Abstract
Background:
In India, anemia is a common and serious health disorder among both sexes and all age groups, with anemia of chronic disease (ACD) being the second most prevalent anemia. Periodontitis is an inflammatory disease of the supporting tissues of the tooth caused by specific microorganisms. An immune response to bacteria and their products induces a major vascular response, offering explanatory mechanisms for the interactions between periodontal infection and a variety of systemic disorders. Therefore, periodontitis results in low-grade systemic inflammation, which may cause lower number of erythrocytes and, consequently, lower hemoglobin concentration.
Materials and Methods:
A total of 100 systemically healthy male patients visiting the outpatient department participated in the study. Of these, 50 patients had healthy periodontium and 50 patients had chronic periodontitis. Clinical parameters and red blood cell parameters of all the patients were assessed at baseline and 6 months after non-surgical periodontal therapy. Statistical analysis using Student's t-test was performed.
Results:
Data analysis revealed that patients with chronic periodontitis showed an improvement in both clinical and red blood cell parameters from baseline to 6 months after non-surgical periodontal therapy.
Conclusion:
From the present study, it can be concluded that like any other chronic condition, chronic periodontitis can lead to ACD. It also provides evidence that non-surgical periodontal therapy can improve the anemic status of patients with chronic periodontitis.
Keywords: Anemia, chronic periodontitis, cytokines, hepcidin, red blood cells
INTRODUCTION
In India, anemia is a common and serious health disorder among both sexes and all age groups, although it has a higher prevalence among women than men.[1]
Anemia of chronic disease (ACD) is the second most prevalent anemia after iron deficiency anemia, and occurs in patients with acute or chronic immune activation. Thus, the condition has been termed “anemia of inflammation.”[2]
ACD is defined as anemia occurring in chronic infections, inflammatory conditions, or a neoplastic disorder, which is not caused by marrow deficiencies or other diseases, and occurring despite the presence of adequate iron stores and vitamins.[3] Typically, ACD is a mild-to-moderate, normochromic/normocytic anemia, and is characterized by decreased serum iron and total iron-binding capacity, with normal or increased iron stores.[3] It is immune driven; cytokines and cells of the reticuloendothelial system induce changes in iron homeostasis, proliferation of erythroid progenitor cells, production of erythropoietin, and life span of red cells, all of which contribute to the pathogenesis of anemia.[2]
Periodontitis is an inflammatory disease of the supporting tissues of the tooth caused by specific microorganisms in a susceptible host. Just as the periodontal tissues mount an immune inflammatory response to bacteria and their products, systemic challenges with these agents also induce a major vascular response.[4] Chronic periodontitis is the most common form of periodontal disease, which progresses relatively slowly and is more common in adults.[5]
It is, therefore, speculated that periodontitis results in low-grade systemic inflammation, which may cause lower number of erythrocytes and, consequently, lower hemoglobin (Hb) concentration.[6,7] However, conflicting results have been reported regarding the association of periodontal disease and anemia.
The purpose of this study is to investigate whether patients with chronic periodontitis have an anemic status and the effect of non-surgical periodontal therapy on red blood cell (RBC) analyses of patients with chronic periodontitis over a 6-month period.
MATERIALS AND METHODS
In this 6-month follow-up randomized controlled double-blind study, male patients of age between 20 and 50 years were selected from the outpatient department. The patient and the pathologist who performed the blood analysis were blinded to the study. The intra-oral examination was done by a single examiner to reduce error. A detailed systemic and family history was recorded. Patients with a history of systemic diseases or conditions that may adversely affect periodontal health were excluded from the study. The exclusion criteria for the study were as follows: (1) female patients; (2) smokers; (3) history of periodontal treatment or use of vitamin, antibiotics, or iron supplements within previous 6 months; (4) individuals who suffered from any acute or chronic medical conditions including diabetes, or viral, fungal, or bacterial infection; and (5) individuals who suffered from malaria or jaundice in the last 1 year [Table 1]. Clinical parameters assessed were probing pocket depth (PPD), clinical attachment loss (CAL), gingival index (GI), and plaque index (PI). Patients diagnosed with chronic periodontitis, having a probing depth of ≥5 mm in >30% sites and CAL ≥ 2 mm at 30% sites,[5] were included in the test group (n = 50). The control group (n = 50) included periodontally healthy male patients with no PPD and CAL [Figures 1 and 2]. Patients were informed regarding the benefits and protocol of the study and a written informed consent was taken from all the patients.
