Abstract
Background
There are conflicting reports on the impact of highly active antiretroviral therapy (HAART) in resolving hematological complications. Whereas some studies have reported improvements in hemoglobin and other hematological parameters resulting in reduction in morbidity and mortality of HIV patients, others have reported no improvement in hematocrit values of HAART-treated HIV patients compared with HAART-naïve patients.
Objective
This current study was designed to assess the impact of HAART in resolving immunological and hematological complications in HIV patients by comparatively analyzing the results (immunological and hematological) of HAART-naive patients and those on HAART in our environment.
Methods
A total of 500 patients participated, consisting of 315 HAART-naive (119 males and 196 females) patients and 185 HAART-experienced (67 males and 118 females) patients. Hemoglobin (Hb), CD4+ T-cell count, total white blood count (WBC), lymphocyte percentage, plateletes, and plasma HIV RNA were determined.
Results
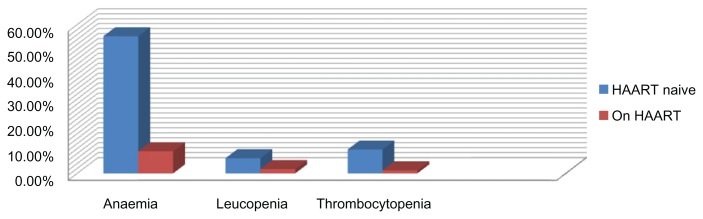
HAART-experienced patients were older than their HAART-naive counterparts. In HAART-naive patients, the incidence of anemia (packed cell volume [PCV] <30%) was 57.5%, leukopenia (WBC < 2.5), 6.1%, and thrombocytopenia < 150, 9.6%; it was, significantly higher compared with their counterparts on HAART (24.3%, 1.7%, and 1.2%, respectively). The use of HAART was not associated with severe anemia. Of HAART-naive patients, 57.5% had a CD4 count < 200 cells/μL in comparison with 20.4% of HAART-experienced patients (P < 0.001). The mean viral load log10 was significantly higher in HAART-naive than in HAART-experienced patients (P < 0.001). Total lymphocyte count < 1.0 was a significant predictor of <CD4 counts < 200 cells/μL in HAART-naïve patients, but this relationship was not observed in HAART-experienced patients.
Conclusion
HAART has the capability of reducing the incidence of anemia, other deranged hematological and immunological parameters associated with disease progression, and death in HIV-infected patients. Total lymphocyte count fails to predict CD4 count < 200 cells/μL in our cohort; thus, its use in the management and monitoring of HIV-infected patients in our settings is not reliable.
Keywords: antiretroviral, lymphocyte, total leukocyte count, CD4, World Health Organization/Aids Clinical Trials Group
Background
Human immunodeficiency virus (HIV) infection has a variety of effects on hematopoiesis. Pancytopenia is part of its natural history.1–4 Cytopenia is a common complication of infection with human immunodeficiency virus type 1 (HIV-1), and, in the course of the disease, more than 70% of the patients develop anemia, frequently requiring transfusion.5 Neutropenia, lymphopenia, and thrombocytopenia may occur indicating that more than one hemopoietic lineage may be impaired. Dysfunction of the bone marrow has been suggested as a possible mechanism; the degree of cytopenia often reflects the severity of the disease.6 HIV-1 infection of marrow stromal cells is sufficient to result in anemia and other cytopaenias.7–9 A decrease in serum erythropoietin levels,10 autoantibodies to erythropoietin, or marrow suppression by opportunistic infections, tumors or various medications,11–14 may also contribute to the cytopenia commonly observed in HIV-infected persons. Highly active antiretroviral therapy (HAART) may ameliorate many of these effects in an indirect manner simply by decreasing the HIV viral burden15–17 and stimulating hematopoietic progenitor cell growth.18 Although HAART use in HIV infection has generally been accepted as the gold standard in the management of HIV patients,19 some antiretroviral drugs (ie, nucleoside analogue reverse transcriptase inhibitors, especially zidovudine) may cause anemia or worsen existing anemia, and, for this reason, zidovudine is usually excluded from the antiretroviral regimen of severely anaemic patients.20 The most troublesome and common toxicities of zidovudine are, notably, anemia and neutropenia.20 There are conflicting reports on the impact of HAART in resolving hematological complications. Whereas some studies have reported improvements in hematocrit and hemoglobin values resulting in reduction in morbidity and mortality of HIV patients,19,21 another study has reported no improvement in hematocrit values of HAART-treated HIV patients compared with HAART-naive patients.22 This current study was designed to assess the impact of HAART in resolving immunological and hematological complications in HIV patients by comparatively analyzing the results (immunological and hematological) of HAART-naive patients and those on HAART in our environment.
