Abstract
Objective
Trauma exposure can precipitate acute/post-traumatic stress responses (AS/PTSD) and disabling cardiovascular disorders (CVD). Identifying acute stress-related physiologic changes that may increase CVD risk could inform development of early CVD-prevention strategies. The endocannabinoid system (ECS) regulates hypothalamic-pituitary-adrenal (HPA) axis response and stress-related cardiovascular function. We examine stress-related endocannabinoid system (ECS) activity and its association with cardiovascular biochemistry/function following acute stress.
Methods
Rodents (n=8-16/group) were exposed to predator odor or saline; elevated plus maze (EPM), blood pressure (BP), serum and cardiac tissue ECS markers, and lipid metabolism were assessed at 24h and 2wks post-exposure.
Results
At 24h the predator odor group demonstrated anxiety-like behavior and had (a) elevated serum markers of cardiac failure/damage (brain natriuretic peptide [BNP]: 275.1 vs. 234.6, p=0.007; troponin-I: 1.50 vs. 0.78, p=0.076), lipogenesis (triacylglycerols [TAG]: 123.5 vs. 85.93, p=0.018), and inflammation (stearoyl delta-9 desaturase activity [SCD-16]: 0.21 vs. 0.07, p<0.001); (b) significant decrease in cardiac endocannabinoid (2-arachidonoyl-sn-glycerol, 2-AG: 29.90 vs. 65.95, p<0.001) and fatty acid ethanolamides (FAE: oleoylethanolamide, OEA: 114.3 vs. 125.4, p=0.047; palmitoylethanolamide, PEA: 72.96 vs. 82.87, p=0.008); and (c) increased cardiac inflammation (IL-1β/IL-6 ratio: 19.79 vs.13.57, p=0.038; TNF-α/IL-6 ratio: 1.73 vs. 1.03, p=0.019) and oxidative stress (thiobarbituric acid reactive substances [TBARS]: 7.81 vs. 7.05, p=0.022), that were associated with cardiac steatosis (higher TAG: 1.09 vs. 0.72, p<0.001). Cardiac lipogenesis persisted, and elevated BP emerged two weeks after exposure.
Conclusions
Acute psychological stress elicits ECS-related cardiac responses associated with persistent, potentially-pathological changes in rat cardiovascular biochemistry/function.
Keywords: Acute stress, cardiovascular status, endocannabinoids, lipogenesis, inflammation, triacylglycerols
Introduction
Acute stress (AS) and post-traumatic stress disorder (PTSD) can increase risk for immediate [1] and long-term development of cardiovascular disorders (CVD) [2, 3]. Despite mounting evidence of a trauma-CVD connection, biological of AS that may presage later development of CVD remain unknown. While medications are being considered for PTSD prevention immediately following trauma exposure [4], identifying early targets for preventing trauma-related CVD, and hence its morbidity/mortality, has received little attention. Yet the early aftermath of trauma remains a potential critical period for just such interventions. If AS produces physiologic changes with enduring CVD-related effects, identifying these early physiologic markers could guide development of new interventions aimed at minimizing the risk of trauma-related mental and physical disease. The significance and urgency of this research is highlighted by CVD's role as the leading cause of death and disability worldwide [5]. In this paper we describe experiments focused on identifying biological markers of early CVD related to acute stress.
Acute stress and cardiovascular disorders
AS can trigger a serious, but reversible short-term cardiomyopathy with no known chronic impact [1], and life-threatening cardiovascular responses (e.g., arrhythmia) associated with chronic disease in individuals with pre-existing CVD (e.g., atherosclerosis) [6]. However, heightened cardiovascular reactivity and delayed cardiovascular recovery following acute laboratory stressors are associated with increased carotid intima-media thickness and higher systolic and diastolic BP—both markers of chronic CVD—even among healthy populations [7]. AS has also been linked to increased CVD in otherwise healthy Americans following collective stress [3]. If AS contributes to subsequent development of CVD in healthy populations, understanding the underlying pathology could support discovery of new therapeutic approaches to early secondary prevention of trauma-related CVD.
Endocannabinoid system, acute stress, and cardiovascular disorders
The endocannabinoid system (ECS) modulates the function of several physiologic systems (e.g., immune, endocrine, and cardiovascular) [8], and regulates glucose/lipid metabolism to conserve and store energy (e.g., lipogenesis) [9]. Anandamide and 2-arachidonoyl-sn-glycerol (2-AG)—two key endocannabinoids—are also central to hypothalamic-pituitary-adrenal (HPA) axis stress response: Anandamide tonically constrains HPA activation by acting in the amygdala, while stress-related increases in 2-AG throughout the limbic system help shut it down [8, 10]. These changes in anandamide and 2-AG also affect sympathetic nervous system activity, serum inflammatory markers, and lipid metabolism with significant implications for cardiovascular function [11]. Indeed, the ECS is actively involved in cardiovascular functions during stress, and it appears to have both cardioprotective and damaging potential [11, 12].
Two fatty acid ethanolamides (FAE) structurally related to anandamide—palmitoylethanolamide (PEA) and oleoylethanolamide (OEA)—are also involved in lipid metabolism and inflammatory response. Although little is known about their role in stress response, these compounds may also have cardioprotective effects: PEA has anti-inflammatory effects in rodents and humans [13, 14], and OEA improves cardiac function following experimental induction of cardiomyopathy [15]. Taken together, the ECS and related FAE regulate many physiologic functions central to cardiovascular health and stress response, making them ideal targets to consider for early intervention strategies.
The current study
Given that the ECS regulates AS and moderates stress-related cardiovascular function, and that OEA and PEA are involved in inflammation and lipid metabolism, we sought to understand the ECS, PEA, and OEA response to AS and their potential roles in subsequent cardiovascular health/function. Our central hypothesis is that AS will trigger not only characteristic anxiety-like behavioral responses, but also changes in ECS, inflammatory, and markers of lipid metabolism that are potentially pathological for cardiovascular function.
Methods
Animals
Two-to-three month old male Sprague-Dawley rats (weight: 250-300g) were trained for hemodynamic parameter measurement and odor exposure procedures for three to five days before starting the experiments. All experiments were conducted between 0800h and 1500h. Baseline hemodynamic parameters were assessed prior to all experiments. All procedures met National Institutes of Health guidelines for the care and use of laboratory animals, and were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine. All experiments were conducted between July 2011 and June 2012.
