Abstract
Background:
A surface smear layer consisting of organic and inorganic material is formed on the root surface following mechanical instrumentation and may inhibit the formation of new connective tissue attachment to the root surface. Modification of the tooth surface by root conditioning has resulted in improved connective tissue attachment and has advanced the goal of reconstructive periodontal treatment.
Aim:
The aim of this study was to compare the effects of citric acid, tetracycline, and doxycycline on the instrumented periodontally involved root surfaces in vitro using a scanning electron microscope.
Settings and Design:
A total of 45 dentin samples obtained from 15 extracted, scaled, and root planed teeth were divided into three groups.
Materials and Methods:
The root conditioning agents were applied with cotton pellets using the Passive burnishing technique for 5 minutes. The samples were then examined by the scanning electron microscope.
Statistical Analysis Used:
The statistical analysis was carried out using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, version 15.0 for Windows). For all quantitative variables means and standard deviations were calculated and compared. For more than two groups ANOVA was applied. For multiple comparisons post hoc tests with Bonferroni correction was used.
Results:
Upon statistical analysis the root conditioning agents used in this study were found to be effective in removing the smear layer, uncovering and widening the dentin tubules and unmasking the dentin collagen matrix.
Conclusion:
Tetracycline HCl was found to be the best root conditioner among the three agents used.
Keywords: Citric acid, scanning electron microscope, tetracycline hydrochloride
INTRODUCTION
Periodontal diseases lead to loss of attachment and exposure of root surface of the teeth to the oral environment. Exposed cementum shows number of changes including accumulation of plaque and calculus on the surface, hypermineralized or demineralized surface areas, loss of collagen cross banding, and contamination with cytotoxin and endotoxins which cause depression in cell growth and viability of fibroblasts thus interfering with the new attachment.[1]
Complete decontamination of the affected root surface is not possible with mechanical methods alone.[2] After hand or ultrasonic scaling of the root surface, a surface smear layer (2-15 μm in thickness) consisting of organic and inorganic material, with particles varying in size from less than 1 μm to more than 15 μm is formed on the root surface[2] which serves as a barrier between the periodontal tissues and the root surface and may inhibit the formation of new connective tissue attachment to the root surface.[3]
For over 90 years, agents of various types have been placed on root surfaces in attempts to modify the diseased tooth structure and for the regeneration of periodontal tissues. Such treatment not only removes the smear layer but also enlarges dentinal tubules into which healing connective tissue can enter. Citric acid, tetracycline hydrochloride, doxycycline hydrochloride, phosphoric acid, sodium hypochlorite, ethylene diamine tetra acetic acid (EDTA), fibronectin, laminin, Cohn's factor, stannous fluoride, etc., are some of the chemical agents that have been used for root conditioning.[4]
Citric acid has been shown to change the surface characteristics of treated root surfaces by removing the smear layer, exposing the dentinal tubules and making the tubules appear wider and with funnel-shaped orifices.[5,6] It partially exposes collagen from radicular dentin which has been shown to improve fibrin linkage and consequently inhibit epithelial downgrowth to stimulate fibrous attachment and migration.[7] It may also act as a potent antibacterial agent.[6]
Tetracyclines are a group of bacteriostatic antimicrobials effective against a wide range of organisms and successfully tested in both animal models and clinical studies. Tetracycline studies demonstrate multiple beneficial properties toward regeneration; enhanced attachment and growth of gingival fibroblasts; good anti-collagenase activity; high substantivity; inhibition of parathyroid hormone-induced bone resorption and anti-inflammatory action. Dentin root surface demineralization by low pH tetracycline increases fibronectin, an extracellular matrix glycoprotein, binding. The adsorbed or bound fibronectin enhances fibroblast attachment and growth while suppressing epithelial cell attachment and growth. Furthermore, the slow release of biologically active tetracycline from the tooth surface occurs for at least 48 hours and up to 14 days.[8]
Doxycycline is effective against suspected causative microflora of periodontitis and has antienzymatic properties. Topical application of doxycycline has shown a long-lasting substantivity on periodontally diseased root surfaces. Antibacterial effect of doxycycline on root conditioned specimens persisted for 14 days.[9]
This study was undertaken to compare the effects of citric acid, tetracycline, and doxycycline on the instrumented periodontally involved root surfaces in vitro using a scanning electron microscope.