Methods
Study area and design
This cross-sectional study was carried out in the Department of Medicine, University of Maiduguri Teaching Hospital, Borno State, from September 2007 through March 2008. This is a 500 bed hospital designated as a Centre of Excellence for infectious diseases and provides primary, secondary and tertiary services for the North Eastern part of Nigeria. It also caters for the neighboring countries such as the Republics of Cameroon, Niger, and Chad. Permission was obtained from the University of Maiduguri Teaching Hospital (UMTH) Ethical Committee. Written informed consent (signed or thumb print) was obtained from patients.
Study population and procedure
A total of 536-HIV positive patients were consecutively recruited into the study. Using a structured, pre-evaluated questionnaire, information was obtained on demographic characteristics, clinical manifestations, medication used, blood transfusions, and sexual and drug use behavior. The study population consisted of HAART-naive patients and patients who had been on HAART for ≥ 12 months. Criteria for HAART initiation for patients on therapy was based on the Centers for Disease Control and Prevention (CDC) criteria.23 HAART use was defined as receipt of two nucleoside reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitor (NNRTI) or one protease inhibitor (PI). The HAART regimen was verified from patient’s clinical records. Pregnant patients and patients on medications that could affect the hematological system (antibiotics, vitamin supplements, or tuberculosis treatment) at the time of sampling were excluded from the study.
Blood sample analysis
The HIV serological reactivity was determined by enzyme-linked immunoassay (ELISA) and confirmed by western blot analysis. Samples for CD4+ T-cell count were collected between 9:00 AM and 10:00 AM and assayed within 6 hours of collection of whole blood using standardized flow cytometric Cyflow machine (Cytec Development Inc, Partec, Germany). Hemoglobin (Hb), total white blood count (WBC), lymphocyte percentage, and plateletes were analyzed using a hematology analyzer (Sysmex® Corporation, Kobe, Japan). Plasma HIV RNA levels were measured using freshly frozen specimens separated within 6 hours of phlebotomy utilizing the Amplicor HIV-1 Monitor Test, version 1.5 (Roche®, Germany), with a minimum cutoff value of 200 copies per mL.
Statistical Analysis
The software program SPSS version 15 (SPSS Inc, Chicago, IL) was used. Results are presented as means ± SEM. Unpaired t tests were used to compare the means of all continuous variables. Categorical data were analyzed using Fisher exact test or Chi-sqaure test for trend. Linear regression was used to test for the degree of association between test parameters. A P value of <0.05 was considered to be statistically significant.
Demographic and social characteristics of the study population
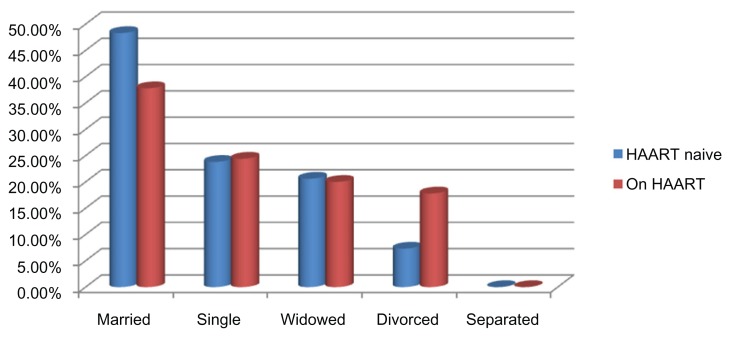
Of the 536 participants that were consecutively recruited into the study, 36 had incomplete or missing records and were excluded from the analysis. Records of 500 subjects were complete, comprising 315 (63%) HAART-naive and 185 (37%) HAART-experienced participants, with median ages of 27.09 ± 16.92 (range, 17–70 years) and 34.43 ± 15.06 (range, 18–67 years), respectively (P < 0.001). Of the 315 HAART-naive participants, 119 (37.8%) were male, and 196 (62.2%), female, with median ages 32.28 ± 17.91 (range, 17–67) years and 24.72 ± 14.89 (range, 17–70) years, respectively (P < 0.001). Of the 185 HAART-experienced participants, 67 (36.2%) were male, while 118 (63.8%) were female, with median ages 38.77 ± 14.81 (range, 20–67) years and 34.08 ± 12.45 (range 18–66) years (P < 0.001), respectively. The age profile as depicted in Table 1 indicates that the majority of the of the participants were <50 years, with the highest proportion (32.1%) from 30 to 39 years of age in HAART-naive participanats, while 40% of HAART-experienced participants were from 40 to 49 years of age. Heterosexual transmission was the presumed risk factor in almost all the study participants. Categorization of the participants based on educational attainment shows that a quarter of the participant had no western education; the majority of the educated had secondary education. Almost half of HAART naïve and one-third of HAART experienced were married, quarter of the studied participants were single in both group, while HAART experienced had more divorced participants as presented in Figure 1. Demographic and social characteristics of the study participants evaluated in this study are presented in Table 1.
Table 1.
