Abstract
Background
Some studies have shown a decline in blood pressure (BP) over the second half of the twentieth century. However, the increasing prevalence of obesity may have opposite effects on recent cohorts.
Method
Using serial BP data from the Fels Longitudinal Study, we examined secular trends in mean BP, the rate of change in BP with age (slopes), and the influence of obesity (i.e., BMI) and height on these trends during young-to-middle adulthood. The study sample consisted of 970 adults, aged 18–40 years, who were born between 1920 and 1979. Participants were grouped into birth decade cohorts and had up to 11 serial measurements of SBP, DBP, and BMI. Sex-stratified mixed longitudinal analyses were used to identify cohort effects on mean BP at ages 19, 29, and 39 years, and on the rate of change in BP with age.
Results
For both sexes, mean SBP did not vary significantly by birth cohort, before and after adjusting for height and BMI. Mean DBP exhibited a U-shaped secular trend even after adjusting for BMI and height that was influenced by age-by-cohort effects. By age 39 years, those born most recently had the highest mean DBP.
Conclusion
There were cohort effects on the rate of change in DBP with age, but not on rate of SBP change. The most recent cohorts had higher rates of DBP change with age compared to the earlier cohorts. The secular trend was partially influenced by the trends in BMI.
Keywords: blood pressure, diastolic, longitudinal, secular trend, systolic, young adulthood
Introduction
The prevalence of stroke and high blood pressure (BP) has declined over the second half of the twentieth century [1–5]. Both SBP and DBP from National Health and Nutrition Examination Surveys (NHANES) I through III decreased by decade, for participants born between the period of 1887 and 1975 [3]. Additionally, more recent birth cohorts have exhibited smaller increases in BP with age than older birth cohorts [3]. Recent increases in childhood [6] and adulthood [7–9] obesity, however, may potentially reverse or attenuate previously observed decreasing trends in BP, as obesity is a significant risk factor for hypertension. Whereas some studies have demonstrated a continuing secular decrease in BP despite the increasing prevalence of obesity [5], others indicate that improvements in the prevalence of hypertension have begun to level off [10]. Although recent evidence indicates that between 2003 and 2006, the increases in childhood obesity have become non-significant [11], the ramifications of increased childhood obesity are expected to continue into the future as obese children tend to grow into obese adults [12–16].
Most of the evidence for secular trends in BP comes from large cross-sectional epidemiological studies [3,10,17]. However, cross-sectional studies of secular trends have several limitations, including potential sampling issues and the fact that cross-sectional data cannot document individual rates of change in BP as each individual is measured only once. Using longitudinal BP data from the Fels Longitudinal Study [18], we examine secular trends in mean BP, the rate of change in BP with age, and the influence of concurrent secular trends in obesity and height on both mean BP and the rate of change in BP with age during early-to-middle adulthood.
Methods
The Fels Longitudinal Study
The Fels Longitudinal Study began in 1929 in southwestern Ohio (Yellow Springs/Dayton). It is the world’s longest running study of human growth, development, and body composition and is composed of mostly white participants residing in the greater Dayton, Ohio area [18]. Participants were not recruited on the basis of having any particular condition or risk factor, rather, these life-long participants were recruited based on a sample of convenience, with the expectation of geographical stability and having attitudes associated with long-term commitment. Measurements were, and continue to be, taken every 2–5 years during adulthood, depending on the participant’s age and proximity to the research center.
At the socioeconomic level, Fels Longitudinal Study participants born prior to 1950 are generally representative of national data, while in more recent years, the lowest socioeconomic quintile has been somewhat under-represented. Sex-stratified prevalence rates of overweight and hypertension in Fels Longitudinal Study participants aged 20–34 and 35–44 years based on the most recent examination since 1999, are similar to NHANES 1999–2002 [19] values. Rates of overweight are slightly lower among Fels Longitudinal Study participants (45.4–71.4 vs. 52.8–71.5%), and hypertension rates are slightly higher (8.3–29.1 vs. 2.7–17.1%) compared to NHANES 1999–2002.
Study sample
Going back to 1929, the Fels Longitudinal Study consists of 1268 participants born between 1920 and 1979 and who were recruited before the age of 40 years. Across the entire 80-year period, 970 (76%) adults (479 men and 491 women) born between 1920 and 1979 were measured between the ages of 18 and 40 years and had at least one measurement of BP, weight, and stature. Participants had visits between February 1947 and December 2009, and had, on average, three serial measurements of BP, weight, and stature. Each participant was categorized into birth cohorts by decade. Due to limited numbers of participants, the oldest two birth cohorts, 1920–1929 and 1930–1939, were combined, resulting in five birth cohorts. Measurements taken during pregnancy were excluded. There were a total of 3263 measurements. All protocols were approved by the Institutional Review Board of Wright State University and informed consent was obtained at each study visit.
