Abstract
Sarcopenia is a loss of muscle protein mass and loss of muscle function. It occurs with increasing age, being a major component in the development of frailty. Current knowledge on its assessment, etiology, pathogenesis, consequences and future perspectives are reported in the present review. On-going and future clinical trials on sarcopenia may radically change our preventive and therapeutic approaches of mobility disability in older people.
Introduction
The aging process involves numerous changes in body composition that affect health amongst which sarcopenia is of clinical and functional significance. Irwin Rosenberg defined sarcopenia in 1989 to describe a recognized age-related decline in muscle mass among the elderly (1, 2). This large and supposedly involuntary loss of muscle tissue in the elderly was considered responsible in part for the age related decline in functional capacity. Since 1989, various definitions of sarcopenia have evolved as our understanding of the aging process and the changes that occur therein progress along with improved body composition measurement techniques and the availability of large representative data sets. Despite this increasing knowledge and improved technology, a worldwide operational definition of sarcopenia applicable across racial/ethnic groups and populations lacks consensus.
One current definition of sarcopenia includes a loss of muscle strength and functional quality in addition to the loss of muscle protein mass, but it is unclear whether a decline in functional capacity results from the loss of muscle mass and/or the qualitative impairment of the muscle tissue (3). For example, after 50 years of age, muscle mass is reported to decline at an annual rate of approximately 1 to 2% (4), but strength declines at 1.5% per year and accelerates to as much as 3% per year after age 60 (5–8). These rates are high in sedentary individuals and twice as high in men as compared to women (9). However, men, on average, have larger amounts of muscle mass and shorter survival than women which implies that sarcopenia is potentially a greater public health concern among women than men (6). Thus, men and women present different trajectories in the decline in skeletal muscle with aging. Men have a gradual decline, while women tend to have a sudden drop in muscle mass and function following menopause. The current prevalence of sarcopenia in populatoins varies depending on the definition used, the limitations of past epidemiological and clinical data from small samples and mixed information from the different measurement techniques employed. For example, based upon Baumgartner et al.’s original definition and data from the New Mexico Elder Health Survey, sarcopenia affects about 20% of men between age 70 and 75 years, about 50% of those over age 80 years, and between 25% and 40% of women have sarcopenia in the same age ranges (10). However, Baumgartner later recognized that these estimates, which were based on a bioelectric impedance equation, might be biased and published revised prevalence estimates based on dual energy x-ray absorptiometry (DXA) ranging from 8.8% in women and 13.5% men aged 60–69 years, and up to 16% in women and 29% in men older than 80 years (11). In a healthy elderly community-dwelling population 70 years of age and older in the French EPIDOS study, only 10 percent of the women had sarcopenia (12) based on the Baumgartner’s index, but cutpoints derived from a different reference group. Using a similar definition, Janssen reported, retrospectively, that 35% of the elderly in the population based NHANES III had a moderated degree of sarcopenia and 10% a severe degree of sarcopenia (13). Findings from a separate study by Melton et al. using yet another definition suggests that sarcopenia affects 6 to 15% of persons over the age of 65 years (14). It is important to note that these studies used different measures of relative muscle mass, reference groups, and cutpoints, so it is difficult to compare prevalence estimates among these study samples. These investigators also reported significant inter-individual variability in the amount of muscle mass and decline in strength, but Kallman et al. (15) reported that 15% of participants aged 60 years and older had no strength decline during 9 years of follow-up.
Numerous epidemiological and clinical studies report that sarcopenia occurs with increasing age, but knowledge of its real prevalence at and across age within and among populations is clearly dependent on an accurate operational definition. In addition, the elderly are an increasing proportion of the general population worldwide, and the impact of their health problems and medical costs highlight the need for a better understanding of sarcopenia as an important health problem for which increased epidemiological and clinical research is warranted. The annual healthcare cost attributable to sarcopenia is estimated at $18 billion in the United States alone (16).