Demographic and social characteristics of the study population.
| HAART naive n = 315 (63%) |
On HAART n = 185 (37%) |
P-value | |
|---|---|---|---|
| Age (years) | |||
| 10–19 | 20 (6.3) | 3 (1.6) | |
| 20–29 | 84 (26.7) | 26 (14.1) | |
| 30–39 | 101 (32.1) | 57 (30.8) | |
| 40–49 | 63 (20) | 74 (40) | |
| 50–59 | 21 (6.7) | 16 (8.7) | |
| 60–69 | 16 (5.1) | 5 (2.7) | |
| 70–79 | 10 (3.2) | 4 (2.2) | |
| Median ± SD | 27.09 ± 16.92 (17–70) | 34.43 ± 15.06 (18–67) | 0.000 |
| Sex | |||
| Male: no (%) | 119 (37.8) | 67 (36.2) | |
| Mean ± SD (min-max) | 32.28 ± 17.91 (17–67) | 38.77 ± 14.81 (20–67) | |
| Female: no (%) | 196 (62.2) | 118 (63.8) | |
| Mean ± SD (min-max) | 24.72 ± 14.89 (17–70) | 34.08 ± 12.45 (18–66) | |
| Risk factor | |||
| Heterosexual | 298 (94.6) | 180 (97.3) | |
| Blood transfusion | 3 (0.95) | 0 | |
| Iv drug use | 0 | 0 | |
| MSM | 0 | 0 | |
| Unkown | 14 (4.4) | 5 (2.7) | |
| Educational status | |||
| No formal education | 67 (21.3) | 33 (17.8) | |
| Quaranic education | 18 (5.7) | 10 (5.4) | |
| Primary education | 54 (17.1) | 31 (16.8) | |
| Secondary education | 119 (37.8) | 73 (39.5) | |
| Tertiary education | 57 (18.1) | 38 (20.5) | |
Figure 1.
Distribution of the participant based on marital status.
Hematological profile, viral load, and cytopenic tendency stratified by HAART use
The mean hemoglobin of 11.89 ± 1.61 (95% confidence interval [CI], 11.64–12.13) in HAART-experienced participants was significantly higher than the mean hemoglobin of 9.76 ± 2.82 (95% CI, 9.45–10.07) in HAART-naive participants (P < 0.001). Using packed cell volume (PCV) <30% to define anemia, 57.5% (180/313) of HAART-naive participants were 3 times at risk of anemia compared with 24.3% (41/169) or HAART-experienced participants (P < 0.001). The mean WBC and the incidence of leukopenia were higher among HAART––naive participants (P < 0.001). The mean total leukocyte count (TLC) of 2.05 ± 1.34 (95% CI, 1.86–2.24) in HAART-experienced participants was significantly higher than the mean TLC of 1.61 ± 1.02 (95% CI, 1.50–1.72) in HAART-naive participants (P < 0.001), consequently HAART-naive participants had 4 times the risk of developing leukopenia (TLC < 2500 μL−1) than HAART-experienced participants (P < 0.001). The mean lymphocyte % of 46.11 ± 12.70 (95% CI, 44.27–47.90) and incidence of lymphopenia (<40%) of 98.3% were significantly higher in HAART-experienced participants than 31.85 ± 12.37 (95% CI, 30.43–33.28) and 73.2% in HAART-naive participants (P < 0.001). The mean neutrophil % of 54.98 ± 15.72 (95% CI, 53.22–56.73) in HAART- naive participants was higher than 38.35 ± 10.49 (95% CI, 36.82–39.87) in HAART-experienced participants (P < 0.001), conversely neutropenia defined as neutrophil % (NC%) % < 60% showed HAART-experienced preponderance (P < 0.001). Although there was no difference in mean platelets counts, the risk of thrombocytopenia (<150 × 103/μL) was 8 times higher in HAART-naive participants (P < 0.001). The mean CD4 count of 347.73 ± 183.85 (95% CI, 320.77–374.70) was significantly higher in HAART-experienced than 238.98 ± 216.57 (95% CI, 214.93–263.03) in HAART-naive participants (P < 0.001), the risk of immunological AIDS (<200 cell/μL) was 3 times in HAART naive than HAART experienced (P < 0.001). The mean viral load log10 was significantly lower in HAART experienced (3.17 ± 1.04) copies/mL than 5.58 ± 2.23 copies/mL in HAART-naive participants (P < 0.001). The haematological profile, viral load and cytopenic tendency of the participants stratified by HAART use is as presented in Table 2. The incidence of cytopenia in the study population is as shown in Figure 2.
Table 2.
