Abstract
Background:
Refractory epilepsy is a significant problem in clinical practice. Sometimes, multiple antiepileptic drugs are required to control the attacks. To avoid various complications ensuring from these drugs, new methods of treatment such as vagus nerve stimulation (VNS) have been recommended. Trigeminal nerve stimulation (TNS) is a new method under evaluation. The purpose of this paper is to determine whether this method is effective or not.
Materials and Methods:
Percutaneous simulation of supraorbital branches of the trigeminal nerve by an electrical device was planned in 18 patients over a six-month period. Participants who fulfilled the research criteria were selected randomly from epileptic patients referred to the clinic. (November 2011-December 2012). T-test was used for data analysis.
Results:
Only eight of 18 patients stayed in the study during all 6 months. A 47.9% reduction in daily seizure frequency was seen in this group (P = 0.022). Other subjects left the study earlier. In this group, seizure frequency increased by 10.6% (P = 0.82).
Conclusions:
The mechanism of the antiepileptic effects of TNS is not yet clear. In animal studies, it is suggested that the trigeminal nucleus and its projection to nucleus tractus solitarius (NTS) and the locus ceruleus, are involved in seizure modulation. Although in comparison with seizure frequency prior to the study there was significant seizure reduction, according to the usual criteria for VNS i.e. 50% seizure frequency reduction, the effect of TNS per se may not yet be adequate for treatment of seizures. Trigeminal nerve stimulation may be an effective “adjuvant” method for treatment of intractable seizure.
Keywords: Refractory epilepsy, TNS (trigeminal nerve stimulation), trigeminal nerve
INTRODUCTION
Epilepsy is a common neurological disorder and the median lifetime prevalence in developed countries has been estimated to be 5.8 per 1000 (range 2.7-12.4). The majority of seizure attacks can be controlled by using one or more antiepileptic drugs (AEDs). However, in 30% of the patients, seizure attacks are not completely controlled with medical therapy only.[1,2,3,9] It seems that these patients have drug-resistant epilepsy. Due to the serious consequences of seizure, such as shortened life span, body injury, mental impairments and social disabilities, full control of epilepsy is of great importance. Therefore, refractory seizures should be controlled by another treatment such as vagus nerve stimulation (VNS) or epileptic surgery, if the patient is willing. VNS and surgery have many complications and are very expensive methods. In VNS[4] and in surgery there is the possibility of consequences such as hoarseness, coughing, throat pain and serious issues such as hemiparesis, memory loss, and language dysfunction respectively. Thus, these methods are limited to a small group of patients only. The newer method - trigeminal nerve stimulation - creates a simple, inexpensive and less complicated treatment for refractory seizures. Now, we present the result of a pilot study of TNS in 18 patients with partial or generalized seizure who were resistant to pharmacotherapy.
MATERIALS AND METHODS
After obtaining approval from the Ethics Committee of Isfahan University of Medicine (Isfahan-Iran, November 2011), we started our open pilot study of TNS on 18 subjects with the inclusion criteria stated below: Age between 18-55 years; three or more partial or generalized seizures per month; use of at least two antiepileptic drugs in sufficient dosages; no serious or progressive medical disorder and no obvious history of cardiac arrhythmia. Subjects also included the patients with structural disorders such as cortical dysplasia, brain tumor and vascular lesions who did not volunteer for surgery or VNS. Participants, who fulfilled our research criteria, were selected randomly from epileptic patients referred to the clinic of neurology (November 2011-December 2012). The purposes and style of the study were clarified for all the patients and their consent was obtained. In this study, we used the “APEX” set for neurostimulation (made in China), which was adjustable for frequency, amplitude, duration and ramp of electrical pulse waves. Stimulation was supplied using the stimulator at 120 Hz, 250 μsec, 5 seconds On and 5 seconds Off. This setting was that used by C.M. DeGiorgio et al, in a pilot study of TNS on seven patients.[5] They used the stimulator at 30 seconds On and 30 seconds Off. However, we preferred the periods of 5 seconds On and 5 seconds Off. Because the duration of the ictal phase in some seizure attacks is less than 30 seconds, we hypothesized that shorter On and Off periods of stimulation are better to control such short lasting episodes of seizure. Power was supplied by 9 volts lithium chargeable batteries. Self-adhesive stimulating electrodes were used for stimulation of the frontal branches of the trigeminal nerve and subjects replaced electrodes weekly. These electrodes were placed on the forehead, just above the eyebrows 2.5 cm from each other. In this site the first branch of the ophthalmic division of the trigeminal nerve is stimulated. Stimulating electrodes could be covered by a cap or hat. Electrodes were connected to the stimulator by a thin soft wire which the patients could hide under clothing. All subjects kept a diary of all seizures during a 4 weeks baseline pre-treatment period. The serum concentration of the antiepileptic drugs was measured and necessary changes in the drug dose were made before beginning the study. Seizure counts, the date and character of each patient were recorded for further comparison. The average daily seizure frequency for all types of seizure was calculated at the end of the 6th month and compared with previously recorded data. In the baseline and the six-month treatment period, the patients received their AEDs as before and any changes in AEDs resulted in elimination from the study. The serum concentration of Phenobarbital in one patient was less than the therapeutic level; so its dosage was increased before the start of the study.