Blood pressure, stature, and weight
Seated BP (mmHg) was obtained through standardized protocols using a standard mercury sphygmomanometer and appropriate cuff sizes [20]. Three measurements of SBP and 5th phase DBP were taken with a 2-min rest between each determination. The second and third BP determinations were used to calculate the mean SBP and DBP. Stature (cm) and weight (kg) were measured using techniques according to the Anthropometric Standardization Reference Manual [21,22], and BMI was calculated as [weight (kg)/stature2 (m2)]. Stature, weight, and BP have been collected since 1929 and the reliability, as measured by intraclass correlation coefficients (ICCs) for both SBP and DBP, is consistently higher than 0.956. The ICCs for stature and weight are consistently higher than 0.999.
Statistical analysis
In order to identify cohort effects (secular trends) in SBP and DBP, we used PROC MIXED (SAS v 9.2; SAS Inc., Cary, North Carolina, USA) to fit sex-stratified mixed longitudinal models with participant-specific random intercept and age effects and an unstructured covariance matrix for these random effects. After testing for quadratic age effects within cohorts, we found that whereas BP increased nonlinearly with age over the entire adult lifespan, it was approximately linear between the ages of 18 and 40 years (the age range common to all the birth cohorts). Thus, age, cohort, and cohort-by-age were included in the model as covariates and the final model was:
where i indexes participants, j indexes observation times, k indexes birth cohorts, b0i and b1i represent the random intercept and age effects for the ith participant, respectively, β0kcohortik and β1kcohortik are the cohort-specific intercepts and age effects, respectively, where cohortik is 1 if participant i is in cohort k and 0 otherwise. Ages were centered at 29 years (the approximate middle of the age range) for ease of interpretation; thus, the intercept β0k represents the estimated mean BP at age 29 years for cohort k. We compared mean BP at ages 19, 29, and 39 years across cohorts. We chose these ages as representative of very early adulthood, young adulthood, and middle adulthood. The rates of increase in BP with age among the five cohorts were also compared using the slopes of the regression equations.
The influence of obesity and height on BP, two factors for which secular trends have been documented and that are known to influence BP, was also investigated by including BMI and height as covariates in the model (with BMI and height centered at their sex-specific means). Thus, the resulting cohort effects estimate the secular trend in BP after removing effects of any concurrent secular trends in obesity and height. We chose to use BMI instead of weight because BMI is less correlated with height.
Pairwise comparisons of mean BP and changes in BP between the five cohorts were two-sided and adjusted for multiple testing using the Holm–Bonferroni procedure with an overall type I error rate of α equal to 0.05, and k equal to 10 tests [23].
Results
Sample characteristics
Sample sizes and BP means by sex and cohort are shown in Table 1 (with individuals possibly represented more than once within their birth cohorts). Note that among women, there appears to be a linear secular trend in SBP, but not among men.
Table 1.
Study sample characteristics including visit information and means (standard deviation) for participants within cohorts
| N adults | Mean no. of visits | Meana SBP (mmHg) | Meana DBP (mmHg) | Meana height (cm) | Meana BMI (kg/m2) | Meana age (years) | Age rangea (years) | |
|---|---|---|---|---|---|---|---|---|
| Men | ||||||||
| 1920–1939 | 106 | 3.4 | 117.3 (10.7) | 74.4 (8.8) | 177.3 (6.0) | 25.4 (3.2) | 30.0 | 18.0–40.0 |
| 1940–1949 | 107 | 3.1 | 114.4 (10.6) | 70.4 (8.8) | 179.5 (6.6) | 24.0 (3.4) | 26.9 | 18.0–40.0 |
| 1950–1959 | 89 | 3.3 | 115.8 (10.7) | 68.1 (10.8) | 180.0 (7.2) | 23.7 (3.8) | 26.5 | 18.0–40.0 |
| 1960–1969 | 90 | 3.7 | 115.8 (10.8) | 71.5 (11.3) | 179.4 (7.4) | 25.0 (4.5) | 27.9 | 18.0–39.9 |
| 1970–1979 | 87 | 4.0 | 116.1 (11.6) | 73.6 (10.7) | 179.9 (7.5) | 24.9 (5.5) | 25.7 | 18.0–39.2 |
| Women | ||||||||
| 1920–1939 | 114 | 2.9 | 103.7 (9.4) | 67.5 (7.6) | 164.7 (5.5) | 21.6 (3.2) | 29.5 | 18.0–40.0 |
| 1940–1949 | 100 | 2.8 | 104.6 (9.7) | 65.9 (7.6) | 165.8 (5.6) | 22.7 (4.5) | 27.6 | 18.0–39.7 |
| 1950–1959 | 95 | 3.0 | 105.6 (9.6) | 65.0 (9.5) | 165.0 (5.7) | 22.9 (3.8) | 27.1 | 18.0–40.0 |
| 1960–1969 | 99 | 3.8 | 106.0 (11.3) | 66.5 (9.9) | 165.9 (6.3) | 25.1 (6.0) | 29.2 | 18.0–40.0 |
| 1970–1979 | 83 | 4.0 | 107.4 (10.4) | 67.4 (9.5) | 165.7 (7.2) | 24.7 (5.4) | 26.2 | 18.0–39.0 |
Values include all available serial measures from each individual.