Hematological profile, viral load, and cytopenic tendency of the study population.
| Characteristics | HAART naive | On HAART | P-value |
|---|---|---|---|
| Participants stratified by HAART use | |||
| Sample size (n) | 315 | 185 | |
| HB (g/dL) | 9.76 ± 2.82 | 11.89 ± 1.61 | 0.000 |
| (95% CI 9.45–10.07) | (95% CI 11.64–1.13) | ||
| PCV < 30% | |||
| TWBC (×103 μL−1) | 5.94 ± 2.91 | 5.05 ± 1.58 | 0.000 |
| (95% CI 5.61–6.26) | (95% CI 4.81–5.29) | 0.029 | |
| <2.5 | 19 (6.1) | 3 (1.7) | |
| TLC (×103 μL−1) | 1.61 ± 1.02 | 2.05 ± 1.34 | 0.000 |
| (95% CI 1.50–1.72) | (95% CI 1.86–2.24) | 0.000 | |
| <1.2 | 94 (32.3) | 11 (10.5) | |
| LC (%) | 31.85 ± 12.37 | 46.11 ± 12.70 | 0.000 |
| (95% CI 30.43–33.28) | (95% CI 44.27–47.90) | 0.000 | |
| <40 | 213 (73.2) | 171 (98.3) | |
| NC (%) | 54.98 ± 15.72 | 38.35 ± 10.49 | 0.000 |
| (95% CI 53.22–56.73) | (95% CI 36.82–39.87) | 0.000 | |
| <60 | 202 (65.4) | 178 (96.7) | |
| PLT (×103 μL−1) | 297.61 ± 127.19 | 291.86 ± 95.91 | 0.000 |
| (95% CI 283.46–311.75) | (95% CI 277.07–306.65) | 0.000 | |
| <150 | 30 (9.6) | 2 (1.2) | 0.000 |
| CD4 count (cells/μL) | 238.98 ± 216.57 | 347.73 ± 183.85 | 0.000 |
| (95% CI 214.93–263.03) | (95% CI 320.77–374.70) | 0.000 | |
| <200 | 168 (53.5) | 37 (20.4) | |
| Viral load log10 | 5.58 ± 2.23 | 3.17 ± 1.04 | 0.000 |
| (95% CI 1.21–2.56) | (95% CI 0.76–1.03) | 0.000 | |
Abbreviations: TWBC, Total white blood count; LYM%, Lymphocyte percent; Neut%, Neutrophil percent; TLC, Total leukocyte count; PLT, Platelets numbers.
Figure 2.
Incidence of cytopenia in the study population.
World Health Organization Clinical Trials Group, anemia toxicity, stratified CD4 count by CDC and TLC grades
Classification of the study population according to the World Health Organization/AIDS Clinical Trials Group (WHO/ACTG) anemia toxicity grades as presented in Table 3 gave a 60.1% and 36.1% calculated incidence of anemia (Hb < 10.5 g/dL−1) in HAART-naive and HAART-experienced participants respectively (x2 = 17.54, P < 0.001). The odds of developing grade 1 anemia in HAART-naive and HAART-experienced participants were similar (P = 0.18); however, HAART-naïve participants were 2 times at risk of developing grade 2 anemia (P < 0.001). Grade 3 and 4 anemia were not seen in HAART-experienced patients.
Table 3.
Study participants stratified by anemia, CD4 count, total leukocyte count, and HAART use.
| Parameter | HAART naive | On HAART | P-value |
|---|---|---|---|
| WHO/ACTG Grade (n) | 313 | 169 | |
| Grade 1 (9.5–10.5) | 68 (21.7) | 46 (27.2) | 0.176 |
| Grade 2 (8.0–9.4) | 44 (14.1) | 15 (8.9) | 0.098 |
| Grade 3 (6.5–7.9) | 41 (13.1) | 0 | 0.000 |
| Grade 4 (<6.5) | 35 (11.2) | 0 | 0.000 |
| CD4 count (CDC) (n) | (314) | (181) | |
| 0–199 | 168 (53.5) | 37 (20.4) | 0.000 |
| 200–499 | 110 (35.0) | 110 (60.8) | 0.000 |
| ≥500 | 36 (11.5) | 34 (18.8) | 0.025 |
| TLC (n) | 286 | 171 | |
| <1 | 59 (20.6) | 26 (15.2) | 0.149 |
| 1.0–2.0 | 140 (48.9) | 57 (33.3) | 0.001 |
| >2.0 | 87 (30.4) | 88 (51.5) | 0.000 |
The Centers for Disease Control and Prevention (CDC) criteria were used to classify the study population into three categories based on CD4 counts: stage 1 (CD4 > 500 cells/μL), stage 2 (CD4 between 200 and 499 cells/μL) and stage 3 (CD4 < 200 cells/μL). The risk of having CD4 count < 200 cells/μL was thrice in HAART-naive participants than those on HAART (P < 0.001), and the chances of having a CD4 count between 200 and 499 cells/μL was 2 times higher in patients on HAART than those who were HAART naive (P = 0.001). No significant difference was observed in the proportion of participants with CD4 counts ≥ 500 cells/μL when HAART-naive participants were compared with HAART-experienced participants (P = 0.025). There was no difference in the proportion of participants with TLC < 1.0103/μL in both groups, HAART-naive participants had a significantly higher proportion of participants with TLC 1.0 to 2.0 (P = 0.001), while HAART-experienced participants displayed twice the preponderance (P < 0.001).
