Abstract
Background
Midodrine and fludrocortisone are considered the first-line pharmacologic treatments for orthostatic hypotension (OH). Although OH is thought to require long-term therapy, it is unknown how long patients remain on treatment (“persistence”).
Methods
We assembled a retrospective cohort of patients with OH aged ≥50 years enrolled in Tennessee Medicaid (1996–2008), and identified new episodes of midodrine and fludrocortisone use. Follow-up continued from first medication fill through treatment discontinuation (90 days without medication), change in treatment, death, hospitalization, loss of enrollment or study end. We compared persistence on treatment using Cox regression models and fludrocortisone as reference. Covariates included demographics, healthcare utilization measurements and co-morbidities.
Results
We identified 1,704 OH patients, who initiated 1,767 episodes of fludrocortisone (1103) or midodrine (664) use. The median age was 69 years, 53% were female and 80% were white. During 738 person years of follow-up, episodes of use ended because of treatment discontinuation in 467 (27% fludrocortisone, 25% midodrine); treatment change in 72 (3% fludrocortisone, 6% midodrine) and death in 53 (3% fludrocortisone, 2% midodrine). Overall median persistence on fludrocortisone and midodrine was 254 (IQR: 119–783) and 259 (IQR: 119–807) days, respectively. The adjusted hazard ratio (aHR) for overall non-persistence on midodrine compared to fludrocortisone was 1.07 (95% CI: 0.90–1.28).
Conclusions
Overall duration of OH treatment with first-line medications was short, and similar for fludrocortisone and midodrine. Further research is warranted to determine the causes of this low persistence.
Keywords: Orthostatic Hypotension, epidemiology, fludrocortisone, midodrine
Introduction
Orthostatic hypotension (OH) is a common yet under-recognized cause of syncope and falls in the elderly population. Each year, OH causes approximately 80,000 hospitalization among US adults aged 75 years or older (Shibao et al. 2007). Orthostatic hypotension is defined as the fall in systolic blood pressure (SBP) of at least 20 mm Hg or diastolic blood pressure (DBP) of at least 10 mm Hg within 3 minutes of upright position or head up tilt.(Freeman et al. 2011) Severely affected patients can stand only for a few minutes before developing disabling pre-syncopal symptoms or syncope.
OH is defined as a persistent or chronic fall in blood pressure upon standing, and for purposes of this study, excludes hypotension caused by acute or transient events associated with rapid loss of blood volume. Most patients diagnosed with OH who fail non-pharmacological measurements are prescribed pharmacological treatment to prevent abrupt reductions in blood pressure on standing and their consequences. The only available treatment guideline, from the European Federation of Neurological Societies (EFNS)(Lahrmann et al. 2006), recommends fludrocortisone, a synthetic aldosterone analog, as the primary therapy for OH. This recommendation is based on case series that reported improvement in upright blood pressure and pre-syncopal symptoms(Campbell et al. 1975;Campbell et al. 1976;Hoehn 1975). Currently off-label use of fludrocortisone for OH among practitioners is common. In the US, the only drug approved for treatment of OH is midodrine, an α1-adrenoreceptor agonist, which has been shown to improve standing blood pressure in clinical trials(Kearney and Moore 2009;Low and Singer 2008). Recently, the US Food and Drug administration recently announced its intention to remove midodrine from the market given the lack of evidence supporting its clinical efficacy in reducing pre-syncopal symptoms (Dhruva and Redberg 2010). Because the full benefit of effective chronic therapies can be achieved only if patients follow their treatment regimens, knowledge about persistence on pharmacological treatments (i.e. duration on continuous treatment) is crucial in the evaluation of medication effects.(Andrade et al. 2006). We, therefore, evaluated the persistence of patients with OH on fludrocortisone and midodrine regimens.
Methods
Cohort Assembly
TennCare is the State-based Medicaid program in Tennessee Subjects enrolled in this program received all their healthcare services including medications free of charge. Using TennCare files we assembled a retrospective cohort of patients with OH. Medication data was available from 1995 through 2008. From 1995 through 2005 the medication data was provided exclusively by TennCare and from 2006 until December 2008, the TennCare medication data was supplemented with Medicare Part D (for those patients that were dually eligible for TennCare and Medicare. In this cohort, we identified all enrollees who were 50 years old or older, had one coded health care encounter for OH (ICD9-CM: 458.0,458.1,458.9,333.0) and filled a prescription for midodrine and/or fludrocortisone.
