Abstract
Background:
Achillea wilhelmsii (A. wilhelmsii) is used in Iraninan folk medicine for the treatment of hypertension; also, in previous reports, the hypotensive and antihypertensive effects of this plant have been indicated. The aim of the present study is to investigate the vasorelaxant effect of the hydroalcholic extract of A. wilhelmsii and its underlying mechanisms in isolated rat aorta.
Materials and Methods:
The effect of the hydroalcholic A. wilhelmsii extract was tested on the contractile response of Wistar rat aorta induced by potassium chloride (KCl) and phenylephrine (PE) using a pressure transducer that is connected to the PowerLab.
Results:
The cumulative concentrations of A. wilhelmsii (0.5-8 mg/ml) induced a vasorelaxation both in endothelium-intact and endothelium-denuded aortas precontracted by high K+ (6 × 10−2 M) or 10−6 M PE. A. wilhelmsii, at a concentration of 4 mg/ml, reduced Ca2+-induced contraction (P < 0.001 vs. control) after PE or KCl had generated a stable contraction in the Ca2+-free solution. Furthermore, after incubation with diltiazem, the vasorelaxant effect of A. wilhelmsii reduced in the endothelium-denuded aortas precontracted by PE or KCl (P < 0.001 vs. control). In contrast, A. wilhelmsii-induced relaxation was not affected by glibenclamide, BaCl2, ruthenium red, methylene blue, or heparin.
Conclusions:
The results showed that A. wilhelmsii had a vasorelaxation effect, which was not endothelium-dependent. The relaxation was mediated by inhibition of extracellular Ca2+ influx through voltage- and receptor-operated Ca2+ channels (VDDCs and ROCCs) in vascular smooth muscle cells.
Keywords: Achillea wilhelmsii, Ca2+ channels, rat aorta, vasorelaxation
INTRODUCTION
High blood pressure is the leading risk factor for mortality around the world, and lowering blood pressure greatly reduces the main risk of developing arterial coronary disease, heart failure, cerebral vascular disease, and renal damage.[1] The interest of the general public in the use of dietary herbs has risen exponentially, due to their presumed low toxicity and good therapeutic performance.
Achillea, is one of the most important genera of the Compositae family and comprises of more than 100 species. The pharmacological effects of the Achillea genus, such as, anti-inflammatory,[2,3] antibacterial,[4,5] antitumor,[6] antispasmodic,[7,8] choleretic,[9] antiulcer,[10] reducing gastric acidity and motility,[11,12] and hepatoprotective,[8] have been reported.
In recent times, evidence of the cardiovascular and vasorelaxant effects of Achillea have been accumulated by several in vivo and in vitro studies.[13,14,15,16,17] Achillea wilhelmsii (A. wilhelmsii) is the major species of the Achillea genus. It is grown in Iran (domestic name: Boomadaran) and widely used in Iranian traditional medicine to treat symptoms associated with gastrointestinal and cardiovascular disorders. Our previous studies have shown the hypotensive and cardiac-depressant effects of A. wilhelmsii.[18,19] A. wilhelmsii contains components including carvacrol, luteolin, apigenin, and 1,8-cineole,[20,21,22] which can influence the vascular smooth muscle tone. In many studies the vasorelaxant effects of carvacrol,[23] luteolin,[24] apigenin,[25] and 1,8-cineole[26] have been demonstrated. However, the mechanism/s of the vasorelaxant effect of A. wilhelmsii has not been clarified. Therefore, the present study has been carried out to examine the effects of the hydroalcholic extract of A. wilhelmsii on the vasomotor tone of the aortic rings, and its possible mechanism of action.
MATERIALS AND METHODS
Chemicals and drugs
All chemicals were of analytical grade (Merck). Phenylephrine hydrochloride (PE Hcl), acetylcholine (ACh), methylene blue, ruthenium red (RR), heparin (HP), and diltiazem were obtained from Sigma (Germany).
Plant material and preparation of the extract
The aerial part of A. wilhelmsii was collected in spring from the Khorasan Province, Neyshabour, Iran, and identified by the Ferdowsi University Herbarium (voucher No. 164-2218-2) and then dried at room temperature. Four hundred grams of the aerial parts of the plant were soaked in ethanol (50%) for 48 hours and filter paper was used to filter the solute after mixing. The solution was then dried using a 40°C oven for 72 hours. The dried extract was dissolved in distilled water to make 0.5, 1, 2, 4, and 8 mg/ml concentrations.
