) cannot increase sufficiently to alleviate triglyceride accumulation in the liver and prevent the occurrence of NAFLD.
Intravascular metabolism of apoB100-containing lipoproteins
Each VLDL particle is produced and secreted with a single embedded molecule of apoB100. Within the liver and shortly after secretion, VLDL particles may also acquire other apolipoproteins, including multiple molecules of apoE, apoCI, apoCII and apoCIII. Upon secretion into the plasma, VLDL particles are directed to muscle and fat tissues, where they acquire an additional complement of apoCII molecules that circulate in association with HDL particles (49, 50). The time-dependent exchange of apoCII from HDL to VLDL also serves to facilitate widespread distribution of triglycerides by the circulation because of the activity of lipoprotein lipase (50).
Lipoprotein lipase (LPL) is a key molecule in the targeting of triglycerides to muscle and fat tissue. It is expressed in capillaries in muscle and fat tissues on the luminal surface of endothelial cells (51). LPL is localized and tethered to the vascular surface of the endothelial cell plasma membrane by non-covalent interactions with glycosyl-phosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1) (52). By virtue of their apolipoprotein contents (i.e. apoB100 and apoCII), VLDL particles bind to and activate LPL. Upon binding, LPL hydrolyzes triglycerides, allowing free fatty acids to be assimilated by the underlying parenchymal cells (52).
Regulation of LPL also influences the extent of tissue uptake of VLDL triglycerides. Both the level of expression and the activity of the protein are regulated (53). This allows VLDL triglycerides to be routed to muscle, as opposed to fat, during fasting. In particular, insulin promotes expression of LPL in adipose tissue by transcriptional upregulation (53, 54), which is diminished in the setting of insulin resistance (53), so that VLDL triglyceride clearance from the plasma is diminished. Under physiological conditions, LPL removes approximately 50% of triglycerides from the core of VLDL particles, at which point conformational changes reduce the binding affinity (50). The rate of lipolysis of chylomicrons and VLDL triglycerides is also controlled by apoCIII. When bound to VLDL, apoCIII inhibits LPL activity (49). As a result, upregulation of apoCIII in the setting of insulin resistance can contribute to hypertriglyceridemia by reducing the overall LPL-mediated metabolism of VLDL particles (25, 49, 55).
LDL formation
Upon dissociating from LPL the exchangeable apolipoproteins of VLDL, including apoCII and apoCIII, transfer spontaneously to HDL (56). In turn, apoE transfers from HDL to VLDL. The presence of ApoE on VLDL identifies it as a remnant particle (57), and renders it a high affinity ligand for hepatic receptors that clear the particle from the circulation (57, 58). However, only approximately 50% of VLDL remnants are cleared by receptors for remnant particles, as discussed below. The other 50% are further metabolized by additional interactions with LPL. This further depletes triglycerides from the core and enriches the particle in cholesteryl esters. These smaller, denser remnant particles are referred to as IDL. Whereas a fraction of IDL particles are taken up into the liver by remnant receptors (59), others interact with hepatic lipase (52). This hydrolyzes most of the remaining triglycerides within the core and further reduces the particle size. As a result of this conformational change, apoE dissociates from the particle, leaving only apoB100. This cholesteryl ester-enriched lipoprotein is defined as LDL (50). Hepatic lipase is upregulated in the setting of insulin resistance (60) and NAFLD (61, 62), and this presumably contributes to increasing the proportion of small dense LDL particles in this metabolic state.
Receptor-mediated clearance of VLDL remnants and LDL particles from the circulation
ApoE-containing remnant particles (i.e. VLDL remnants and IDL) are cleared from the plasma by hepatocytes when they become small enough to pass through the fenestrated endothelium of the liver and become trapped within the Space of Disse by electrostatic interactions with large extracellular heparan sulfate proteoglycans (HSPG) (57, 63). There, hepatic lipase remodels the particles, rendering them an optimal size to interact with cell surface receptors. The receptors that bind and take up apoE-containing remnant particles include the LDL receptor, the LDL-receptor related protein (LRP)-1 and HSPG. LRP-1 and HSPG may also cooperate to take up the particles, so that there are effectively four pathways for remnant clearance.