Sarcopenia is different from starvation and cachexia that are also associated with loss of muscle mass but for which the causes and therapeutic approaches are different. During starvation, protein-energy deficiency results in a loss of fat and muscle mass (17, 18), but they are reversible with replenishment. Cachexia results in both fat and muscle mass loss, but it accompanies chronic diseases such as cancer, AIDS or rheumatoid arthritis (17, 19). Sarcopenia is thought to reflect mainly an age-related decreased in the synthesis of muscle protein rather than an excess catabolic process associated with disease or from a reduced caloric intake, although some have hypothesized that low grade, chronic inflammation with increased protein degradation may contribute (20–22). Sarcopenia is assumed to be a major component in the development of frailty (23, 24), but that assumption depends on the operational definition used.
Operational definition of sarcopenia
Recognition of the need for a consensus operational definition of sarcopenia occurred in 1995 (25), but an operational definition requires the availability of accurate and valid measurements of specific body compositional criteria that can be collected easily in large epidemiological and clinical studies. One reason for the divergent prevalence of sarcopenia within and between groups of elderly is due in part to the practical difficulties and changes in assessing muscle mass that have occurred over the past 20 years. In 1989, the primary method for measuring body composition was underwater weighing or hydrodensitometry which was not accommodating for many of the elderly (26, 27). Unlike osteoporosis for which there is consensus regarding clinical symptoms and measurement methodology using DXA, body composition methodology has changed considerably during the past two decades from underwater weighing to the availability of fan-beam DXA machines that can quantify muscle, (bone and fat) mass in a few minutes. This twenty years period has also seen the introduction of very precise body tissue measurement methods such as magnetic resonance imaging and less valid predictive techniques such as bioelectrical impedance. In addition, the size, characteristics and demographics of the world’s elderly population have changed considerably. Many published studies of sarcopenia over the last twenty years have used different samples of convenience of varying size and numerous measurement methods based on differing underlying assumptions and criteria, so that some studies were comparable and others only informative. Thus, despite relative agreement among clinicians and epidemiologists on a theoretical definition of sarcopenia, development of a consensus operational definition useful across clinics, studies and populations has not occurred (28).
Baumgartner et al. (10) as suggested by Heymsfield (29) summed the muscle mass of the four limbs from a DXA scan as appendicular skeletal muscle mass (ASM), and defined a skeletal muscle mass index (SMI) as ASM/height2 (as kg/m2). Individuals with a SMI two standard deviations below the mean SMI of a middle-age reference male and female population from the Rosetta study (9) were defined as gender-specific cutpoints for sarcopenia. This definition has been used by several authors (10, 12, 14), but a limitation may be the ability of DXA to distinguish water retention or fat tissue infiltration within muscle or soft tissue. There are few published data to date, however, for the impact of variation in these on sarcopenia prevalence estimates. Chen et al. (30), and others (31), report strong correlations (r > 0.94) between DXA and MRI measures of skeletal muscle mass, indicating that this increasingly available method is useful for cross-sectional studies and screening. Kim et al. estimated that fatty infiltration of muscle can inflate skeletal muscle mass estimates from DXA by 1 to 8% (31). This amount of measurement error probably translates to only a small error in misclassification of individuals in a population as sarcopenic. It is likely that the use of different measures of muscle mass (e.g. total vs appendicular skeletal muscle), reference populations, and cutpoints have larger effects. Another potential limitation is that this approach does not account for the joint effects of fat mass or body weight. Most obese adults have increased muscle mass in addition to a high fat mass but a low muscle mass in relation to their total body weight (32); while thin elderly have a high proportion of muscle mass in relation to their total body weight. Thus, the SMI potentially misclassifies the obese elderly with a high SMI and mobility and functional limitations and the thin elderly with a low SMI and none or a few mobility or functional limitations. Thus, Baumgartner’s original estimates for the association of SMI with functional outcomes in the New Mexico Elderly Health Survey were adjusted for body fatness (10). Some investigators have taken a different approach, and tried to build control for fat mass or body weight into the index, rather than adjusting statistically for these when analyzing associations with other variables. For example, Janssen et al. used muscle mass relative to weight rather than stature (33). To address these limitations, others have recommended that fat mass be considered in operational definitions of sarcopenia. In a later study, Baumgartner cross-classified subjects by SMI and percent body fat and reported that associated disability was strongest in those who were both sarcopenic and obese (11). Newman et al. used a residual method, to adjust muscle mass for fat mass in addition to height (32), and found that this index had stronger associations with lower extremity functional limitation (32) and disability than sarcopenia defined by the SMI alone (34). From a statistical point of view, it makes little difference when studying associations of sarcopenia with other outcomes whether adjustment for fat mass or body weight is made through an a priori adjustment to the index, or by regression adjustment during analysis. The approach taken by Baumgartner (11) in cross-classifying subjects by SMI and a measure of adiposity, however, is distinctly different in that it combines the joint effects of sarcopenia and obesity in a single categorical variable.
