Abstract
Curcumin, the yellow pigment of turmeric found in Southeast Indian food, is one of the most popular phytochemicals for cancer prevention. Numerous reports have demonstrated modulation of multiple cellular signaling pathways by curcumin and its molecular targets in various cancer cell lines. To identify a new molecular target of curcumin, we used shape screening and reverse docking to screen the protein data bank against curcumin. Cyclin dependent kinase 2 (CDK2), a major cell cycle protein, was identified as a potential molecular target of curcumin. Indeed, in vitro and ex vivo kinase assay data revealed a dramatic suppressive effect of curcumin on CDK2 kinase activity. Furthermore, curcumin induced G1 cell cycle arrest, which is regulated by CDK2 in HCT116 cells. Although the expression levels of CDK2 and its regulatory subunit, cyclin E, were not changed, the phosphorylation of Rb, a well-known CDK2 substrate, was reduced by curcumin. Because curcumin induced cell cycle arrest, we investigated the anti-proliferative effect of curcumin on HCT116 colon cancer cells. In this experiment, curcumin suppressed HCT116 cell proliferation effectively. To determine if CDK2 is a direct target of curcumin, CDK2 expression was knocked down in HCT116 cells. As expected, HCT116 sh-CDK2 cells exhibited G1 arrest and reduced proliferation. Because of the low levels of CDK2 in HCT116 sh-CDK2 cells, the effects of curcumin on G1 arrest and cell proliferation were not substantial relative to HCT116 sh-control cells. From these results, we identified CDK2 as a direct target of curcumin in colon cancer cells.
Keywords: CDK2, colon cancer, curcumin, HCT116, cell cycle
Introduction
Curcumin or diferuloylmethane is a yellow pigment and a constituent of turmeric extracted originally from Curcuma longa (Linn). In China and India, curcumin is widely used as a coloring and flavoring agent. The chemotherapeutic and chemopreventive effects of curcumin have been reported (1–7). Additionally, oral intake of curcumin reportedly showed a beneficial effect against precancerous lesions in Phase I and Phase II clinical trials (8, 9). Furthermore, some investigators have suggested several target proteins and signaling pathways affected by curcumin, including the AMPK-COX-2 (10), JNKs (11), E2F4 (12) and cyclin D1-CDK4 signaling pathways (13). Although numerous proteins have been suggested as targets of curcumin, CDK2 has not been reported as a direct target of curcumin in colon cancer.
Aberrant growth is a characteristic of cancer cells (14). Research data clearly indicate that cancer cell proliferation is much greater and less controlled than normal cell growth (15). Cyclin-dependent kinases (CDKs), which are serine/threonine protein kinases, are essential kinases mediating the cell cycle (16). Thus far, nine CDKs have been identified and each regulates specific points of the cell cycle (16, 17). For example, G1 is mediated by CDK4, CDK6 and CDK2; the S phase is regulated by CDK2; and the G2/M phase is controlled by CDK1 (16, 17). The kinase activity of CDKs is regulated by cyclins and different CDKs are required by various cyclins for their kinase activity (18). In the G1 phase, cyclins D1, D2, and D3 interact with CDK4 and CDK6 (19). In the G1 to S phase transition, cyclin E binds to CDK2 (20, 21) and during the S phase, the cyclin A/CDK2 complex regulates cell cycle (21). To promote the M phase, cyclin A binds to CDK1 in the late G2 and early M phase (22, 23). Deregulation of growth is characteristic of cancer cells and cell cycle proteins are highly activated in these cells (24). Cyclin E-dependent kinase activity was reported to be substantially higher in colorectal cancers and CDK2 expression levels are also high in colorectal adenomas (25). We found that three colon cancer cell lines, HCT116, HCT15 and DLD-1, show dramatically increased expression of CDK2 compared to “normal” (see footnote) human colon epithelial cells. This suggests that CDK2 could be a potential therapeutic target for colon cancer treatment.
Herein, we used computational modeling to identify CDK2 as a potential target of curcumin. Both in vitro and ex vivo kinase assay data confirmed that curcumin effectively suppressed CDK2 kinase activity. Because CDK2 is highly activated in colon cancer, we selected the HCT116 colon cancer cell model to evaluate the effects of curcumin on cell cycle. We found that curcumin caused G1 cell cycle arrest, which is regulated by CDK2. Furthermore, curcumin exhibited a dramatic anti-proliferation effect on 3 different colon cancer cell lines and knocking down expression of CDK2 confirmed that CDK2 is a direct target of curcumin. To our knowledge, this is the first report to show that CDK2 is a direct target of curcumin and could be a novel target of curcumin in preventing or treating colon cancer.
Materials and Methods
Chemicals
Curcumin and fetal bovine serum (FBS) were purchased from Sigma-Aldrich (St. Louis, MO). McCoy’s 5A was obtained from Thermo Fisher Scientific (Fremont, CA). Penicillin/streptomycin were purchased from Invitrogen (Grand Island, NY). Antibodies against CDK2, CDK4, phosphorylated Rb (Thr821) and cyclin E were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and the antibody against phosphorylated c-Myc was purchased from Cell Signaling Biotechnology (Beverly, MA). Secondary antibodies to detect rabbit and mouse IgG and conjugated to HRP were obtained from Zymed (San Francisco, CA).
Cell culture
All cell lines were obtained from American Type Culture Collection (Manassas, VA). The cells were cytogenetically tested and authenticated before being frozen. Each vial of frozen cells was thawed and maintained for a maximum of 8 weeks. The frozen vials were available for each cell line to ensure that all cell-based experiments were performed on cells that had been tested and in culture for 8 weeks or less. Human colonic epithelial cells (HCEC) were cultured in basal media (HyClone, Logan, UT) supplemented with epidermal growth factor (25 ng/ml), insulin (10 µg/ml), gentamicin sulfate (50 µg/ml), transferrin (2 µg/ml), hydrocortisone (1 µg/ml), sodium selenite (5 nM), and 2% cosmic calf serum (HyClone). HCT116 human colon cancer cells were cultured in McCoy’s 5A medium supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA) and 1% penicillin/streptomycin. HCT15 and DLD-1 human colon cancer cells were cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin.
Shape screening
Shape screening was performed using the Phase module (26, 27) in Schrödinger Suite 2011 (28) by comparing the volumetric similarity between curcumin and each compound from the Protein Data Bank (PDB) ligand database. The structure of curcumin was downloaded from the PubChem Compound database (http://www.ncbi.nlm.nih.gov/pccompound) and further processed using the ConfGen module (29) in Maestro (30). The best 3-dimensional (3–D) conformation of the lowest potential energy from ConfGen was selected as the query molecule structure. The ligand database was downloaded from the PDB (31) in March 2010. Shape screening was performed directly with this database without further modification. During the screening process, polar hydrogens were included and one alignment per ligand was retained. Only the molecules with a ShapeSim (i.e., the overall similarities between curcumin and screened compounds) score of less than 0.7 were eliminated.