The effects of insulin on the LDL receptor are complex (42). Whereas insulin decreases mRNA levels of the LDL receptor, it does not change LDL receptor protein levels. This appears to be because insulin upregulates expression of proprotein convertase subtilisin/kexin type 9 (PCSK9), a circulating protein that binds and degrades the LDL receptor (64). Complementary evidence suggests that LRP-1- and HSPG-mediated-uptake of VLDL remnants may be increased by insulin action (42). In the setting of insulin resistance, increased hepatic uptake of triglyceride-containing remnant lipoproteins may contribute to hepatic steatosis (6, 65).
In contrast to apoE-containing remnant particles, LDL particles are only taken up by hepatic cells by the LDL receptor (66). Moreover, LDL particles interact only weakly with the receptor, which results in prolonged lifespan of LDL within the plasma (days) compared with remnant particles (hours) (66). The consequence of this is that LDL cholesterol is the predominant form of cholesterol in human plasma. Approximately 70% of LDL receptors are localized to the liver, with the remainder expressed on adrenocortical, gonadal and smooth muscle cells, as well as macrophages and lymphocytes.
Upon binding the LDL receptor, LDL particles are removed from the plasma by receptor-mediated endocytosis (66, 67). In this pathway, both the lipoprotein particle and receptor are taken up into an endocytic vesicle that is delivered to lysosomes, where the particle dissociates from the receptor and is hydrolyzed. Whereas apoB100 is degraded, cholesteryl esters are hydrolyzed to form free cholesterol, which is delivered to the ER via lysosomes by the activity of the Niemann-Pick Type C (NPC) proteins 1 and 2 (68). The LDL receptor is separated and returned to the cell surface (66, 67).
An increase in the cholesterol content of the ER due to the receptor-mediated uptake of LDL cholesterol leads to a well-characterized homeostatic response that prevents cytotoxicity due to cholesterol accumulation (67). Cholesterol reduces the activity of HMG-CoA reductase, which is the rate-limiting enzyme in cholesterol biosynthesis. Excess cholesterol in the ER is also converted to cholesteryl esters by the activity of ACAT2 and stored within neutral lipid droplets (20). Downregulation of LDL receptor expression in turn reduces the uptake of additional cholesterol from the plasma. SREBP2 plays a key role in orchestrating this response to cholesterol (69). SREPB2 is a membrane-bound transcription factor that promotes the transcription of cholesterol biosynthetic genes including HMG-CoA reductase, as well as that of the LDL receptor. SREPB2 resides in the ER and is activated by proteolytic cleavage when cholesterol levels in the ER are low. In the setting of excess cholesterol within the ER, SREPB2 is maintained in its inactive form (70).
Lipidomic analyses of samples from liver biopsies of NALFD patients demonstrate increased concentrations of unesterified cholesterol that are not associated with increases in concentrations of esterified cholesterol (71). Subsequent analyses of mRNA and protein expression revealed upregulation of SREBP2 along with HMG-CoA reductase (72). However, LDL receptor expression was decreased by an unclear mechanism, and this was associated with increased plasma LDL concentrations. These findings suggest that reduced uptake of LDL cholesterol in NAFLD contributes to atherogenic dyslipidemia.
Reverse cholesterol transport
Whereas most cell types are competent to synthesize enough cholesterol to serve their own needs, only the liver possesses the enzymatic and cellular machinery to degrade and eliminate cholesterol by conversion to bile salts and secretion into bile (73). HDL particles in the plasma function to scavenge excess cellular cholesterol and transport it to the liver (74, 75). This process, often referred to as reverse cholesterol transport (76, 77), begins with the production of nascent HDL particles by the liver (78). The particles mature within the intravascular space, take up excess cholesterol from cells and then return to the liver where they interact with the SR-BI receptor, which selectively removes lipids, but not protein from HDL (79).