All definitions of sarcopenia are arbitrary and open to criticism. An operational definition needs to differentiate those with sarcopenia from those not affected, needs to define standards and be applicable across populations. Some have argued, on the other hand, that the lack of association between the SMI definition of sarcopenia and disability suggests that population-specific thresholds are needed (12), and that ethnicity along with age or frailty should be considered when setting a cutpoint for sarcopenia. The use of a country-specific reference group such as the middle-age population from the Rosetta study (9) is questionable for world-wide application. The best reference data set should include the measurements that are correlated with and can discriminate sarcopenia and have a broad ethnic, racial and age representation. Recently, Janssen et al. used data from the National Health and Nutrition Survey III to try to improve prevalence estimates in the United States. In addition, receiver operating curves were used to determine the sex-specific skeletal mass cutpoint below which the risk of physical disability significantly increased (13). Women who’s SMI was below 5.75 kg/m2 muscle mass had an increased risk for physical disability (OR=3.31, 95%CI; 1.91–5.73) and men who’s SMI was below 8.50 kg/m2 had an increased risk for physical disability of (OR=4.71, 95%CI; 2.28–9.74). It is important to note that in this study total skeletal muscle mass was predicted using a bioelectric impedance equation. However, these new cut points were closely similar to those first proposed by Baumgartner et al. (10). It is important to stress here that consideration additionally needs to be given the method used to measure the function outcome. Differences among studies in the strength of association of various definitions of sarcopenia with functional status are a function of the methods used to define functional status or disability and their sensitivity and precision.
As note earlier, a limitation of current methods of measuring muscle mass in defining sarcopenia is systematic errors introduced by the age-related increase in fatty infiltration of muscle tissue. Intra-muscular adipose tissue infiltration, measured by magnetic resonance imaging (MRI) (35) and computed tomography (CT), improves our understanding of fat infiltration and muscle tissue impairment, but they are costly and often inaccessible (36). Kim et al. published methods for correcting DXA muscle mass for fatty infiltration as measured by MRI, but these remain to be validated in other populations (31). Ultrasound can accurately measure cross-sectional thicknesses and areas in subcutaneous adipose and muscles tissues (37, 38), and bioelectrical impedance can provide estimates of muscle mass, but these can have large errors and are sample and population specific (39–42). These measurement techniques can not actually be used as screening tools in ambulatory clinical settings. An advantage of ultrasound is that it can also measure changes in tendon insertion angle (angle of pannation) (43). Tendons play a major role in the development of muscle strength. Anthropometry has been used with limited success to detect sarcopenia in ambulatory settings (12, 44, 45). Changes in body weight loss are insufficient because an increase in fat mass can obscure a loss in muscle tissue leading to the condition referred to as “sarcopenic obesity” (10), and nearly 30% of men and 10% of women older than 80 years in the U.S. are reported to have sarcopenic-obesity (11, 28, 46). Calf circumference is significantly correlated to muscle mass, but this correlation is low and explains only a small part of the variance (12). Calf circumference has been related to activity levels and can indicate decreases in muscle in the legs, especially those muscles used in balance, but it is more representative of muscle mass in very ill, frail or dying elderly patients than in healthy or obese elderly (44). Moreover, calf circumference can be confounded by subcutaneous fat and in the general healthy or obese community-dwelling elderly (25, 47), thus due to this low sensitivity, calf circumference is a poor screening tool for sarcopenia (12).