CD4 count categories stratified by World Health Organization AIDS Clinical Trials Group grades and Centers for Disease Control and Prevention criteria
Analysis of the three categories of CD4 counts, WHO/ACTG anemia toxicity and defined TLC grades are as depicted in Table 4. Based on the analysis of the three categories of CD4 counts and TLC in HAART-naive participants, when TLC was <1000 μL−1, there was a gradual increase in the proportion of patients within the three CD4 categories, from 1.7% in stage 1, 6.8% in stage 2, and to a peak percentage of 91.5% in stage 3 (P < 0.001). With a TLC between 1000 and 2000 μL−1, a steady increase was maintained across all the three stages. A similar proportion of patients within the CD4 stages 1 and 3 was observed with a higher proportion of 48.3% in stage 2 when the TLC was >2000 μL−1.
Table 4.
Analysis of the study population stratified by HAART use, TLC, anemia, and CD4 count.
| Variable | CD4 counts | P-value | ||
|---|---|---|---|---|
|
|
||||
| Stage 3 (0–199) | Stage 2 (200–499) | Stage 1 (≥500) | ||
| HAART naive | ||||
| TLC | ||||
| <1.0 | 54 (91.5) | 4 (6.8) | 1 (1.7) | 0.000 |
| 1.0–2.0 | 70 (50.0) | 58 (41.4) | 12 (8.6) | 0.000 |
| >2.0 | 24 (27.6) | 42 (48.3) | 21 (24.1) | 0.000 |
| Anemia | ||||
| 9.5–10.5 | 44 (64.7) | 18 (26.5) | 6 (8.4) | 0.000 |
| 8.0–9.4 | 26 (59.1) | 16 (36.4) | 2 (4.5) | 0.000 |
| 6.5–7.9 | 24 (60.0) | 13 (32.5) | 3 (7.5) | 0.000 |
| <6.5 | 27 (77.1) | 7 (20.0) | 1 (2.9) | 0.000 |
| On HAART | ||||
| TLC | ||||
| <1.0 | 4 (15.4) | 19 (70.1) | 3 (11.5) | 0.000 |
| 1.0–2.0 | 21 (36.8) | 26 (45.6) | 10 (17.5) | 0.005 |
| >2.0 | 11 (12.5) | 50 (56.8) | 21 (23.9) | |
| Anaemia | ||||
| 9.5–10.5 | 5 (10.9) | 32 (69.6) | 9 (19.6) | 0.000 |
| 8.0–9.4 | 6 (40.0) | 8 (53.3) | 1 (6.7) | 0.020 |
| 6.5–7.9 | 0 | 0 | 0 | |
| <6.5 | 0 | 0 | 0 | |
Taking grades of anemia into account, a steady increase in the proportion of participants was observed from stage 1 through stage 3 across all grades.
In patients on HAART, when the TLC was <1000 μL−1, 1000 to 2000 μL−1, and >2000 μL−1, patients within category 2 had the highest proportion of participants than category 1 and 3 across all grades. The pattern of incidences of grades 1 and 2 was not consistent, and grades 3 and 4 were absent in HAART-experienced participants.
Discussion
Hematological abnormalities are among the most common complications of HIV, which involves all lineages of blood cells.24 Establishing the presence of abnormal hematological manifestations in HAART-naive HIV patients and those on HAART and performing a comparative analysis within the two study groups gives a clear pointer to the overall impact of the treatment regimen in HIV patients. The majority of the study population was <50 years of age, the most productive segment of the society. These findings are in agreement with previous reports by Akinsete et al25 and Erhabor26 and as well as other researchers.27 The observation of a higher HIV prevalence among young Nigerians is worrisome since the economically viable section of Nigeria’s population is most affected by the scourge. Heterosexual transmission as the presumed risk factor in this report is a pointer to the fact that youths are more sexually active and are more prone to high risk behaviors such as maintenance of multiple sex partners that make them vulnerable. Intravenous drug abuse and men that have sex with men (MSM) as risk factors for HIV infection were not observed in our cohort; however, 19 participants (3.8%) in our study within the ages of adolescence and young adulthood were unaware of how they contracted their HIV infection and were not sexually exposed, which could be attributable to prevalent harmful traditional practices such as female genital mutilation, tattooing, group circumcision, and scarification marking performed for adolescent and young adults in our environment.
Results from this study showed that HIV infection may result in deranged hematological indices regardless of age and sex that is ameliorated by use of HAART. The majority of the HIV positive patients were female among both HAART-naive and HAART—experienced participants.