Potential cohort members were required to have at least 180 days of continuous enrollment in TennCare before entering the cohort, to allow the collection of baseline characteristics. They were also required to have at least 1 prescription filled during this baseline period to assure access to medication benefits. We excluded patients with baseline diagnoses for conditions that have the potential to produce acute and transient OH. To limit potential sources of poor persistence, we excluded patients with established serious life-threatening diseases identified during baseline. These conditions were solid organ transplantation, end stage renal disease and hemodialysis.
Cohort members were followed from the date when the selection criteria were met to the end of the study (December 31st 2008), date of death, all-cause hospitalization or loss of enrollment from TennCare, whichever came first.
New episodes of midodrine and fludrocortisone use
A new episode of medication use started on the date when a prescription for midodrine or fludrocortisone was filled after 180 days without exposure to midodrine or fludrocortisone. Medication use was assessed using the days of drug supply recorded in the pharmacy files. Under TennCare, most prescriptions have a maximum of 30 days of drug supply. Within episodes of use, we allowed gaps in medication use (defined as less than or equal to 90 days without medication available). An episode of new medication use ended on the date of death, loss of enrollment, medication discontinuation or change in therapy (from midodrine to fludrocortisone or vice versa), date of hospitalization or end of study period, whichever came first. Patients initiating both drugs simultaneously were excluded from this assessment.
Using new episodes of medication use, we evaluated the overall persistence on midodrine and fludrocortisone, which represents the combination of 2 outcomes: 1) time to drug discontinuation, i.e. cessation of therapy for at least 90 days; and 2) time to switching to a different OH regimen. Although no standard definition exists to define OH discontinuation, we applied a conservative 90-day threshold to define drug discontinuation in this study.(Andrade, Kahler, Frech, & Chan 2006)
Statistical Analyses
Factors potentially affecting treatment persistence were measured during the 180 baseline days. Baseline covariate information included: 1) demographics; 2) health care utilization data, including number of hospitalizations, outpatient and emergency room visits, and number of different medication prescriptions filled; and, 3) specific dichotomous variables, indicating the utilization of sympatholytic agents, direct acting vasodilators, beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, alpha-adrenoreceptor blockers, diuretics, nitrates, antidiabetics, opioids, antidepressants, antipsychotics, antiarrhythmics, benzodiazepines. We also assessed clinical diagnoses including obesity, hypertension, hyperlipidemia, cigarette smoking, osteoporosis, history of fracture, dementia, cancer, chronic obstructive pulmonary disease (COPD), depression, peripheral neuropathy, Parkinson’s disease and other causes of Parkinsonism, autonomic neuropathy, multiple system atrophy, thyroid disease, excessive alcohol consumption, arthritis, arthrosis, diabetes, atrial fibrillation, arrhythmias, myocardial infarction, obstructive coronary disease, transient ischemic attack, cerebrovascular disease, peripheral artery disease, valve disease, syncope, and congestive heart failure.
We used Cox proportional hazards regression models to compare persistence between study medications and estimated hazard ratios and 95% confidence intervals after adjustment for demographics, healthcare utilization and co-morbidities listed before. Patients with OH could contribute more than 1 episode of use of the same regimen, as long as they met our selection criteria and definition of new user. A new set of covariates was obtained for each subsequent episode and we accounted for multiple episodes per patient, calculating robust standard errors. Model assumptions were verified using a global test and Schoenfeld residuals. We accounted for the extensive list of baseline covariates in our study using propensity scores. For this strategy, we first calculated the probability of midodrine use conditional on the study covariates using a logistic regression model, and then included the propensity score as a linear term in the final outcome model. In our study, the exposures were common but the outcomes infrequent relative to the number of covariates. In this scenario, propensity score methods yield more accurate estimates than those obtained by including all covariates in a multivariable model or by variable selection procedures.(Arbogast and Ray 2011)
Analyses were performed in Stata version 11.0 (StataCorp., College Station, TX). The study protocol was approved by the Institutional Review Board of Vanderbilt University Medical Center and informed consent was waived.