Secular trends in blood pressure
The fixed effect parameter estimates (Table 2) indicate that there are cohort effects in mean SBP for women and in mean DBP for both men and women. There are also age-by-cohort effects on SBP in men and for DBP in both men and women.
Table 2.
Fixed effect parameters estimates (standard error of mean) from mixed models adjusting for age, cohort, and cohort-by-age effects
| Men
|
Women
|
|||
|---|---|---|---|---|
| SBP (mmHg) | DBP (mmHg) | SBP (mmHg) | DBP (mmHg) | |
| Intercept | 117.74 (1.02)*** | 76.26 (0.84)*** | 107.41 (0.93)*** | 68.72 (0.77)*** |
| 1920–1939a | 0.07 (1.37) | −2.48 (1.13)* | −2.48 (1.26)* | −0.63 (1.05) |
| 1940–1949a | −1.61 (1.38) | −5.09 (1.13)*** | −1.34 (1.30) | −2.03 (1.08) |
| 1950–1959a | −1.52 (1.44) | −6.87 (1.18)*** | −1.19 (1.30) | −2.73 (1.08)* |
| 1960–1969a | −2.06 (1.40) | −4.43 (1.15)** | −1.31 (1.26) | −2.30 (1.04)* |
| 1970–1979 | reference | |||
| Ageb | 0.53 (0.09)*** | 0.80 (0.09)*** | 0.17 (0.09) | 0.60 (0.08)*** |
| Age 31920–1939c | −0.22 (0.12) | −0.54 (0.12)*** | 0.14 (0.12) | −0.39 (0.11)** |
| Age 31940–1949c | −0.19 (0.13) | −0.64 (0.12)*** | 0.00 (0.13) | −0.67 (0.11)*** |
| Age 31950–1959c | −0.34 (0.12)* | −0.21 (0.12) | 0.08 (0.12) | −0.34 (0.10)* |
| Age 31960–1969c | −0.18 (0.12) | −0.03 (0.12) | 0.18 (0.11) | −0.20 (0.10) |
| Age 31970–1979 | reference | |||
Parameter estimates (SE).
1970–1979 is referent group.
Age centered at 29 years.
Age 31970–1979 is referent group.
P<0.05;
P<0.001;
P<0.0001.
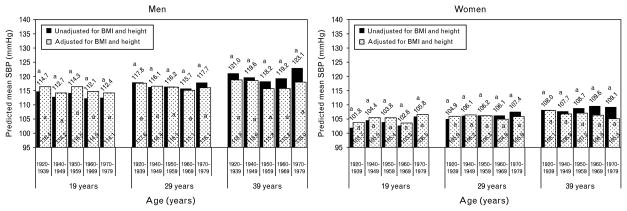
The predicted mean SBP and DBP values by cohort for this unadjusted model are plotted in Figs 1 and 2 respectively at ages 19, 29, and 39 years (black bars). Men had greater mean SBP (Fig. 1, black bars) and DBP (Fig. 2, black bars) than women in all cohorts, at every age. After adjusting for multiple tests, there were no cohort differences in mean SBP at any age for either men or women, as indicated by the similar letters (Fig. 1, black bars).
Fig. 1.
Predicted mean SBP before (black bars) and after adjusting for BMI and height (white dotted bars). Means with different letters (a,b,c) are significantly (P <0.05) different from each other, within each model and age group, stratified by sex, after adjusting for multiple testing using Holm–Bonferroni.
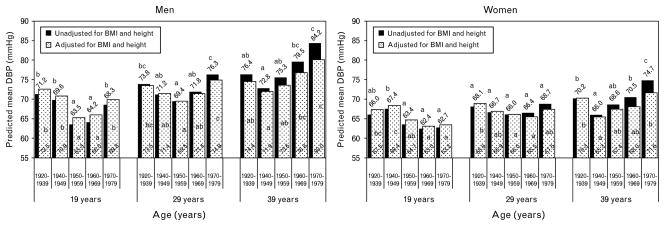
Fig. 2.
Predicted mean DBP before (black bars) and after adjusting for BMI and height (white dotted bars). Means with different letters (a,b,c) are significantly (P <0.05) different from each other, within each model and age group, stratified by sex, after adjusting for multiple testing using Holm–Bonferroni.
There were, however, significant differences in mean DBP between cohorts at all ages for both sexes except women at age 29 years as indicated by the different letters in Fig. 2 (black bars). For men at each age (19, 29, and 39 years) and for women at age 39 years, the cohort differences appear to follow a U-shaped trend across birth cohorts. Differences in DBP between cohorts, however, differ by age (due to the age-by-cohort effect).