MATERIALS AND METHODS
For the present study, 15 single rooted anterior teeth indicated for extraction due to advanced chronic periodontitis were selected from the outpatient department, Department of Periodontology and Oral Implantology.
Freshly extracted single rooted human teeth with proximal attachment loss of 5 mm or more, no history of root planing or prophylaxis in the past 6 months, no history of acute pain or swelling, absence of any restoration or caries were selected.
Preparation of the specimen
A total of 45 dentin samples were obtained from the periodontally affected mid root region of 15 extracted teeth Following extraction, the teeth were washed and cleaned using a soft bristle brush and stored in distilled water
Scaling and root planing of root surfaces was done to obtain smooth, shiny hard surface
Samples were obtained from the cervical two thirds of the root by making two parallel grooves 0.5 mm depth with circular disc under copious water irrigation
The first groove was positioned horizontally at the CEJ and the second groove was made parallel to the first and apical to it. The portion of tooth above and below these grooves was sectioned off
Three longitudinal root sections were then prepared from each tooth by cutting the cervical two thirds of the root into two halves first and then splitting one of the halves into two more halves by cutting perpendicular to the first cut
The three samples from each tooth were stored in individual containers containing distilled water.
Root conditioning agents
The experimental groups were divided into A, B, and C based on the type of conditioning done
A – Application of saturated citric acid (pH 1) for 5 minutes. Saturated citric acid solution was prepared by slowly adding anhydrous citric acid powder to 50 ml of distilled water using a magnetic stirrer until no more crystals dissolved in the solution
B – Application of tetracycline HCl (pH 1.6). Tetracycline HCl (250 mg/ml) was made by mixing 500 mg in 2 ml of sterile water
C – Application of doxycycline (pH 2.2). Doxycycline HCl solution (100 mg/ml) was made by mixing doxycycline HCl powder (100 mg) in sterile water (1 ml).
Application of the solutions
Application of the respective agents on the sample was done passively with cotton pellets saturated with the agent that were changed every 30 seconds for a total period of 5 minutes
Following treatment, samples were rinsed with distilled water for 20 seconds and air dried.
Scanning photomicrographs of the root surfaces were taken at ×2000 and ×6000 magnification [Figures 1–6]. The specimens were examined for total number of tubules, number of patent tubules, percentage of patent tubules, and diameter of tubules and the results were statistically analyzed [Table 1 and Graphs 1–4].
Figure 1.

Surface morphology of root specimen treated with citric acid (pH 1) at a magnification of ×2000
Figure 6.

Surface morphology of root specimens treated with doxycycline (pH 2.2) at a magnification of ×6000
Table 1.
Total number of tubules, number of patent tubules, percentage of patent tubules, and diameter of tubules in root sections in the three experimental groups
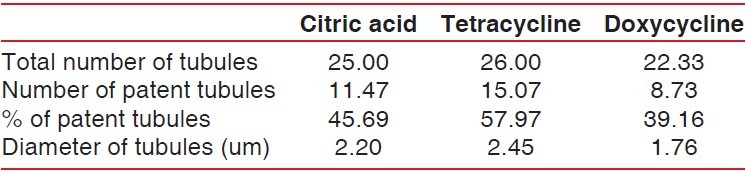
Graph 1.
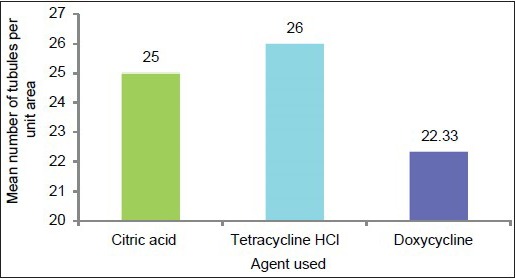
Graphic representation of mean number of tubules per unit area in the three study groups
Graph 4.
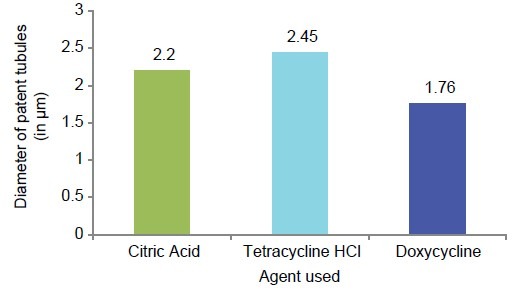
Graphic representation of mean diameter of tubules (μm) in the three study groups
Figure 2.