Results
Orthostatic Hypotension Cohort
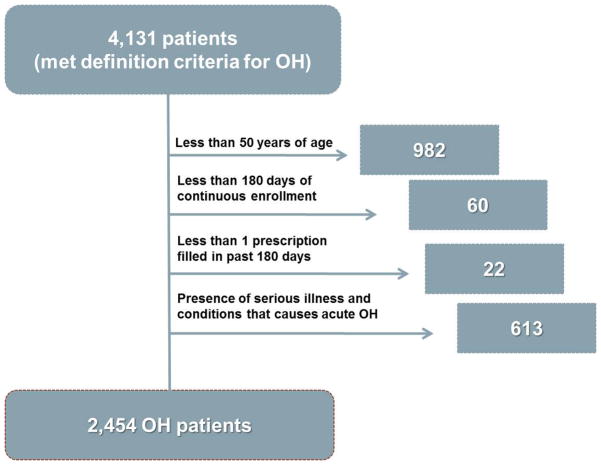
During the study period 4,131 TennCare enrollees met our OH definition (i.e. filled a prescription for midodrine/fludrocortisone and had a coded healthcare encounter with a diagnosis of OH during the previous 180 days). We excluded 982 (24%) persons who were younger than 50 years of age, 60 persons with less than 180 days of continuous enrollment prior to the cohort entry, 22 persons with no prescription filled before cohort entry and 613 persons because of serious illness or conditions that cause acute and transient OH. Our final cohort included 2,454 patients with OH (Figure 1).
Figure 1.
Orthostatic hypotension (OH) cohort assembly.
New Episodes of Fludrocortisone or Midodrine Use
We identified 1,103 episodes of new use of fludrocortisone and 664 episodes of new use of midodrine therapy. The median age was 69 for both medication groups. The distributions of gender and race were similar between fludrocortisone or midodrine users. Midodrine users had more prescriptions filled for other drugs compared with fludrocortisone users. The median number of different medications used during the baseline 180 days was 14 (interquartile range [IQR], 9–22) for midodrine and 13 (range, 8–19) for fludrocortisone.
The number of outpatient visits during baseline was similar between exposures groups. Similarly, the number of hospital admissions and emergency room visits in the past 30 days were similar between groups. However, the prevalence of hypertension, hyperlipidemia, depression, diabetes, coronary artery disease, arrhythmia, congestive heart failure and syncope was higher in patients initiating midodrine than in patients initiating fludrocortisone (Table 1).
Table 1.
Baseline Profile of Orthostatic Hypotension Drug Users
| Fludrocortisone n=1103 |
Midodrine n=664 |
P value | |
|---|---|---|---|
| Age* (yrs, interquartile range [IQR]) | 69 (61–78) | 69 (60–78) | 0.3847 |
| Gender (%) | |||
| Female | 53.04 | 54.07 | 0.674 |
| Race (%) | |||
| White | 80.15 | 80.27 | 0.147 |
| Black | 10.61 | 12.65 | |
| Other | 9.25 | 7.08 | |
| Healthcare use during baseline | |||
| Nursing home residents (%) | 16.86 | 18.22 | 0.465 |
| Disability (%) | 50.86 | 50.9 | 0.986 |
| Number of outpatient visits (median, IQR) | 4 (1–9) | 4 (1–9) | 0.3036 |
| Medication use during the past 6 months* (median, IQR) | 13 (8–19) | 14 (9–22) | 0.0005 |
| Hospital admission in the past 30 days (%) | 63.46 | 67.17 | 0.114 |
| Hospital admission in past 31 days-6 months (%) | 56.94 | 60.24 | 0.172 |
| Emergency admission in past 30 days † (%) | 46.69 | 52.11 | 0.027 |
| Emergency admission in past 31 days-6 months (%) | 56.39 | 55.27 | 0.646 |
| Co-morbidities (%) group | |||
| Cardiovascular conditions | |||
| Myocardial infarction | 11.42 | 14.76 | 0.041 |
| obstructive coronary artery disease † | 39.35 | 46.99 | 0.002 |
| Congestive heart failure † | 22.94 | 30.87 | 0 |
| Hypertension † | 58.75 | 66.72 | 0.001 |
| Hyperlipidemia † | 24.12 | 30.27 | 0.004 |
| Atrial fibrillation | 15.68 | 17.02 | 0.461 |
| Arrhythmia † | 33.27 | 40.21 | 0.003 |
| Atrial flutter | 15.68 | 17.02 | 0.461 |
| Syncope † | 36.63 | 44.73 | 0.001 |
| Transient ischemic attack | 15.87 | 13.1 | 0.113 |
| Peripheral artery disease | 10.34 | 14.01 | 0.02 |
| Endocrine conditions | |||
| Obesity | 2.72 | 4.52 | 0.043 |
| Diabetes † | 34.45 | 43.22 | 0 |
| Osteoporosis | 9.7 | 7.38 | 0.096 |
| Fracture | 11.42 | 11.9 | 0.763 |
| Thyroid disease | 15.41 | 14.91 | 0.776 |
| Neurological conditions | |||
| Dementia | 15.41 | 16.27 | 0.634 |