In both sexes, when comparing between the earliest and latest birth cohorts (1920–1939 vs. 1970–1979), at age 19 years, the latest birth cohort has marginally lower mean DBP (68.3 vs. 71.2 mmHg in men and 62.7 vs. 66.0 mmHg in women). However, this slight secular improvement disappears at age 39, by which time (age) the latest cohort has significantly higher DBP (84.2 vs. 76.4 mmHg in men and 74.1 vs. 70.2 mmHg in women). Thus, the magnitude and direction of the overall secular trend in DBP depends on the period of adulthood considered. Participants born more recently, on average, start adulthood (at age 19) with marginally lower DBP than those born in earlier cohorts, but enter middle adulthood (at age 39) with significantly higher DBP.
With respect to the U-shaped secular trend, the cohort with the lowest predicted mean DBP (nadir) among men and women is most often the 1950–1959 birth cohort. The secular increase from the nadir is statistically significant (P <0.05) for men at all ages and for women at age 39 years. Also, in men, the difference between DBP at the nadir and the 1970–1979 cohort becomes larger at older ages. For example, the difference in mean DBP between the 1950–1959 and 1970–1979 cohorts is 4.8 mmHg (P <0.05) at age 19 years, 6.9 mmHg at age 29 years, and is 11.5 mmHg at age 39 years (Fig. 2, black bars). Among women, the trend is less clear, with significant mean differences in the nadir and peak occurring only in women at age 39 years. Thus, the magnitude of the secular increase in DBP, particularly from the nadir, is larger during middle adulthood than it is during younger adulthood.
Secular trends in the rate of change in blood pressure with age
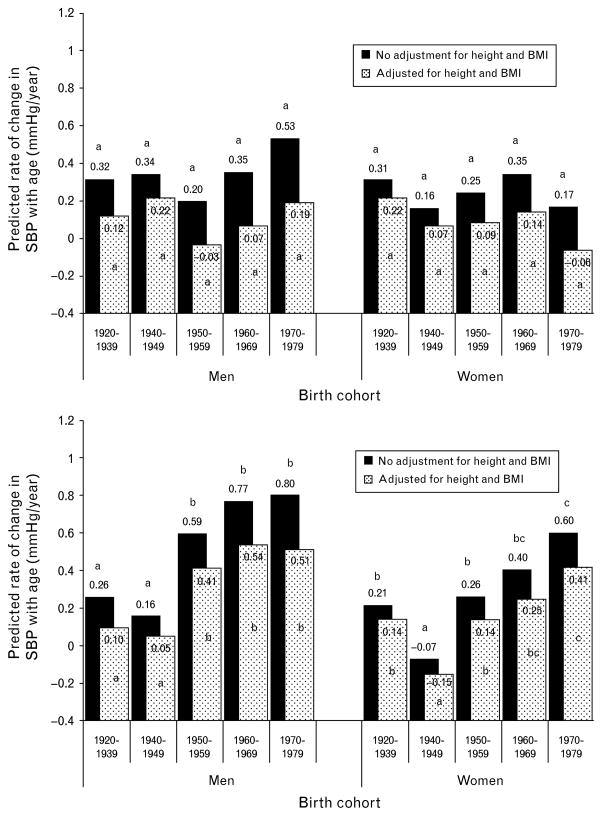
The rates of increase in BP from age 18 to 40 years are shown in Fig. 3 (black bars). The estimated cohort-specific rates ranged from 0.16 to 0.53 mmHg/year for SBP (Fig. 3, top half black bars), and −0.07 to 0.80 mmHg/year for DBP (Fig. 3, bottom half black bars). After adjusting for multiple comparisons, rates of change in SBP were not significantly different between cohorts for either men or women.
Fig. 3.
Predicted changes in SBP and DBP with age before (black bars) and after (white dotted bars) adjusting for BMI and height. Rates with different letters (a,b,c) are significantly (P <0.05) different from each other, within each model and age group, stratified by sex, after adjusting for multiple testing using Holm–Bonferroni.
There were significant cohort differences in the rate of change in DBP with age among both men and women (Fig. 3, bottom half, black bars). In both men and women, the 1940–1949 cohort had the slowest rate of DBP change with age. Among men, each of the last three cohorts (1950–1959, 1960–1969, and 1970–1979) had significantly greater rates of change in DBP than each of the two earliest cohorts (1920–1939 and 1940–1949; P <0.05, adjusted for multiple comparisons). Among women, the latest cohort (1970–1979) had a significantly greater rate of change in DBP than the three earliest birth cohorts (P <0.05, adjusted for multiple comparisons). The most recent cohort (1970–1979) had the highest rates of change with age, with mean rates of increase of 0.80 and 0.60 mmHg/year for men and women, respectively. These rates are approximately three times faster than the rate of DBP increase in the first (1920–1939) cohort.
Influence of adiposity and height on secular trends in blood pressure
As shown in Table 3, BMI is significantly positively associated with BP. Because BMI was centered at sex-specific mean values of 24.6 and 23.5 kg/m2 for men and women, respectively, in cohorts and ages wherein BMI tends to be less than these sex-specific values, predicted mean BP increases after adjusting for BMI, as mean BP is predicted at a higher BMI. Conversely, cohorts and ages with BMIs that tend to be greater than 24.6 and 23.5 kg/m2 for men and women, respectively, have lower predicted BP. Height was significant for SBP in men, but not in women or for DBP in either men or women.