Animals
The experiment was conducted using 91 male Wistar rats (weighing 200-250 g). The animals were kept in a 22 ± 2°C temperature with a 12 hour light/dark cycle and fed with a standard diet and drinking tap water. All experiments were conducted in accordance with the internationally accepted principles for laboratory animal use and care and, with institutional guidelines.
Preparation of rat aortas
After anesthesia with Ketamin (50 mg/kg), the animals were decapitated by guillotine. The chest was opened and the descending thoracic aorta was rapidly dissected out and immersed in chilled Krebs solution (composition (mM): NaCl 118.5, KCl 4.74, MgSO 4 1.18, NaHCO3 24.9, CaCl 2 2.5, and glucose 10. pH = 7.4) and gassed with carbogen (95% O2, 5% CO2). After the perivascular tissue was carefully removed, aortic rings approximately 5 mm in length were cut. The aortic rings were suspended in organ chambers containing 10 ml Krebs’ solution at 37° C, pH = 7.4, and aerated with 95% O2 + 5% CO2. After a resting tension of 2 g, the vessel segments were allowed to equilibrate for one hour. Changes in tension were recorded by isometric transducers connected to a data acquisition system (AD instrument, Australia). When required, the endothelium was removed by gently rubbing the intimal space with a thin metal rod. The absence of a functional endothelium was verified by the inability of ACh (10−5 M) to induce the relaxation of rings precontracted with PE (10−6 M).
Experimental procedure
Aortic contraction induced by Phenylephrine and Potassium Chloride
In this series of experiments, 10−6 M PE or 6 × 10−2 M KCl were used to induce a steady contraction in rings with the endothelium intact or denuded, and A. wilhelmsii was added cumulatively (0.5, 1, 2, 4, and 8 mg/ml). The A. wilhelmsii extract induced relaxation in the aortic rings, which was calculated as a percentage of the relaxation in response to PE and KCl.
A. wilhelmsii extract induced relaxation, the roles of influx of Ca2+ and Ca2+ channels
In the first set of these experiments, an attempt was made to verify that the relaxation induced by A. wilhelmsii involved Ca2+ influx. The endothelium-denuded aortic rings were washed four to five times with Ca2+-free Krebs’ solution (containing 5 × 10−5 M EGTA) before PE (10−6 M) or KCl (6 × 10−2 M) was applied, to produce a steady contraction, and then Ca2+ was added cumulatively to obtain a concentration-response curve (10−5 to 10−2 M). In the second set of experiments, the aim was to evaluate the roles of voltage-dependent calcium channels in extract-induced relaxation. Endothelium-denuded aortic rings were exposed to diltiazem (10−5 M), an L-type Ca2+ channel inhibitor, for 30 minutes, before the application of PE (10−6 M) or KCl (6 × 10−2 M), to induce a steady contraction; subsequently the A. wilhelmsii extract (4 mg/ml) was added to evoke a relaxation.
A. wilhelmsii extract induced relaxation and intracellular sources of Ca2+
In this set of experiments, the aim was to clarify whether the relaxation induced by A. wilhelmsii was related to the inhibition of intracellular Ca2+ release. Endothelium-denuded aortic rings were exposed to diltiazem (10−5 M) and Ruthenium red (10−5 M), a ryanodine receptor inhibitor (RR)[27] or heparin (50 mg/l). An IP3 receptor inhibitor (HP)[28] was added 30 minutes before the application of PE (10−6 M) to induce a steady contraction; subsequently the A. wilhelmsii extract (4 mg/ml) was added to evoke a relaxation in a separate experimental group.
A. wilhelmsii extract induced relaxation and guanylate cyclase
To examine the role of guanylate cyclase in the extract-induced relaxation, the aortic rings were rinsed and exposed to 10-5 M methylene blue, an inhibitor of the cGMP-mediated pathway, for 30 minutes before the application of 10−6 M PE to induce a steady contraction, and finally the effects of the cumulative concentrations of the extract (0.5, 1, 2, 4 and 8 mg/ml) were evaluated for 25 minutes.
A. wilhelmsii extract induced relaxation and K+ channels
To examine the role of K+ channels in the extract induced relaxation, the aortic rings were rinsed and exposed to glibenclamide (10-5 M), an inhibitor of the ATP-dependent K+ channels (KATP) and barium chloride (4 × 10−3 M), an inhibitor of the inward rectifier K+ channels (KIR), for 30 minutes before the application of 10−6 M PE, to induce a steady contraction, and finally the effects of the cumulative concentrations of the extract (0.5, 1, 2, 4 and 8 mg/ml) were evaluated for 25 minutes.