Table 1.
Demographic characteristics of the study population
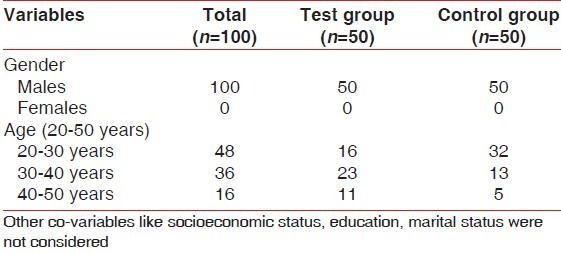
Figure 1.
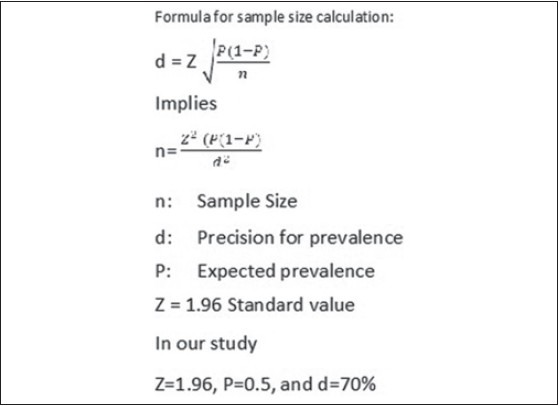
Formula for sample size estimation
Figure 2.
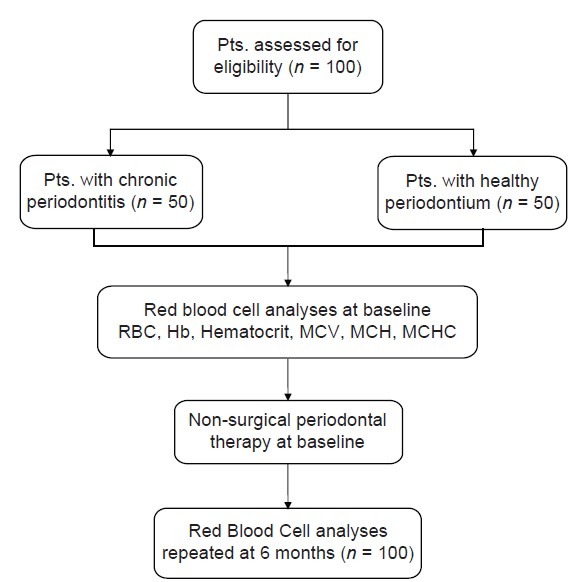
Study flowchart
Red blood cell analysis
At baseline: All the blood samples of the test and control groups were assessed at the pathology laboratory. Ten milliliters of venous blood samples were collected by venipuncture under aseptic conditions in the antecubital fossa. The blood was transferred into ethylenediaminetetraacetic acid containing bulbs. The samples were processed within 4 h of collection in an automated hematology analyzer and semi-automated (ok) 3-part differential cell counter ACT-8 Beckham Coulter. The parameters assessed were RBC count, Hb concentration, hematocrit value, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC).
Non-surgical periodontal therapy consisting of scaling and root planing was performed on patients in the test group (n = 50) and oral prophylaxis was performed in the control group (n = 50). Oral hygiene instructions were given to the patient. Patients were advised to use 0.2% chlorhexidine mouthwash, 10 ml twice daily for 15 days.
At 6 months: Patients of the test and control groups were recalled. Clinical parameters and RBC parameters were re-assessed and oral prophylaxis was performed. Oral hygiene instructions were given to the patient.
Statistical analysis
Student's t-test was performed with appropriate Microsoft Excel software for a comparative analysis of outcome parameters between test and control groups at baseline and 6 months. Means and standard deviations of all the parameters were calculated for both groups. To illustrate the differences between groups, two-tailed P value was used, which was considered statistically significant if <0.05.
RESULTS
The mean and standard deviation of all blood and clinical parameters in the test and control groups are presented in Tables 2 and 3, respectively.
Table 2.