Table 3.
Fixed effect parameters estimates (standard error of mean) of mixed models additionally adjusted for secular trends in BMI and height
| Men
|
Women
|
|||
|---|---|---|---|---|
| SBP | DBP | SBP | DBP | |
| Intercept | 116.07 (0.95)*** | 74.89 (0.77)*** | 105.89 (0.84)*** | 67.46 (0.73)*** |
| 1920–1939a | 1.56 (1.28) | −1.41 (1.03) | 0.02 (1.15) | 1.42 (1.00) |
| 1940–1949a | 0.35 (1.29) | −3.52 (1.04)** | 0.33 (1.16) | −0.60 (1.01) |
| 1950–1959a | 0.08 (1.34) | −5.43 (1.08)*** | 0.39 (1.17) | −1.42 (1.01) |
| 1960–1969a | −0.92 (1.30) | −3.47 (1.04)* | −0.99 (1.12) | −1.94 (0.97)* |
| 1970–1979 | reference | |||
| Ageb | 0.19 (0.09)* | 0.51 (0.09)*** | −0.06 (0.09) | 0.41 (0.08)*** |
| Age 31920–1939c | −0.07 (0.12) | −0.42 (0.11)** | 0.28 (0.12)* | −0.27 (0.10)* |
| Age 31940–1949c | 0.02 (0.12) | −0.46 (0.12)** | 0.13 (0.12) | −0.57 (0.11)*** |
| Age 31950–1959c | −0.23 (0.12) | −0.10 (0.11) | 0.15 (0.11) | −0.27 (0.10)* |
| Age 31960–1969c | −0.13 (0.11) | 0.02 (0.11) | 0.21 (0.11) | −0.16 (0.09) |
| Age 31970–1979 | reference | |||
| BMId | 1.09 (0.08)*** | 0.88 (0.07)*** | 0.82 (0.07)*** | 0.65 (0.06)*** |
| Heighte | 0.15 (0.05)* | 0.03 (0.04) | −0.02 (0.06) | −0.03 (0.05) |
Parameter estimates (SE).
1970–1979 is referent group.
Age centered at 29 years.
Age 31970–1979 is referent group.
BMI centered at 24.6 kg/m2 for men and 23.5 kg/m2 for women.
Height centered at 179.3 cm for men and 165.5 cm for women.
P<0.05;
P<0.001;
P<0.0001.
Predicted BP means after adjusting for BMI and height at ages 19, 29, and 39 years are shown in Figs 1 and 2 for this adjusted model (white dotted bars), with the means from the unadjusted models shadowed behind (black bars). The BMI adjustment does not alter any relationship between birth cohorts for SBP (Fig. 1, dotted white bars), although it does attenuate cohort differences in predicted mean SBP in both men and women at later ages (age >29 years). Interestingly, although the secular trend is not significant at age 39 years among women, predicted mean SBP increases slightly across cohorts when unadjusted for BMI and height (black bars), whereas it decreases slightly after adjustment (white dotted bars), thus demonstrating that raw SBP increases in this group are very much due to the increases in BMI and height over time.
For DBP (Fig. 2, dotted white bars), the BMI and height adjustment attenuates some cohort differences (e.g., women at 39 years) and magnifies others (e.g., women at age 19 and 29 years). Although adjustment for BMI and height changes the magnitude of mean DBP in men, it does not alter the significant differences in cohorts. In women at age 19 and 29 years, the significance patterns change, due to an increase in the predicted mean DBP for the 1920–1939 cohort. Adjusting for BMI and height also changes the magnitude and significance of the difference in the last two cohorts among women at age 39 years such that the 1960–1969 and 1970–1979 are no longer significantly higher than the 1940–1949 cohort.
Secular trends in the rate of change in BP with age before (black bars) and after adjusting for BMI and height (dotted white bars) are shown in Fig. 3. Adjusting for BMI and height did not change the cohort differences in the rate of change in SBP (top half) and DBP (bottom half) for either men or women, although all rates of change with age were attenuated.
Discussion
Several studies have shown that mean SBP, mean DBP, and the prevalence of hypertension are decreasing [2–5,10,17] over time, whereas others have reported an opposite trend in the prevalence of hypertension [24,25] and DBP [7,26,27]. Still others have found a U-shaped trend in mean SBP [27,28] and mean DBP [28], or no significant trend in mean SBP [5,25] or DBP [5,25,27]. Our data indicate that there is no secular trend in SBP and a curvilinear (U-shaped) secular trend in DBP, even after adjusting for BMI and height.