HDL production by the liver
ApoA-I is the principal determinant of HDL structure and function. HDL particles characteristically contain apoA-II, but its function is less well understood (80). Unlike VLDL particles that are assembled intracellularly, HDL particles are formed within the plasma at the surface of hepatocytes (77). Lipid-poor apoA-I that is secreted by the liver (81) or dissociates from other plasma lipoproteins (82, 83) interacts with ATP-binding cassette (ABC) A1 (84), which is expressed in the hepatocyte plasma membrane (79). This interaction lipidates apoA-I with phospholipids and unesterified cholesterol from the plasma membrane (79) to form small disk-shaped HDL particles that are known as nascent or preβ-HDL (83). In the setting of insulin resistance, hyperinsulinemia reduces HDL biogenesis, both by promoting the phosphorylation and degradation of ABCA1 and by reducing the specific activity of the protein (85).
HDL maturation in plasma and uptake of cellular cholesterol
In order to maximize the efficiency of HDL as an acceptor of excess cellular cholesterol, preβ-HDL particles mature to form spherical particles. This is mediated by the activities of lecithin:cholesterol acyltransferase (LCAT) and phospholipid transfer protein (PLTP). LCAT, which circulates in plasma, binds disk-shaped HDL and catalyzes the conversion of cholesterol cholesteryl esters (79): a fatty acid is removed from a phospholipid molecule and esterified to the free hydroxyl group of cholesterol. Due to their hydrophobicity, cholesteryl esters relocate to the HDL core (50), causing the particle to become spherical. PLTP removes phospholipids from the surface of VLDL remnants, which are present in excess due to the activity of LPL, reducing the size of the core. These phospholipids are relocated to the surface of HDL particles, which are expanding in size due to the activity of LCAT. A direct role for LCAT in altered HDL metabolism in the setting of insulin resistance is unclear (86, 87).
There are several mechanisms by which HDL particles can remove excess cholesterol from cells, including picking up cholesterol desorbed from the plasma membrane, as well as that transferred to HDL by the ATP-binding cassette class proteins (e.g., ABCA1 and ABCG1) (84, 88, 89). Whereas spherical HDL particles are most efficient, immature preβ-HDL particles can also remove excess cholesterol. These pathways are important in protecting macrophages within the artery from becoming overloaded with cholesterol (90). In the plasma of human subjects, the capacity of HDL to efflux cholesterol is reduced under atherogenic conditions (91), most likely due to mechanisms at the cellular level as well as by variations in HDL function.
Reverse cholesterol transport is enhanced by the activity of cholesteryl ester transfer protein (CETP), which also circulates in the plasma. CETP catalyzes the transfer of cholesteryl ester molecules from HDL particles to apoB100-containing lipoproteins, including VLDL, LDL and remnants in exchange for a triglyceride molecule (92, 93). This process allows apoB100-containing remnant particles to be utilized for the delivery of additional cholesterol originating from non-hepatic cells to the liver. In the setting of increased plasma triglyceride concentrations, CETP activity significantly reduces HDL cholesterol content and promotes the formation of triglyceride-enriched LDL particles, which become remodeled by lipases to become smaller and denser. CETP activity is increased in the plasma of patients with NAFLD and the metabolic syndrome, and its activity is associated with the content of plasma VLDL cholesterol (62). This contribution of CETP to the atherogenic lipid profile has provided an important rationale for the interest in targeting this protein in clinical practice (94).