Reverse Docking
To enhance the reliability of the evaluation of the potential targets of curcumin from shape screening, reverse docking using the Glide module (32, 33) in Schrödinger was also used. For this calculation, only the potential kinase targets were considered. The crystal structure of each protein kinase bound with the potential identified ligand from the PDB was downloaded. Each raw PDB structure was then converted into an all-atom, fully prepared receptor model structure using Protein Preparation Wizard module in Maestro. The docking receptor grid of each potential target was prepared using Glide’s Receptor Grid Generation (32, 33). Docking of the curcumin molecule into each receptor gird was performed using Glide in the XP (Extra Precision) mode (34). All generated conformations of curcumin from ConfGen were used for rigid-protein docking. In all other respects, the default settings were used. The best scoring energy was selected from the best docking of different initial conformations of curcumin to each kinase target.
Molecular modeling
The docking structure of curcumin and CDK2 was generated by the Induced Fit Docking (IFD) protocol (35) in the Schrödinger Suite 2011. All default settings were used, except that the XP (Extra Precision) scoring function was used (34). Briefly, the grid box and center were settled by default using the ligand in the ATP binding site and no constraints were defined. During the Glide docking process, the van der Waals radii of protein and ligand were scaled by a factor of 0.5. In addition, only the residues within 5 Å of the ligand were refined for Prime active site optimization. The binding pose with the lowest score was considered as the correct binding structure and used as the representative docking structure.
CDK1, CDK2, and CDK4 kinase assays
The CDK1, CDK2, and CDK4 kinase assays were performed using active recombinant CDK1, CDK2, and CDK4 enzymes (Millipore, Bedford, MA) and evaluating the ability of each kinase to transfer the radiolabeled phosphate from [γ-32P] ATP according to the manufacturer’s instructions. Briefly, active recombinant CDK1, CDK2, or CDK4 (100–200 ng) and curcumin were incubated at 30°C for 15 min in assay buffer (20 mM MOPS (pH 7.2), 25 µM β-glycerol phosphate, 5 mM EGTA, 1 mM sodium orthovanadate and 1 mM DTT). Myelin basic protein (20 µg) as substrate was added to each mixture and then incubated at 30°C for 15 min with a [γ-32P] ATP solution in a magnesium acetate-ATP cocktail buffer (Upstate Biotechnology Inc., Lake Placid, NY). Then the reaction mixture was transferred onto p81 paper and the p81 paper was washed 3 times for 5 min each with 0.75% phosphoric acid,. The radiolabeled phosphate was measured using a scintillation counter.
For the ex vivo CDK2 immunoprecipitation and kinase assays, 20 µl of Protein A/G Plus Agarose (Santa Cruz, CA) were added to cell lysates containing 600 µg protein and then incubated for 1 h at 4°C. After centrifugation at 12,000 rpm for 5 min at 4°C, an antibody against CDK2 (30 µg) was added to the supernatant fraction of the mixture, and then rocked overnight at 4°C. These mixtures were centrifuged at 12,000 rpm for 5 min at 4°C and washed twice. Curcumin was added at various concentrations and the kinase assay was performed in a manner similar to the in vitro kinase assay.
Western blot analysis
HCT116 colon cancer cells were cultured for 48 h, and in order to synchronize the cell cycle, the cells were incubated in serum free McCoy’s 5A media for 12 h. After synchronization, the cells were treated or not treated with various concentrations of curcumin for 30 min. Then the cells were disrupted with lysis buffer [10 mM Tris (pH 7.5), 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton X-100, 1 mM dithiothreitol (DTT), 0.1 mM phenylmethylsulfonyl fluoride (PMSF), and 10% glycerol and protease inhibitor cocktail tablet]. The protein concentration was determined using a dye-binding protein assay kit (Bio-Rad Laboratories, Philadelphia, PA) as described by the manufacturer. The proteins were separated electrophoretically using a 10% SDS-polyacrylamide gel. After separation, the proteins were transferred to an Immobilon-P membrane (Millipore Corporation, St. Charles, MO). The membrane was blocked with 5% fat-free milk for 1 h and incubated with the specific primary antibody at 4°C overnight. After hybridization with an HRP-conjugated secondary antibody, the protein bands were detected using a chemiluminescence detection kit (GE healthcare, Pittsburgh, PA).
Preparation of curcumin-Sepharose 4B beads
CNBr-activated Sepharose 4B beads, used in the current experiments, bind and immobilize chemicals at available hydroxyl (–OH) groups. Curcumin has 3 hydroxyl groups and therefore can be easily coupled with Sepharose beads. For activation, the Sepharose 4B beads (0.3 g) were washed with 30 ml of 1 mM HCl 3 times for 5 min each by gentle inversion and then incubated with 3 mg of curcumin or DMSO in coupling buffer [0.1 M NaHCO3 (pH 8.3) and 0.5 M NaCl (pH 8.3)] at 4°C overnight. The mixtures were washed 5 times with coupling buffer and incubated with blocking buffer [0.1 M Tris-HCl (pH 8.0)] at 4°C overnight. The excess of uncoupled curcumin was removed by washing buffer [0.1 M acetate buffer (pH 4.0)] and 0.1 M Tris-HCl buffer (pH 8.0) containing 0.5 M NaCl (pH 8.0) 3 times and then resuspended in 1 ml of PBS.
Cell-based pull-down assay
HCT116 colon cancer cells were disrupted with lysis buffer [50 mM Tris (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.5% Nonidet P-40, 0.02 mM PMSF, and 1× protease inhibitor mixture] and proteins extracted. Proteins (500 µg) were incubated with Sepharose 4B beads (as a negative control) or curcumin-Sepharose 4B beads (100 µl of 50 % slurry) in reaction buffer [50 mM Tris (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40, 2 mg/ml BSA, 0.02 mM PMSF, and 1× protease inhibitor mixture] at 4°C overnight. The mixture was washed 5 times with 1 ml of washing buffer [50 mM Tris (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40, and 0.02 mM PMSF] and proteins bound to the beads were analyzed by Western blotting.
ATP and curcumin competition assay
Recombinant CDK2 (200 ng) was incubated with ATP (0, 1, 10, or 100 µM) at 4°C for 1 h. Then 100 µl of Sepharose 4B (negative control) or curcumin-Sepharose 4B beads were added to the mixture and incubated at 4°C overnight. The samples were then washed with washing buffer (above) and proteins analyzed by Western blotting.