Experimental design
Acute stress exposure
As our interests ultimately lie in understanding the link between acute mental stress and physical disease, we used a well-established model of traumatic stress known to invoke acute anxiety-like behavior—predator odor [16]. This model mimics brief exposure to a life-threatening experience and is more natural and consistent with the animals’ threat perceptions than foot/tail shock or restraint. It reliably produces increased levels of stress-related hormones (adrenocorticotropin hormone [ACTH], corticosterone [the rodent counterpart of cortisol]), autonomic nervous system activation, elevated HR and BP, and behaviors suggesting acute psychological symptoms consistent with traumatic stress exposure [16].
Rats were exposed for 10 min to physiological saline (odor-free control substance) or 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), a pheromone secreted by the red fox, a natural predator of the rat. Procedures were specifically designed to prevent persistent exposure to the predator scent. Odor exposure was conducted in a fume hood to prevent predator odor from circulating through and contaminating the behavioral testing and animal housing areas. A sterile gauze square (5 × 5 cm) was impregnated with 35 μl of either saline or TMT in the fume hood and transported to the animal's home cage in a covered Petri dish [17]. The cover was removed and replaced with wire mesh. Saline-exposed rats were tested first, followed by TMT-exposed rats the next day to minimize risk of TMT contaminating the control group and allow thorough cleaning of the odor-exposure room. TMT exposure was done on a different floor of the building from the one housing the rats. Following odor exposure, the odorant container was removed and animals in both experimental groups were placed in new clean cages, with new cage lids, food, and water bottles (stored outside the odor testing room) to ensure termination of odor exposure [18].
Experiment 1. Biological markers in response to AS and cardiovascular function at 24h
Rats were randomly assigned to two groups: Control group (10-min saline exposure; n=10) and TMT group (10-min TMT exposure; n=12). Hemodynamic parameters were measured twice: before (baseline) and 24h after odor exposure (before sacrificing). 24h after exposure, blood was collected under light anesthesia (isoflurane) into BD Vacutainer SST™ 5 ml tubes (Becton, Dickinson and Company, Franklin Lakes, NJ) and rats killed by decapitation. Heart, liver, lungs, adrenal glands, kidney, hypothalamus, hypophysis and remaining brain were harvested and kept at −80°C. Blood was centrifuged at 2000×g at 4°C for 30 min to obtain serum, which was stored at −80°C.
Experiment 2. Early behavioral response to acute stress and effects on cardiovascular and biological markers over time
Rats were randomly assigned to two groups: Control group (10-min saline exposure; n=8) and TMT group (10-min TMT exposure; n=16). The day before odor exposure, baseline hemodynamic parameters were measured. 24h after odor exposure, the elevated plus maze (EPM) test was conducted followed by hemodynamic parameter measurement. EPM and hemodynamic measurements were performed again 1-wk and 2-wk after odor exposure. Rats were killed at 2-wk and tissues harvested as described above. To control for the effects of exposure to a strong odor, we repeated the EPM component of this experiment at 24h with a second control group exposed to butyric acid (BA), a noxious, but non-threatening odor (3 exposure groups: saline, BA, TMT; n=8). Odor exposure procedures were identical to those in Experiment 1.
Measures
Heart rate (HR), systolic (SBP), diastolic (DBP) and mean BP were measured by volume pressure recording technology using the Non-Invasive Blood Pressure System CODA-6 (Kent Scientific Corporation, CODA, Torrington, CT). Anxiety-like behavior was assessed individually for 5 minutes with a standardized EPM protocol. Triacylglycerols (TAG), free fatty acids (FFA), OEA, PEA, anandamide, 2-AG, 2-palmitoylglycerol (2-PG), 2-oleoylglycerol (2-OG) and ceramides were extracted from tissues and serum and quantified using liquid chromatography mass spectrometry (LC/MS). N-acylphosphatidylethanolamine-specific phospholipase D (NAPEPLD) activity (necessary for anandamide, OEA, PEA production), fatty acid amide hydrolase (FAAH) activity (degrades anandamide, OEA), N-acylethanolamine-hydrolyzing acid amidase (NAAA) activity (degrades PEA), monoacylglycerol lipase (MGL) activity (degrades 2-AG), and diacylglycerol lipase (DGL) activity (necessary for 2-AG production) were each measured in cardiac tissue. Total RNA was extracted from heart and mRNA expression of genes related to inflammation, oxidative stress, and lipid metabolism was determined by quantitative RT-PCR with Taqman® PCR Master Mix in a Mx3000P plate thermocycler and normalized to β-actin expression. Measurement details are provided in Supplemental Digital Content (SDC) 1.
Statistical Analysis
Results are expressed as mean ± standard error of the mean (s.e.m.). Student's t-test examined whether group differences were statistically significant at p< 0.05 level. One-way ANOVA, followed by Bonferroni test, was used to assess statistical differences among saline, TMT, and BA groups. The non-parametric Mann-Whitney U-test evaluated group differences for the highly variable EPM indices. Pearson test was used to examine correlations between parameters. Analyses were conducted using GraphPad Prism software (GraphPad Software; San Diego, CA).
Results
24-hours post TMT exposure
Anxiety-like behavior
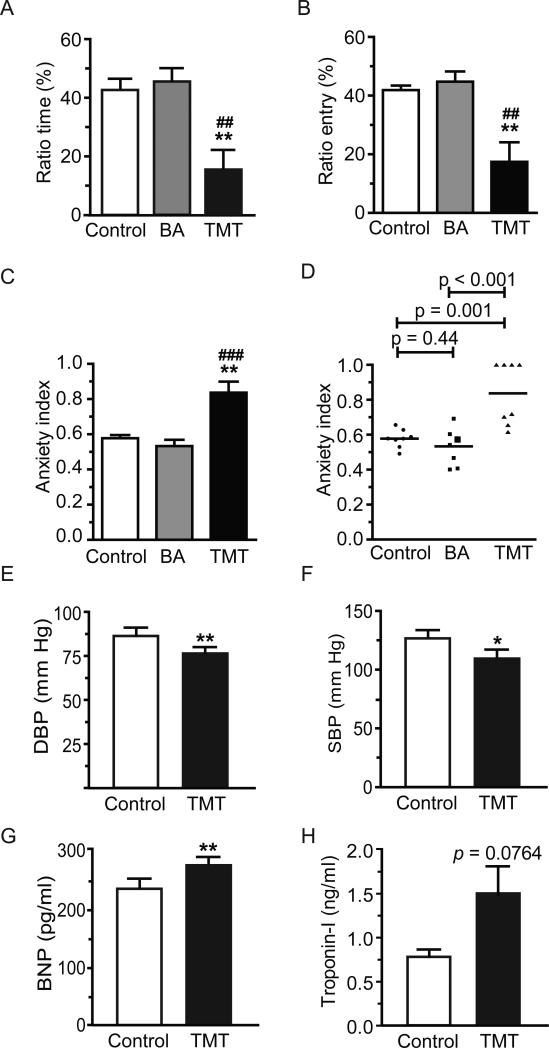
At 24h post-exposure, TMT elicited significant anxiety-like behavior in the EPM: Relative to control and BA-exposed rats, TMT-exposed rats showed significantly reduced ratio time (time spent in open arms/time in closed arms, Figure 1A, p=0.003 and p=0.001 respectively), with no significant difference between control and BA-exposed animals (p=0.23). Ratio entry (number of open arm entries/closed arm entries) was also lower in TMT versus control and BA-exposed rats (Figure 1B, p=0.003 and p=0.007 respectively), with no control versus BA-exposed group differences (p=0.34). TMT-exposed rats had significantly higher anxiety index than control and BA-exposed animals (Figure 1C, p=0.001 and p<0.001 respectively), with no control versus BA-exposed group differences (p=0.44). Anxiety index variability comparing TMT with control and BA groups is shown in Figure 1D. As there was no behavioral indication of anxiety in the BA-exposed group, the remaining tests were conducted with control and TMT-exposed animals only.