It is possible that the different results across previous studies result from small snapshots of long-term secular trends in BP. Due to limited ranges of ages and birth years, some studies may have only captured the first or last part of a curvilinear trend. Some cross-sectional studies, which consist of cohorts estimated by comparing population means at different time periods, ignore periods (cohorts) that are intermediate to the earliest and latest periods [29,30]. Such linear trends are, thus, based on the difference between the first and last cohorts and show a decreasing (linear) trend when actually, the beginnings of a curvilinear trend is starting to appear, particularly between the penultimate and most recent cohorts. For example, Knuiman et al. [29] report a decreasing linear trend in DBP from 1966 to 1981. The 1978 and 1981 cohorts, however, are on an ‘upswing’ and have higher DBP means than the lowest (1975) cohort [29]. This also occurs in the study by Sjol et al. [30] of BP in a Danish population, in which at any given age and BP-percentile grouping, mean BP is lowest in 1987 (from 1964) and begins to increase by 1991 [30]. In both of these studies, BP was lower in more recent cohorts compared to the earliest cohorts; however, the most recent cohorts did not have the lowest observed BP over the entire period studied.
Typically, in westernized countries, SBP increases with age, whereas DBP peaks at approximately 50–60 years of age and then declines [3,31]. Previous studies suggest that the increase in BP with age is slower or less steep in more recent cohorts [3]. In contrast, our data indicate there are no significant differences between birth cohorts with respect to increases in SBP with age, and that the increase in DBP with age is steeper in more recent cohorts compared to earlier cohorts, even after adjusting for BMI.
We found that BMI did not explain all of the increasing trends in DBP. Adjusting for BMI, however, did alter the magnitude of predicted mean SBP, DBP, and decreased the rate of change in BP with age. One study found that despite increasing prevalence of overweight and obesity, decreases in BP were evident [5]. Our study contradicts this study [5] and others [10] that show improved (lower) cardiovascular disease risk factor profiles. Adjusting for BMI also attenuated age-related differences (Figs 1 and 2). BMI tends to be higher at older ages. After adjusting for BMI, there appears to be less difference in mean BP because both age groups are set to the same BMI. This attenuation in age-related differences can also be observed in the rate of change in BP with age (Fig. 3), wherein the rate of increase is less steep after adjusting for BMI and height.
Because antihypertensive medication use has been posited as a possible reason for decreases in BP and hypertension over time [3], we also examined the potential role of antihypertensive medication separately (not shown). In our study sample, the prevalence of hypertensive medication use in all cohorts was less than 7% (data not shown). We found no differences in our comparisons of BP between cohorts, presumably due to low rates of antihypertensive medication use before 40 years of age.
Our findings have public health implications because increases in BP are occurring in what should be the healthiest segment of the adult population [26]. Generally, elevated SBP is associated with arterial stiffness and loss of elasticity (distensibility), whereas DBP is thought to reflect peripheral resistance [32,33]. Whereas many have found SBP to be more highly related to vascular disease [34], wherein SBP and isolated systolic hypertension is more prevalent in the elderly, DBP is more important as a risk factor for cardiovascular disease among individuals less than 50 years of age [32,33,35,36]. Moreover, DBP is still an important risk factor for other vascular problems as elevated DBP but not SBP, was recently found to be an important risk factor for poor cognition [37]. Another point of concern is that our data confirm that this segment of the population is unlikely to be using antihypertensive medication or to have hypertension [26]; yet, it also shows that young, ostensibly healthy adults are influenced by significant age-related increases in BP. These findings suggest that in the near future, if intervention efforts to lower DBP are ignored, the prevalence of hypertension in young-to-middle adulthood may increase due to DBP staging (wherein DBP levels are at a higher stage than SBP) [32,33]. Indeed, among normotensive adults from the Minnesota Heart Study, mean DBP significantly increased in the 2000–2002 cohort compared to the 1980–1982 cohort, whereas among hypertensive individuals, mean DBP decreased [27], presumably due to the use of antihypertensive medication.
Very few studies are available to examine long-term secular trends with serial data. Strengths of this study include the use of longitudinal data to determine individual changes in BP with age. Furthermore, our study includes participants over a wide range of birth years (1920–1979). It is possible that we would not have detected the curvilinear secular trend in DBP with a smaller range of birth cohorts. The 22-year age range of observations also allowed us to observe that the secular trend in BP differs at different stages of early adulthood.
There are some limitations to this study. The sample size within each cohort is relatively small compared to other surveys using national data [3,10,24,25,38]. However, though small in participant number, our study uses serial data to estimate secular trends in BP as well as changes in BP with age. Another limitation to this study is the generalizability of this study due to the population and age range. This study can, at most, be generalized to US-based non-Hispanic white adults of young-to-middle age who are, for the most part, normotensive. We were not able to comprehensively examine the effects of diet [34,39–43], physical activity [44,45], and smoking [45,46], as these data were not collected over the entire study. We were, however, able to examine the influence of other factors, including concurrent BMI and height. Regardless of the role that any of these unmeasured behavioral factors may have played, our study shows that DBP and its rate of increase with age have been worsening for over 30 years.