Statistical analysis
All data are expressed as mean ± S.E.M. Student's t-test was used to compare the data. Curves were compared using one-way ANOVA followed by the Tukey's test. P-values less than 0.05 were considered to be statistically significant.
RESULTS
Effect of A. wilhelmsii on phenylephrine and potassium chloride contracted aorta
Figure 1 shows the effect of the cumulative concentrations of the A. wilhelmsii extract (0.5, 1, 2, 4 and 8 mg/ml) on aortic smooth muscle contracted by KCl (6 × 10−2 M) and PE (10-6 M) in the intact and denuded endothelium.
Figure 1.
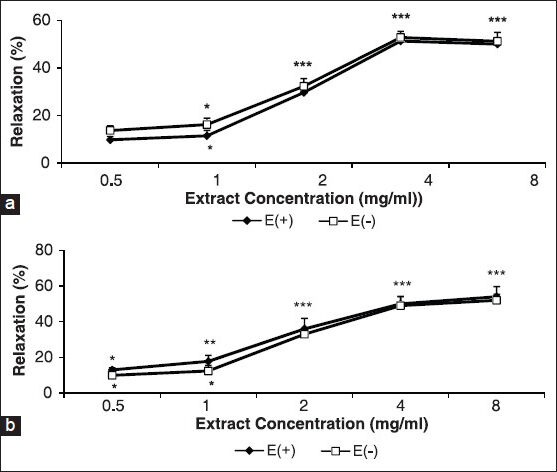
Effect of cumulative concentrations of A. wilhelmsii extract (0.5, 1, 2, 4, and 8 mg/ml) on phenylephrine (PE) (10−6 M) (a) and KCl (6 × 10−2 M) (b) precontracted rat aortic rings with (E+) or without (E-) endothelium. Data are expressed as mean ± S.E.M., using the unpaired t-test (n = 7); *P < 0.05, **P < 0.01, ***P < 0.001, compared to the baseline
Extract-induced vasorelaxation incidents at 0.5, 1, 2, 4, and 8 mg/ml in intact and denuded rings contracted with KCl were 13 ± 1.2%, 17.8 ± 3.4%, 36 ± 5.9%, 50 ± 4.2%, and 54 ± 4.3%, and 10 ± 2.4%, 12.4 ± 3.1%, 33 ± 3%, 49 ± 5%, and 52 ± 3.4%, respectively [Figure 1a] compared to PE, where they were, 9.8 ± 1.4%, 11.5 ± 2.3%, 29.7 ± 3.1%, 51.3 ± 2.6, and 50 ± 2.4% and 13.7 ± 2.01, 16.2 ± 2.7, 32.3 ± 3.2%, 52.8 ± 2.6%, and 51.2 ± 3.7%, respectively [Figure 1b].
Effect of A. wilhelmsii on extracellular Ca2+-induced contraction and Ca2+ channels
The cumulative addition of Ca2+ in a Ca2+-free medium containing PE or KCl induced a concentration-dependent contraction of aortic rings. Pre-incubation of the rings with 4 mg/ml of A. wilhelmsii significantly inhibited the Ca2+-induced contraction in both KCl [Figure 2a] and PE [Figure 2b] constricted rings. In the endothelium-denuded rings pretreated for 30 minutes with diltiazem (10−5 M) and subsequently contracted by PE or KCl, the relaxant effect of A. wilhelmsii (4 mg/ml) was significantly reduced (P < 0.001) [Figure 3].
Figure 2.