The main effect from a loss of muscle mass is reduced muscle strength, which is an important factor to consider in defining sarcopenia. Muscle strength is measured with simple and complex equipment. Grip strength is a simple estimator of total muscle strength (48) but is not useful in those with hand arthritis. Leg muscle strength, assessed as maximal lower extremity muscle strength or power is a good measure of functional status, but it is properly measured with a dynamometer (e.g. Biodex or Kin Com) by a trained technician and is strongly associated with measures of mobility (49). Including a measure of muscle strength in an operational definition is logical as several authors have reported that muscle strength, more than muscle mass, is independently associated with physical performance (50). Moreover, different factors, such as physical activity or hormones, can underlie and contribute in varying degree to the loss of muscle strength and loss of muscle mass. The loss of muscle quality, an important component of the definition of sarcopenia, supposes an assessment of both muscle strength and muscle mass.
Muscle power is strongly related to functional limitation more than muscle mass or muscle strength (51). Muscle power is strength multiplied by speed, and it is defined as muscle work (muscle strength multiplied by a distance) divided by time. Muscle power declines considerably with aging (52) and at a higher rate than muscle strength. A muscle power assessment is probably closer to a theoretical definition of sarcopenia than muscle strength alone, but muscle power does not include a quantitative measure. Using muscle power to define sarcopenia (53) could include, muscle contraction speed and a measure of muscle quality in addition to muscle strength. Muscle mass is strongly correlated to muscle strength and power, but the same amount of muscle mass is able to produce different levels of strength and power. Defining sarcopenia on muscle strength or muscle power has several other limitations. Osteoarthritis and other co-morbidities common with old age can induce underestimates of strength and power from pain affecting individual performance. Loss of muscle strength in the upper or lower limbs can have separate causes and be associated with different outcomes. Moreover, isokinetic, isometric, concentric or eccentric muscle strength are different aspects of muscle strength that are probably not affected at the same level during the process of sarcopenia. A significant loss of strength with isokinetic testing at high angular velocity (49, 54, 55) and a relative preservation of eccentric strength (56, 57) is reported with aging, but a decrease in strength is more important in isometric or concentric strength than in eccentric strength (5). Thus, which measures of muscle strength should be used to define sarcopenia is unclear at this time based upon available data. Research is needed to validate the parameters (muscle mass, muscle strength, muscle quality) that could be used to define sarcopenia. An operational definition of sarcopenia should include components that contribute to physical function, but these components may be under different mechanisms or treated by different approaches. However, estimates of the prevalence of sarcopenia in populations with complex and variable combinations of muscle mass, strength and power measurements has not yet been proven. Moreover, a clinical tool may not be useful in epidemiology, and visa versa.
Etiology and Pathogenesis of sarcopenia
Multiple risk factors and mechanisms contribute to the development of sarcopenia (28, 58). Lifestyle behaviors such as physical inactivity, smoking and poor diet, as well as aged-related changes in hormones and cytokine levels are important risk factors. Postulated mechanisms include alterations in muscle protein turnover, muscle tissue remodeling, the loss of alpha-motor-neurons, and muscle cell recruitment and apoptosis (28). Genetic susceptibility also plays a role and explains individual and group differences in rates of sarcopenia. The relative influences of these factors on sarcopenia components such as muscle mass, muscle strength and muscle quality are not well-understood (59). Each factor in the etiology and pathogenesis of sarcopenia potentially contributes differently to the loss of muscle mass, strength and/or quality. In treatment for sarcopenia, it could be argued that improving muscle strength or muscle power is more relevant clinically for the outcomes of disability or mobility than increasing muscle mass; however increasing muscle mass is more important for other outcomes such as protein stores or thermogenesis. The notion that muscle strength and muscle mass are differentially affected by various treatment modalities is supported by experimental and clinical findings (59–64). While behavioral treatments, such as exercise increase muscle mass and strength, pharmacologic treatments, such as growth hormone, increase mass without a significant change in strength.