The female preponderance in this study confirms the World Health Organization (WHO) report that HIV/AIDS affects females most severely in sub-Saharan Africa.28,29 The significant difference in age between HAART-experienced and HAART-naive patients further supports the proposition that HIV/AIDS infection may be acquired at an early age and most probably during the reproductive stage of life. The strict eligibility criteria for HAART initiation, CD4 < 200 cells/μL or the presence of AIDS- defining illnesses (clinical AIDS) irrespective of CD4,30 may also explain why most of the patients on HAART were older than their HAART-naive counterparts in that there could be a time lag between the time a patient tests positive for HIV and the time treatment is initiated, and treatment, once started, is continued throughout the life of the patient.
The incidence of cytopenia in this study was significantly higher in HAART-naive patients than in patients on HAART. Consistent with this report, since the introduction of HAART, several studies have examined the prevalence of anemia, indicating that the prevalence of severe anemia has declined while mild to moderate anemia remains relatively common with use of HAART,4,16 HIV-1 infection of marrow stromal cells is sufficient to result in anemia and other cytopaenias.7–9 A decrease in serum erythropoietin levels,10 auto-antibodies to erythropoietin, or marrow suppression by opportunistic infections, tumors, or various medications10–13 may also contribute to the anemia commonly observed in HIV-infected persons. HAART may ameliorate many of these effects in an indirect manner simply by decreasing the HIV viral burden.15–17 A study18 has shown that HAART was associated with an increase in hematopoietic progenitor cell growth. Although the mean platelet count was similar in both HAART-naive and HAART-experienced participants, the relative risk of developing thrombocytopenia in HAART-naive populations was 8 times that of the HAART-experienced. This finding is in sharp contrast to studies conducted previously31,32 that reported a similar thrombocytopenic incidence in HAART-naive and HAART- experienced patients. Taking into account participants’ levels of immunosuppression based on CD4 counts, it was shown that the incidence of grades 1 to 4 anemia among HAART-naive participants with severe, moderate, and mild immunosuppression declined significantly with increased CD4 levels. Although the linear relationship between level of immunity and grades of anemia was lost in HAART-experienced patients, severe grades (3 and 4) were not present in patients on HAART as a result of declines in the prevalence of opportunistis infections and immune-reconstitution, consistent with other reports.15,33 The laboratory parameter most studied as a potential alternative to CD4 count is TLC. In places where CD4 count testing is not available, the WHO recommends considering treatment for WHO stage 2 disease if TLC is <1200 μL−1. The use of TLC is not recommended in asymptomatic patients.34 Evidence frequently cited to support the use of a TLC cutoff of 1200 μL−1 (for stage 2 disease) includes a South African cohort study in which TLC < 1250 μL−1 was found to be an equivalent predictor of disease progression compared with CD4 count < 200 cells/mm3.35 Our study questions this cutoff because 27.6% of HAART-naive and 12.5% of HAART-experienced participants in this report had CD4 counts < 200 cells/μL. A previous study in Nigeria reported that a third of patients with CD4 counts < 200 cells/μL had TLC > 1200 μL−1.36 This may imply that an acceptable reproducible cut has been elusive. Further, in an analysis of WHO stage 2 patients in an antiretroviral therapy (ART) program (n = 51 281), CD4 counts and TLC counts were significantly positively correlated.30 Using TLC < 1200 μL−1 as a predictor of CD4 count < 200 cells/μL resulted in 31.5% sensitivity, 96.0% specificity, 95.9% positive predictive value, and 31.6% negative predictive value. Increasing the cutoff value to 1900 μL−1 resulted in 67.0% sensitivity, 67.9% specificity, 86.3% positive predictive value, and 40.4% negative predictive value. Taken together, TLC < 1200 μL−1 was a poor predictor of CD4 count < 200 cells/μL, and over half of patients with CD4 count < 200 cells/μL would have been inappropriately excluded by TLC-guided treatment with a cutoff of 1200 μL−1.37 Thus, TLC is of limited usefulness for guiding initiation of ART: some patients with CD4 count < 200 cells/μL will not be started on ART because their TLC will be >1200 μL−1. Some investigators have suggested that incorporating the hemoglobin level, and perhaps body mass index or platelet count, will improve the accuracy of TLC,36,37 but it remains to be seen if additional parameters such as these will provide more useful information on a consistent basis.
Conclusion
HAART has the capability of reducing the incidence of anemia, other deranged hematological and immunological parameters associated with disease progression, and death in HIV-infected patients. Total lymphocyte count fails to predict CD4 count < 200 cells/μL in our cohort; thus, its use in the management and monitoring of HIV-infected patients in our settings is not reliable. In agreement with WHO and other reports, females of reproductive age are at a greater risk of becoming HIV-infected than males, and this could perpetuate documented social complications associated with HIV infection. Continued efforts to educate the vulnerable regarding social lifestyle and possible risk factors associated with HIV cannot be over-emphasized.