Figure 1.
Rats exposed to TMT demonstrated significantly more anxiety-like behavior at 24h post-exposure than rats exposed to saline or butyric acid (BA): (A) time spent in the open arms (ratio time); (B) open arms entries (ratio entries); (C) anxiety index; and (D) scatter plot of anxiety index variability. Horizontal lines represent the mean of each group (n = 8-16). TMT also decreased BP and induced biochemical changes consistent with heart failure and myocardial damage: (E) diastolic BP; (F) systolic BP; (G) serum brain natriuretic peptide (BNP) levels, and (H) serum troponin-I levels. Data are expressed as mean ± s.e.m. (n = 10-16). * p<0.05; ** p<0.01.
Hemodynamic parameters and cardiovascular markers
Baseline HR, DBP and SBP did not differ between control and TMT-exposed rats (p=0.53, p=0.24, p=0.10, respectively). DBP and SBP were significantly reduced in TMT-exposed rats 24h after exposure (Figure 1E, p=0.009; Figure 1F, p=0.011); HR was not significantly affected by TMT exposure (p=0.11).
Serum brain natriuretic peptide (BNP), a marker of heart failure [19], was significantly elevated 24h after TMT exposure (Figure 1G, p=0.007). Serum troponin-I, a marker of myocardial damage [20], was higher in TMT-exposed rats, but the difference only approached statistical significance (Figure 1H, p=0.076).
Cardiac lipid metabolism
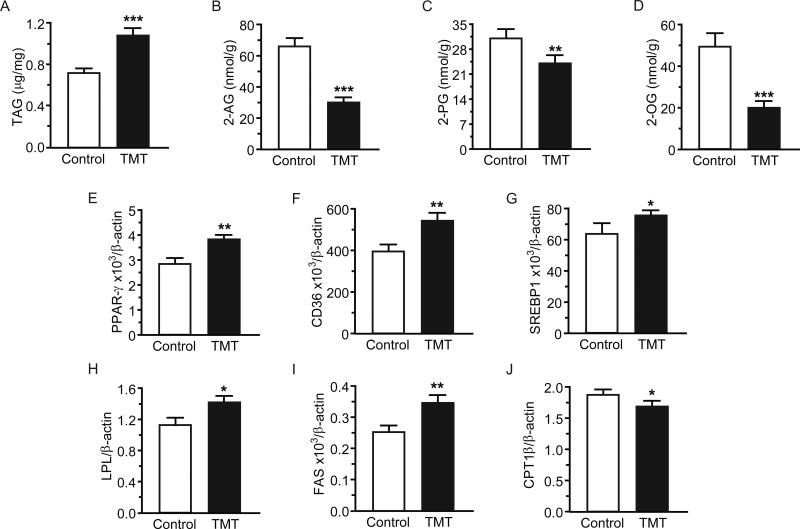
Relative to the control group, TMT-exposed animals had higher cardiac TAG levels and lower 2-AG, 2-PG and 2-OG levels 24h post-exposure (Figure 2A, p<0.001; Figure 2B, p<0.001; Figure 2C, p=0.010; Figure 2D, p<0.001, respectively). Cardiac TAG and 2-AG levels were significantly and negatively correlated (r=−0.76, p<0.001). Cardiac monoacylglycerol lipase (MGL) activity and expression were elevated (p=0.008, p<0.001, respectively), which may help explain reduced 2-AG levels. Diacylglycerol lipase (DGL) activity was not affected by TMT exposure (p=0.13) but expression of DGL-β increased (p=0.010).
Figure 2.
Acute TMT exposure increased triacylglycerol (TAG) content in the heart and modified the cardiac expression of enzymes and receptors involved in lipid metabolism regulation at 24h. Heart content of: (A) TAG; (B) 2-AG; (C) 2-PG; and (D) 2-OG. mRNA expression of: (E) peroxisome proliferator-activated receptor gamma (PPAR-γ); (F) cluster of differentiation 36 (CD36); (G) sterol regulatory element-binding protein-1 (SREBP-1); (H) lipoprotein lipase (LPL); (I) fatty acid synthase (FAS); and (J) carnitine palmitoyltransferase-1β (CPT-1β). Data are expressed as mean ± s.e.m. (n = 10-12). * p<0.05; ** p<0.01; *** p<0.001.
We examined whether TMT exposure induced changes in expression of lipid metabolism-related genes in the heart. Expression of several genes associated with lipogenesis was higher in TMT-exposed than in control animals. These included: peroxisome proliferator-activated receptor-gamma (PPAR-γ; Figure 2E, p=0.002), cluster of differentiation 36 (CD36; Figure 2F, p=0.001), sterol regulatory element-binding protein-1 (SREBP-1; Figure 2G, p=0.036), lipoprotein lipase (LPL; Figure 2H, p=0.0115), and fatty acid synthase (FAS; Figure 2I, p=0.001). Carnitine palmitoyltransferase-1β mRNA levels (CPT-1β), an enzyme involved in β-oxidation, were reduced (Figure 2J, p=0.027). Supplemental Table 1 in SDC2 shows heart levels of anandamide, FFA, ceramides and estimated enzyme activities at 24h.