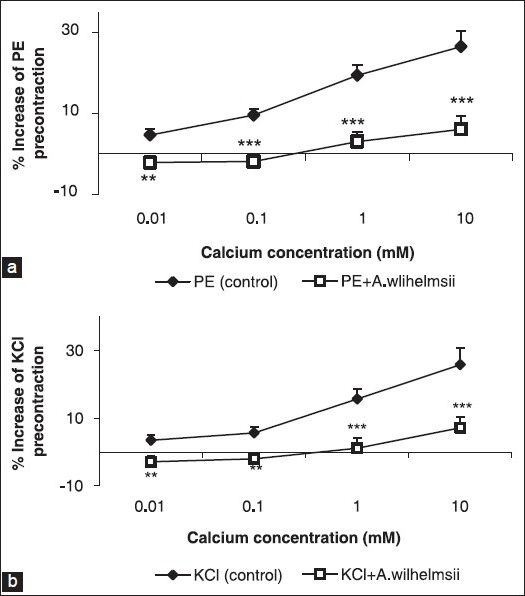
Effect of A. wilhelmsii extract at 4 mg/ml on the Ca2+-induced (10−5 to 10−3 M) contraction of rat aortic rings without endothelium, pretreated with phenylephrine (PE) (10−6 M) (a) and KCl (6 × 10−2 M) (b). Data are expressed as mean ± S.E.M., using unpaired t-test (n = 7); **P < 0.01, ***P < 0.001 compared to the control
Figure 3.
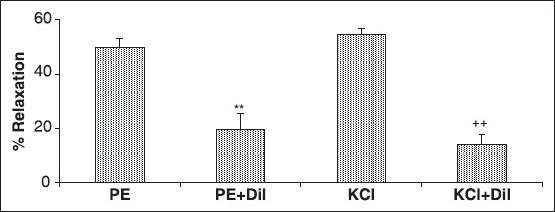
Effects of A. wilhelmsii extract, at 4 mg/ml, on the endothelium-denuded rat aortic rings contracted with phenylephrine (PE) (10−6 M) or KCl (6 × 10−2 M), after diltiazem (10−5 M) pretreatment. Data are expressed as mean ± S.E.M., using one way ANOVA (n = 7); ***P < 0.001 compared to PE, +++P < 0.001 compared to KCl
Effect of A. wilhelmsii on intracellular sources of Ca2+
The results of 30 minutes of pre-incubation of the endothelium-denuded aortic rings with diltiazem and heparin or RR, with subsequent contraction by PE showed that the relaxant effect of A. wilhelmsii (4 mg/ml) was the same as diltiazem [Figure 4].
Figure 4.
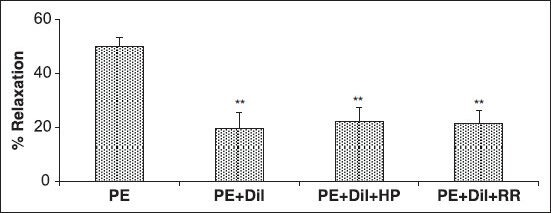
Effects of A. wilhelmsii extract at 4 mg/ml on endothelium-denuded rat aortic rings contracted with phenylephrine (PE) (10−6 M), after diltiazem (10−5 M), heparin (50 mg/l), and Ruthenium red (10−5 M) pretreatment. Data are expressed as mean ± S.E.M., using one way ANOVA (n = 7). +Dil: Denuded endothelium pretreated with diltiazem, +Dil + HP: denuded endothelium pretreated with diltiazem and heparin, +Dil+RR: denuded endothelium pretreated with diltiazem and Ruthenium red. ***P < 0.001 compared to PE
Effect of A. wilhelmsii on guanylate cyclase
The relaxant effect of the cumulative concentrations of A. wilhelmsii showed no difference when compared with A. wilhelmsii alone after 30 minutes of pre-incubation of the aortic rings with methylene blue, with subsequent contraction by PE [Figure 5a].
Figure 5.
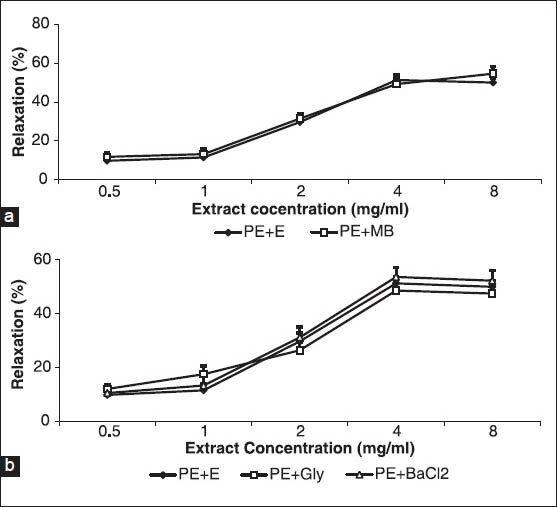
Effect of cumulative concentrations of A. wilhelmsii extract (0.5, 1, 2, 4 and 8 mg/ml) on rat aortic rings contracted with phenylephrine (PE) (10−6 M) after being pretreated with a guanylate cyclase inhibitor methylene blue (10-5 M)(a) and K+ channel inhibitor, glibenclamide (10−5 M) and barium chloride (4 × 10−3 M)(b). Data are expressed as means ± S.E.M., using unpaired t-test (a) and one way ANOVA (b) (n = 7). +En: With endothelium, MB: Methylene blue, Gly: glibenclamide, BaCl2: Barium chloride
Effect of A. wilhelmsii on K+ channels
After 30 minutes of pre-incubation of the aortic rings with glibenclamide or barium chloride and with a subsequent contraction by PE, the relaxant effect of the cumulative concentrations of A. wilhelmsii showed no difference when compared with A. wilhelmsii alone [Figure 5b].