| Peripheral Neuropathy | 13.69 | 15.06 | 0.424 |
| Parkinsonism | 9.43 | 8.43 | 0.48 |
| Autonomic Neuropathy | 6.35 | 7.83 | 0.233 |
| Multiple System Atrophy | 4.9 | 4.07 | 0.419 |
| Other chronic conditions | |||
| Cancer | 19.58 | 21.99 | 0.225 |
| COPD | 39.98 | 41.27 | 0.595 |
| Depression | 22.39 | 27.56 | 0.014 |
| Excessive alcohol consumption | 3.72 | 4.07 | 0.712 |
| Cigarette smoking† | 16.5 | 19.13 | 0.159 |
| Arthrosis/joint disease | 26.47 | 27.26 | 0.718 |
| Other medication use during last year (%) | |||
| Sympatholytic agents | 4.35 | 3.92 | 0.658 |
| Direct acting vasodilators | 1.36 | 1.66 | 0.616 |
| Beta-blockers † | 25.39 | 35.84 | 0 |
| Calcium channel blockers | 21.03 | 20.93 | 0.96 |
| Ace-inhibitors and ARB † | 31.55 | 36.45 | 0.035 |
| Alpha-blockers | 3.17 | 3.01 | 0.85 |
| Diuretics † | 38.17 | 49.55 | 0 |
| Nitrates | 22.48 | 23.49 | 0.624 |
| antidiabetics † | 26.38 | 32.68 | 0.005 |
| Opioids † | 55.39 | 63.1 | 0.001 |
| Antidepressants | 52.04 | 58.43 | 0.009 |
| Antipsychotics | 14.51 | 16.27 | 0.318 |
| Antiarrhythmics | 8.25 | 9.94 | 0.227 |
| Benzodiazepines | 33.82 | 30.87 | 0.202 |
Median, (IQR) and P values for Mann-Whitney test for marked row/section.
Proportions and P values for X2 tests, unless otherwise specified.
Patients with OH frequently used opioids, antidepressants, benzodiazepines, beta-blockers and diuretics during baseline. Opioid use was higher among new midodrine users compared with fludrocortisone users (63% vs. 55%, respectively, P<0.001). Similarly, antidepressants, diuretics, antihypertensives, beta-blockers and antidiabetics were more commonly used by new midodrine users compared with fludrocortisone users.
Overall Persistence on OH drugs
Of 1,767 episodes of fludrocortisone or midodrine use, 467 episodes ended because of discontinuation (26%) or change (4%) in the original regimen. Furthermore, 3% of fludrocortisone and 2% of midodrine episodes ended because of death. Approximately 43% of the new episodes of medication use had only 1 prescription filled, 16% had two prescriptions filled and 41% had 3 or more prescriptions filled. The overall median persistence (duration of treatment for 50% of the patients) on fludrocortisone and midodrine was 254 (IQR: 119–783) and 259 (IQR: 119–807) days, respectively. The propensity score adjusted hazard ratio (aHR) for non-persistence on midodrine compared to fludrocortisone was 1.07 (95% CI: 0.90–1.28). Adjustment for deciles of propensity scores yielded virtually identical results (not shown) (Table 2).
Table 2.
Risk of non-persistence on treatments for orthostatic hypotension
| Risk of Discontinuation or Change in Treatmet | |||||
|---|---|---|---|---|---|
| Treatment | Number of episodes of use | Number of events | Median duration on treatment (IQR) | Unadjusted Hazard Ratio (95% confidence interval) | Adjusted Hazard Ratio (95% confidence interval) |
| Fludrocortisone | 1103 | 329 | 254 (119–783) | Reference | Reference |
| Midodrine | 664 | 210 | 259 (119–807) | 1.04 (0.89, 1.24) | 1.07 (0.90, 1.28) |
| Risk of Discontinuation | |||||
| Fludrocortisone | 1103 | 298 | 268 (119, 816) | Reference | Reference |
| Midodrine | 664 | 169 | 304 (133, 865) | 0.93 (0.77, 1.11) | 0.97 (0.80, 1.18) |
The median time to discontinuation of OH treatments (considering changes in therapy as censored events) for those who started on fludrocortisone was 268 days and the median time for discontinuation for those starting on midodrine was 304 days. The aHR for discontinuing midodrine compared to fludrocortisone was 0.97 (95%CI 0.80–1.18).