Lack of physical activity
Inactivity is an important contributor to the loss of muscle mass and strength at any age (44, 65, 66). Inactivity results from bed rest studies indicate that a decrease in muscle strength occurs before a decrease in muscle mass (47), and low levels of physical activity result in muscle weakness that, in turn, results in reduced activity levels, loss of muscle mass and muscle strength. Thus, physical activity should be protective for sarcopenia, but some studies suggest that the amount of protection depends on the type of activity. Aerobic activities such as walking, running, cycling or swimming increase maximal oxygen consumption (VO2max), improve muscle quality (muscle strength/muscle mass), neuromuscular adaptation, and muscle function and are associated with decreased morbidity and mortality independent of body fat. Aerobic exercise does not contribute as much to muscle hypertrophy as resistive exercises, but they stimulate muscle protein synthesis (67), satellite cell activation and increased muscle fibers area (68, 69). A possible important aspect of aerobic exercises is that they reduce body fatness, including intramuscular fat, which is important for improving the functional role of muscle relative to body weight. In contrast, muscle mass, strength, and muscle quality (strength adjusted for muscle mass) are reported to improve significantly with resistance training in older people (20). Robust evidence in several studies indicate that resistance training such as weight lifting increases myofibrillar muscle protein synthesis (70, 71), muscle mass and strength (72–79) even in the frail elderly. Strength gains results from a combination of improved muscle mass and quality and neuronal adaptation (innervations, activation pattern). However, sarcopenia is observed in master athletes who maintain resistance training activities throughout their lifetimes (80, 81). Whether aerobic training can reduce, prevent or treat sarcopenia is an important practical question because resistance training is less appealing to many sedentary elders. Leisure physical activity is not enough to prevent the decline in muscle mass (82), but aerobic and resistance activities improve balance, fatigue, pain release, cardiovascular risk factors, and appetite. Thus, promoting an active lifestyle can prevent the functional effects of sarcopenia, but resistance training is the best approach to prevent and treat sarcopenia, although both training modalities contribute to the maintenance and improvement of muscle mass and strength in the elderly.
Loss of neuro-muscular function
The neurological contribution to sarcopenia occurs through a loss of alpha motor-neuron axons (83). Decreased electrophysiological nerve velocity, related to the dropout of the largest fibers, reduces internodal length and segmental demyelization occurs with the aging process (5), but the role of demyelization in sarcopenia seems minor (84), but the central drive that contributes to a decrease in voluntary strength is supposed to be preserved (85). The progressive denervation and reinervation process observed during aging (86–88) and resulting in fiber type grouping (89) is the potential primary mechanism involved during the development of sarcopenia. From cross-sectional findings, the decline in motor neurons starts after the seventh decade (90) with a loss of alpha motor-neurons in the order of 50% (91), and this affects the lower extremities with their longer axons more than the upper limbs (5). The remaining alpha-motor-neurons enlarge their own motor unit territory by capturing the denerved fibers, but the increase of the motor unit size (89) and the reduction in alpha-motor-neuron number and in motor unit numbers (8, 92) results in a decline in coordinated muscle action and a reduction in muscle strength. Reinnervation contributes to the final differentiation of nerve fibers and the repartition between the type I fibers (slow, oxidative fibers) and the type II fibers (fast, glycolitic fibers). The average type II fibers area is diminished with age by 20 to 50% while the type I is diminished by 1 to 25% (91). In terms of number, half of the type I and type II fibers remain at 90 years of age compared to young adults in post-mortem whole muscle cross sectional studies (55, 93).