Cell proliferation assay
To evaluate the effect of curcumin on proliferation, HCT116 cells were seeded (4×103 cells per well) in 96-well plates for 24 h. Cells were then treated with curcumin for 24, 48, or 72 h. Cell proliferation was analyzed using Cell Titer96 Aqueous One Solution (Promega, Madison, WI) by incubating with 20 µl of MTS solution for 1 h at 37°C in a 5% CO2 incubator. The absorbance was read at 492 nm.
Cell cycle analysis
HCT116 cell cycle was evaluated using a published method (36) with slight modifications. After arresting the cells in the G0 phase, the cells were treated with various concentrations of curcumin for 9 h. After fixing with ethanol, cells were stained with propidium iodide and the cell cycle phase was determined by flow cytometry.
Results
Computer modeling and binding between curcumin and CDK2
To identify potential targets of curcumin, we performed both ligand-based and structure-based in silico screening using the computer modeling methods described in Materials and Methods. Based on the structure of curcumin, we first screened the PDB ligand library to identify compounds that showed a shape similarity score above 0.7. The proteins co-crystallized with the potentially identified ligands could possibly bind to curcumin. In order to enhance the reliability of the evaluation of these potential targets of curcumin identified from shape screening, we then performed structure-based reverse docking studies using curcumin and the crystal structures of these proteins and discovered CDK2 as a potential kinase target of curcumin.
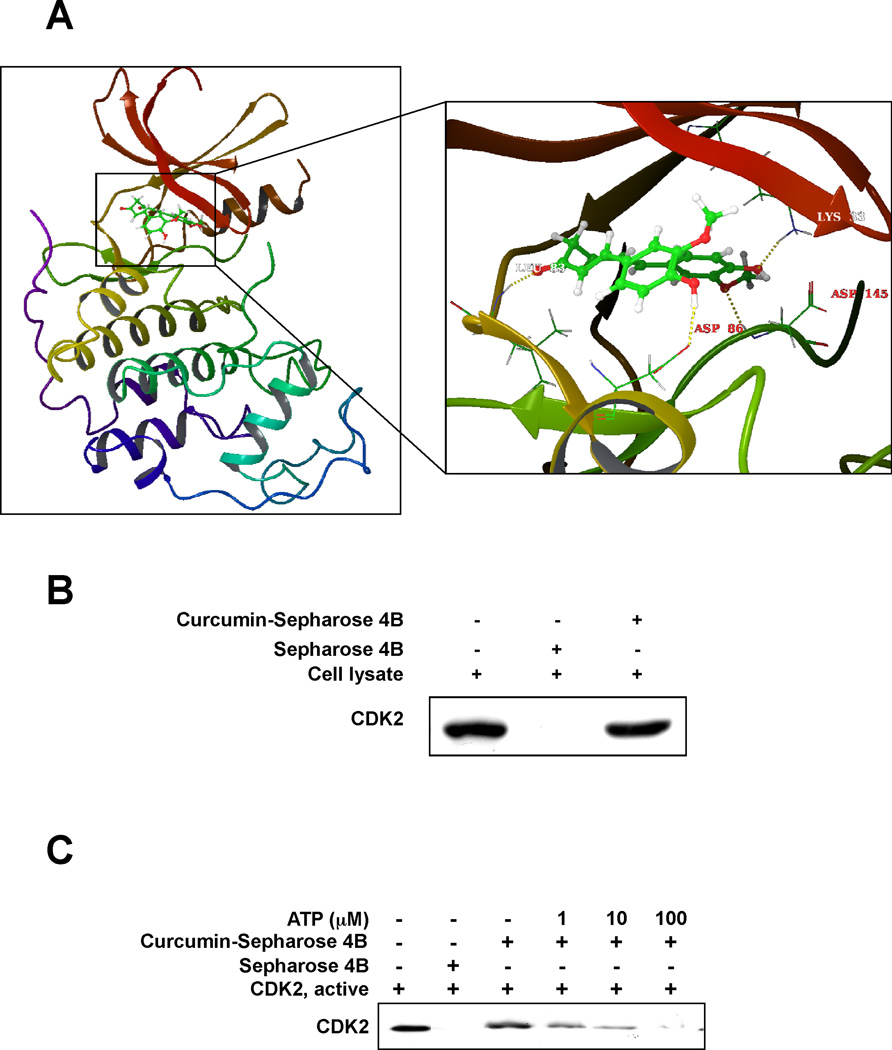
To predict the binding conformation between curcumin and CDK2, we performed flexible-ligand flexible-protein docking using the IFD (Induced Fit Docking) Module (35). Induced fit docking has an advantage over the regular Glide docking (33) because of its ability to capture the ligand-induced conformational changes in the receptor active site (37). From the IFD, the binding affinity between curcumin and CDK2 was predicted to be very good with a score of -12.69 kcal/mol. The computational model of the curcumin-CDK2 complex is shown in Fig. 1A. Curcumin formed 4 hydrogen bonds with CDK2–two are involved with the backbone atoms of the hinge loop residue Leu83 and the activation loop residue Asp145, and the other two are formed with the side chain atoms of Asp86 and Lys33. In addition, several residues around the ATP binding pocket, including Ile10, Val64, Phe80, Phe82, Leu83, and Leu134, showed some hydrophobic interactions with the carbons of curcumin (data not shown). These computational results indicate that curcumin should exhibit ATP-competitive inhibitory effects against the CDK2 kinase.
Fig. 1.
Curcumin directly binds to CDK2 in an ATP-competitive manner. (A) The interaction between curcumin and several residues in the ATP binding pocket of CDK2. Curcumin forms hydrogen bonds with Lys33, Leu83, Asp86 and Asp145, respectively. Note: the α-helices are drawn as cylinders and the β-strands as arrows. Curcumin is shown in stick model and protein residues are shown in line model. (B) Curcumin directly binds with CDK2 in HCT116 colorectal cancer cells. The binding of curcumin with CDK2 in HCT116 cells was detected by immunoblotting with a specific CDK2 antibody: lane 1 (input control), whole-cell lysates from HCT116 cells; lane 2 (control), lysates from HCT116 cells; and lane 3, whole-cell lysates from HCT116 cells precipitated with curcumin-Sepharose 4B beads. (C) Curcumin competes with ATP for binding with CDK2. Active CDK2 was incubated with ATP at different concentrations (0, 1, 10, or 100 µM) with 50 µl of curcumin-Sepharose 4B beads or 50 µl of Sepharose 4B (as a negative control) beads in reaction buffer. After washing, the pulled-down CDK2 proteins were detected by Western blotting.