Footnotes
Author Contributions
Conceived and designed the experiments: BD, IK, AH. Analysed the data: BD, IK, AH. Wrote the first draft of the manuscript: BD, IK, AH, AD, MAS. Contributed to the writing of the manuscript: BD, IK, AD, MAS. Agree with manuscript results and conclusions: BD, IK, AH, AD, MAS. Made critical revisions and approved final version: BD, IK, AH. All authors reviewed and approved of the final manuscript.
Funding
Author(s) disclose no funding sources.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Bernstein ZP, Gworek MA, Small BM. Hematologic abnormalities in patients with the acquired immunodeficiency syndrome. J Med. 1989;20:177–92. [PubMed] [Google Scholar]
- 2.Frontiera M, Myers AM. Peripheral blood and bone marrow abnormalities in the acquiredimmunodeficiency syndrome. West J Med. 1987;147(2):157–60. [PMC free article] [PubMed] [Google Scholar]
- 3.Ganser A. Abnormalities of hematopoiesis in the acquired immunodeficiency syndrome. Blut. 1988;56(2):49–53. doi: 10.1007/BF00633460. [DOI] [PubMed] [Google Scholar]
- 4.Kaslow RA, Phair JP, Friedman HB, et al. Infection with the human immunodeficiency virus: Clinical manifestations and their relationship to immune deficiency. Ann Intern Med. 1987;107(4):474–80. doi: 10.7326/0003-4819-107-4-474. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson MA, Pereperi L, Volberdin PA, Porteoenus D, Toy PT, Feigal D. Red cell transfusion therapy for anaemia in patients with AIDS and ARC: incidence associated factors, and outcome. Transfusion. 1990;30:133–7. doi: 10.1046/j.1537-2995.1990.30290162898.x. [DOI] [PubMed] [Google Scholar]
- 6.Koka PS, Jamieson BD, Brooks DG, et al. HIV-1 induced hematopoietic inhibition is independent of productive infection of progenitor cells in vivo. J Virol. 1999;73:9089–97. doi: 10.1128/jvi.73.11.9089-9097.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moses AU, Williams S, Henevild ML, et al. Human immunodeficiency Virus infection of bone marrow endothelium reduces induction of stromal Hematopoietic growth factors. Blood. 1996;87:919–25. [PubMed] [Google Scholar]
- 8.Bahner I, Kerans K, Coutinho S, et al. Infection of human marrow stroma by HIV-1 is both required and sufficient for HIV-1 induced haematopoietic suppression in vitro: demonstration by gene modification of primary human stroma. Blood. 1997;90:1787–98. [PubMed] [Google Scholar]
- 9.Scadden DT, Zeira M, Woon A, et al. Human immunodeficiency virus infection of human bone marrow stromal fibroblasts. Blood. 1990;76:317–22. [PubMed] [Google Scholar]
- 10.Spivak JL, Barnes DC, Fuchs E, et al. Serum immunoreactive erythropoietin in HIV infected patients. JAMA. 1989;261:3104–7. [PubMed] [Google Scholar]
- 11.Frickhofen N, Abkowitz JL, Safford M, et al. persistent B19 parvovirus infection in patients infected with human immunodeficiency virus type 1: a treatable cause of anaemia in AIDS. Ann Intern Med. 1990;113:926–33. doi: 10.7326/0003-4819-113-12-926. [DOI] [PubMed] [Google Scholar]
- 12.Creag-Kirk, Dio P, Andrew E, et al. Survival experience among patients with AIDS receiving zidovudine-follow-up of patients in a compassionate plea program. JAMA. 1988;260:3009–19. [PubMed] [Google Scholar]
- 13.Seneviratne LC, Tulpule A, Espina BM, et al. Clinical, immunologic, and pathologic correlates of bone marrow involvement in 291 patients with AIDS related lymphoma. Blood. 2001;98:2358–63. doi: 10.1182/blood.v98.8.2358. [DOI] [PubMed] [Google Scholar]
- 14.Sipsas NV, Kokori SI, Ionnidis JPA, et al. Circulating autoantibodies to erythropoietin are associated with human immunodeficiency virus type I related anaemia. J Infect Dis. 1999;180:2044–7. doi: 10.1086/315156. [DOI] [PubMed] [Google Scholar]
- 15.Semba RD, Shah N, Vlahov D. Improvement of anaemia among HIV infected injection drug users receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;26:315–9. doi: 10.1097/00126334-200104010-00003. [DOI] [PubMed] [Google Scholar]
- 16.Mellors JW, Kingsley LA, Ronaldo CR, et al. Quantification of HIV-RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122:573–9. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Walker A, Peto T, Darbyshire J. Markers of HIV infection in the Concorde trial. Concord Co-ordinating committee. QJM. 1998;91:423–38. doi: 10.1093/qjmed/91.6.423. [DOI] [PubMed] [Google Scholar]