Hematological parameters at baseline and 6 months in the test group
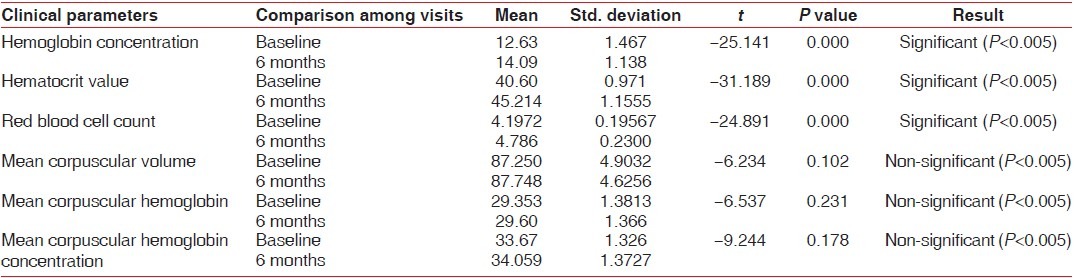
Table 3.
Hematological parameters at baseline and 6 months in the control group
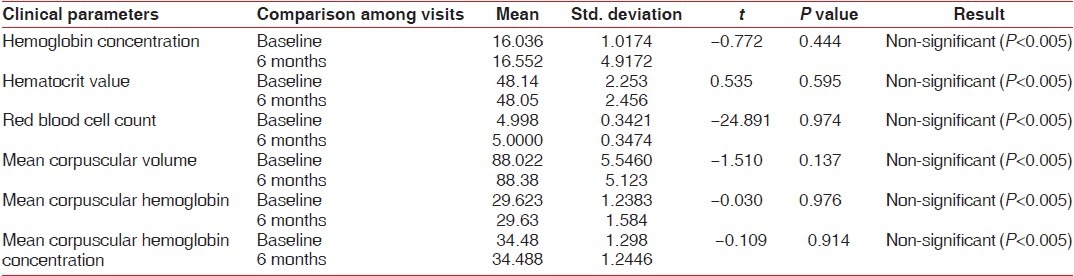
Hb concentration, hematocrit value, and RBC count in the test group at baseline were below the normal reference range [Table 2, Graphs 1–3], whereas in the control group, they was well within the normal reference range [Table 3, Graphs 1–3]. This suggested that patients with chronic periodontitis had Hb concentration, hematocrit value, and RBC count below the normal reference range at baseline, as compared to the controls at baseline. At 6 months after non-surgical periodontal therapy, Hb concentration, hematocrit value, and RBC count in the test group showed a statistically significant increase, indicating that non-surgical periodontal therapy has a positive effect on the anemic status of the patients [Tables 2 and 3, Graphs 1–3].
Graph 1.
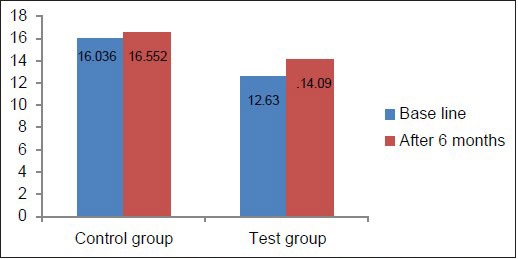
Comparison of Hb at baseline and 6 months in the test and control groups
Graph 3.
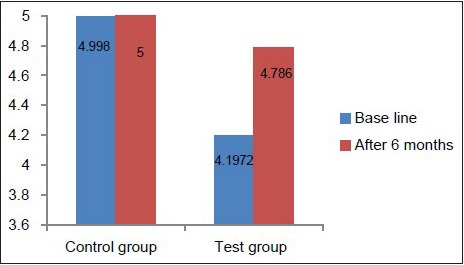
Comparison of RBC count at baseline and 6 months in the test and control groups
Graph 2.
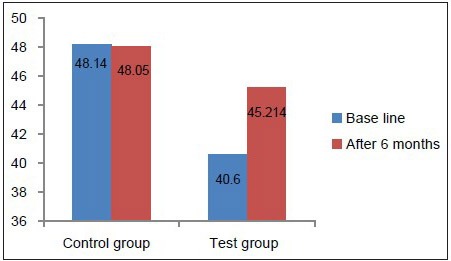
Comparison of hematocrit value at baseline and 6 months in the test and control groups
MCV, MCH, and MCHC values at baseline in the test as well as the control group were well within the normal reference range [Tables 2 and 3, Graphs 4–6], indicating that patients with chronic periodontitis had ACD which is a normocytic, normochromic anemia.
Graph 4.