Because the computer modeling data suggested that curcumin could bind to the ATP pocket of CDK2 (Fig. 1A), we evaluated the binding between curcumin and CDK2 in HCT116 colorectal cancer cell lysates. CDK2 was pulled down by curcumin-Sepharose 4B beads, but was not detected in Sepharose 4B beads (Fig. 1B). To confirm that curcumin binds to the ATP pocket of CDK2, various concentrations of ATP were pre-mixed with a recombinant CDK2 protein before the addition of curcumin-Sepharose 4B or Sepharose 4B beads. The binding between CDK2-curcumin was dose-dependently reduced by ATP (Fig. 1C). The results indicate that curcumin directly binds to the ATP pocket of CDK2.
Curcumin selectively suppresses CDK2 kinase activity
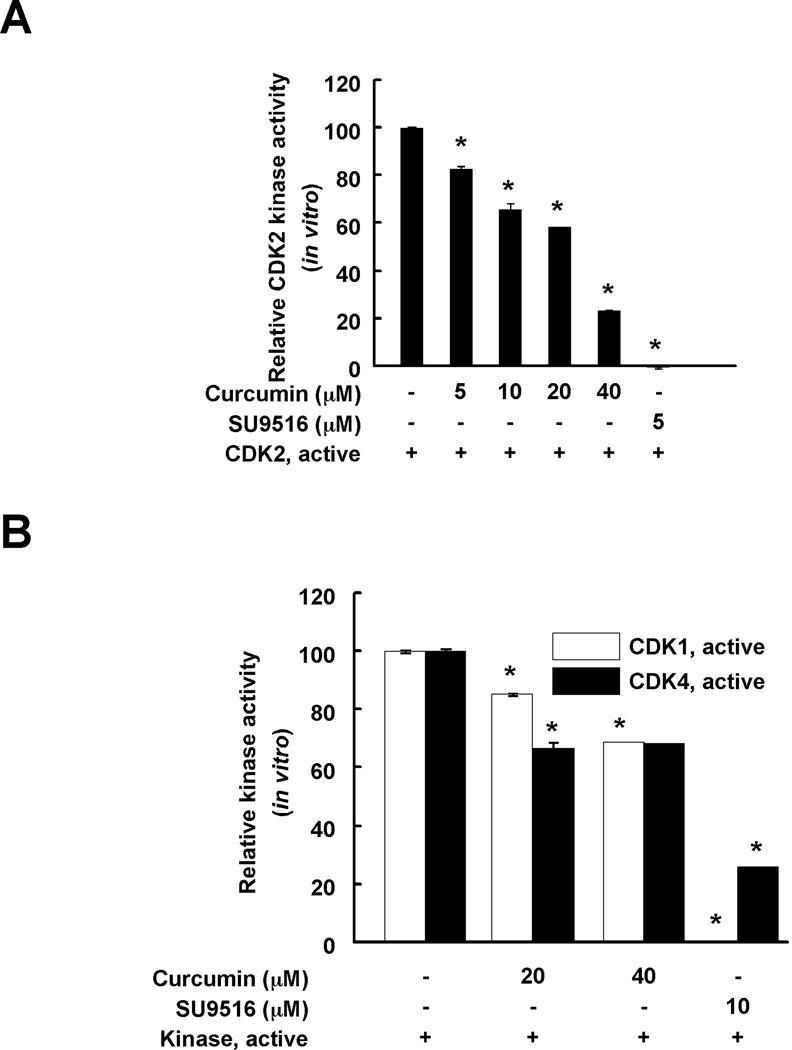
The effect of curcumin on CDK2 kinase activity was evaluated using in vitro kinase assays. The kinase assay data revealed that curcumin suppressed CDK2 kinase activity in a dose-dependent manner. Curcumin at 5, 10, 20, or 40 µM reduced CDK2 kinase activity by 17.5, 34.3, 58.2, or 77.0%, respectively (Fig. 2A). SU9516, a well-known CDK2 inhibitor also decreased CDK2 kinase activity (positive control). To investigate the effect of curcumin on CDK2 in HCT116 colorectal cancer cell lysates, CDK2 kinase was immunoprecipitated using a specific CDK2 antibody. Curcumin showed a similar inhibitory effect on immunoprecipitated-CDK2 kinase activity (Supplementary Figure 1). In addition, the effect of curcumin on CDK1 and CDK4 kinase activity was measured. The inhibitory effect of curcumin on CDK1 and CDK4 kinase activity was less than the effect on CDK2. Even curcumin at 40 µM did not decrease activity below 60% (Fig. 2B). Taken together, curcumin strongly affects CDK2 kinase activity compared to CDK1 or CDK4 kinase activity.
Fig. 2.
Curcumin effectively suppresses CDK2 kinase activity in vitro compared with CDK1 or 4 kinase activity. The effect of curcumin on CDK2 (A) or CDK1 and CDK4 (B) in vitro kinase activity was examined as described in "Materials and Methods". Data are represented as means ± S.D. as determined from 3 independent experiments. The asterisk (*) indicates a significant (p < 0.001) decrease in kinase activity compared to untreated control.
Curcumin decreases proliferation of colon cancer cells
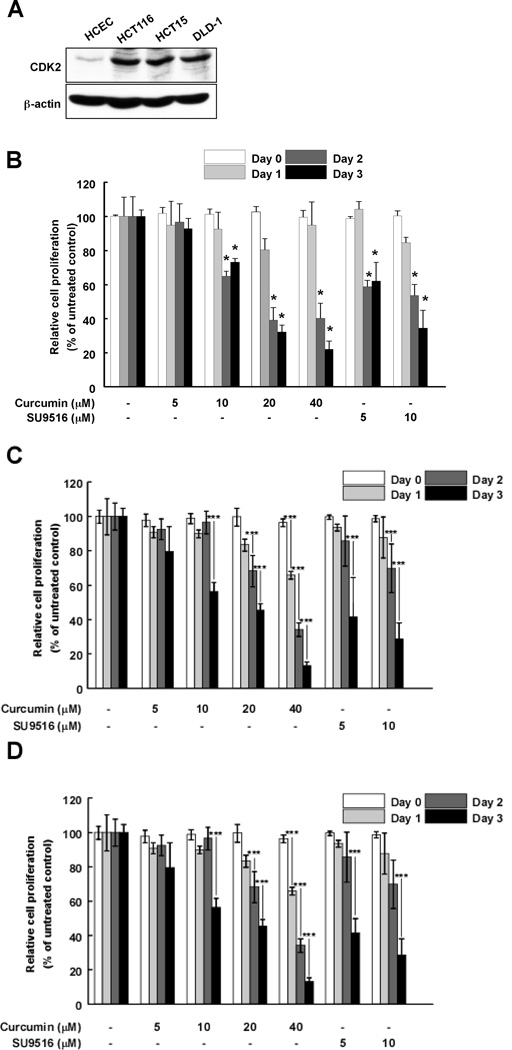
CDK2 is an important protein in the cell cycle. The cyclin E/Cdk2 complexes induce mammalian cells to enter the S phase by phosphorylating specific substrates such as Rb. Thus, many chemotherapeutic and chemopreventive agents have been developed targeting CDK2 (38). In particular, cyclin E-dependent kinase activity and the kinase activity and expression level of the CDK2 protein are substantially higher in colorectal cancers and in 90% of colorectal adenomas (38). Here, we investigated the endogenous expression level of CDK2 in colorectal cancer cells (HCT116, HCT15 and DLD-1) and “normal” (see footnote) human colon epithelial cells (HCEC). Our results (Fig. 3A) show that the expression level of CDK2 is much higher in colon cancer cells, including HCT116, HCT15 and DLD-1 cells, compared to HCEC cells. Thus, we hypothesized that curcumin could suppress proliferation of colon cancer cells by inhibiting CDK2 kinase activity. The effect of curcumin was evaluated on the three colon cancer cell lines and results indicated that curcumin substantially reduced proliferation of HCT116 (Fig. 3B), HCT15 (Fig. 3C) and DLD-1 (Fig. 3D) colon cancer cells. Curcumin did not exhibit any significant effects on the proliferation or viability of “normal” (see footnote) human colon epithelial cells (data not shown).