During aging, the number of satellite cells and their recruitment ability (72) decrease with a greater decrease in type II than type I fibers. Satellite cells are myogenic stem cells that can differentiate to new muscle fibers and new satellite cells if activated during the process of regeneration (94), but this regeneration may lead to imbalance and the number of type II muscle fibers may decline following damage. Muscles of elderly subjects are vulnerable to damage and recover poorly after trauma (95). Moreover, the separation between fast and slow fibers may not be as clear as in young muscle (86). Aging is associated with increased co-expression of the two myosin heavy chain (MHC) isoforms determining the fast or slow fiber characteristics. All these mechanisms contribute to the age-related loss of muscle mass, strength and contractility.
Further research is needed to understand better the contribution and role of neuro-muscular impairment during the onset of sarcopenia. Underlying mechanisms involved in the rate of motor unit loss such as physical activity, oxidative stress, genetics or hormones are not clearly delineated, and lack of external stimuli may be one of the reasons for the lack of regeneration (94). For example, the attenuated rise of heat shock protein (HSP) after exercise in the elderly could result in lower muscle protein synthesis (96). It is also unclear whether the loss of motor-neurons is one of the first stages leading to sarcopenia. Fiber regeneration may be altered before reinnervation contributes to their final differentiation into type I or II fibers. Recent studies suggest that sarcopenic muscle fibers express a regenerative phenotype such as increased expression of myogenic regulatory factors, MRFs, increased precursor cell proliferation, high content of the embryonic myosin heavy chain isoform than an expected denerved phenotype. In contrast to the deinnervated pathological conditions, the ubiquitin proteosome pathway is down-regulated in sarcopenic fibers (97).
Altered endocrine function
There is evidence linking age-related hormonal changes to the loss of muscle mass and muscle strength. Insulin, estrogens, androgens, growth hormone, prolactin, thyroid hormones, catecholamines and corticosteroids are involved in the etiology and pathogenesis of sarcopenia, but controversy persists regarding their respective roles and effects on skeletal muscle in adulthood and old age.
Insulin
Sarcopenia may be accompanied by a progressive increase in body and intramyocellular fat mass which are associated with an increased risk of insulin resistance (14). Insulin’s role in the etiology and pathogenesis of sarcopenia could be important even if its effect on muscle synthesis remains controversial (98–100). Insulin selectively stimulates skeletal muscle mitochondrial protein synthesis (101), but it is unclear if the anabolic effect of insulin on muscle synthesis is impaired with advancing age. Compared to young adults, increases in insulin levels after glucose and amino acids ingestion results in a lower protein synthesis (98), and a reduced effect on mitochondrial function in elderly (102). The normal increase in protein synthesis in response to insulin seems impaired in the aging muscle cell, due to alterations in signaling systems for translation initiation (103). The weight gain that frequently occurs during middle age results in a decline in the anabolic action of insulin, potentially predisposing to sarcopenia (104), but the presence of amino acids, especially for high intakes, may stimulate the anabolic effect of insulin (105).
Estrogens
There are conflicting data on the effects of estrogens on sarcopenia. Epidemiological and interventional studies suggest that estrogens prevents the loss of muscle mass (59, 106, 107), as their decline with age increase the levels of proinflammatory cytokines suspected to be involved in the sarcopenia process such as tumor necrosis factor alpha (TNFα) and interleukin 6 (Il-6) (21, 108). However, none of five recent clinical trials reported an increase muscle mass after hormone replacement therapy (HRT) (109). Effects of estrogens on muscle strength and function are also controversial (59, 110). In the Health, Aging and Body Composition Study, estrogen replacement was associated with higher quadriceps cross-sectional area but not with knee extensor strength (110). However, three recent clinical HRT trials reported an increase in muscle strength (see (109) for review). Estrogens increase the level of sex hormone binding globulin which reduces the level of serum free testosterone (111), thus HRT should decrease rather than increase muscle mass (112). Both these mechanisms may play a marginal role involving estrogen during the development of sarcopenia. Estrogen’s association with strength training does not seem to produce any anabolic effect on muscle mass or muscle strength (113).