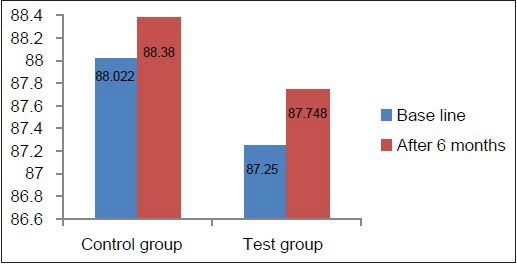
Comparison of MCV at baseline and 6 months in the test and control groups
Graph 6.
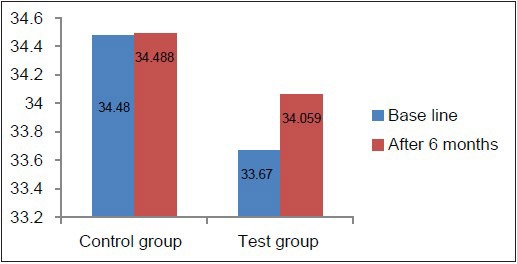
Comparison of MCHC at baseline and 6 months in the test and control groups
Graph 5.
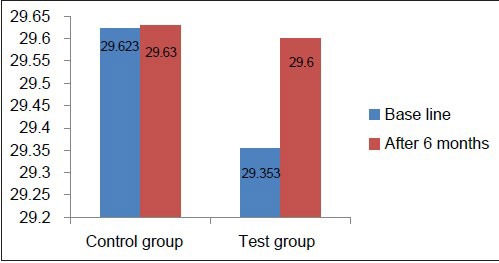
Comparison of MCH at baseline and 6 months in the test and control groups
Clinical parameters measured showed a statistically significant decrease from baseline to 6 months after non-surgical periodontal therapy in the test group [Table 4, Graphs 7–10]. Clinical parameters in the control group were within the normal reference range at baseline and 6 months [Table 5, Graphs 7–10].
Table 4.
Clinical parameters at baseline and 6 months in the test group
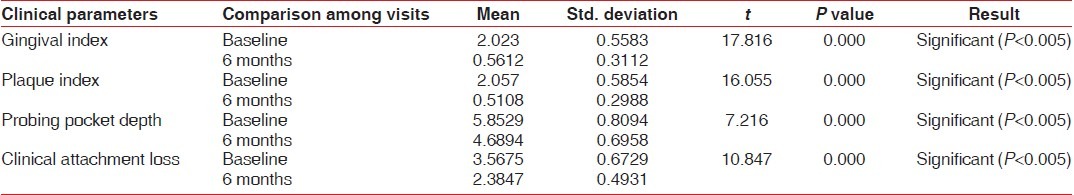
Graph 7.
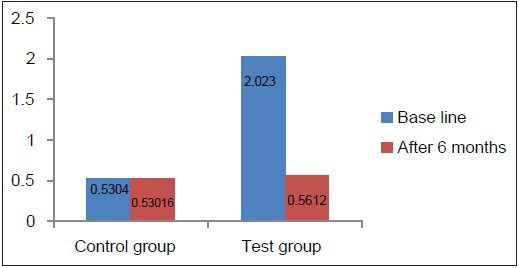
Comparison of GI at baseline and 6 months in the test and control groups
Graph 10.
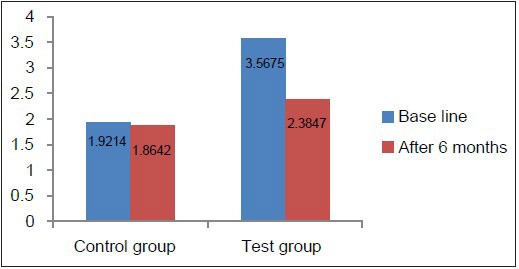
Comparison of CAL at baseline and 6 months in the test and control groups
Table 5.
Clinical parameters at baseline and 6 months in the control group
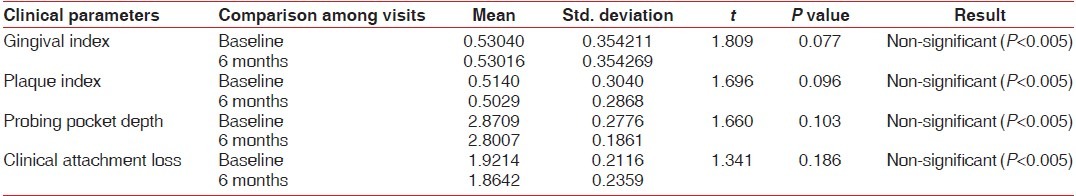
Graph 8.