Fig. 3.
CDK2 is highly expressed in colon cancer cells and curcumin selectively suppresses HCT116 cell proliferation. (A) The CDK2 expression level is much higher in colon cancer cells (HCT116, HCT15, and DLD-1) compared to “normal” (see footnote) colon cells (HCEC). Cells were harvested and CDK2 expression was visualized using a specific antibody. Curcumin reduces proliferation of HCT116 (B), HCT15 (C), and DLD-1 (D) colon cancer cells. Each colon cancer cell line was seeded (1×103 cells per well) in 96-well plates and then cells were treated with curcumin (0, 5, 10, 20 or 40 µM) at 6 h after seeding. Cell growth was determined at 1, 2, or 3 days using the MTS assay as described in "Materials and Methods". Data are represented as the means ± S.D. as determined from 3 independent experiments. The asterisk (*) indicates a significant (p < 0.001) decrease in activity compared to untreated control.
Curcumin induces G1 cell cycle arrest and reduces phosphorylation of Rb in HCT116 colorectal cancer cells
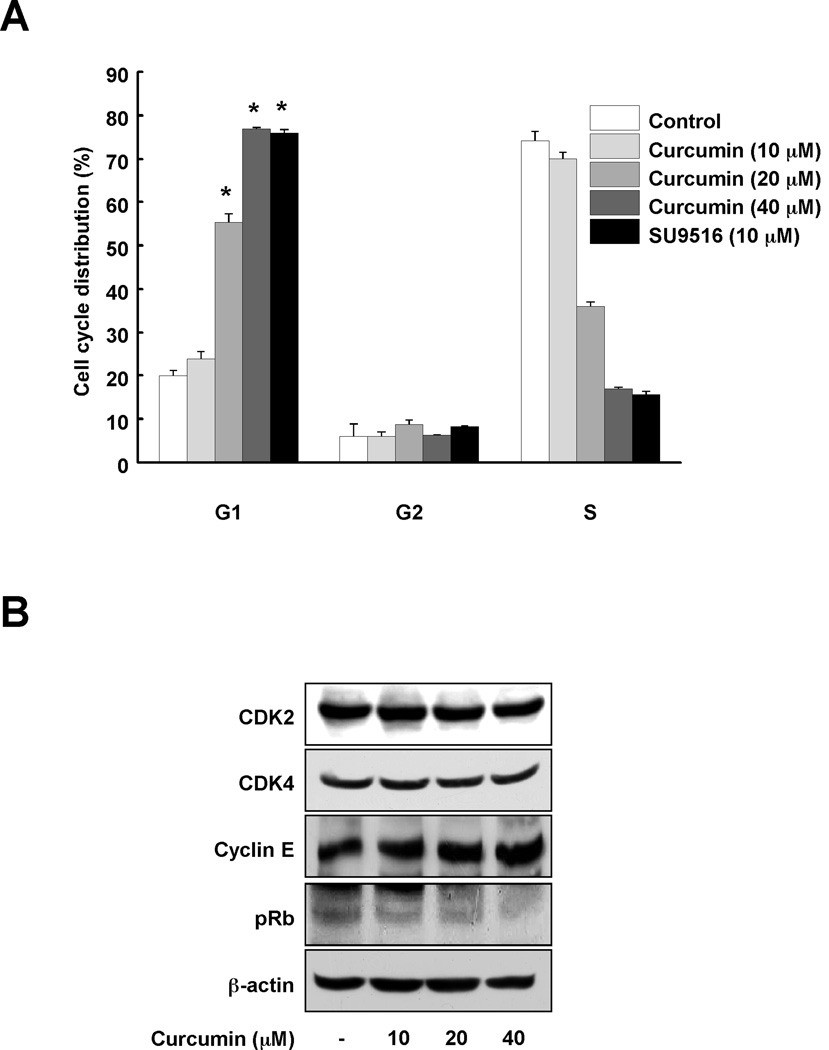
Because curcumin inhibited CDK2 kinase activity and decreased proliferation of colon cancer cells, we hypothesized that curcumin might affect the G1 cell cycle phase, which is regulated by CDK2. Curcumin induced G1 cell cycle arrest in a dose-dependent manner (Fig. 4A). The well-known CDK2 inhibitor, SU9516, was used as a positive control. In addition, phosphorylation of Rb was reduced by curcumin (Fig. 4B). Because the expression levels of CDK2, CDK4 and cyclin E were not changed by curcumin, the effect of curcumin on Rb is likely due to the inhibitory effect of curcumin on CDK2 kinase activity.
Fig. 4.
Curcumin induces G1 arrest in HCT116 colon cancer cells. (A) The effect of curcumin on cell cycle was investigated using flow cytometry. HCT116 colon cancer cells were cultured until they reached confluence in 96-well plates and then synchronized in G0 phase by serum deprivation. After 9 h of treatment with curcumin (0, 10, 20, or 40 µM) or SU9516 (10 µM), cell cycle phases were analyzed. Data are represented as means ± S.D. as determined from 3 independent experiments and the asterisk (*) indicates a significant difference (p < 0.001) compared to untreated group. (B) To determine the effect of curcumin on cell cycle proteins, Western blotting was performed using specific antibodies. After synchronizing the cells for 12 h, cells were treated with the indicated concentration of curcumin for 30 min. Data are representative of 3 independent experiments that gave similar results.