Hepatic uptake of HDL cholesterol
Upon return to the liver, HDL particles bind to scavenger receptor, class B type I (SR-BI) (95) on the hepatocyte plasma membrane (96). SR-BI mediates selective lipid uptake, whereby HDL cholesterol, but not apolipoproteins, is transported into the cell (79). ApoA-I remains in the plasma and can initiate the formation of additional HDL particles. Uptake is enhanced when hepatic lipase first binds and modifies the HDL particle (18). HDL particles that are enriched in triglyceride due to the activity of CETP, such as in the setting of insulin resistance, and then modified by hepatic lipase are more rapidly taken up into cells (97). Although the mechanisms are not completely understood (97), this effect may contribute to reducing plasma HDL cholesterol, but may also include the disassociation of apoAI from HDL particles as hepatic lipase remodels the particles, which results in its rapid clearance from the plasma.
CONCLUSIONS
Lipoprotein metabolism is a precisely regulated process that includes the orchestration of the delivery of triglyceride molecules to muscle and adipose tissue and the removal of excess cholesterol from cells. In the setting of NAFLD and insulin resistance, multiple perturbations in lipoprotein metabolism are observed, which collectively result in a pattern of atherogenic dyslipidemia characterized by high concentrations of plasma triglycerides, low HDL cholesterol and small dense LDL particles. Considering that CVD is the most common cause of death in NAFLD patients, the understanding and treatment of dyslipidemia is essential to the overall management of these patients.
ACKNOWLEDGEMENTS
This work was supported in part by research grants DK56626, DK48873, and HL58541 from the National Institutes of Health.
ABBREVIATIONS
- ABC
ATP-binding cassette
- ACAT
acyl-coenzyme A:cholesterol acyltransferase
- Apo
apolipoprotein
- CETP
cholesteryl ester transfer protein
- ChREBP
carbohydrate response element binding protein
- CVD
cardiovascular disease
- ER
endoplasmic reticulum
- ERAD
proteasome-mediated ER-associated degradation
- FIT
fat inducing transmembrane
- GPIHBP1
glycosyl-phosphatidylinositol-anchored high-density lipoprotein-binding protein
- HDL
high density lipoprotein
- HSPG
heparan sulfate proteoglycans
- IDL
intermediate density lipoprotein
- LCAT
lecithin:cholesterol acyltransferase
- LDL
low density lipoprotein
- LDLr
LDL receptor
- LPL
lipoprotein lipase
- LRP
LDL-receptor related protein
- LXR
liver X receptor
- MESA
Multi-Ethnic Study of Atherosclerosis
- MTP
microsomal triglyceride transfer protein
- NAFLD
nonalcoholic fatty liver disease
- NPC
Niemann-Pick Type C
- PCSK9
proprotein convertase subtilisin/kexin type 9
- PLTP
phospholipid transfer protein
- PNPLA3
patatin-like phospholipase domain-containing 3
- SR-BI
scavenger receptor, class B type I
- SREBP
sterol regulatory element binding protein
- VLDL
very low density lipoprotein
REFERENCES
- 1.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 2.Vanwagner LB, Bhave M, Te HS, Feinglass J, Alvarez L, Rinella ME. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology. 2012;56:1741–1750. doi: 10.1002/hep.25855. [DOI] [PubMed] [Google Scholar]
- 3.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatrath H, Vuppalanchi R, Chalasani N. Dyslipidemia in patients with nonalcoholic fatty liver disease. Semin. Liver Dis. 2012;32:22–29. doi: 10.1055/s-0032-1306423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treeprasertsuk S, Leverage S, Adams LA, Lindor KD, St Sauver J, Angulo P. The Framingham risk score and heart disease in nonalcoholic fatty liver disease. Liver Int. 2012;32:945–950. doi: 10.1111/j.1478-3231.2011.02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol. Metab. 2011;22:353–363. doi: 10.1016/j.tem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: Cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology. 2006;131:934–945. doi: 10.1053/j.gastro.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 8.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 9.Musso G, Cassader M, Gambino R. Diagnostic accuracy of adipose insulin resistance index and visceral adiposity index for progressive liver histology and cardiovascular risk in nonalcoholic fatty liver disease. Hepatology. 2012;56:788–789. doi: 10.1002/hep.25677. [DOI] [PubMed] [Google Scholar]