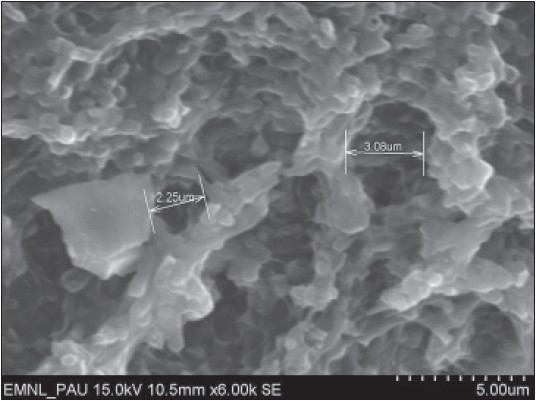
Surface morphology of root specimen treated with citric acid (pH 1) at a magnification of ×6000
Figure 3.

Surface morphology of root specimens treated with tetracycline (pH 1.6) at a magnification of ×2000
Figure 4.
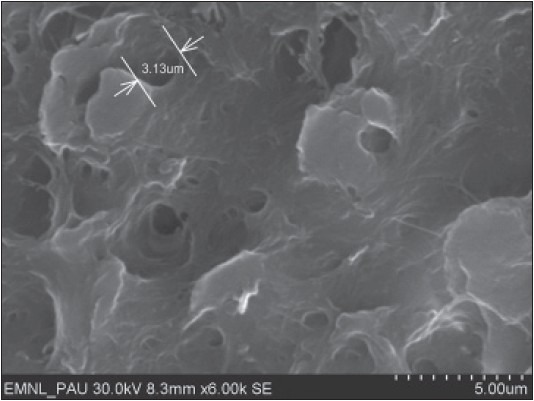
Surface morphology of root specimens treated with tetracycline (pH 1.6) at a magnification of ×6000
Figure 5.
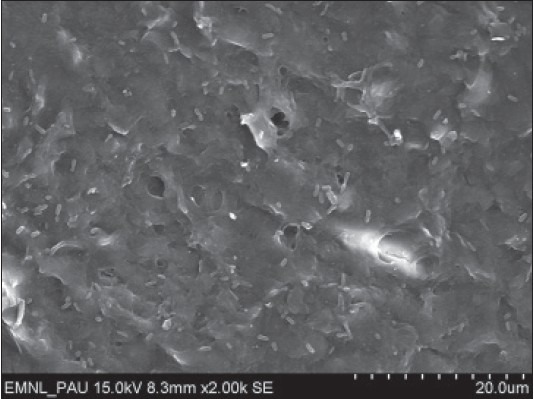
Surface morphology of root specimens treated with doxycycline (pH 2.2) at a magnification of ×2000
Graph 2.
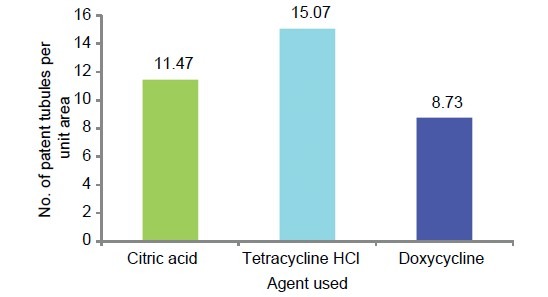
Graphic representation of mean number of patent tubules per unit area in the three study groups
Graph 3.
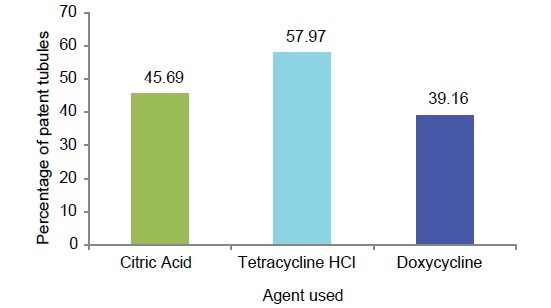
Graphic representation of percentage of patent tubules in each group
OBSERVATION AND RESULTS
Scaled and root planed tooth surfaces after treatment with root conditioning agents for 5 minutes appeared smooth to undulating with numerous well-defined round to oval dentinal tubule orifices. Tubule orifices were irregular in shape, several being flared or funnel shaped. Some of the tubular orifices were occluded.