The effect of curcumin is reduced in HCT116-sh-CDK2 cells
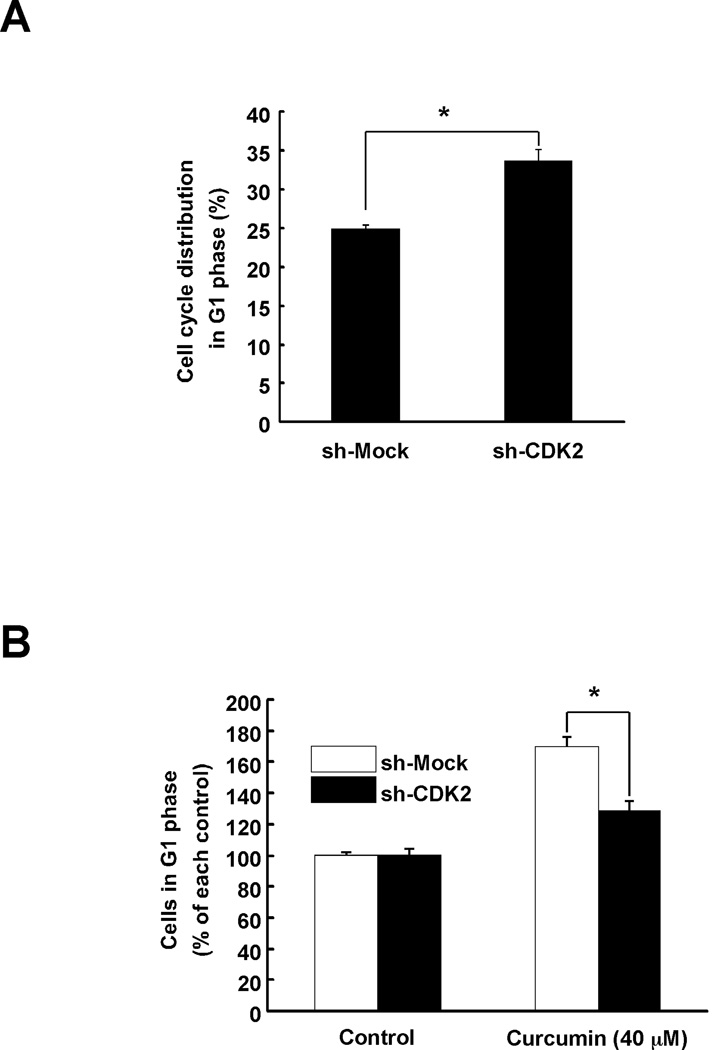
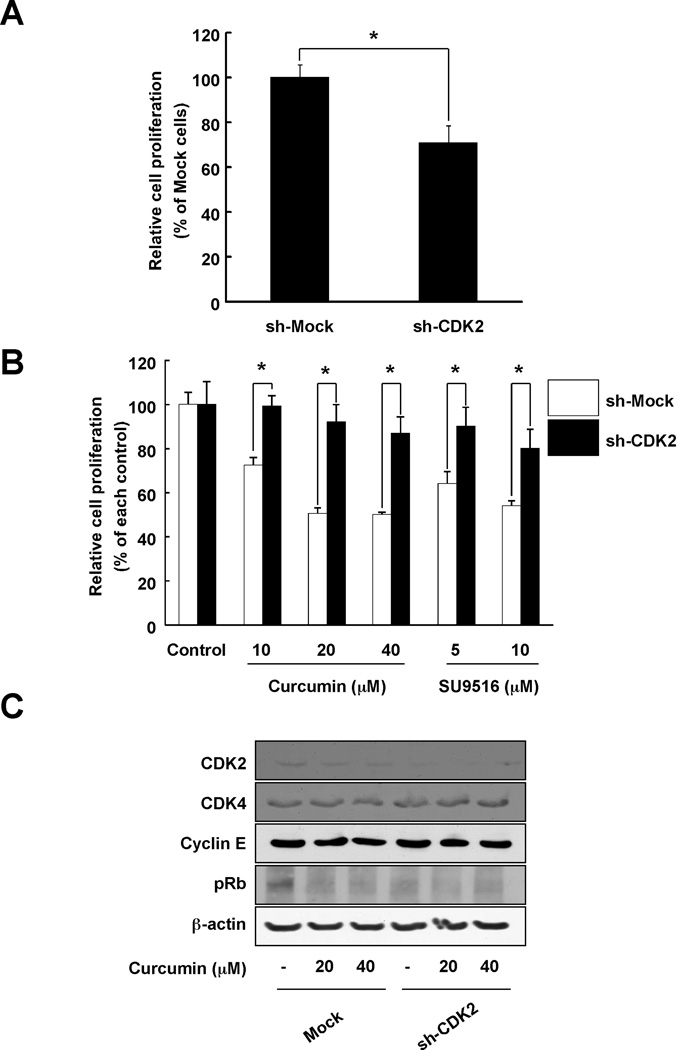
Many proteins have been reported as a target of curcumin. However, no reports demonstrate that CDK2 is a direct protein target of curcumin. To confirm that CDK2 is a direct target of curcumin, we knocked down the expression of CDK2 in HCT116 colon cancer cells. Cells expressing sh-CDK2 HCT116 cells showed a greater number of cells in G1 phase compared to sh-Mock cells (Fig. 5A). However, curcumin had less effect on inducing cell cycle arrest in sh-CDK2 cells compared to sh-Mock (Fig. 5B). sh-CDK2 HCT116 cells also exhibited a greater reduction in proliferation (Fig. 6A) and in addition, curcumin had less effect on proliferation in these cells (Fig. 6B). Next, the phosphorylation of Rb, a substrate of CDK2, was compared between sh-Mock HCT116 and sh-CDK2 HCT116 cells. Curcumin could suppress Rb phosphorylation in cells expressing sh-Mock whereas the effect was much less in sh-CDK2 cells (Fig. 6C). Taken together, we suggest that CDK2 is involved in the anti-proliferative effects of curcumin in colon cancer cells.
Fig. 5.
The effect of curcumin on cell cycle in sh-CDK2-transfected HCT116 colon cancer cells. (A) HCT116 cells stably expressing knockdown of CDK2 were established and G1 phase was arrested in sh-CDK2-transfected HCT116 cells. Cell cycle was analyzed using flow cytometry. (B) The effect of curcumin on G1 phase arrest was less in sh-CDK2-transfected HCT116 colon cancer cells compared to sh-Mock-transfected cells. After 9 h of curcumin treatment, cell cycle phases were analyzed. Data are shown as means ± S.D. as determined from 3 independent experiments and the asterisk (*) indicates a significant (p < 0.001) difference compared to sh-Mock-transfected HCT116 cells.