- 10.Gupte P, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, Patel N, et al. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J. Gastroenterol. Hepatol. 2004;19:854–858. doi: 10.1111/j.1440-1746.2004.03312.x. [DOI] [PubMed] [Google Scholar]
- 11.Tolman KG, Fonseca V, Tan MH, Dalpiaz A. Narrative review: hepatobiliary disease in type 2 diabetes mellitus. Ann. Intern. Med. 2004;141:946–956. doi: 10.7326/0003-4819-141-12-200412210-00011. [DOI] [PubMed] [Google Scholar]
- 12.Assy N, Kaita K, Mymin D, Levy C, Rosser B, Minuk G. Fatty infiltration of liver in hyperlipidemic patients. Dig. Dis. Sci. 2000;45:1929–1234. doi: 10.1023/a:1005661516165. [DOI] [PubMed] [Google Scholar]
- 13.Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, Hirschhorn JN, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–1987. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lomonaco R, Chen J, Cusi K. An Endocrine Perspective of Nonalcoholic Fatty Liver Disease (NAFLD) Ther. Adv. Endocrinol. Metab. 2011;2:211–225. doi: 10.1177/2042018811419157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFilippis AP, Blaha MJ, Martin SS, Reed RM, Jones SR, Nasir K, Blumenthal RS, et al. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;227:429–436. doi: 10.1016/j.atherosclerosis.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Small DM. The Physical Chemistry of Lipids. From Alkanes to Phospholipids. New York: Plenum Press; 1986. p. 672. [Google Scholar]
- 17.Jonas A. Lipoprotein structure. In: Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins and Membranes. 4th eds. Amsterdam: Elsevier; 2002. 483-404. [Google Scholar]
- 18.Cohen DE. Lipoprotein metabolism and cholesterol balance. In: Arias IM, Alter H, Boyer JL, Cohen DE, Fausto N, Shafritz DA, Wolkoff AW, editors. Biology and Pathobiology. 5th ed. Oxford: Wiley Blackwell; 2009. pp. 271–285. [Google Scholar]
- 19.Iqbal J, Hussain MM. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009;296:E1183–E1194. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudel LL, Lee RG, Parini P. ACAT2 is a target for treatment of coronary heart disease associated with hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2005;25:1112–1118. doi: 10.1161/01.ATV.0000166548.65753.1e. [DOI] [PubMed] [Google Scholar]
- 21.Rutledge AC, Su Q, Adeli K. Apolipoprotein B100 biogenesis: a complex array of intracellular mechanisms regulating folding, stability, and lipoprotein assembly. Biochem. Cell Biol. 2010;88:251–267. doi: 10.1139/o09-168. [DOI] [PubMed] [Google Scholar]
- 22.Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J. Biol. Chem. 2002;277:17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 23.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 24.Chamberlain JM, O'Dell C, Sparks CE, Sparks JD. Insulin suppression of apolipoprotein B in McArdle RH7777 cells involves increased sortilin 1 interaction and lysosomal targeting. Biochem. Biophys. Res. Commun. 2013;430:66–71. doi: 10.1016/j.bbrc.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao Z, Wang Y. Apolipoprotein C-III and hepatic triglyceride-rich lipoprotein production. Curr. Opin. Lipidol. 2012;23:206–212. doi: 10.1097/MOL.0b013e328352dc70. [DOI] [PubMed] [Google Scholar]
- 26.Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, et al. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 1992;258:999–1001. doi: 10.1126/science.1439810. [DOI] [PubMed] [Google Scholar]