The multiple comparisons Bonferroni test was applied to compare the three groups. The mean number of total tubules per unit area in the tetracycline HCl-treated group was higher than in the doxycycline group and the citric acid group. The mean difference in total number of tubules in specimens treated with tetracycline and doxycycline was highly significant (P = 0.001). Similarly, the mean difference in total number of tubules in specimens treated with citric acid and doxycycline was significant (P < 0.05), but the mean difference in total number of tubules in specimens treated with citric acid and tetracycline was not significant (P > 0.05) [Table 2 and Graph 5].
Table 2.
Mean comparison of total number of tubules between all the three study groups
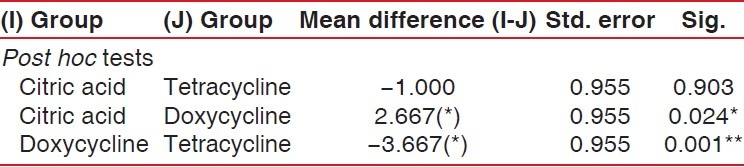
Graph 5.
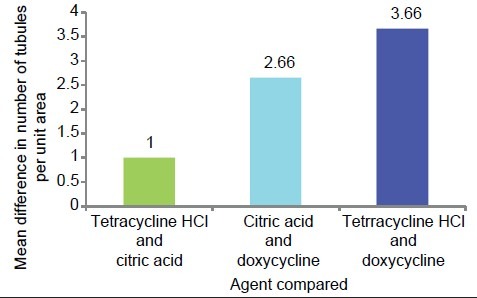
Graphic representation of group-wise mean difference of total number of tubules per unit area
The multiple comparisons Bonferroni test for the comparison of number of patent tubules per unit area between the three study groups showed high significance (P < .001) results [Table 3 and Graph 6].
Table 3.
Mean comparison of number of patent tubules per unit area between all the three study groups
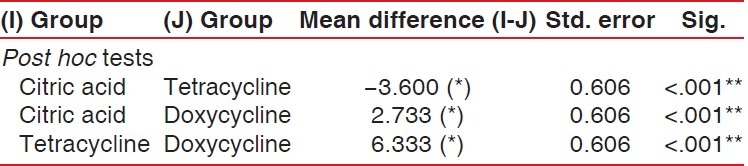
Graph 6.
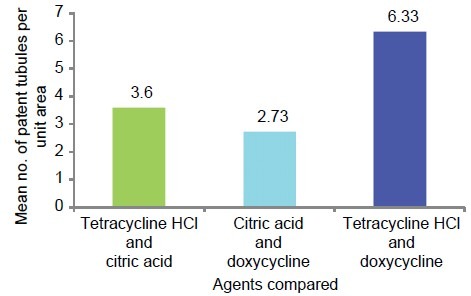
Graphic representation of group-wise mean difference of patent number of tubules per unit area
The multiple comparisons Bonferroni test for the comparison of percentage of patent tubules between the three study groups showed high significance (P < .001) results [Table 4 and Graph 7].
Table 4.
Mean comparison of percentage of patent tubules between all the three study groups
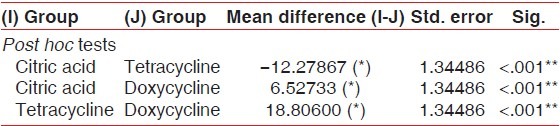
Graph 7.
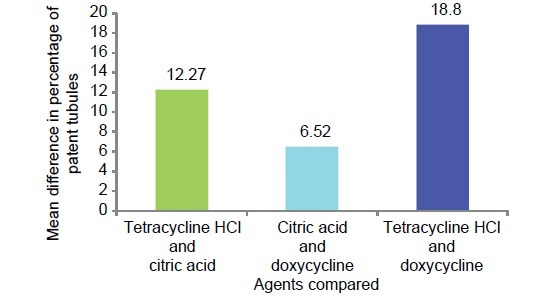
Graphic representation of group-wise mean difference of percentage of patent tubules
The mean diameter of tetracycline HCl-treated specimens was higher than the citric acid group and the doxycycline group. The mean difference of tubule diameter in citric acid-treated specimens and tetracycline-treated specimens was not significant (P > .05).