RESULTS
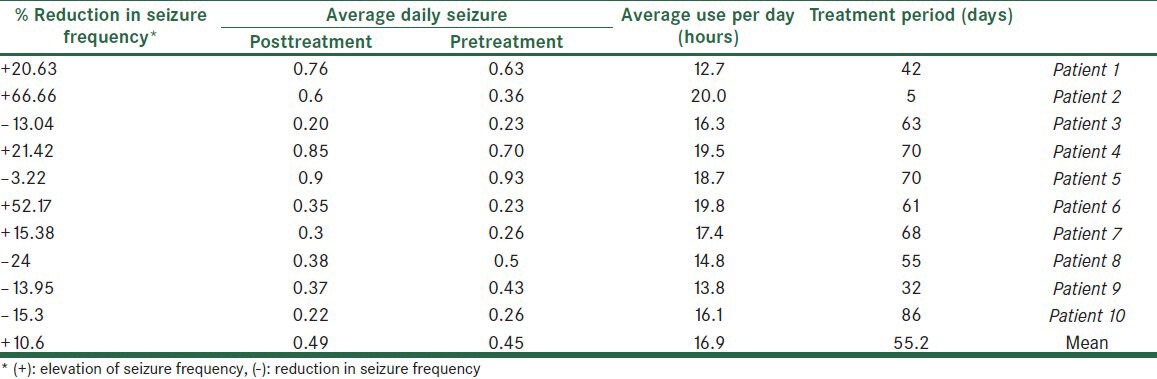
Eighteen subjects completed the study criteria (8 male and 10 female). Eight of the patients had complex partial seizure (CPS), one simple partial and three generalized tonic colonic (GTC) epilepsy. Six subjects had GTC + CPS. Only eight patients (44.4%) completed the six-month period. Other patients used the neurostimulator for 42-86 days. Interference of the external device with work (in two patients), difficulty in the constant use of the device (in four patients), fear of attracting the attention of others or ridicule (in four patients) and doubting the efficacy of the study (in two patients), were the most important reasons for leaving the study. The intensity of stimulation was adjustable between 1-8 scales. Many patients did not tolerate intensity scales greater than two. Among the participants, the sensation of pressure on the head (27.7%), pain (22.2%), skin reaction (16.6%) and tingling (16.6%) were the most common adverse effects. Close observation of the subjects in the first six hours of stimulation did not demonstrate the changes in the blood pressure or the cardiac rhythm; however, when using the device at home, some patients reported a feeling of transient palpitation and light headedness. The device was used to average 16.7 and 16.9 hours per day respectively in Group 1 and Group 2 (P = 0.07). Eight patients who completed the six-month study period, showed a mean 47.9% reduction in their daily seizure frequency at the end of six month [Table 1]. In the patients that left the study before completion of 6 months (Group 2) average daily seizure was 10.6 % more than pretreatment period (P = 0.82, Table 2). Two patients from Group 1 and one patient from Group 2 reported a significant improvement in their mood and quality of life; although this finding must be confirmed by the objective tools. Finally, five patients (27.7%) tended to use the device in the presented form, and another subjects hoped to use the device, if the method is used to facilitate.
Table 1.
Summary of results in eight patients who completed 6 months of follow-up
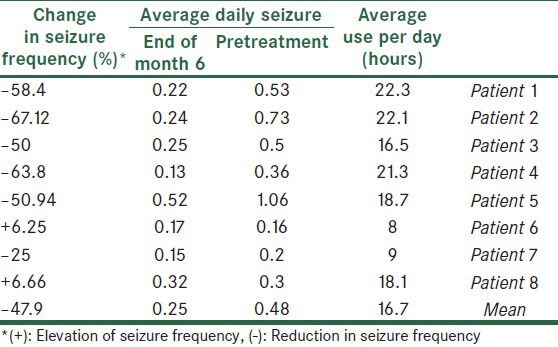
Table 2.