Fig. 6.
The effect of curcumin on proliferation and cell cycle protein expression in sh-CDK2-transfected HCT116 colon cancer cells. (A) Proliferation was relatively lower in sh-CDK2-transfected HCT116 cells compared to sh-Mock-transfected HCT116 cells. HCT116 cells (1×103 cells) were seeded in 96-well plates and after 72 h, proliferation was measured using the MTS assay as described in "Materials and Methods". (B) The effects of curcumin on cell proliferation were less in sh-CDK2-transfected HCT116 cells compared to sh-Mock-transfected cells. sh-Mock- and sh-CDK2-transfected HCT116 cells were treated with curcumin (0, 10, 20, or 40 µM) and growth was determined at 3 days using the MTS assay as described in "Materials and Methods". Data are represented as means ± S.D. as determined from 3 independent experiments. For A and B, the asterisk (*) indicates a significant difference (p < 0.001) compared to sh-Mock-transfected HCT116 cells. (C) Curcumin had no effect on cell cycle proteins in sh-CDK2-transfected HCT116 colon cancer cells. sh-Mock- and sh-CDK2-transfected HCT116 cells were treated with the indicated concentrations of curcumin for 30 min. Cell cycle protein expression was determined using specific antibodies. Data are representative of 3 independent experiments that gave similar results.
Discussion
Curcumin is a compound that targets multiple molecular and cellular pathways in vitro as well as in animal models, suggesting that curcumin is an attractive compound for chemoprevention or chemotherapy. However, the molecular mechanism of curcumin activity against cancer cells is not understood. In the current study, we showed an interaction between curcumin and CDK2 through the ATP pocket of CDK2. Additionally, we demonstrated an inhibitory effect of curcumin on CDK2 activity. Similar to SU9516, a known CDK2 inhibitor, curcumin also could inhibit CDK1/4 activity (Fig. 2B). However the inhibitory effect of curcumin on CDK1 was less effective than that against CDK2. Although the inhibitory effect of curcumin on CDK4 seemed to be fairly substantial, it did not act in a dose-dependent manner. Computational docking model data consistently showed that the binding affinity of CDK2 on curcumin was stronger than its binding with CDK1 or 4 (Supplementary Figure 2). Taken together, we considered CDK2 as a more specific molecular target of curcumin than CDK1 or 4.
Based on the idea that cell cycle proteins are activated in cancer cells, we found that CDK2 expression in 3 colon cancer cell lines, HCT116, HCT15 and DLD-1, was much higher than in “normal” (see footnote) HCEC cells (Fig. 3A). Thus, the specific anti-proliferative effect of curcumin on cancer cells is likely due to the high CDK2 expression level. To support this hypothesis, we used knockdown CDK2 HCT116 cells to show that curcumin could induce G1 arrest and inhibit proliferation. The effect of curcumin was relatively lower in sh-CDK2 HCT116 cells compared with sh-Mock HCT116 cells. This suggested that the inhibitory effect of curcumin is dependent on the level of CDK2 expression. Although curcumin has a CDK2-dependent inhibitory effect on G1 arrest and cell growth, it substantially inhibited cell growth (Fig. 6B) and phosphorylation of Rb (Fig. 6C) and induced G1 arrest (Fig. 5B) in sh-CDK2 cells. Moreover the effect of sh-CDK2 on G1 arrest (Fig. 5A) is much less than that of curcumin (Fig. 4A). These results indicate that curcumin has CDK2-dependent effects as well as CDK2-independent effects on G1 phase and cell growth of HCT116 cells. Currently, curcumin targets multiple molecular and cellular pathways in various cancers. In the present study, we did not address the CDK2-independent effects of curcumin or try to identify the molecule involved in the CDK2-independent effects of curcumin. This question remains to be investigated.
Previous studies have reported several targets of curcumin’s anti-carcinogenic effects, including NF-κB (39), STAT3 (40), EGFR (41), PI3-K (42), and ERK1/2 (43). One group suggested curcumin inhibits human multiple myeloma cell proliferation by interfering STAT3 phosphorylation (40). Others reported that curcumin (up to 10 µM) reduced EGFR and IGF-1R phosphorylation (41). However, no one has reported a direct target of curcumin.
Here, we report CDK2 as a direct target of curcumin, which could contribute to curcumin’s positive preventive or therapeutic effects against colorectal cancer. However, to verify the direct binding mode between curcumin and CDK2, point mutation studies and X-ray crystallographic studies will be needed. Additionally, although in vitro data support an anti-proliferative effect of curcumin, in vivo studies will be required for full confirmation of the effect of curcumin.
Supplementary Material
Acknowledgements
This work was supported by The Hormel Foundation, National Institutes of Health grants CA027502 (Z. Dong), CA120388 (Z. Dong), R37 CA081064 (Z. Dong), and NIESH ES016548 (Z. Dong), the National Leap Research Program (No. 2010-0029233 K.W. Lee) through the National Research Foundation, the Global Frontier Project grant (NRFM1AXA002-2012M3A6A4054949 K.W. Lee) of National Research Foundation funded by the Ministry of Education, Science and Technology of Korea, and WCU (World Class University) program (R31-10056 K.W. Lee) through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology.
We thank Nicki Brickman at The Hormel Institute, University of Minnesota for assistance in submitting our manuscript.
Footnotes
Authors declare no conflict of interest.
Footnote: Normal human colon epithelial cells (HCEC) comprise an immortalized epithelial cells derived from human colon biopsies with properties similar to normal colon epithelial cells.