- 27.Berriot-Varoqueaux N, Aggerbeck LP, Samson-Bouma M, Wetterau JR. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu. Rev. Nutr. 2000;20:663–697. doi: 10.1146/annurev.nutr.20.1.663. [DOI] [PubMed] [Google Scholar]
- 28.Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N. Engl. J. Med. 2007;356:148–156. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- 29.Goh VJ, Silver DL. The lipid droplet as a potential therapeutic target in NAFLD. Semin. Liver Dis. 2013 doi: 10.1055/s-0033-1358521. in press. [DOI] [PubMed] [Google Scholar]
- 30.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 33.Chamoun Z, Vacca F, Parton RG, Gruenberg J. PNPLA3/adiponutrin functions in lipid droplet formation. Biol. Cell. 2013;105:219–233. doi: 10.1111/boc.201200036. [DOI] [PubMed] [Google Scholar]
- 34.Li JZ, Huang Y, Karaman R, Ivanova PT, Brown HA, Roddy T, Castro-Perez J, et al. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J. Clin. Invest. 2012;122:4130–4144. doi: 10.1172/JCI65179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pirazzi C, Adiels M, Burza MA, Mancina RM, Levin M, Stahlman M, Taskinen MR, et al. Patatin-like phospholipase domain-containing 3 (PNPLA3) I148M (rs738409) affects hepatic VLDL secretion in humans and in vitro. J. Hepatol. 2012;57:1276–1282. doi: 10.1016/j.jhep.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 36.Tanoli T, Yue P, Yablonskiy D, Schonfeld G. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J. Lipid Res. 2004;45:941–947. doi: 10.1194/jlr.M300508-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.McGowan MP, Tardif JC, Ceska R, Burgess LJ, Soran H, Gouni-Berthold I, Wagener G, et al. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS One. 2012;7:e49006. doi: 10.1371/journal.pone.0049006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan M, Cederbaum AI, Zhang YL, Ginsberg HN, Williams KJ, Fisher EA. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J. Clin. Invest. 2004;113:1277–1287. doi: 10.1172/JCI19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brodsky JL, Fisher EA. The many intersecting pathways underlying apolipoprotein B secretion and degradation. Trends Endocrinol Metab. 2008;19:254–259. doi: 10.1016/j.tem.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher EA. The degradation of apolipoprotein B100: multiple opportunities to regulate VLDL triglyceride production by different proteolytic pathways. Biochim. Biophys. Acta. 2012;1821:778–781. doi: 10.1016/j.bbalip.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strong A, Ding Q, Edmondson AC, Millar JS, Sachs KV, Li X, Kumaravel A, et al. Hepatic sortilin regulates both apolipoprotein B secretion and LDL catabolism. J. Clin. Invest. 2012;122:2807–2816. doi: 10.1172/JCI63563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haas ME, Attie AD, Biddinger SB. The regulation of ApoB metabolism by insulin. Trends Endocrinol. Metab. 2013 doi: 10.1016/j.tem.2013.04.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013;48:434–441. doi: 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu X, So J-S, Park J-G, Lee A-H. Transcriptional control of hepatic lipid metabolism by SREBP and ChREBP. Semin. Liver Dis. 2013 doi: 10.1055/s-0033-1358523. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Sparks CE, Sparks JD. Hepatic steatosis and VLDL hypersecretion. Curr. Opin. Lipidol. 2012;23:395–397. doi: 10.1097/MOL.0b013e3283555afa. [DOI] [PubMed] [Google Scholar]
- 47.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henkel A, Green RM. The unfolded protein response in fatty liver disease. Semin. Liver Dis. 2013 doi: 10.1055/s-0033-1358522. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shachter NS. Apolipoproteins C-I and C-III as important modulators of lipoprotein metabolism. Curr. Opin. Lipidol. 2001;12:297–304. doi: 10.1097/00041433-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Fielding PE, Fielding CJ. Dynamics of lipoprotein transport in the human circulatory system. In: Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins and Membranes. 4th ed. Amsterdam: Elsevier; 2002. pp. 527–552. [Google Scholar]