In summary, our study shows that mean SBP and the rate of change in SBP among 18–40-year olds have not changed significantly for individuals born between 1920 and 1979. For DBP, however, there is a secular trend in both mean DBP and rate of DBP increase with age. Among those born most recently, mean DBP is lower at the beginning of adulthood (age 19 years) compared to the earliest cohort. However, the rate of change in DBP with age has increased in recent cohorts such that by middle adulthood (age 39 years), those born most recently have higher DBP than those born in the earliest cohort. These secular changes are only partially attributable to concurrent increasing secular trends in obesity. The secular trends observed during early-to-middle adulthood may result in an earlier onset, and higher incidence, of hypertension, and thus, adults in this age range may require additional effort in reducing both BP and further cardiovascular disease events.
Acknowledgments
We thank the participants of the Fels Longitudinal Study for their continued participation and research staff at the Lifespan Health Research Center for their assistance. This work was supported by a National Institutes of Health grant (R01-HD12252).
Abbreviations
- ΔDBP
change in diastolic blood pressure
- ΔSBP
change in systolic blood pressure
- BP
blood pressure
- ICCs
intraclass correlation coefficients
- NHANES
National Health and Nutrition Examination Survey
Footnotes
There are no conflicts of interest.
References
- 1.McCarron P, Smith GD, Okasha M. Secular changes in blood pressure in childhood, adolescence and young adulthood: systematic review of trends from 1948 to 1998. J Hum Hypertens. 2002;16:677–689. doi: 10.1038/sj.jhh.1001471. [DOI] [PubMed] [Google Scholar]
- 2.Antikainen RL, Jousilahti P, Tuomilehto J. Trends in the prevalence of isolated systolic hypertension in the middle-aged population in 1972–1992. J Hum Hypertens. 1999;13:485–491. doi: 10.1038/sj.jhh.1000859. [DOI] [PubMed] [Google Scholar]
- 3.Goff DC, Howard G, Russell GB, Labarthe DR. Birth cohort evidence of population influences on blood pressure in the United States, 1887–1994. Ann Epidemiol. 2001;11:271–279. doi: 10.1016/s1047-2797(00)00224-6. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Garrison RJ, Dannenberg AL. Secular blood pressure trends in normotensive persons: the Framingham study. Am Heart J. 1993;125:1154–1158. doi: 10.1016/0002-8703(93)90129-w. [DOI] [PubMed] [Google Scholar]
- 5.Sheffer R, Calderon-Margalit R. Trends in overweight, obesity and blood pressure among Israeli working adults implications for public health. Eur J Public Health. 2008;18:121–125. doi: 10.1093/eurpub/ckm083. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Beydoun MA. The obesity epidemic in the United States: gender, age, socioeconomic, racial/ethnic, and geographic characteristics – a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 8.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207:928–934. doi: 10.1016/j.jamcollsurg.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 12.Hallstrom B, Jonsson AC, Nerbrand C, Norrving B, Lindgren A. Stroke incidence and survival in the beginning of the 21st century in southern Sweden: comparisons with the late 20th century and projections into the future. Stroke. 2008;39:10–15. doi: 10.1161/STROKEAHA.107.491779. [DOI] [PubMed] [Google Scholar]
- 13.Thorvaldsen P, Davidsen M, Bronnum-Hansen H, Schroll M. Stable stroke occurrence despite incidence reduction in an aging population: stroke trends in the Danish monitoring trends and determinants in cardiovascular disease (MONICA) population. Stroke. 1999;30:2529–2534. doi: 10.1161/01.str.30.12.2529. [DOI] [PubMed] [Google Scholar]
- 14.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653–658. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 15.Huang TT, Johnson MS, Figueroa-Colon R, Dwyer JH, Goran MI. Growth of visceral fat, subcutaneous abdominal fat, and total body fat in children. Obes Res. 2001;9:283–289. doi: 10.1038/oby.2001.35. [DOI] [PubMed] [Google Scholar]
- 16.Salbe AD, Weyer C, Lindsay RS, Ravussin E, Tataranni PA. Assessing risk factors for obesity between childhood and adolescence: I. Birth weight, childhood adiposity, parental obesity, insulin, and leptin. Pediatrics. 2002;110:299–306. doi: 10.1542/peds.110.2.299. [DOI] [PubMed] [Google Scholar]
- 17.Kastarinen MJ, Nissinen AM, Vartiainen EA, Jousilahti PJ, Korhonen HJ, Puska PM, Tuomilehto JO. Blood pressure levels and obesity trends in hypertensive and normotensive Finnish population from 1982 to 1997. J Hypertens. 2000;18:255–262. doi: 10.1097/00004872-200018030-00003. [DOI] [PubMed] [Google Scholar]
- 18.Roche AF. Growth, maturation, and body composition: the Fels Longitudinal Study 1929–1991. New York: Cambridge University Press; 1992. [Google Scholar]