- 18.Isgro A, Mezzaroma I, Aiuti A, et al. Recovery of hematopoietic activity in bone marrow from human immunodeficiency virus type 1 infected patients during highly active anti-retroviral therapy. AIDS Res Hum Retroviruses. 2000;16:1471–9. doi: 10.1089/088922200750005994. [DOI] [PubMed] [Google Scholar]
- 19.Odunukwe N, Idigbe O, Kanki P, et al. Haematological and biochemical response to treatment of HIV-1 infection with a combination of nevirapine + stavudine + lamivudine in Lagos, Nigeria. Turk J Haematol. 2005;22:125–31. [PubMed] [Google Scholar]
- 20.Fischl MA, Parker CB, Pettinelli C, et al. A randomized controlled trial of reduced daily dose of zidovudine in patients with the acquired immunodeficiency syndrome. The AIDS Clinical Trial Group. N Engl J Med. 1990;323:1009–14. doi: 10.1056/NEJM199010113231501. [DOI] [PubMed] [Google Scholar]
- 21.Gea-Banacloche JC, Lane HC. Immune reconstitutionin HIV-1 infections. AIDS. 1999;13:525–38. [Google Scholar]
- 22.Omoregie R, Egbeobauwaye A, Ogefere H, Omokaro EU, Ekeh CC. Prevalence of Antibodies to HAART Agents among HIV Patients in Benin city, Nigeria. African Journal of Biomedical Research. 2008;11:33–7. [Google Scholar]
- 23.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA. 1993;269:729–30. [PubMed] [Google Scholar]
- 24.Kirchhoff F, Silvestri G. Is Nef the elusive cause of HIV-associated hematopoietic dysfunction? J Clin Invest. 2008;118:1622–5. doi: 10.1172/JCI35487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akinsete I, Akanmu AS, Okanny CC. Trends in HIV-seropositivity among visa applicants in Lagos, Nigeria: A five-year survey 1992–6. Niger Postgrad Med J. 1998;5:69–72. [Google Scholar]
- 26.Erhabor O. The geometrically increasing prevalence of HIV infection and its attendant social implication in Port Harcourt, Nigeria. Lab News J. 2001;14:27–8. [Google Scholar]
- 27.Amballi AA, Ajibola A, Ogun SA, et al. Demographic pattern and haematological profile in people living with HIV/AIDS in a university teaching hospital. Scientific Research and Essays. 2007;2(8):315–8. [Google Scholar]
- 28.Dabis F, Ekpini ER. HIV-1/AIDS and maternal and child health in Africa. Lancet. 2002;359(9323):2097–104. doi: 10.1016/S0140-6736(02)08909-2. [DOI] [PubMed] [Google Scholar]
- 29.Joint United Nations Programme on HIV/AIDS and World Health Organization. AIDS Epidemic Update: Dec 2004. Geneva, Switzerland: UNAIDS; 2004. [Google Scholar]
- 30.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents in Resource-Limited Settings: Towards Universal Access. Recommendations for a Public Health Approach. Geneva, Switzerland: World Health Organization; 2006. 2006 revision. [Google Scholar]
- 31.Pechere M, Samii K, Hirschel B. HIV-related thrombocytopenia. N Engl J Med. 1993;328(24):1785–6. doi: 10.1056/NEJM199306173282414. [DOI] [PubMed] [Google Scholar]
- 32.Owiredu WKBA, Quaye L, Amidu N, Addai-Mensah O. Prevalence of anaemia and immunological markers among Ghanaian HAART-naïve HIV-patients and those on HAART. Afr Health Sci. 2011;11(1):2–15. [PMC free article] [PubMed] [Google Scholar]
- 33.Entonu PE, Agwale SM. A review of the epidemiology, prevention and treatment of human immunodeficiency virus infection in Nigeria. Braz J Infect Dis. 2007;11(6):579–90. doi: 10.1590/s1413-86702007000600011. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. ARV treatment guidelines and technical and operational recommendations for ART (2006) [Accessed Aug 17, 2007]. Available at: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf.
- 35.Post FA, Wood R, Maartens G. CD4 and total lymphocyte counts as predictors of HIV disease progression. QJM. 1996;89:505–8. doi: 10.1093/qjmed/89.7.505. [DOI] [PubMed] [Google Scholar]
- 36.Akinola NO, Olasode O, Adediran IA, et al. The search for a predictor of CD4 cell count continues: Total lymphocyte count is not a substitute for CD4 cell count in the management of HIV-infected individuals in a resource-limited setting. Clin Infect Dis. 2004;39:579–81. doi: 10.1086/422722. [DOI] [PubMed] [Google Scholar]
- 37.Akanbi M, Taiwo B, Welty L, et al. Sensitivity of total lymphocyte count as a predictor of CD4 count in ARV naive HIV patient at JUTH, Jos, Nigeria. Proceedings from the XVI International AIDS Conference; Aug 13–18, 2006; Toronto, Ontario. Abstract MOPE0151. [Google Scholar]