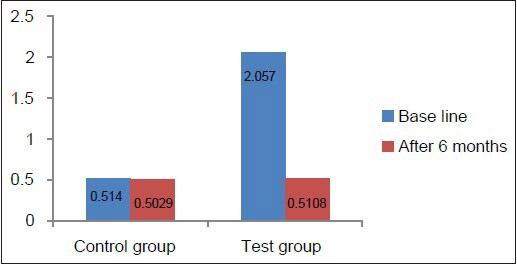
Comparison of PI at baseline and 6 months in the test and control groups
Graph 9.
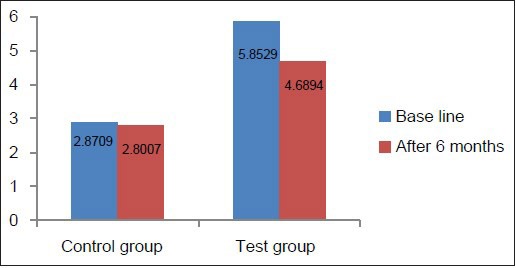
Comparison of PPD at baseline and 6 months in the test and control groups
DISCUSSION
In the present study, the Hb concentration, hematocrit value, RBC count, MCV, MCH, and MCHC in the control group at baseline and 6 months were in the normal reference range. These results are in accordance with the results of the studies conducted by Hutter et al.,[6] Gokhale et al.,[8] and Naik et al.[5]
The Hb concentration, hematocrit value, and RBC count in the test group at baseline were below the normal reference range, whereas the MCV, MCH, and MCHC were in the normal reference range. These results are in accordance with the results of the studies conducted by Lainson et al.,[7] Hutter et al.,[6] Gokhale et al.,[8] and Naik et al.[5] Aljohani et al.[9] and Wakai et al.[10] in their studies found no correlation between Hb levels and periodontal status, when they compared Hb levels in patients with periodontitis of different severity, whereas in the present study, Hb level in periodontitis patients versus healthy controls was compared.
After non-surgical periodontal therapy which included scaling and root planing, a statistically significant increase in the Hb concentration, hematocrit value, and RBC count in the test group at 6 months was seen. These results are in accordance with the results of the studies conducted by Hutter et al.,[6] Gokhale et al.,[8] Naik et al.,[5] and Pradeep et al.[4] According to these authors and a few studies[11,12,13,14,15,16,17,18] in the past, periodontitis patients have elevated levels of acute phase proteins, interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF). These inflammatory mediators are shown to suppress mature erythroid progenitors[19] and inhibit in vitro colony formation by erythroid burst-forming units and erythroid colony-forming units from normal human marrow.[20,21,22] Inhibition of erythropoietin, the hormone responsible for erythropoiesis, was also seen.[23] This leads to decrease in RBC count.
Some studies also showed a disturbance in iron regulatory system. In vitro stimulation of fresh human hepatocytes with IL-6 showed strong induction of hepcidin, a liver hormone which caused disturbance in iron regulatory mechanism.[24] Hepcidin causes ferroportin internalization and degradation, thereby decreasing iron transfer into blood plasma from the duodenum, from macrophages involved in recycling senescent erythrocytes, and from iron-storing hepatocytes, with the iron stores being adequate. This response restricts the iron supply to erythropoietic precursors, and hence, a decrease in Hb count was seen.[25]
The GI, PI, CAL, and PPD values showed a significant improvement in the test group from baseline to 6 months after non-surgical periodontal therapy consisting of scaling and root planing. These results are in accordance with the study results of Pradeep et al.[4]
Epidemiological studies suggest that periodontitis is associated with an increased risk for systemic diseases like cardiovascular diseases, cerebrovascular ischemia, atherosclerosis, and preterm low birth of infants.[26]
In India, anemia is more prevalent in females because of poor nutrition, increased menstrual losses, high incidence of tropical and intestinal infections, and other miscellaneous factors. Iron deficiency anemia is the most common type of anemia seen in India. Females are also prone to hormonal imbalance during puberty, during the reproductive phase, and toward menopausal age. The microbial flora and host immune response are altered leading to exaggerated response of the periodontal tissues to local factors.[27] Therefore, to eliminate bias, only male patients were included in the study.[8]
Smokers were also excluded from the study. Various studies[28,29,30] have evaluated the PPD, CAL, PI, GI, and radiographic bone measurements of smokers and non-smokers, and found a positive correlation between smoking and these clinical parameters. Studies also indicate elevated concentrations of TNF and IL-6 as a consequence of smoking.[31]
The present study shows an association between chronic periodontitis and signs of anemia, which suggests a measurable effect of periodontitis on the systemic condition of the patient.