Anti-inflammatory lipid mediators and cardiac inflammation
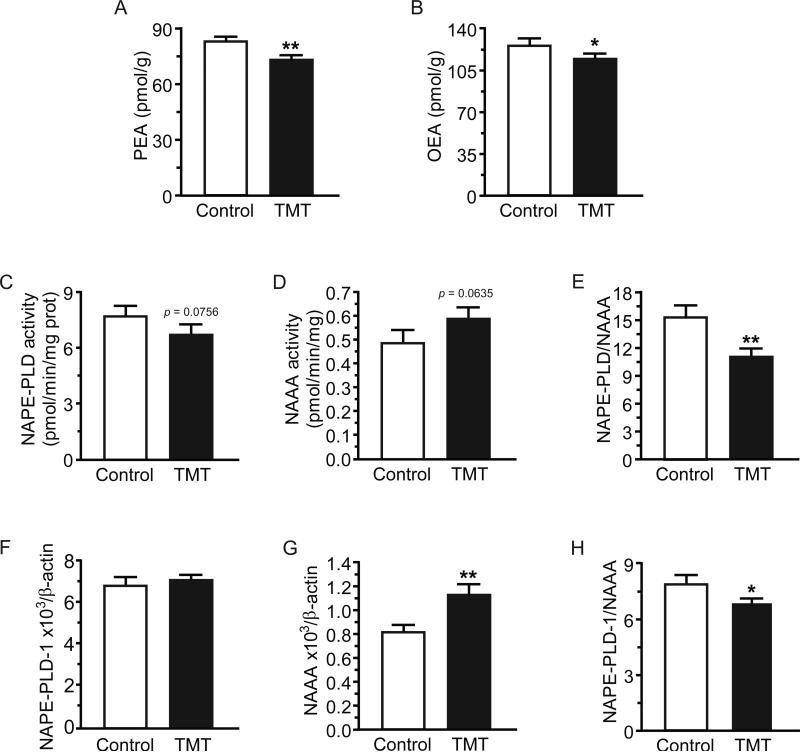
Cardiac levels of PEA and OEA were significantly reduced 24h after TMT exposure (Figure 3A, p=0.008; Figure 3B, p=0.047, respectively). NAPE-PLD activity trended lower (Control 7.73 ± 0.40, TMT 6.70 ± 0.38 [pmol/min/mg prot]; Figure 3C, p=0.076), while NAAA activity trended higher (Control 0.49 ± 0.04, TMT 0.59 ± 0.03 [pmol/min/mg prot]; Figure 3D, p=0.064) making the NAPE-PLD/NAAA ratio significantly lower (Figure 3E, p=0.003) in TMT-exposed versus control animals. This suggests that TMT exposure may lead to decreased production and increased degradation of PEA and OEA—two anti-inflammatory lipid mediators [21]. Similarly, TMT did not affect NAPEPLD mRNA transcription, but enhanced NAAA transcription (Control 6.88 ± 0.31, TMT 7.14 ± 0.20 [arbitrary units], Figure 3F, p=0.481; Control 0.82 ± 0.03, TMT 1.12 ± 0.07 [arbitrary units], Figure 3G, p=0.002, respectively), resulting in a significantly reduced NAPE-PLD/NAAA mRNA ratio in the TMT group (Figure 3H, p=0.032). FAAH activity trended lower (p=0.050) in TMT versus control animals, while FAAH expression was not affected by TMT (p=0.11).
Figure 3.
Acute TMT exposure induced an inflammatory phenotype in the heart at 24h by decreasing palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) levels. Heart levels of: (A) PEA; (B) OEA; (C) N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD) activity; (D) N-acylethanolamine-hydrolyzing acid amidase (NAAA) activity; and (E) the ratio NAPE-PLD to NAAA activity. mRNA expression of (F) NAPE-PLD1 and (G) NAAA in heart with (H) ratio NAPE-PLD1 to NAAA mRNA. Data are expressed as mean ± s.e.m. (n = 10-12). * p<0.05; ** p<0.01.
Supplemental Table 2 in SDC3 shows anandamide, 2-AG, PEA, and OEA, levels in central and peripheral tissues relevant to stress response and cardiovascular function/regulation. TMT exposure increased hypothalamic 2-AG and PEA and reduced pituitary anandamide and PEA. TMT decreased 2-AG in lungs, increased it in the liver, but did not affect anandamide, PEA, or OEA in either the lung or liver. TMT exposure increased adrenal gland PEA, but no other differences in kidney or adrenal gland emerged.
Serum TAG and inflammation
Serum TAG were elevated 24h-post TMT exposure (see Supplemental Figure 1A, p=0.018 in SDC4). Several markers of inflammation were also increased by TMT: 20:4ω6/22:6ω3 ratio (Control 0.47 ± 0.05, TMT 1.19 ± 0.04 [nmol/ml]; Control 0.14 ± 0.02, TMT 0.3 ± 0.01 [nmol/ml], respectively; see Supplemental Figure 1B, p< 0.001 in SDC4); 16:1ω3/16:0 ratio (Control 0.19 ± 0.02, TMT 0.54 ± 0.05 [nmol/ml]; Control 2.62 ± 0.17, TMT 2.64 ± 0.11 [nmol/ml], respectively; see Supplemental Figure 1C, p<0.001 in SDC4), a surrogate of stearoyl delta-9 desaturase activity (SCD-16); and 20:3ω6/18:2ω6 ratio (Control 0.046 ± 0.006, TMT 0.103 ± 0.004 [nmol/ml]; Control 5.03 ± 0.40, TMT 4.83 ± 0.19 [nmol/ml], respectively; see Supplemental Figure 1D, p<0.001 in SDC4), a surrogate of delta-6 desaturase activity (D6D).
Supplemental Table 3 in SDC5 shows serum levels of endocannabinoids, FAE, FFA, estimated enzyme activities, and ceramides. TMT exposure increased most FFA with significantly higher total FFA, unsaturated FFA, and poly-unsaturated fatty acid (PUFA), suggesting marked changes in lipid metabolism.