- 51.Merkel M, Eckel RH, Goldberg IJ. Lipoprotein lipase: genetics, lipid uptake, and regulation. J. Lipid Res. 2002;43:1997–2006. doi: 10.1194/jlr.r200015-jlr200. [DOI] [PubMed] [Google Scholar]
- 52.Young SG, Zechner R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 2013;27:459–484. doi: 10.1101/gad.209296.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009;297:E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 54.Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med. 2002;80:753–769. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]
- 55.Cohn JS, Patterson BW, Uffelman KD, Davignon J, Steiner G. Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C-III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. J. Clin. Endocrinol. Metab. 2004;89:3949–3955. doi: 10.1210/jc.2003-032056. [DOI] [PubMed] [Google Scholar]
- 56.Cooper AD. Hepatic uptake of chylomicron remnants. J Lipid Res. 1997;38:2173–2192. [PubMed] [Google Scholar]
- 57.Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40:1–16. [PubMed] [Google Scholar]
- 58.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 59.Packard CJ, Shepherd J. Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol. 1997;17:3542–3556. doi: 10.1161/01.atv.17.12.3542. [DOI] [PubMed] [Google Scholar]
- 60.Lewis GF, Murdoch S, Uffelman K, Naples M, Szeto L, Albers A, Adeli K, et al. Hepatic lipase mRNA, protein, and plasma enzyme activity is increased in the insulin-resistant, fructose-fed Syrian golden hamster and is partially normalized by the insulin sensitizer rosiglitazone. Diabetes. 2004;53:2893–2900. doi: 10.2337/diabetes.53.11.2893. [DOI] [PubMed] [Google Scholar]
- 61.Miksztowicz V, Lucero D, Zago V, Cacciagiu L, Lopez G, Gonzalez Ballerga E, Sorda J, et al. Hepatic lipase activity is increased in non-alcoholic fatty liver disease beyond insulin resistance. Diabetes Metab. Res. Rev. 2012;28:535–541. doi: 10.1002/dmrr.2312. [DOI] [PubMed] [Google Scholar]
- 62.Lucero D, Zago V, Lopez GI, Graffigna M, Lopez GH, Fainboim H, Miksztowicz V, et al. Does non-alcoholic fatty liver impair alterations of plasma lipoproteins and associated factors in metabolic syndrome? Clin. Chim Acta. 2011;412:587–592. doi: 10.1016/j.cca.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 63.Schneider WJ. Lipoprotein receptors. In: Vance DE, editor. Biochemistry of Lipids, Lipoproteins and Membranes. Amsterdam: Elsevier; 2002. pp. 553–572. [Google Scholar]
- 64.Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade. J. Lipid Res. 2012;53:2515–2524. doi: 10.1194/jlr.R026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang ZG, Robson SC, Yao Z. Lipoprotein metabolism in nonalcoholic fatty liver disease. J. Biomed. Res. 2013;27:1–13. doi: 10.7555/JBR.27.20120077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 67.Goldstein JL, Brown MS. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab. 2008;8:512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 72.Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, Kellum J, et al. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15:665–674. doi: 10.1016/j.cmet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen DE. Pathogenesis of gallstones. In: Zakim D, Boyer TD, editors. Hepatology: A Textbook of Liver Disease. 4 ed. Philadelphia, PA: W.B. Saunders; 2002. pp. 1713–1743. [Google Scholar]
- 74.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 75.Silver DL, Jiang XC, Arai T, Bruce C, Tall AR. Receptors and lipid transfer proteins in HDL metabolism. Ann. N.Y. Acad. Sci. 2000;902:103–102. doi: 10.1007/978-4-431-68424-4_20. [DOI] [PubMed] [Google Scholar]