This study also suggested that non-surgical periodontal therapy had a positive effect on the Hb count, RBC count, and hematocrit value at the end of 6 months.
Decreased MCV values suggest microcytosis which is most commonly caused by iron deficiency anemia and elevated levels of MCV suggest macrocytosis caused by vitamin deficiency. In this study, MCV values were in the normal reference range for both chronic periodontitis group and periodontally healthy group at baseline as well as 6 months post non-surgical periodontal therapy. This suggested that the anemic status was not due to iron deficiency or vitamin deficiency, as the MCV values decrease or increase in such conditions.
A decrease in the MCH value is seen in microcytic anemia caused due to iron deficiency, whereas an increase in MCH value is seen in macrocytic anemia caused due to vitamin deficiency. The present study shows MCH values within the reference range for both the groups at baseline and 6 months, indicating the anemia to be normocytic as seen in ACD.[4]
Though the mean Hb count is lower than the normal values, MCHC is within the normal reference ranges in both the groups at baseline and 6 months, indicating that the Hb concentration per volume of packed red cell is normal. MCHC within the normal reference range indicates normochromic anemia, as seen in ACD.[4]
The limitation of this study was that an analysis of serum ferritin levels and the soluble serum transferring receptor concentration, or a bone marrow examination was necessary to quantify the iron stores and definitely distinguish between ACD and iron deficiency anemia. Another limitation of the study was the small sample size. Therefore, further studies with a larger sample size should be conducted and the serum ferritin levels must also be assessed.
CONCLUSION
A correlation was found between chronic periodontitis and decreased levels of Hb, hematocrit value, and RBC count, suggesting that anemia is induced by inflammation caused in patients suffering from periodontal disease. The study also provides evidence that non-surgical periodontal therapy can improve the anemic status of the patients. Further cohort studies with a larger sample size are needed to find an association between chronic periodontitis and anemia and the effect of periodontal treatment on the anemic status of these patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Malhotra P, Kumari S, Kumar R, Varma S. Prevalence of anemia in adult rural population of North India. J Assoc Physicians India. 2004;52:18–20. [PubMed] [Google Scholar]
- 2.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 3.Means RT, Jr, Krantz SB. Progress in Understanding the pathogenesis of the anemia of chronic disease. Blood. 1992;80:1639–47. [PubMed] [Google Scholar]
- 4.Pradeep AR, Anuj S. Anemia of chronic disease and chronic periodontitis: Does periodontal therapy have an effect on anemic status? J Periodontol. 2011;82:388–94. doi: 10.1902/jop.2010.100336. [DOI] [PubMed] [Google Scholar]
- 5.Naik V, Acharya A, Deshmukh VL, Shetty S, Shirhatti R. Generalized, severe, chronic periodontitis is associated with anemia of chronic disease: A pilot study in urban Indian males. J Invest Clin Dent. 2010;1:139–43. doi: 10.1111/j.2041-1626.2010.00028.x. [DOI] [PubMed] [Google Scholar]
- 6.Hutter JW, van der Velden U, Varoufaki A, Huffels RA, Hoek FJ, Loos BG. Lower numbers of erythrocytes and lower levels of haemoglobin in periodontitis patients compared to control subjects. J Clin Periodontol. 2001;28:930–6. doi: 10.1034/j.1600-051x.2001.028010930.x. [DOI] [PubMed] [Google Scholar]
- 7.Lainson PA, Brady PP, Fraleigh CM. Anemia, a systemic cause of periodontal disease? J Periodontol. 1968;39:35–8. doi: 10.1902/jop.1968.39.1.35. [DOI] [PubMed] [Google Scholar]
- 8.Gokhale SR, Sumanth S, Padhye AM. Evaluation of blood parameters in patients with chronic periodontitis for signs of anemia. J Periodontol. 2010;81:1202–6. doi: 10.1902/jop.2010.100079. [DOI] [PubMed] [Google Scholar]
- 9.Aljohani HA. Association between hemoglobin level and severity of chronic periodontitis. JKAU: Med Sci. 2009;17:53–64. [Google Scholar]
- 10.Wakai K, Kawamura T, Umemura O, Hara Y, Machida J, Anno T, et al. Associations of medical status and physical fitness with periodontal disease. J Clin Periodontol. 1999;26:664–72. doi: 10.1034/j.1600-051x.1999.261006.x. [DOI] [PubMed] [Google Scholar]