- 19.National Center for Health Statistics (NCHS) Health, United States, 2004 with chartbook on trends in the health of Americans. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 20.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 21.Lohman T. Applicability of body composition techniques and constants for children and youths. Exerc Sports Sci Rev. 1986;14:325–357. [PubMed] [Google Scholar]
- 22.Lohman TG, Roche AF, Martorell R, editors. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Publishers Inc; 1988. [Google Scholar]
- 23.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 24.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 25.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 26.Kumanyika SK, Landis JR, Matthews YL, Weaver SL, Harlan LC, Harlan WR. Secular trends in blood pressure among adult blacks and whites aged 18–34 years in two body mass index strata, United States, 1960–1980. Am J Epidemiol. 1994;139:141–154. doi: 10.1093/oxfordjournals.aje.a116976. [DOI] [PubMed] [Google Scholar]
- 27.Luepker RV, Arnett DK, Jacobs DR, Jr, Duval SJ, Folsom AR, Armstrong C, Blackburn H. Trends in blood pressure, hypertension control, and stroke mortality: the Minnesota Heart Survey. Am J Med. 2006;119:42–49. doi: 10.1016/j.amjmed.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 28.Rosengren A, Eriksson H, Hansson PO, Svardsudd K, Wilhelmsen L, Johansson S, et al. Obesity and trends in cardiovascular risk factors over 40 years in Swedish men aged 50. J Intern Med. 2009;266:268–276. doi: 10.1111/j.1365-2796.2009.02116.x. [DOI] [PubMed] [Google Scholar]
- 29.Knuiman MW, Jamrozik K, Welborn TA, Bulsara MK, Divitini ML, Whittall DE. Age and secular trends in risk factors for cardiovascular disease in Busselton. Aust J Public Health. 1995;19:375–382. doi: 10.1111/j.1753-6405.1995.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 30.Sjol A, Thomsen KK, Schroll M. Secular trends in blood pressure levels in Denmark 1964–1991. Int J Epidemiol. 1998;27:614–622. doi: 10.1093/ije/27.4.614. [DOI] [PubMed] [Google Scholar]
- 31.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 32.Tin LL, Beevers DG, Lip GY. Systolic vs diastolic blood pressure and the burden of hypertension. J Hum Hypertens. 2002;16:147–150. doi: 10.1038/sj.jhh.1001373. [DOI] [PubMed] [Google Scholar]
- 33.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 34.Franklin SS, Lopez VA, Wong ND, Mitchell GF, Larson MG, Vasan RS, Levy D. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:243–250. doi: 10.1161/CIRCULATIONAHA.108.797936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perry HM, Jr, Miller JP, Baty JD, Carmody SE, Sambhi MP. Pretreatment blood pressure as a predictor of 21-year mortality. Am J Hypertens. 2000;13:724–733. doi: 10.1016/s0895-7061(99)00214-9. [DOI] [PubMed] [Google Scholar]
- 36.Schillaci G, Pirro M, Mannarino E. Assessing cardiovascular risk: should we discard diastolic blood pressure? Circulation. 2009;119:210–212. doi: 10.1161/CIRCULATIONAHA.108.827931. [DOI] [PubMed] [Google Scholar]
- 37.Tsivgoulis G, Alexandrov AV, Wadley VG, Unverzagt FW, Go RC, Moy CS, et al. Association of higher diastolic blood pressure levels with cognitive impairment. Neurology. 2009;73:589–595. doi: 10.1212/WNL.0b013e3181b38969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 39.Casagrande SS, Wang Y, Anderson C, Gary TL. Have Americans increased their fruit and vegetable intake? The trends between 1988 and 2002. Am J Prev Med. 2007;32:257–263. doi: 10.1016/j.amepre.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Kant AK, Graubard BI. Secular trends in patterns of self-reported food consumption of adult Americans: NHANES 1971–1975 to NHANES 1999–2002. Am J Clin Nutr. 2006;84:1215–1223. doi: 10.1093/ajcn/84.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cummins RO. Recent changes in salt use and stroke mortality in England and Wales. Any help for the salt-hypertension debate? J Epidemiol Community Health. 1983;37:25–28. doi: 10.1136/jech.37.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen SJ, Siega-Riz AM, Popkin BM. Trends in energy intake in U.S. between 1977 and 1996: similar shifts seen across age groups. Obes Res. 2002;10:370–378. doi: 10.1038/oby.2002.51. [DOI] [PubMed] [Google Scholar]
- 44.Ekblom B, Engstrom LM, Ekblom O. Secular trends of physical fitness in Swedish adults. Scand J Med Sci Sports. 2007;17:267–273. doi: 10.1111/j.1600-0838.2006.00531.x. [DOI] [PubMed] [Google Scholar]
- 45.Posner BM, Franz MM, Quatromoni PA, Gagnon DR, Sytkowski PA, D’Agostino RB, Cupples LA. Secular trends in diet and risk factors for cardiovascular disease: the Framingham Study. J Am Diet Assoc. 1995;95:171–179. doi: 10.1016/S0002-8223(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 46.Bjorkelund C, Andersson-Hange D, Andersson K, Bengtsson C, Blomstrand A, Bondyr-Carlsson D, et al. Secular trends in cardiovascular risk factors with a 36-year perspective: observations from 38- and 50-year-olds in the Population Study of Women in Gothenburg. Scand J Prim Healthcare. 2008;26:140–146. doi: 10.1080/02813430802088403. [DOI] [PMC free article] [PubMed] [Google Scholar]