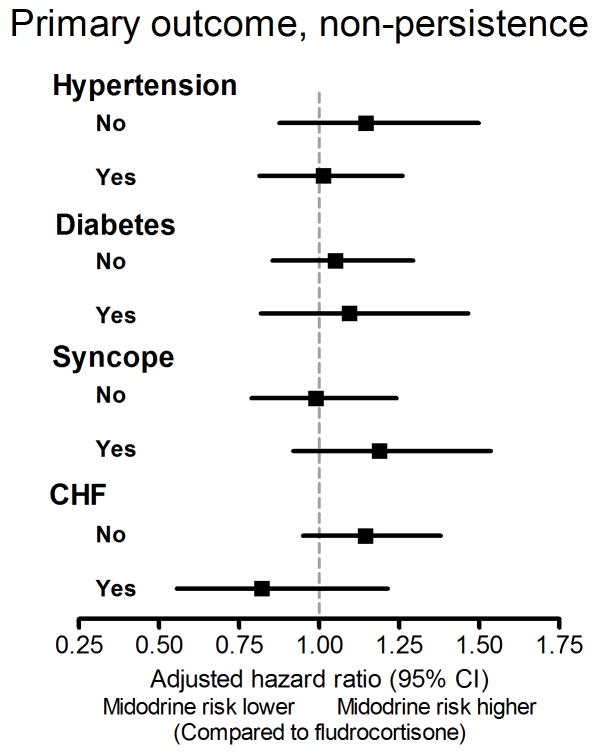
Because the prevalence of hypertension, diabetes, congestive heart failure and syncope was higher in patients initiating midodrine compared to fludrocortisone, we performed a stratified analysis of overall persistence and calculated the aHR for non-persistence in subgroup of patients based on these different diagnoses. The results of these subgroup analyses are presented in Figure 2.
Figure 2.
Stratified analyses for non-persistence by subgroups based on different diagnosis.
CHF, congestive heart failure
We also assessed the concomitant use of diuretics and alpha blockers with OH drugs by determining the number of concurrent refills. These medications have been shown to increase the risk of developing or worsening OH. Of note, 264 patients (24%) on fludrocortisone and 221 (33%) on midodrine had concurrent prescriptions for diuretics. In addition 21 (2%) and 11 (2%) patients on fludrocortisone and midodrine, respectively, had concurrent prescriptions for alpha-blockers. Furthermore, we examined the concurrent prescriptions for anti-hypertensive agents with OH drugs. Eighteen percent of patients filled a prescription for beta-blockers, 10% for calcium channel blockers, 18% for ACE and ARB.
Discussion
Although fludrocortisone and midodrine are commonly used for the treatment of OH in the US, little is known about the effectiveness and safety of these medications in routine clinical practice. Persistence on these treatments can be used as one measure of long-term effectiveness. In a large cohort of Medicaid enrollees with OH, overall persistence on fludrocortisone or midodrine was short and similar for both drugs.
Our study provides an initial assessment of OH medication use not restricted to specialized care settings. Persistence on both fludrocortisone and midodrine was poor. This is unexpected for drugs that are meant to treat a chronic condition such as OH. There may be different explanations for these observations. It could be possible that our findings reflects a gap in knowledge about use of these agents for the treatment of OH in clinical practice, particularly because we observed the use of these agents in combination with other pharmacologic agents that antagonize their actions, i.e. midodrine combined with alpha-blockers, or fludrocortisone combined with diuretics. Likewise, non-persistence of treatment could be related to the development of adverse events. Again, to some degree this could be due to knowledge gap by providers about the appropriate use of these agents, which would explain the high incidence of heart failure in patients who were prescribed fludrocortisone. Finally we cannot exclude the possibility that our results are explained by the lack of efficacy of these drugs in reducing orthostatic-related symptoms or by the presence of adverse effects.