Similarly, the mean difference of tubule diameter in citric acid-treated specimens and doxycycline-treated specimens was not statistically significant (P >.05) but the mean difference between tetracycline and doxycycline was statistically significant (P < 0.05) [Table 5 and Graph 8].
Table 5.
Mean comparison of diameter of tubules between all the three study
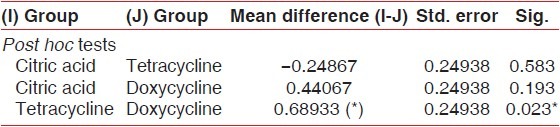
Graph 8.
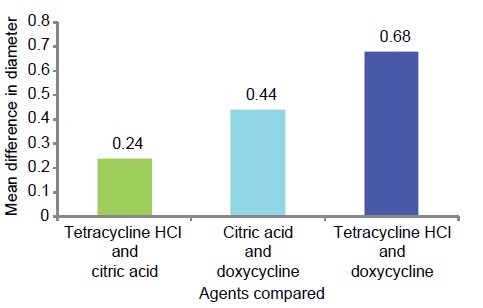
Graphic representation of group-wise mean difference of diameter of patent tubules (μm)
DISCUSSION
The single, most common primary etiological factor in periodontal disease is bacterial plaque. Periodontitis-affected root surfaces undergo histologic, physical, chemical, and immunochemical changes that may make them poor base for attachment and proliferation of fibroblasts. Root biomodification has been advocated as an effective way to remove the smear layer and condition the roots for better regeneration. Root biomodification uses acid substances or chelating agents to remove the smear layer and expose root collagen fibers. Independent of being conditioned, the collagen from the root surface attaches to fibrin present in a clot, preventing epithelial downgrowth, and forms a scaffold for cell development and mature collagen fiber attachment.[10]
Citric acid, tetracycline, and tetracycline derivatives such as minocycline HCl, doxycycline HCl, are frequently used as root conditioners, since their acid pH partially demineralizes the planed root surfaces, eliminating the smear layer, opening and widening the dentin tubules, and exposing various components (i.e. type I collagen, proteoglycans, fibronectin, growth factors) of cementum extracellular matrix or dentin extracellular matrix.[11] Several in vitro studies have suggested that root surface modifications by tetracycline HCl or by citric acid could influence the behavior of periodontal fibroblasts, improving their attachment and spreading.[12] Some of the mechanisms by which this can occur include (i) induced cementogenesis, (ii) collagen splicing, (iii) fibronectin fibrin-collagen binding thereby inhibiting epithelial apical migration, and (iv) enhanced fibroblast chemotaxis, migration, and attachment.[12,13,14,15] Other investigations have reported similar findings even though treatment variation existed in their experimental technique.[1] Contrastingly, Polson and Hanes (1987) were not able to demonstrate any fiber attachment to periodontitis-affected cementum following citric acid treatment. Differences between studies likely relate to basic biomechanical factors of periodontal wound healing overriding the experimental condition in the study by Polson and Hanes.[6]
Mechanical instrumentation of the root surfaces was done in order to remove the hypermineralized surface layer present on the periodontitis-affected roots and obtain a smooth glass-like surface.[16]
Application of the respective root conditioners on the sample was done passively with cotton pellets saturated with the agent that were changed every 30 seconds for a total period of 5 minutes. The “Passive Burnishing Technique” for application of root conditioner has been preferred over burnishing technique as the latter may itself form smear layer which partially or completely obliterate the dentinal tubule openings.[10] Changing pellets every 30-seconds helped to apply a constant concentration of the drug over the application interval. It has been suggested that this procedure enhances a chemical/mechanical action which chemically loosens inorganic material and surface debris, thereby exposing underlying dentin to demineralizing action of fresh acid.[16]