DISCUSSION
In these experiments we found that A. wilhelmsii evoked relaxation in aortic rings precontracted by KCl and PE, regardless of the presence or absence of the endothelium. This indicated that the action of A. wilhelmsii to induce relaxation was directly on the vascular smooth muscle cells (VSMCs) and not on the endothelium-derived vasodilator factors, such as, NO and prostacyclin.
Ca2+ is a critical factor in the excitation-contraction coupling in smooth muscle cells.[29,30] Influx of extracellular Ca2+ through receptor-operated Ca2+ channels (ROCCs), voltage-dependent Ca2+ channels (VDCCs), and release of Ca2+ from the sarcoplasmic reticulum by activation of 1,4,5 triphosphate inositol (IP3) and ryanodine receptors (RYR),[31,32,33] result in increased intracellular Ca2+, which causes contraction.
Our results showed that the relaxing activity of A. wilhelmsii is evident after both PE- and KCl-induced contraction. PE is an alpha adrenergic agonist that induces VSMC contractions by a Ca2+ influx through the ROCCs and by the release of intracellular Ca2+ from the sarcoplasmic reticulum after activation of IP3 receptors (IP3R).[31,33,34] By contrast, the contraction elicited by KCl mainly results from the influx of extracellular Ca2+ induced by depolarization of the cell membrane and subsequent opening of the VDCCs.[32]
To determine whether A. wilhelmsii modified the extracellular Ca2+ influx, experiments were conducted on rings contracted with PE or KCl in a Ca2+-free Krebs solution, in which Ca2+ was added subsequently. Our data reporting that A. wilhelmsii decreased Ca2+-induced contractions after both PE- and KCl-induced contractions argue that the blockade of both ROCCs and VDCCs are a part of the vasodilating effects of A. wilhelmsii. These results were verified by PE- and KCl-induced contractions in the presence of diltiazem, in which the vasorelaxant effect of A. wilhelmsii decreased significantly.
The results of this study are consistent with the previous findings in the gastrointestinal or vascular smooth muscles. Previous studies on the effect of various species of Achillea genus on gastrointestinal smooth muscle have revealed its antispasmodic effects.[7,35] Yaeesh and colleagues showed that A. millefolium inhibits calcium channels and its antispasmodic effect contributes to the antagonistic roles on VDCCs.[8] The vasorelaxant effect of A. millefolium, which is mainly mediated by calcium channel inhibition was also shown.[16] Furthermore, we previously demonstrated the cardiac depressant and hypotensive effects of A. wilhelmsii[19] and the negative inotropic and chronotropic effect of A. millefolium in isolated heart.[17]
To investigate whether A. wilhelmsii could exert its vasorelaxant effects by interfering with the calcium release from the intracellular source the experiments were conducted on rings precontracted with PE. It is well known that the PE-induced release of intracellular calcium is attributable to the receptor-mediated formation of IP3.[36] Heparin and RR did not affect the vasorelaxant effect of A. wilhelmsii. These results indicate the IP3 signaling pathway and ryanodine receptors have any role in the vasorelaxant effect of A. wilhelmsii.
It is well established that cGMP provides the signal that elicits vascular relaxation via cGMP-dependent protein kinase signaling. Activation of the soluble guanylate cyclase (sGC) in the VSMCs results in an increase in intracellular cGMP levels, which elicits cGMP-dependent protein kinase (PKG) signaling. It has been reported that PKG inhibits Ca2+ influx, augments Ca2+ sequestration, and decreases the sensitivity of contractile elements to Ca2+.[37] Our results showed that Methylene blue did not reduce the relaxation induced by A. wilhelmsii, therefore, it suggests that the vascular relaxation evoked by A. wilhelmsii was not mediated by cGMP signaling. It is in favor of the endothelium-independent vasorelaxant effect of A. wilhelmsii.