- 11.Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Perodontol. 2001;72:1221–7. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- 12.Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35:277–90. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 13.Mealey BL, Klokkevold PR. Periodontal medicine. In: Newman MG, Takei HH, Carranza FA, editors. Carranza's Clinical Periodontology. 9th ed. Philadelphia: Saunders; 2002. pp. 229–44. [Google Scholar]
- 14.D’Aiuto F, Parkar M, Andreou G, Suvan J, Brett PM, Ready D, et al. Periodontitis and systemic inflammation: Control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83:156–60. doi: 10.1177/154405910408300214. [DOI] [PubMed] [Google Scholar]
- 15.D’Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. 2005;84:269–73. doi: 10.1177/154405910508400312. [DOI] [PubMed] [Google Scholar]
- 16.Ide M, Jagdev D, Coward PY, Crook M, Barclay GR, Wilson RF. The short-term effects of treatment of chronic periodontitis on circulating levels of endotoxin, C-reactive protein, tumor necrosis factor-alpha, and interleukin-6. J Periodontol. 2004;75:420–8. doi: 10.1902/jop.2004.75.3.420. [DOI] [PubMed] [Google Scholar]
- 17.Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72:1221–7. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- 18.Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000;71:1528–34. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- 19.Johnson CS, Keckler DJ, Topper MI, Braunschweiger PG, Furmanski P. In vivo hematopoietic effects of recombinant interleukin-la in mice: Stimulation of granulocytic, monocytic, megakaryocytic, and early erythroid progenitors, suppression of late erythroid progenitors, and reversal of erythroid suppression with erythropoietin. Blood. 1989;73:678–83. [PubMed] [Google Scholar]
- 20.Maury CP, Andersson LC, Teppo AM, Partanen S, Juvonen E. Mechanism of the anaemia in rheumatoid arthritis: Demonstration of raised interleukin- lß concentrations in anaemic patients and of interleukin 1 mediated suppression of normal erythropoiesis and proliferation of human erythroleukemia (HEL) cells in vitro. Ann Rheum Dis. 1988;47:972–8. doi: 10.1136/ard.47.12.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Means RT, Jr, Dessypris EN, Krantz SB. Inhibition of human erythroid colony-forming units by interleukin-1 is mediated by gamma interferon. J Cell Physiol. 1992;150:59–64. doi: 10.1002/jcp.1041500109. [DOI] [PubMed] [Google Scholar]
- 22.Zoumbos NC, Djeu JY, Young NS. Interferon is the suppressor of hematopoiesis generated by stimulated lymphocytes. J Immunol. 1984;133:769–74. [PubMed] [Google Scholar]
- 23.Faquin WC, Schneider TJ, Goldberg MA. Effect of inflammatory cytokines on hypoxia-induced erythropoietin production. Blood. 1992;79:1987–94. [PubMed] [Google Scholar]
- 24.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acutephase protein. Blood. 2003;101:2461–3. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 25.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–33. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scannapieco FA. Position paper. Periodontal disease as a potential risk factor for systemic diseases. J Periodontol. 1998;69:841–50. [PubMed] [Google Scholar]
- 27.Amar S, Chung KM. Effects of hormonal variation on the periodontium in women. Periodontol 2000. 1994;6:79–87. doi: 10.1111/j.1600-0757.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 28.Bergstrom J. Tobacco smoking and risk for periodontal disease. J Clin Periodontol. 2003;30:107–13. doi: 10.1034/j.1600-051x.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 29.Haffajee AD, Socransky SS. Relationship of cigarette smoking to attachment level profiles. J Clin Periodontol. 2001;28:283–95. doi: 10.1034/j.1600-051x.2001.028004283.x. [DOI] [PubMed] [Google Scholar]
- 30.Erdemir EO, Nalcaci R, Caglayan O. Evaluation of systemic markers related to anemia of chronic disease in the peripheral blood of smokers and non-smokers with chronic periodontitis. Eur Dent J. 2008;2:102–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Tappia PS, Troughton KL, Langley-Evans SC, Grimble RF. Cigarette smoking influences cytokine production and antioxidant defenses. Clin Sci. 1995;88:485–9. doi: 10.1042/cs0880485. [DOI] [PubMed] [Google Scholar]