References
- 1.Kuttan G, Kumar KB, Guruvayoorappan C, Kuttan R. Antitumor, anti-invasion, and antimetastatic effects of curcumin. Advances in experimental medicine and biology. 2007;595:173–184. doi: 10.1007/978-0-387-46401-5_6. [DOI] [PubMed] [Google Scholar]
- 2.Thangapazham RL, Sharma A, Maheshwari RK. Beneficial role of curcumin in skin diseases. Advances in experimental medicine and biology. 2007;595:343–357. doi: 10.1007/978-0-387-46401-5_15. [DOI] [PubMed] [Google Scholar]
- 3.Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278–287. doi: 10.1038/sj.onc.1209019. [DOI] [PubMed] [Google Scholar]
- 4.Shehzad A, Khan S, Shehzad O, Lee YS. Curcumin therapeutic promises and bioavailability in colorectal cancer. Drugs Today (Barc) 46:523–532. doi: 10.1358/dot.2010.46.7.1509560. [DOI] [PubMed] [Google Scholar]
- 5.Ryu MJ, Cho M, Song JY, Yun YS, Choi IW, Kim DE, et al. Natural derivatives of curcumin attenuate the Wnt/beta-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochemical and biophysical research communications. 2008;377:1304–1308. doi: 10.1016/j.bbrc.2008.10.171. [DOI] [PubMed] [Google Scholar]
- 6.Huang MT, Smart RC, Wong CQ, Conney AH. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-Otetradecanoylphorbol- 13-acetate. Cancer research. 1988;48:5941–5946. [PubMed] [Google Scholar]
- 7.Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer research. 1991;51:813–819. [PubMed] [Google Scholar]
- 8.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer research. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 9.Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer prevention research (Philadelphia, Pa. 4:354–364. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YK, Park SY, Kim YM, Park OJ. Regulatory effect of the AMPK-COX-2 signaling pathway in curcumin-induced apoptosis in HT-29 colon cancer cells. Annals of the New York Academy of Sciences. 2009;1171:489–494. doi: 10.1111/j.1749-6632.2009.04699.x. [DOI] [PubMed] [Google Scholar]
- 11.Collett GP, Campbell FC. Curcumin induces c-jun N-terminal kinase-dependent 19 apoptosis in HCT116 human colon cancer cells. Carcinogenesis. 2004;25:2183–2189. doi: 10.1093/carcin/bgh233. [DOI] [PubMed] [Google Scholar]
- 12.Kim KC, Lee C. Curcumin Induces Downregulation of E2F4 Expression and Apoptotic Cell Death in HCT116 Human Colon Cancer Cells; Involvement of Reactive Oxygen Species. Korean J Physiol Pharmacol. 14:391–397. doi: 10.4196/kjpp.2010.14.6.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukhopadhyay A, Banerjee S, Stafford LJ, Xia C, Liu M, Aggarwal BB. Curcumininduced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21:8852–8861. doi: 10.1038/sj.onc.1206048. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen-Ba G, Vasseur P. Epigenetic events during the process of cell transformation induced by carcinogens (review) Oncology reports. 1999;6:925–932. doi: 10.3892/or.6.4.925. [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell proliferation. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nurse P, Masui Y, Hartwell L. Understanding the cell cycle. Nature medicine. 1998;4:1103–1106. doi: 10.1038/2594. [DOI] [PubMed] [Google Scholar]
- 17.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 18.Pines J. Cyclins and cyclin-dependent kinases: a biochemical view. The Biochemical journal. 1995;308(Pt 3):697–711. doi: 10.1042/bj3080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 20.Dulic V, Lees E, Reed SI. Association of human cyclin E with a periodic G1-S phase protein kinase. Science (New York, NY. 1992;257:1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- 21.Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Molecular and cellular biology. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King RW, Jackson PK, Kirschner MW. Mitosis in transition. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 23.Arellano M, Moreno S. Regulation of CDK/cyclin complexes during the cell cycle. The international journal of biochemistry & cell biology. 1997;29:559–573. doi: 10.1016/s1357-2725(96)00178-1. [DOI] [PubMed] [Google Scholar]
- 24.Tubiana M. Kinetics of cell proliferation and cancer: introduction (author's transl) Bulletin du cancer. 1978;65:407–415. [PubMed] [Google Scholar]
- 25.Cam WR, Masaki T, Shiratori TY, Kato N, Okamoto M, Yamaji Y, et al. Activation of cyclin E-dependent kinase activity in colorectal cancer. Digestive diseases and sciences. 2001;46:2187–2198. doi: 10.1023/a:1011962915280. [DOI] [PubMed] [Google Scholar]
- 26.Phase version 3.3. New York, NY: Schrödinger, LLC; 2011. [Google Scholar]
- 27.Dixon SL, Smondyrev AM, Knoll EH, Rao SN, Shaw DE, Friesner RA. PHASE: a new engine for pharmacophore perception, 3D QSAR model development, and 3D database screening: 1. Methodology and preliminary results. J Comput Aided Mol Des. 2006;20:647–671. doi: 10.1007/s10822-006-9087-6. [DOI] [PubMed] [Google Scholar]
- 28.Schrödinger Suite Schrödinger, LLC. 2011 [Google Scholar]
- 29.Confgen version 2.3. New York, NY: Schrödinger, LLC; 2011. New York, NY. [Google Scholar]
- 30.Maestro version 9.2. New York, NY: Schrödinger, LLC; 2011. [Google Scholar]
- 31.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The Protein Data Bank. Nucleic acids research. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glide version 5.7. New York, NY: Schrödinger, LLC; 2011. [Google Scholar]
- 33.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. Journal of medicinal chemistry. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 34.Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, et al. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. Journal of medicinal chemistry. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 35.Schrödinger Suite 2011 Induced Fit Docking protocol; Glide version 5.7, Schrödinger, LLC, New York, NY, 2011; Prime version 3.0. New York, NY: Schrödinger, LLC; 2011. [Google Scholar]
- 36.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. Journal of the National Cancer Institute. 1997;89:1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 37.Sherman W, Day T, Jacobson MP, Friesner RA, Farid R. Novel procedure for modeling ligand/receptor induced fit effects. J Med Chem. 2006;49:534–553. doi: 10.1021/jm050540c. [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Liu K, Huang Z, Park CM, Thimmegowda NR, Jang JH, et al. A chrysin derivative suppresses skin cancer growth by inhibiting cyclin-dependent kinases. The Journal of biological chemistry. 2013;288:25924–25937. doi: 10.1074/jbc.M113.464669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] The Journal of biological chemistry. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 40.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 41.Reddy S, Rishi AK, Xu H, Levi E, Sarkar FH, Majumdar AP. Mechanisms of curcumin- and EGF-receptor related protein (ERRP)-dependent growth inhibition of colon cancer cells. Nutrition and cancer. 2006;55:185–194. doi: 10.1207/s15327914nc5502_10. [DOI] [PubMed] [Google Scholar]
- 42.Squires MS, Hudson EA, Howells L, Sale S, Houghton CE, Jones JL, et al. Relevance of mitogen activated protein kinase (MAPK) and phosphotidylinositol-3- kinase/protein kinase B (PI3K/PKB) pathways to induction of apoptosis by curcumin in breast cells. Biochemical pharmacology. 2003;65:361–376. doi: 10.1016/s0006-2952(02)01517-4. [DOI] [PubMed] [Google Scholar]
- 43.Lev-Ari S, Vexler A, Starr A, Ashkenazy-Voghera M, Greif J, Aderka D, et al. Curcumin augments gemcitabine cytotoxic effect on pancreatic adenocarcinoma cell lines. Cancer investigation. 2007;25:411–418. doi: 10.1080/07357900701359577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.