Summary of results in ten patients who left the study before 6 months of follow-up
DISCUSSION
The small sample size in this open pilot study makes data analysis difficult; however, reduction in the daily seizure frequency in the patients who completed the six-month study, demonstrates that the trigeminal nerve stimulation can be an effective method for the treatment of intractable seizure, if properly performed. Comparing Tables 1 and 2 shows that those patients who have used the device for more days, have the most benefit; but whether TNS has long-term or accumulative effects in addition to its immediate effects on seizure control is not clear and larger studies is required. Elevated frequency of seizures in Group 2 may be incidental; because this elevation had not statistical significance. The mechanism of the antiepileptic effects of TNS is poorly understood. Animal studies demonstrate that the trigeminal nucleus and its projection to nucleus tractus solitarius (NTS) and locus ceruleus, are involved in seizure modulation.[2,5] In one study by Dr. Christopher M. DeGiorgio et al., at UCLA[2] it has been demonstrated that trigeminal nerve stimulation can reduce the seizure activity induced by intraperitoneal injection of pentylenetetrazole. They concluded that cranial nerve stimulation (TNS and VNS) can increase the seizure activity threshold with multiple mechanisms. They suggested that neuronal activation during TNS results in the suppression of simultaneous neuronal firing due to seizure activity[2,5,8] (perhaps because of the non-excitatory period). In addition some authors such as Krahl SE[6] and Readt R,[7] have suggested the role of neurotransmitters such as norepinephrine in the suppression of seizure activity by VNS, but whether noradrenergic effects are also present during TNS is not clear. As previously mentioned, 3 of 8 patients reported a great improvement in their mood symptoms which supports the previously proposed anti-depression effects of TNS,[2] but in this study the anti-depression effects of TNS was not assessed and depression criteria were not determined for any patient; so, mood improvement in these subjects is only a subjective judgment. This pilot study shows that TNS has mild antiepileptic effects. Although there was a significant reduction in seizure frequency after the study, according to the usual criteria for VNS, 50% of seizure frequency reduction, the effect of TNS only may not yet be adequate for treatment of seizure. Trigeminal nerve stimulation may be an effective “adjuvant” method for the treatment of intractable seizure. Many patients may not want to use an external device that looks strange. This issue caused some patients to leave the present study. So, it would be better if implantable electrodes were used. Some advantages of TNS are as follows: it is a noninvasive method, non-expensive and easy to use; moreover no important adverse effects have been reported.
ACKNOWLEDGMENT
We thank Dr. H Akhlaghinia for language editing.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Begley CE, Famulari M, Annegers JF, Lairson DR, Reynolds TF, Coan S, et al. The cost of epilepsy in United States: An estimate from population- Based clinical and survey data. Epilepsia. 2000;41:342–51. doi: 10.1111/j.1528-1157.2000.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 2.DeGiorgio CM, Fanselow EE, Schrader LM, Cook IA. Trigeminal nerve stimulation: Seminal animal and human study for epilepsy and depression. NeurosurgClin N Am. 2011;22:449–56. doi: 10.1016/j.nec.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Najafi MR, Saadatnia M, Saffarifard A, Keyhanian K, Davoudi V. Epidemiology of restless legs syndrome in the Iranian population. Sleep Biol Rhythms. 2011;9:56–9. [Google Scholar]
- 4.Ramsay RE, Uthman BM, Augustinsson LE, Upton AR, Naritoku D, Willis J, et al. Vagus nerve stimulation for treatment of partial seizures: Safety, side effects, and tolerability. First International Vagus Nerve stimulation study group. Epilepsia. 1994;35:627–36. doi: 10.1111/j.1528-1157.1994.tb02483.x. [DOI] [PubMed] [Google Scholar]
- 5.DeGiorgio CM, Shewmon A, Murray D, Whitehurst T. Pilot study of trigeminal nerve stimulation for epilepsy: A proof- of- concept trial. Epilepsia. 2006;47:1213–5. doi: 10.1111/j.1528-1167.2006.00594.x. [DOI] [PubMed] [Google Scholar]
- 6.Krahl SE, Clark KB, Smith DC, Browning RA. locus ceruleus lesions suppress the seizure- attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39:709–14. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 7.Raedt R, Clinckers R, Mollet L, Vonck K, El Tahry R, Wyckhuys T, et al. Increased hyppocampal noradrenalin is a biomarker for efficacy of vagus nerve stimulation in a limbic seizure model. J Neurochem. 2011;117:461–9. doi: 10.1111/j.1471-4159.2011.07214.x. [DOI] [PubMed] [Google Scholar]
- 8.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: A meta-analytic approach. Epilepsia. 2010;51:883–90. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.French JA. Refractory epilepsy: Clinical overview. Epilepsia. 2007;48(Suppl1):3–7. doi: 10.1111/j.1528-1167.2007.00992.x. [DOI] [PubMed] [Google Scholar]