SEM analysis of the conditioned root surfaces revealed that removal of smear layer by tetracycline HCl (250 mg/ml) and citric acid was better than doxycycline (100 mg/ml). This could be attributed to the lower pH of tetracycline HCl (pH 1.6) and citric acid (pH 1) as compared to doxycycline HCl (pH 2.2). Removal of the smear layer was near total following a 5 minute application of citric acid, tetracycline, and doxycycline group except for few areas which were covered by debris. This debris may be (i) fragments of enamel, cementum, or dentin chipped off during instrumentation; (ii) foreign material that contaminated the surface during preparation of the specimen for SEM; (iii) precipitation artifacts resulting from interactions between buffer and fixative materials or between the specimen and these materials; or (iv) a combination of the above (Lasho, O’Leary and Kafrawy).[1]
A maximum mean total number of tubules were found in tetracycline HCl-treated specimens followed by citric acid-treated specimens followed by doxycycline. Lafferty TA, Gher ME and Gray JL also reported that conditioning with tetracycline HCl and citric acid produced comparable surface characteristics when used on periodontally diseased human root surfaces.[17] This is probably because pH of citric acid (pH 1) and tetracycline HCl (pH 1.6) was acidic enough to expose similar and considerable number of dentinal tubules whereas pH of doxycycline (pH 2.2) was less acidic as compared to the other two agents. These results are in accordance with studies done by Madison and Hokett and Shetty, Dinesh and Seshan.[8,4]
Tetracycline-treated specimens had the highest number of patent tubules when compared to citric acid and doxycycline. Doxycycline-treated specimens had the least number of patent tubules among the three agents used. These results are in agreement with earlier studies.[8,18,19] On the contrary, Sterrett JD, Simmons J, Whitford G, and Russell CM found that citric acid solution (pH 1.6) was more effective than tetracycline (150 mg/ml) in demineralizing dentin which could be attributed to the lesser concentration (150 mg/ml) of tetracycline used.[15]
The diameter of tetracycline HCl conditioned specimens was significantly more than doxycycline. The diameter of citric acid-treated specimens was more than doxycycline-treated specimens but less than tetracycline-treated specimens. Garberoglio and Braimstrom (1976), Tronstad (1973), Hanes, O’Brien, and Garnick (1987) have also shown that in addition to removing the smear layer these agents also enlarge the openings of the dentinal tubules as demonstrated by the increase in tubule diameter following treatment with them.[3] This enlargement or widening of the tubule orifice can be attributed to the preferential demineralization of the peritubular dentin by these agents.
In the present study, it was found that root conditioning in all the three experimental groups helped in the removal of smear layer, exposure of dentinal tubules, and also widening of dentinal tubule orifices in vitro. The type and degree of instrumentation of the specimens before acid treatment and the angulation of the electron beam may have affected the appearance of the root surface and dentinal tubular orifices slightly.
CONCLUSION
The root conditioning agents used in this study were found to be effective in removing the smear layer, uncovering and widening the dentin tubules, and unmasking the dentin collagen matrix. However, among the three groups, the results were best in tetracycline HCl conditioned group showing that the 250 mg/ml concentration of tetracycline HCl is best suited to be used as root conditioning agent. Hence, the application of tetracycline HCl as a root conditioner might have a significant role in periodontal wound healing and futuristic new attachment in vivo.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lasho DJ, O’Leary TJ, Kafrawy AH. A scanning electron microscope study of the effects of various agents on instrumented periodontally involved root surfaces. J Periodontol. 1983;54:210–20. doi: 10.1902/jop.1983.54.4.210. [DOI] [PubMed] [Google Scholar]
- 2.Polson AM, Frederick GT, Ladenheim S, Hanes PJ. The production of a root surface smear layer by instrumentation and its removal by citric acid. J Periodontol. 1984;55:443–6. doi: 10.1902/jop.1984.55.8.443. [DOI] [PubMed] [Google Scholar]