Besides Ca2+ channels, K+ channels contribute to the regulation of the membrane potential in electrically excitable cells, including VSMCs.[38] Membrane hyperpolarization due to an efflux of K+ results from the opening of the K+ channels in the VSMCs. This effect is followed by the closure of voltage-dependent Ca2+ channels, leading to the reduction in Ca2+ entry and vasodilation.[32] VSMCs express both KATP and KIR.[39,40] In the present study, the blockade of the KATP or KIR channel with glibenclamide or BaCl2, respectively, did not affect the relaxing properties of A. wilhelmsii, which indicated that these K+ channels may not be involved in A. wilhelmsii-induced vasorelaxation.
Moreover, A. wilhelmsii contains important ingredients, such as, carvacrol, luteolin, apigenin, and 1,8 cineole, which can influence the vascular smooth muscle tone. In many studies the antispasmodic and vasorelaxant effects of carvacrol,[23,41,42] luteolin,[24,43] apigenin,[25] and 1, 8 cineole[26,44] have been demonstrated. Luteolin has a vasorelaxant effect by the inhibition of sarcolemmal Ca2+ channels, release from the intracellular Ca2+ stores, and activation of K+ channels.[23]
Taken together, this study demonstrates that A. wilhelmsii exhibits vasodilating activity. The relaxant effect does not depend on the presence of the endothelium. The extract acts directly on VSMCs and relaxation is mainly related to the inhibition of extracellular Ca2+ influx through ROCCs and VDCCs.
ACKNOWLEDGMENT
The authors would like to thank the Research Affairs of the Mashhad University of Medical Sciences for their financial support.
Footnotes
Source of Support: Research Affairs of Mashhad University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 2.Benedek B, Kopp B, Melzig MF. Achillea millefolium L.s.l - is the anti-inflammatory activity mediated by protease inhibition. J Ethnopharmacol. 2007;113:312–7. doi: 10.1016/j.jep.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Elmann A, Mordechay S, Erlank H, Telerman A, Rindner M, Ofir R. Anti-neuroinflammatory effects of the extract of Achillea fragrantissima. BMC Complement Altern Med. 2011;11:98. doi: 10.1186/1472-6882-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecchini C, Silvi S, Cresci A, Piciotti A, Caprioli G, Papa F, et al. Antimicrobial efficacy of Achillea ligustica All.(Asteraceae) essential oils against reference and isolated oral microorganisms. Chem Biodivers. 2012;9:12–24. doi: 10.1002/cbdv.201100249. [DOI] [PubMed] [Google Scholar]
- 5.Stojanovic G, Radulovic N, Hashimoto T, Palic R. In vitro antimicrobial activity of extracts of four Achillea species: The composition of Achillea clavennae L. (Asteraceae) extract. J Ethnopharmacol. 2005;101:185–90. doi: 10.1016/j.jep.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Csupor-Loffler B, Hajdu Z, Zupko I, Rethy B, Falkay G, Forgo P, et al. Antiproliferative effect of flavonoids and sesquiterpenoids from Achillea millefolium s.l. on cultured human tumour cell lines. Phytother Res. 2009;23:672–6. doi: 10.1002/ptr.2697. [DOI] [PubMed] [Google Scholar]
- 7.Karamenderes C, Apaydin S. Antispasmodic effect of Achillea nobilis L.subsp. sipylea (O. Schwarz) Bassler on the rat isolated duodenum. J Ethnopharmacol. 2003;84:175–9. doi: 10.1016/s0378-8741(02)00296-9. [DOI] [PubMed] [Google Scholar]
- 8.Yaeesh S, Jamal Q, Khan AU, Gilani AH. Studies on hepatoprotective, antispasmodic and calcium antagonist activities of the aqueous-methanol extract of Achillea millefolium. Phytother Res. 2006;20:546–51. doi: 10.1002/ptr.1897. [DOI] [PubMed] [Google Scholar]