Oxidative stress and inflammation
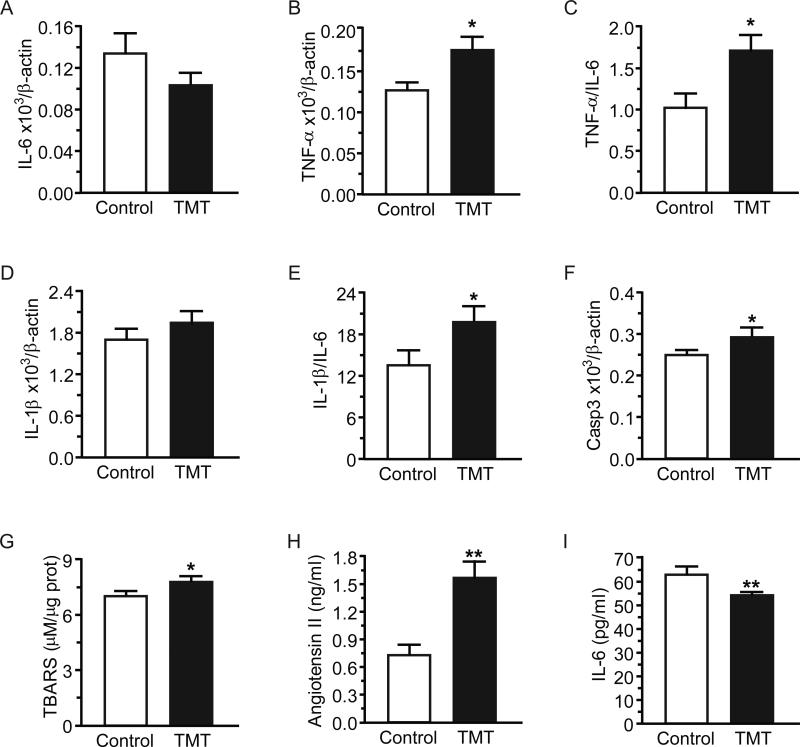
In heart tissue, TMT produced no significant changes in IL-6 mRNA levels at 24h (Control 0.134 ± 0.018, TMT 0.103 ± 0.010 [arbitrary units]), but TNF-α mRNA (Control 0.127 ± 0.007, TMT 0.1761 ± 0.016 [arbitrary units]) and the TNF-α/IL-6 ratio were increased in TMT versus control animals (Figure 4A, p=0.14; Figure 4B, p=0.014; Figure 4C, p=0.019, respectively). IL-1β expression was unaffected by TMT (Control 1.702 ± 0.1333, TMT 1.934 ± 0.1822 [arbitrary units]), however the IL-1β/IL-6 ratio was significantly elevated in TMT-exposed rats (Figure 4D, p=0.34; Figure 4E, p=0.038, respectively). Cardiac caspase-3 mRNA, a marker of apoptosis [22], and TBARS, an oxidative stress marker [23] were increased by TMT (Figure 4F, p=0.048; Figure 4G, p=0.022, respectively). In serum, TMT increased angiotensin-II, a peptide linked to oxidative stress [24-26] and apoptosis [27], and it also decreased IL-6 (Figure 4H, p=0.002; Figure 4I, p=0.006, respectively).
Figure 4.
Acute TMT exposure induced oxidative stress and inflammation at 24h. Heart mRNA expression of: (A) interleukin-6 (IL-6); (B) tumor necrosis factor-α (TNF-α); (C) ratio TNF-α to IL-6 mRNA; (D) interleukin-1β (IL-1β); (E) ratio IL-1β to IL-6; (F) caspase-3; and (G) thiobarbituric acid reactive substances (TBARS) content in the heart. Serum levels of (H) angiotensin-II and (I) IL-6. Data are expressed as mean ± s.e.m. (n = 10-12). * p<0.05; ** p<0.01.
1- and 2-weeks post TMT exposure
Hemodynamic parameters and anxiety-like behavior
TMT did not affect HR, DBP and SBP 1-wk post-exposure (p=0.62; p=0.57; p=0.91, respectively). However, unlike the TMT-related hypotension observed at 24h, TMT-exposed rats had significantly higher DBP and SBP than controls 2-wk after exposure (Figure 5A, p=0.008; Figure 5B, p=0.007, respectively), and HR trended lower, although the difference did not reach statistical significance (p=0.065). Anxiety-like behavior was significantly elevated in the TMT group through 1-week, but not at 2-weeks, post-exposure in keeping with previous research (see Supplemental Figures 2A-2D in SDC6).
Figure 5.
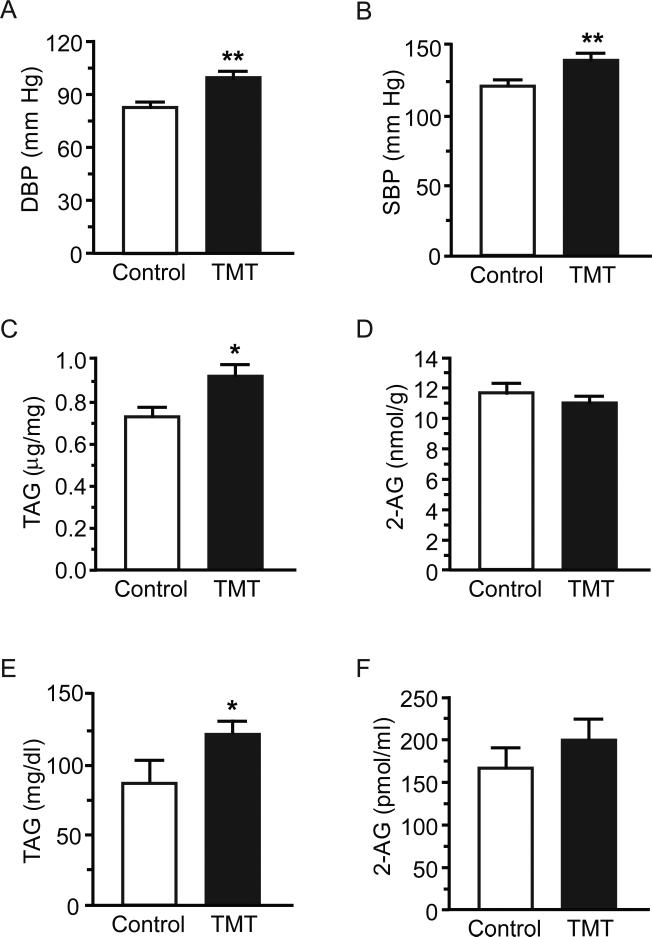
Acute TMT exposure increased BP and triacylglycerol (TAG) content in heart and serum at 2-wk: (A) diastolic BP; (B) systolic BP; (C) heart levels of TAG; (D) heart content of 2-AG; (E) serum levels of TAG; and (F) serum levels of 2-AG. Data are expressed as mean ± s.e.m. (n = 8-16). ** p<0.01.