- 3.Hanes PJ, O’Brien NJ, Garnick JJ. A morphological comparison of radicular dentin following root planing and treatment with citric acid or tetracycline HCl. J Clin Periodontol. 1991;18:660–8. doi: 10.1111/j.1600-051x.1991.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 4.Shetty B, Dinesh A, Seshan H. The comparative effects of tetracyclines and citric acid on dentin root surface of periodontally involved human teeth: A scanning electron microscope study. J Indian Soc Periodontol. 2008;12:8–15. doi: 10.4103/0972-124X.44090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wen CR, Caffesse RG, Morrison EC, Nasjleti CE, Parikh UK. In vitro effects of citric acid application techniques on dentin surfaces. J Periodontol. 1992;63:883–9. doi: 10.1902/jop.1992.63.11.883. [DOI] [PubMed] [Google Scholar]
- 6.Polson AM, Hanes PJ. Cell and fiber attachment to demineralized dentin. J Clin Periodontol. 1987;14:357–65. doi: 10.1111/j.1600-051x.1987.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 7.Rompen EH, Goffinet GH, Nusgens B. Human periodontal ligament fibroblast behavior on chemically conditioned dentine: An in vitro study. J Periodontol. 1999;70:1144–52. doi: 10.1902/jop.1999.70.10.1144. [DOI] [PubMed] [Google Scholar]
- 8.Madison JG, Hokett SD. The effects of different tetracyclines on the root surface of instrumented, periodontally involved human teeth: A comparative scanning electron microscope study. J Periodontol. 1997;68:739–45. doi: 10.1902/jop.1997.68.8.739. [DOI] [PubMed] [Google Scholar]
- 9.Demirel K, Baer PN, McNamara TF. Topical application of doxycycline on periodontally involved root surfaces in vitro: Comparative analysis of subtantivity on cementum and dentin. J Periodontol. 1991;62:312–6. doi: 10.1902/jop.1991.62.5.312. [DOI] [PubMed] [Google Scholar]
- 10.Leite FR, Sampaio JE, Zandim DL, Dantas AA, Leite ER, Leite AA. Influence of root-surface conditioning with acid and chelating agents on clot stabilization. Quintessence Int. 2010;41:341–9. [PubMed] [Google Scholar]
- 11.Vanheusden AJ, Goffinet G, Zahedi S, Nusgens B, Lapiѐre CM, Rompen EH. In vitro stimulation of human gingival epithelial cell attachment to dentin by surface conditioning. J Periodontol. 1999;70:594–603. doi: 10.1902/jop.1999.70.6.594. [DOI] [PubMed] [Google Scholar]
- 12.Register AA, Burdick FA. Accelerated reattachment with cementogenesis to dentin, demineralized in situ I. Optimum range. J Periodontol. 1975;46:646–55. doi: 10.1902/jop.1975.46.11.646. [DOI] [PubMed] [Google Scholar]
- 13.Garrett JS, Crigger M, Egelberg J. Effects of citric acid on diseased root surfaces. J Periodont Res. 1978;13:155–63. doi: 10.1111/j.1600-0765.1978.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 14.Polson AM, Proye MP. Effect of root surface alterations on periodontal healing. II. Citric acid treatment of the denuded root. J Clin Periodontol. 1982;9:441–54. doi: 10.1111/j.1600-051x.1982.tb02105.x. [DOI] [PubMed] [Google Scholar]
- 15.Misra V, Mehrotra KK, Dixit J, Maitra SC. Effect of a carbon dioxide laser on periodontally involved root surfaces. J Periodontol. 1999;70:1046–52. doi: 10.1902/jop.1999.70.9.1046. [DOI] [PubMed] [Google Scholar]
- 16.Isik AG, Tarim B, Hafez AA, Yalcin FS, Onan U, Cox CF. A comparative scanning electron microscopic study on the characteristics of demineralized dentin root surface using different tetracycline HCl concentrations and application times. J Periodontol. 2000;71:219–25. doi: 10.1902/jop.2000.71.2.219. [DOI] [PubMed] [Google Scholar]
- 17.Lafferty TA, Gher ME, Gray JL. Comparative SEM study on the effect of acid etching with tetracycline HCl or citric acid on instrumented periodontally involved human root surfaces. J Periodontol. 1993;64:689–93. doi: 10.1902/jop.1993.64.8.689. [DOI] [PubMed] [Google Scholar]
- 18.Leite FR, Sampaio JE, Zandim DL, Dantas AA, Leite ER, Leite AA. Influence of root-surface conditioning with acid and chelating agents on clot stabilization. Quintessence Int. 2010;41:341–9. [PubMed] [Google Scholar]
- 19.Ashok KP, Shobha PM. A comparative scanning electron microscope study on the effect of acid etching with citric acid, tetracycline hydrochloride and EDTA on instrumented, periodontally involved root surfaces. Indian J Stomatol. 2010;1:61–6. [Google Scholar]