- 9.Benedek B, Geisz N, Jager W, Thalhammer T, Kopp B. Choleretic effects of yarrow (Achillea millefolium s.l.) in the isolated perfused rat liver. Phytomedicine. 2006;13:702–6. doi: 10.1016/j.phymed.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Potrich FB, Allemand A, da Silva LM, Dos Santos AC, Baggio CH, Freitas CS, et al. Antiulcerogenic activity of hydroalcoholic extract of Achillea millefolium L.: Involvement of the antioxidant system. J Ethnopharmacol. 2010;130:85–92. doi: 10.1016/j.jep.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Niazmand S, Khooshnood E, Derakhshan M. Effects of Achillea wilhelmsii on rat's gastric acid output at basal, vagotomized, and vagal-stimulated conditions. Pharmacogn Mag. 2010;6:282–5. doi: 10.4103/0973-1296.71791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niazmand S, Khoshnood E. The effects of Achillea wilhelmsii extract on eat's gastric motility at basal and vagal stimulated conditions. Iran J Basic Med Sci. 2011;14:151–7. [Google Scholar]
- 13.Asgary S, Naderi GH, Sarrafzadegan N, Mohammadifard N, Mostafavi S, Vakili R. Antihypertensive and antihyperlipidemic effects of Achillea wilhelmsii. Drugs Exp Clin Res. 2000;26:89–93. [PubMed] [Google Scholar]
- 14.Dall’Acqua S, Bolego C, Cignarella A, Gaion RM, Innocenti G. Vasoprotective activity of standardized Achillea millefolium extract. Phytomedicine. 2011;18:1031–6. doi: 10.1016/j.phymed.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 15.de Souza P, Gasparotto A, Jr, Crestani S, Stefanello ME, Marques MC, da Silva-Santos JE, et al. Hypotensive mechanism of the extracts and artemetin isolated from Achillea millefolium L. (Asteraceae) in rats. Phytomedicine. 2011;18:819–25. doi: 10.1016/j.phymed.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Khan AU, Gilani AH. Blood pressure lowering, cardiovascular inhibitory and bronchodilatory actions of Achillea millefolium. Phytother Res. 2011;25:577–83. doi: 10.1002/ptr.3303. [DOI] [PubMed] [Google Scholar]
- 17.Niazmand S, Saberi Z. The chronotropic and inotropic effects of aqueous-ethanolic extract of Achillea millefolium on rat's isolated heart. Pharmacologyonline. 2010;3:791–8. [Google Scholar]
- 18.Niazmand S, Esparham M, Rezaee SA, Harandizadeh F. Hypotensive effect of Achillea wilhelmsii aqueous-ethanolic extract in rabbit. Avicenna J Phytomed. 2011;1:51–6. [Google Scholar]
- 19.Niazmand S, Esparham M. Cardiovascular effects of aqueous-ethanolic extract of Achillea Wilhelmsii in rabbit. Pharmacologyonline. 2011;1:818–25. [Google Scholar]
- 20.Afsharypuor S, Asgary S, Lockwood GB. Constituents of the essential oil of Achillea wilhelmsii from Iran. Planta Med. 1996;62:77–8. doi: 10.1055/s-2006-957810. [DOI] [PubMed] [Google Scholar]
- 21.Dokhani S, Cottrell T, Khajeddin J, Mazza G. Analysis of aroma and phenolic components of selected Achillea species. Plant Foods Hum Nutr. 2005;60:55–62. doi: 10.1007/s11130-005-5100-9. [DOI] [PubMed] [Google Scholar]
- 22.Javidian K, Miri R, Sadeghpour H. Compsition of the volatile oil of Achillea wilhelmsii C. Koch from Iran. Daru. 2004;12:63–6. [Google Scholar]
- 23.Peixoto-Neves D, Silva-Alves KS, Gomes MD, Lima FC, Lahlou S, Magalhaes PJ, et al. Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fundam Clin Pharmacol. 2010;24:341–50. doi: 10.1111/j.1472-8206.2009.00768.x. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Xia Q, Wang X, Song J, Bruce IC. Luteolin induces vasorelaxion in rat thoracic aorta via calcium and potassium channels. Pharmazie. 2005;60:444–7. [PubMed] [Google Scholar]
- 25.Jin BH, Qian LB, Chen S, Li J, Wang HP, Bruce IC, et al. Apigenin protects endothelium-dependent relaxation of rat aorta against oxidative stress. Eur J Pharmacol. 2009;616:200–5. doi: 10.1016/j.ejphar.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Lahlou S, Figueiredo AF, Magalhaes PJ, Leal-Cardoso JH. Cardiovascular effects of 1,8-cineole, a terpenoid oxide present in many plant essential oils, in normotensive rats. Can J Physiol Pharmacol. 2002;80:1125–31. doi: 10.1139/y02-142. [DOI] [PubMed] [Google Scholar]