- 76.Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. J. Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 77.Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 2012;32:2813–2820. doi: 10.1161/ATVBAHA.112.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, et al. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zannis VI, Chroni A, Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J. Mol. Med. 2006;84:276–294. doi: 10.1007/s00109-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 80.Blanco-Vaca F, Escola-Gil JC, Martin-Campos JM, Julve J. Role of apoA-II in lipid metabolism and atherosclerosis: advances in the study of an enigmatic protein. J Lipid Res. 2001;42:1727–1739. [PubMed] [Google Scholar]
- 81.Kiss RS, McManus DC, Franklin V, Tan WL, McKenzie A, Chimini G, Marcel YL. The lipidation by hepatocytes of human apolipoprotein A-I occurs by both ABCA1-dependent and - independent pathways. J. Biol. Chem. 2003;278:10119–10127. doi: 10.1074/jbc.M300137200. [DOI] [PubMed] [Google Scholar]
- 82.Webb NR, Cai L, Ziemba KS, Yu J, Kindy MS, van der Westhuyzen DR, de Beer FC. The fate of HDL particles in vivo after SR-BI-mediated selective lipid uptake. J. Lipid Res. 2002;43:1890–1898. doi: 10.1194/jlr.m200173-jlr200. [DOI] [PubMed] [Google Scholar]
- 83.Rye KA, Barter PJ. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler Thromb Vasc Biol. 2004;24:421–428. doi: 10.1161/01.ATV.0000104029.74961.f5. [DOI] [PubMed] [Google Scholar]
- 84.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 85.Nonomura K, Arai Y, Mitani H, Abe-Dohmae S, Yokoyama S. Insulin down-regulates specific activity of ATP-binding cassette transporter A1 for high density lipoprotein biogenesis through its specific phosphorylation. Atherosclerosis. 2011;216:334–341. doi: 10.1016/j.atherosclerosis.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 86.Ng DS. The role of lecithin:cholesterol acyltransferase in the modulation of cardiometabolic risks - a clinical update and emerging insights from animal models. Biochim. Biophys. Acta. 2012;1821:654–659. doi: 10.1016/j.bbalip.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 87.Tzotzas T, Desrumaux C, Lagrost L. Plasma phospholipid transfer protein (PLTP): review of an emerging cardiometabolic risk factor. Obes. Rev. 2009;10:403–411. doi: 10.1111/j.1467-789X.2009.00586.x. [DOI] [PubMed] [Google Scholar]
- 88.Rothblat GH, de la Llera-Moya M, Atger V, Kellne-Weibel G, Williams DL, Phillips MC. Cell cholesterol efflux: integration of new and old observations provides new insights. J. Lipid Res. 1999;40:781–796. [PubMed] [Google Scholar]
- 89.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 90.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–2455. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 91.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barter PJ, Brewer HB, Jr, Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:160–167. doi: 10.1161/01.atv.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- 93.Charles MA, Kane JP. New molecular insights into CETP structure and function: a review. J. Lipid Res. 2012;53:1451–1458. doi: 10.1194/jlr.R027011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hewing B, Fisher EA. Rationale for cholesteryl ester transfer protein inhibition. Curr. Opin. Lipidol. 2012;23:372–376. doi: 10.1097/MOL.0b013e328353ef1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 96.Silver DL. A Carboxyl-terminal PDZ-interacting Domain of Scavenger Receptor B, Type I Is Essential for Cell Surface Expression in Liver. J. Biol. Chem. 2002;277:34042–34047. doi: 10.1074/jbc.M206584200. [DOI] [PubMed] [Google Scholar]
- 97.Xiao C, Watanabe T, Zhang Y, Trigatti B, Szeto L, Connelly PW, Marcovina S, et al. Enhanced cellular uptake of remnant high-density lipoprotein particles: a mechanism for high-density lipoprotein lowering in insulin resistance and hypertriglyceridemia. Circ. Res. 2008;103:159–166. doi: 10.1161/CIRCRESAHA.108.178756. [DOI] [PubMed] [Google Scholar]