2-week cardiac and serum TAG
Cardiac TAG content remained elevated at 2-wk in TMT-exposed versus control animals (Figure 5C, p=0.020), while 2-AG returned to baseline (Figure 5D, p=0.29); 2-OG also returned to baseline but 2-PG increased (p=0.64; p=0.034, respectively). Cardiac TAG and DBP levels were positively correlated at 2-wk (SBP: r = 0.38, p=0.067; DBP: r = 0.40, p=0.049). Serum TAG also remained elevated relative to controls (Figure 5E, p=0.040), while 2-AG did not differ between control and TMT animals (Figure 5F; p=0.39). 2-PG and 2-OG returned to baseline (p=0.91; p=0.15 respectively).
2-week serum markers of lipid metabolism, inflammation, and oxidative stress
Supplemental Table 4 in SDC7 shows 2-wk serum levels of FAE, FFA, estimated enzyme activities, and ceramides. Anandamide, PEA, and OEA were not affected by TMT at 2-wk. All saturated FFA, which were elevated 24h-post TMT exposure, returned to baseline by 2-wks, except for 16:0 which was unchanged at 24h but increased at 2-wk. TMT elicited a persistent elevation of most PUFA. Thus, TMT significantly increased serum total FFA and total unsaturated FFA. TMT also appeared to increase SCD-18 activity and reduce elongase activity, thereby creating a persistent inflammatory state. Supplemental Table 5 in SDC8 shows detailed cardiac lipid composition 2-wk after TMT. Cardiac TBARS trended higher in TMT animals but differences were not statistically significant (p=0.088). Serum BNP returned to baseline in TMT rats 2-wk after TMT exposure (p=0.20).
Discussion
Our goal was to examine whether acute exposure to a life-threatening event elicits cardiovascular effects associated with changes in ECS, inflammatory, and/or lipid metabolism using an animal model of acute stress. The rodents in our study demonstrated anxiety-like behavior suggesting the validity of our predator odor model of AS. These behavioral changes were also consistent with the pattern of ECS changes in the brain—decreased anandamide in the pituitary, increased 2-AG in the hypothalamus—as lower anandamide signaling is associated with anxiety-like behavioral responses to stress [8]. However, our model not only produced anxiety-like behavior consistent with a stress response, but also identified changes in cardiac ECS, inflammation, and TAG that are consistent with elevated risk for the development of CVD—potentially atherogenic inflammation and lipogenesis. The elevated cardiac TAG levels persisted over two weeks and were associated with altered cardiovascular function (i.e., significantly elevated DBP at 2-wks) in the absence of an ongoing behavioral response, as TMT-related anxiety-like behavior dissipated after 1-wk post-exposure. The single exposure to acute psychological stress also triggered biochemical changes associated with oxidative stress and apoptosis. In sum, we provide evidence of an association between early acute stress-related ECS activity and persistent, potentially-pathological change in cardiovascular biochemistry and function.
This study expands prior research addressing AS-related CVD in several ways. First, it suggests that AS may elicit biochemical changes that contribute to subsequent—not just immediate—development of CVD. Consistent with research on acute-onset stress cardiomyopathy [1], we document early hemodynamic and cardiovascular changes consistent with heart failure (BP, BNP), cardiomyocyte damage or death (troponin-I, caspase-3 mRNA expression) and oxidative stress (TBARS, angiotensin-II). We also provide new evidence of persistent alterations in inflammatory markers (SCD-18, elongase activity), cardiac and serum lipid metabolism (TAG, FFA), and hemodynamic measurements (BP) in acutely stressed, but otherwise healthy rodents.
Second, although acute stress was previously linked to serum cholesterol changes in humans [28, 29], our findings offer insight into early AS physiology that may promote stress-related CVD. That is, AS-related cardiovascular changes in ECS and FAE activity appear consistent with atherogenic inflammation and lipogenesis (e.g., elevated TAG) [30-32]. Importantly, the cardioprotective, anti-inflammatory role of ECS CB2 activity, which decreases atherosclerotic inflammation [12], may have been reduced through the higher MGL activity and lower cardiac 2-AG documented following AS, with cardiac 2-AG and TAG levels strongly and negatively correlated. Further examination of the pathways linking AS with these ECS-related changes is needed. Stress-response pathways involving glucocorticoid release [33], reninangiotensin-aldosterone system activity [25, 34, 35], and FAE (OEA, PEA) activity [36] are important candidates for this work.
Our findings also add to the growing clinical literature on cardiometabolic disease by suggesting that ECS activity may play a role in cardiac steatosis, a condition that increases risk of heart failure in diabetic patients [37, 38]. Although the ECS has recently emerged as an important player in cardiovascular pathology and protection, its role in the development of cardiac steatosis remains unknown [12]. However, human and rodent studies have described an obesity-related cardiac lipotoxicity characterized by elevated cardiomyocyte TAG, contractile dysfunction, and non-ischemic heart failure [38]. Similarly, our TMT-exposed rats initially had lower BP and elevated cardiac TAG, along with higher serum BNP and cardiac TNF-α mRNA—both known markers of heart failure. The fact that these consistent findings result from very different forms of stress—a chronic disease associated with hyperglycemia, hyperinsulinemia, obesity, and an upregulated ECS versus an acute psychological stress—suggests the possibility that there are subclinical ECS-related pathologic responses to acute stress that (a) may not be reversed when acute stress abates and (b) may increase long-term risk for CVD by promoting cardiac lipogenesis, steatosis, and failure. Considered more broadly, our findings raise questions about whether intermittent AS experienced over one's lifetime contributes incrementally to the development of CVD with subclinical changes that progressively increase risk for a major cardiovascular event, and how these changes might compare to the cardiovascular impact of chronic stress exposure. Indeed, recent epidemiologic work demonstrates that psychological distress has a dose-response association with subsequent cardiovascular mortality—the lowest levels of subclinical distress predicted a 25% increased risk over eight years [39]. Documenting trajectories of acute stress-related changes in ECS activity, lipogenesis, oxidative stress, and inflammation over an extended period, starting in the first 24h, could clarify how AS affects subsequent CVD development and facilitate identification of an optimal time for testing early CVD-prevention interventions.