Recently published guidelines for the treatment of OH suggest that the first step should be the removal of any medication that could precipitate or contribute to OH. Among common offenders are alpha-blockers commonly used to treat symptoms due to prostate hyperplasia, diuretics and tricyclic antidepressants (Shibao et al. 2013). If OH persists and the patient is symptomatic, pharmacological treatment is indicated, and fludrocortisone and midodrine are considered first line therapies for symptomatic OH (Lahrmann, Cortelli, Hilz, Mathias, Struhal, & Tassinari 2006). Because these conditions are rare, there is limited evidence based on long-term randomized controlled clinical trials to support these treatment options. In the case of fludrocortisone, the recommendation is based on case series published in the 1970’s that reported improvement in upright blood pressure and pre-syncopal symptoms (Campbell, Ewing, & Clarke 1975;Campbell, Ewing, & Clarke 1976;Hoehn 1975). Considering that fludrocortisone produces a transient increase in sodium retention and plasma volume, the potential development or exacerbation of heart failure in a susceptible population such as the elderly is of concern. Midodrine is the only drug approved by The Food and Drug Administration for the treatment of OH. The approval was based on randomized clinical trials demonstrating improvement in standing systolic blood pressure in OH patients.
Our study is the first to describe the clinical characteristics of the patients in the community actually receiving these drugs. In our cohort, patients with OH had multiple co-morbidities; more than 50% had a hospital or emergency admission during baseline. More than half had a diagnosis of hypertension, a known risk factor for OH; more than a third had a diagnosis of coronary artery disease, arrhythmias, diabetes mellitus or COPD and approximately one fourth of patients had a diagnosis of heart failure. Our patients filled an average 13 different medications for the treatment of these co-morbidities. Some of these medications, such as diuretics and alpha blockers increase the risk of OH. Thus, it is surprising that they were prescribed with concurrent OH medications, particularly with medication that opposed the pharmacological effect of OH drugs. Indeed, approximately 27% of patients with OH filled a prescription for a diuretic while on treatment with OH drugs (24% on fludrocortisone and 32% on midodrine). Similarly, 2% filled prescriptions for alpha-blockers. These findings suggest that prescribing physicians need treatment guidelines regarding the treatment of orthostatic hypotension.
We also explored the concomitant use of other anti-hypertensive agents with OH drugs, 18% of patients filled a prescription for beta-blockers, 10% for calcium channel blockers, 18% for ACE and ARB. Even though there is a conventional belief that lowering and controlling blood pressure with anti-hypertensive medications may exacerbate OH, existing data do not support this theory(Gangavati et al. 2011). Withholding antihypertensive treatment often worsen OH through pressure diuresis.
Our findings must be interpreted in light of several limitations. First, patients with OH were identified through computerized diagnosis codes and not clinical criteria. However, our evaluation was restricted to patients who had compatible diagnosis and started specific OH therapies excluding patients with conditions causing acute or transient causes of OH. Second, TennCare databases lack clinical information, such as functional status, which may determine channeling selected patients to a particular OH therapy. Although our statistical analyses accounted for a number of confounders (e.g. medical conditions, health care utilization), residual differences between patients using these two therapies cannot be completely ruled out. Furthermore, specific reasons for treatment discontinuation or poor persistence could not be evaluated using our resources. Third, we measured medication exposure using pharmacy records but use of the study medications was not measured directly. Nevertheless, tracking filled prescriptions from computerized pharmacy records has a good correlation with patient self-reports of medication use and is a valid method to measure drug utilization (Levy et al. 2003;McKenzie et al. 2000;Steiner and Prochazka 1997). Finally, because TennCare enrollees represent a Medicaid population, these results may not be extrapolated directly to other populations such as patients cared for in specialized autonomic dysfunction centers. However, Medicaid covers a significant proportion of the US population that is typically excluded from premarketing studies and for whom limited information on medications’ effectiveness and safety is available.
In summary, our study suggests that the duration of treatment with medications for OH was short, and similar for fludrocortisone and midodrine. Further research is warranted to assess if low persistence is explained by lack of knowledge of prescribing physicians, low effectiveness or poor safety profile of these medications. Our results also highlight the limitations inherent in using conservative inclusion/exclusion criteria in clinical trials that are not reflective of patients in the community who will actually receive these medications.