- 27.Maggi CA, Patacchini R, Perretti F, Tramontana M, Manzini S, Geppetti P, et al. Sensory nerves, vascular endothelium and neurogenic relaxation of the guinea-pig isolated pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:78–84. doi: 10.1007/BF00178976. [DOI] [PubMed] [Google Scholar]
- 28.Mohanty MJ, Li X. Stretch-induced Ca(2+) release via an IP(3)-insensitive Ca(2+) channel. Am J Physiol Cell Physiol. 2002;283:C456–62. doi: 10.1152/ajpcell.00057.2002. [DOI] [PubMed] [Google Scholar]
- 29.Lohn M, Furstenau M, Sagach V, Elger M, Schulze W, Luft FC, et al. Ignition of calcium sparks in arterial and cardiac muscle through caveolae. Circ Res. 2000;87:1034–9. doi: 10.1161/01.res.87.11.1034. [DOI] [PubMed] [Google Scholar]
- 30.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: Sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium. 2003;34:211–29. doi: 10.1016/s0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 31.Imtiaz MS, Katnik CP, Smith DW, van Helden DF. Role of voltage-dependent modulation of store Ca2+ release in synchronization of Ca2+ oscillations. Biophys J. 2006;90:1–23. doi: 10.1529/biophysj.104.058743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 33.Thorneloe KS, Nelson MT. Ion channels in smooth muscle: Regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005;83:215–42. doi: 10.1139/y05-016. [DOI] [PubMed] [Google Scholar]
- 34.McCarron JG, Bradley KN, MacMillan D, Muir TC. Sarcolemma agonist-induced interactions between InsP3 and ryanodine receptors in Ca2+ oscillations and waves in smooth muscle. Biochem Soc Trans. 2003;31:920–4. doi: 10.1042/bst0310920. [DOI] [PubMed] [Google Scholar]
- 35.Lemmens-Gruber R, Marchart E, Rawnduzi P, Engel N, Benedek B, Kopp B. Investigation of the spasmolytic activity of the flavonoid fraction of Achillea millefolium s.l. on isolated guinea-pig ilea. Arzneimittelforschung. 2006;56:582–8. doi: 10.1055/s-0031-1296755. [DOI] [PubMed] [Google Scholar]
- 36.Eckert RE, Karsten AJ, Utz J, Ziegler M. Regulation of renal artery smooth muscle tone by alpha1-adrenoceptors: Role of voltage-gated calcium channels and intracellular calcium stores. Urol Res. 2000;28:122–7. doi: 10.1007/s002400050149. [DOI] [PubMed] [Google Scholar]
- 37.Gollasch M, Nelson MT. Voltage-dependent Ca2+ channels in arterial smooth muscle cells. Kidney Blood Press Res. 1997;20:355–71. doi: 10.1159/000174250. [DOI] [PubMed] [Google Scholar]
- 38.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44:65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- 39.Cortes SF, Rezende BA, Corriu C, Medeiros IA, Teixeira MM, Lopes MJ, et al. Pharmacological evidence for the activation of potassium channels as the mechanism involved in the hypotensive and vasorelaxant effect of dioclein in rat small resistance arteries. Br J Pharmacol. 2001;133:849–58. doi: 10.1038/sj.bjp.0704147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35:173–8. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baser KH. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr Pharm Des. 2008;14:3106–19. doi: 10.2174/138161208786404227. [DOI] [PubMed] [Google Scholar]
- 42.Boskabady MH, Jandaghi P. Relaxant effects of carvacrol on guinea pig tracheal chains and its possible mechanisms. Pharmazie. 2003;58:661–3. [PubMed] [Google Scholar]
- 43.Qian LB, Wang HP, Chen Y, Chen FX, Ma YY, Bruce IC, et al. Luteolin reduces high glucose-mediated impairment of endothelium-dependent relaxation in rat aorta by reducing oxidative stress. Pharmacol Res. 2010;61:281–7. doi: 10.1016/j.phrs.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Nascimento NR, Refosco RM, Vasconcelos EC, Kerntopf MR, Santos CF, Batista FJ, et al. 1,8-Cineole induces relaxation in rat and guinea-pig airway smooth muscle. J Pharm Pharmacol. 2009;61:361–6. doi: 10.1211/jpp/61.03.0011. [DOI] [PubMed] [Google Scholar]