Finally, given the known sex differences in human [40] and animal [41] stress response, our findings may be sex specific as we studied only young male rats. AS cardiomyopathy is most common in post-menopausal women, suggesting that age-by-sex differences may be relevant for understanding AS-related cardiovascular response. Moreover, the stronger behavioral response to predator odor in female (vs. male) mice may reflect a unique pattern of vulnerability to acute stress [41]. It is important to replicate our study with young females and older male/female rats to identify how age-related differences and sex hormones may affect AS-related ECS, FAE, and inflammatory responses.
Contributions and limitations
Using a controlled experimental model, we provide evidence of persistent cardiac steatosis induced by a single exposure to predator odor (life threat) in otherwise healthy rodents. We document changes in the expression of genes involved in ECS signaling, lipid metabolism, inflammatory response, and oxidative stress that are associated with AS exposure. In so doing, we highlight pathways that may link AS with persistent pathology in the heart that may contribute to subsequent development of CVD.
Nonetheless, we acknowledge limitations of this study. We did not identify the specific biochemical pathway(s) by which AS-related ECS or FAE changes affect cardiovascular function. Understanding the specific mechanisms underlying these relationships is essential for developing clinical applications. We have included biological measures at only two time points: 24h and 2wk, with no early measures between stress exposure and 24h. To fully understand the time course for acute stress physiology and optimal time for considering an intervention, earlier assessments are needed that track the trajectories of biomarkers over the first 24h. Similarly, although the 2-wk time frame we used in rats is consistent with approximately one human year [42], cardiac biochemistry and function should be examined over several weeks post TMT-exposure to fully understand the pathological impact of these findings. Extending the study to the equivalent of a few years (6-8 weeks) would give us a better idea of the degree to which these changes will affect long-term cardiac function.
In conclusion, we offer new evidence linking the ECS—a system known to affect both AS response and cardiovascular function—with persistent, potentially pathological cardiovascular change soon after AS exposure. Our findings draw from the growing literature on AS cardiomyopathy, ECS-related AS and cardiovascular regulation, and stress-related CVD to suggest new ways of understanding how AS may affect the development of subsequent CVD. Collectively, our observations indicate that the ECS and FAE OEA/PEA must be seriously considered as central players in the physiologic processes linking psychological stress and physical disease.
Supplementary Material
Acknowledgements
We would like to thank Dr. Guillermo Moreno-Sanz and Dr. Kwang-Mook Jung for their valuable scientific advice and Opeyemi Oluyemi for his technical help.
Sources of funding: This study was funded by the Robert Wood Johnson Foundation (RWJF) Nurse Faculty Scholars grant 68046 to E. Alison Holman, the intra-mural research program of the National Institute of Health's (NIH) Institute of Diabetes and Digestive and Kidney Diseases NIDDK-073955, and NIH Institute on Alcohol Abuse and Alcoholism grant NIAAA-RL1 AA017538 to Daniele Piomelli. These agencies supported the work of Drs. Holman (RWJF) and Guijarro (NIH) for this project and provided funding for laboratory supplies as needed. The agencies were not involved in determining the content or preparation of this manuscript.
Acronyms
- 2-AG
2-arachidonoyl-sn-glycerol
- 2-OG
2-oleoylglycerol
- 2-PG
2-palmitoylglycerol
- AS
Acute stress
- BA
Butyric acid
- BNP
Brain natriuretic peptide
- BP
Blood pressure
- CVD
Cardiovascular disorders
- DBP
Diastolic blood pressure
- DGL
Diacylglycerol lipase
- ECS
Endocannabinoid system
- EPM
Elevated plus maze
- FAAH
Fatty acid amide hydrolase
- FAE
Fatty acid ethanolamides
- FFA
Free fatty acids
- HPA
Hypothalamic-pituitary-adrenal axis
- HR
Heart rate
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- MGL
Monoacylglycerol lipase
- NAAA
N-acylethanolamine-hydrolyzing acid amidase
- NAPE-PLD
N-acylphosphatidylethanolamine-specific phospholipase D
- OEA
Oleoylethanolamide
- PEA
Palmitoylethanolamide
- PPAR
Peroxisome proliferator-activated receptor
- PTSD
Post-traumatic stress disorder
- PUFA
Polyunsaturated fatty acid
- SBP
Systolic blood pressure
- SCD-16
Stearoyl delta-9 desaturase activity
- SDC
Supplemental digital content
- TAG
Triacylglycerols
- TBARS
Thiobarbituric acid reactive substances
- TMT
2,5-dihydro-2,4,5-trimethylthiazoline (predator odor)
- TNF-α
Tumor necrosis factor-α
Footnotes
Conflict of Interest: The authors have nothing to disclose.
References
- 1.Wittstein IS. Stress cardiomyopathy: A syndrome of catecholamine-mediated myocardial stunning? Cell Mol Neurobiol. 2012;32:847–857. doi: 10.1007/s10571-012-9804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boscarino JA. A prospective study of ptsd and early-age heart disease mortality among vietnam veterans: Implications for surveillance and prevention. Psychosom Med. 2008;70:668–676. doi: 10.1097/PSY.0b013e31817bccaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holman EA, Silver RC, Poulin M, Andersen J, Gil-Rivas V, McIntosh DN. Terrorism, acute stress, and cardiovascular health: A 3-year national study following the september 11th attacks. Arch Gen Psychiatry. 2008;65:73–80. doi: 10.1001/archgenpsychiatry.2007.6. [DOI] [PubMed] [Google Scholar]
- 4.Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in iraq and post-traumatic stress disorder. New Engl J Med. 2010;362:110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 6.Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9:360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 7.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 8.Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuro-psychopharmacol Biol Psychiatry. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dipatrizio NV, Piomelli D. The thrifty lipids: Endocannabinoids and the neural control of energy conservation. Trends Neurosci. 2012;35:403–411. doi: 10.1016/j.tins.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Sullivan SE, Kendall PJ, Kendall DA. Endocannabinoids and the cardiovascular response to stress. J Psychopharmacol. 2012;26:71–82. doi: 10.1177/0269881111408457. [DOI] [PubMed] [Google Scholar]