Acknowledgments
Funding source: C.S. was supported by American Heart Association, Clinical Research Program 10CRP4310026, National Institute of Health (NIH) grant K23 HL103976-02 and by PHRMA Foundation Career Development Award. The study was supported by American Heart Association, Clinical Research Program 10CRP4310026, Vanderbilt CTSA grant 1UL1 RR000445 from NACTS/NIH and R03 AG042981, NIH. Dr. Lipsitz was supported by NIH grant AG025037 and holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife. Dr. Italo Biaggioni is consultant for Chelsea Therapeutics and Shire. Dr. Italo Biaggioni has received research funds through the Vanderbilt University School of Medicine from Chelsea Therapeutics to conduct the droxidopa multicenter study.
We are indebted to the Tennessee Bureau of TennCare of the Department of Finance and Administration, which provided the data.
C.S. was supported by American Heart Association, Clinical Research Program 10CRP4310026, National Institute of Health (NIH) grant K23 HL103976-02 and by PHRMA foundation Career Development Award.
The study was supported by American Heart Association, Clinical Research Program 10CRP4310026, Vanderbilt CTSA grant 1UL1 RR000445 from NACTS/NIH and R03 AG042981, NIH.
Dr. Lipsitz was supported by NIH grant AG025037 and holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife.
Footnotes
All remaining authors declared no conflict of interest.
All authors had access to the data. All the authors declared that they met criteria for authorship including acceptance of responsibility for the scientific content of the manuscript.
References
- Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–574. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- Arbogast PG, Ray WA. Performance of disease risk scores, propensity scores, and traditional multivariable outcome regression in the presence of multiple confounders. Am J Epidemiol. 2011;174(5):613–620. doi: 10.1093/aje/kwr143. [DOI] [PubMed] [Google Scholar]
- Campbell IW, Ewing DJ, Clarke BF. 9-a-fluorohydrocortisone in the treatment of postural hypotension in diabetic autonomic neuropathy. Diabetes. 1975;24:381–384. doi: 10.2337/diab.24.4.381. [DOI] [PubMed] [Google Scholar]
- Campbell IW, Ewing DJ, Clarke BF. Therapeutic experience with fludrocortisone in diabetic postural hypotension. British Medical Journal. 1976;1(6014):872–874. doi: 10.1136/bmj.1.6014.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhruva SS, Redberg RF. Accelerated approval and possible withdrawal of midodrine. JAMA. 2010;304(19):2172–2173. doi: 10.1001/jama.2010.1695. [DOI] [PubMed] [Google Scholar]
- Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz IJ, Schondorf R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci. 2011;161(1–2):46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, Lipsitz LA. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59(3):383–389. doi: 10.1111/j.1532-5415.2011.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM. Levodopa-induced postural hypotension. Treatment with fludrocortisone. Archives of Neurology. 1975;32:50–51. doi: 10.1001/archneur.1975.00490430072013. [DOI] [PubMed] [Google Scholar]
- Kearney F, Moore A. Pharmacological options in the management of orthostatic hypotension in older adults. Expert Rev Cardiovasc Ther. 2009;7(11):1395–1400. doi: 10.1586/erc.09.130. [DOI] [PubMed] [Google Scholar]
- Lahrmann H, Cortelli P, Hilz M, Mathias CJ, Struhal W, Tassinari M. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13(9):930–936. doi: 10.1111/j.1468-1331.2006.01512.x. [DOI] [PubMed] [Google Scholar]
- Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10(2):67–71. [PubMed] [Google Scholar]
- Low PA, Singer W. Management of neurogenic orthostatic hypotension: an update. Lancet Neurol. 2008;7(5):451–458. doi: 10.1016/S1474-4422(08)70088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie DA, Semradek J, McFarland BH, Mullooly JP, McCamant LE. The validity of medicaid pharmacy claims for estimating drug use among elderly nursing home residents: The Oregon experience. J Clin Epidemiol. 2000;53(12):1248–1257. doi: 10.1016/s0895-4356(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Shibao C, Grijalva CG, Raj SR, Biaggioni I, Griffin MR. Orthostatic hypotension-related hospitalizations in the United States. Am J Med. 2007;120(11):975–980. doi: 10.1016/j.amjmed.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Shibao C, Lipsitz LA, Biaggioni I. ASH position paper: evaluation and treatment of orthostatic hypotension. J Clin Hypertens(Greenwich) 2013;15(3):147–153. doi: 10.1111/jch.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]