- 12.Montecucco F, Di Marzo V. At the heart of the matter: The endocannabinoid system in cardiovascular function and dysfunction. Trends Pharmacol Sci. 2012;33:331–340. doi: 10.1016/j.tips.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Berdyshev E, Boichot E, Corbel M, Germain N, Lagente V. Effects of cannabinoid receptor ligands on lps-induced pulmonary inflammation in mice. Life Sci. 1998;63:PL125–129. doi: 10.1016/s0024-3205(98)00324-5. [DOI] [PubMed] [Google Scholar]
- 14.Richardson D, Pearson RG, Kurian N, Latif ML, Garle MJ, Barrett DA, Kendall DA, Scammell BE, Reeve AJ, Chapman V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2008;10:R43. doi: 10.1186/ar2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su HF, Samsamshariat A, Fu J, Shan YX, Chen YH, Piomelli D, Wang PH. Oleylethanolamide activates ras-erk pathway and improves myocardial function in doxorubicin-induced heart failure. Endocrinology. 2006;147:827–834. doi: 10.1210/en.2005-1098. [DOI] [PubMed] [Google Scholar]
- 16.Cohen H, Kozlovsky N, Alona C, Matar MA, Joseph Z. Animal model for ptsd: From clinical concept to translational research. Neuropharmacology. 2012;62:715–724. doi: 10.1016/j.neuropharm.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Hotsenpiller G, Williams JL. A synthetic predator odor (tmt) enhances conditioned analgesia and fear when paired with a benzodiazepine receptor inverse agonist (fg-7142). Psychobiology. 1997;25:83–88. [Google Scholar]
- 18.Hebb AL, Zacharko RM, Gauthier M, Drolet G. Exposure of mice to a predator odor increases acoustic startle but does not disrupt the rewarding properties of vta intracranial self-stimulation. Brain Res. 2003;982:195–210. doi: 10.1016/s0006-8993(03)03008-7. [DOI] [PubMed] [Google Scholar]
- 19.Palazzuoli A, Caputo M, Calabro A, Nuti R. Clinical impact of bnp and other emerging biomarkers in heart failure evaluation and management. Minerva Cardioangiol. 2012;60:183–194. [PubMed] [Google Scholar]
- 20.Vassiliadis E, Barascuk N, Didangelos A, Karsdal MA. Novel cardiac-specific biomarkers and the cardiovascular continuum. Biomarker Insights. 2012;7:45–57. doi: 10.4137/BMI.S9536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- 22.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 23.Pryor WA. The antioxidant nutrients and disease prevention--what do we know and what do we need to find out? Am J Clin Nutr. 1991;53:391S–393S. doi: 10.1093/ajcn/53.1.391S. [DOI] [PubMed] [Google Scholar]
- 24.Chen AF, Chen DD, Daiber A, Faraci FM, Li H, Rembold CM, Laher I. Free radical biology of the cardiovascular system. Clin Sci (Lond) 2012;123:73–91. doi: 10.1042/CS20110562. [DOI] [PubMed] [Google Scholar]
- 25.de Cavanagh EMV, Inserra F, Ferder L. Angiotensin ii blockade: A strategy to slow ageing by protecting mitochondria? Cardiovasc Res. 2011;89:31–40. doi: 10.1093/cvr/cvq285. [DOI] [PubMed] [Google Scholar]
- 26.Benigni A, Cassis P, Remuzzi G. Angiotensin ii revisited: New roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang C, Wang Y, Liu H, Li N, Sun Y, Liu Z, Yang P. Ghrelin protects h9c2 cardiomyocytes from angiotensin ii-induced apoptosis through the endoplasmic reticulum stress pathway. J Cardiovasc Pharmacol. 2012;59:465–471. doi: 10.1097/FJC.0b013e31824a7b60. [DOI] [PubMed] [Google Scholar]
- 28.Stoney CM, Bausserman L, Niaura R, Marcus B, Flynn M. Lipid reactivity to stress: Ii. Biological and behavioral influences. Health Psychol. 1999;18:251–261. doi: 10.1037//0278-6133.18.3.251. [DOI] [PubMed] [Google Scholar]
- 29.Stoney CM, West SG, Hughes JW, Lentino LM, Finney ML, Falko J, Bausserman L. Acute psychological stress reduces plasma triglyceride clearance. Psychophysiology. 2002;39:80–85. doi: 10.1017/S0048577202010284. [DOI] [PubMed] [Google Scholar]
- 30.Talayero BG, Sacks FM. The role of triglycerides in atherosclerosis. Curr Cardiol Rep. 2011;13:544–552. doi: 10.1007/s11886-011-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruby MA, Nomura DK, Hudak CS, Mangravite LM, Chiu S, Casida JE, Krauss RM. Overactive endocannabinoid signaling impairs apolipoprotein e-mediated clearance of triglyceride-rich lipoproteins. Proc Nat Acad Sci. 2008;105:14561–14566. doi: 10.1073/pnas.0807232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajesh M, Batkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horvath B, Holovac E, Cinar R, Liaudet L, Mackie K, Hasko G, Pacher P. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes. 2012;61:716–727. doi: 10.2337/db11-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism. 2011;60:1500–1510. doi: 10.1016/j.metabol.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Hongo M, Ishizaka N, Furuta K, Yahagi N, Saito K, Sakurai R, Matsuzaki G, Koike K, Nagai R. Administration of angiotensin ii, but not catecholamines, induces accumulation of lipids in the rat heart. Eur J Pharmacol. 2009;604:87–92. doi: 10.1016/j.ejphar.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Saavedra JM, Sanchez-Lemus E, Benicky J. Blockade of brain angiotensin ii at1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: Therapeutic implications. Psychoneuroendocrinology. 2011;36:1–18. doi: 10.1016/j.psyneuen.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solorzano C, Zhu C, Battista N, Astarita G, Lodola A, Rivara S, Mor M, Russo R, Maccarrone M, Antonietti F, Duranti A, Tontini A, Cuzzocrea S, Tarzia G, Piomelli D. Selective n-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc Nat Acad Sci. 2009;106:20966–20971. doi: 10.1073/pnas.0907417106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lingvay I, Raskin P, Szczepaniak LS. The fatty hearts of patients with diabetes. Nat Rev Cardiol. 2009;6:268–269. doi: 10.1038/nrcardio.2009.30. [DOI] [PubMed] [Google Scholar]
- 38.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 39.Russ TC, Stamatakis E, Hamer M, Starr JM, Kivimaki M, Batty GD. Association between psychological distress and mortality: Individual participant pooled analysis of 10 prospective cohort studies. Br Med J. 2012;345:e4933. doi: 10.1136/bmj.e4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- 41.Adamec R, Holmes A, Blundell J. Vulnerability to lasting anxiogenic effects of brief exposure to predator stimuli: Sex, serotonin and other factors-relevance to ptsd. Neurosci Biobehav Rev. 2008;32:1287. doi: 10.1016/j.neubiorev.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinn R. Comparing rat's to human's age: How old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.