Growth hormone and Insulin-like Growth Hormone 1
Insulin-like Growth Factor-1 (IGF-1) and Growth Hormone (GH) decline with age (114) and are potential contributors to sarcopenia. GH replacement therapy lowers fat mass, increases lean body mass and improves blood lipid profile. IGF-1 activates satellite cell proliferation and differentiation, and increases protein synthesis in existing fibers (115). There is also evidence that IGF-1 acts in muscle tissue by interacting with androgens (116), but there are conflicting results on its effect on muscle strength despite the apparent increase in muscle mass (117–122). Methods used to assess muscle mass, such as anthropometry, BIA or DXA, are unable to distinguish aqueous from non-aqueous components of the muscle mass. Theoretically, this can be accomplished with CT or MRI, only a few studies of GH or IGF1 have used these methods. One study reported that GH increased muscle strength only when coupled with a weight training program (122). The aging muscle is capable of synthesizing IGF-1, but it may be less sensitive to IGF-1 and could have an attenuated ability to synthesize an isoform of IGF-1 promoting satellite cell proliferation (123). Exercise may reverse the resistance of aging muscle to IGF-1 (123).
Testosterone
Testosterone levels gradually decreases in elderly men at a rate of 1% per year (124), and epidemiological studies suggest a relationship between low levels of testosterone in elderly and loss of muscle mass, strength and function. The increase in sex hormone binding globulin levels with age results in lower levels of free or bioavailaible testosterone (125). Clinical and experimental studies support the hypothesis that low testosterone predict sarcopenia with low testosterone resulting in lower protein synthesis and a loss of muscle mass (126). Testosterone induces in a dose-dependent manner an increase numbers of satellite cells which is a major regulating factor of satellite muscle cell function (116). When administrated to hypogonadal subjects or elderly subjects with low levels, testosterone (127–134) increased muscle mass, muscle strength and protein synthesis. However, inconclusive results are reported from studies evaluating the effectiveness of testosterone therapy on muscle strength and function in community-dwelling population (see chapter therapeutic perspective below and (135) for references).
Dehydroepiandrostedione (DHEA)
Blood levels of dehydroepiandrostedione, another anabolic steroid hormone, decrease dramatically with age and are significantly lower in very old men as compared to young men (136). Despite evidence that DHEA supplementation results in an increase of blood testosterone levels in women and an increase of IGF-1 in men, few studies have reported an effect on muscle size, strength or function (137).
Vitamin D and Parathyroid Hormone (PTH)
With aging 25(OH) vitamin D levels decline (138). Several cross sectional studies have reported the association between low 1,25OH vitamin D and low muscle mass, low muscle strength, decreased balance and increased risk of falls (139–143). One recent longitudinal epidemiological study reported an independent association between low serum vitamin D and sarcopenia (144). Several explanations to this association are possible. Nuclear 1,25OHvitamin D has been described in muscle cells (145) and low levels of vitamin D have shown to decreasing muscle anabolism (146). Low vitamin D may also influence muscle protein turn-over through reduced insulin secretion (147). Low levels of vitamin D are associated with raised PTH but, previous studies suggest that high PTH is independently associated with sarcopenia (59, 144) and increased risk of falling (148). An independent association between high PTH blood levels and number of falls in nursing-home residents was recently reported (149). PTH may modulate muscle tissue functioning through an increase in intracellular calcium (148) or an induced pro-inflammatory pathway (144). It is important that 25(OH) vitamin D levels be measured in all older persons with muscle loss and if the value is less that 30ng/ml, vitamin D should be replaced (63, 150–152).
High level of cytokines
Chronic medical conditions, such as COPD, heart failure and cancer are highly prevalent in elderly and are associated with an increased serum level of pro-inflammatory cytokines and loss of body weight, including lean mass. This condition can occur in younger adults or elderly persons and is called cachexia. This acute hyper-catabolism differs from the long-term age-related process that leads to sarcopenia. However, aging is also associated with a more gradual, chronic, increased production of pro-inflammatory cytokines, particularly Il-6 and Il-1, by peripheral blood mononuclear cells (153). There is some evidence that increased fat mass and reduced circulating levels of sex hormones with aging contribute to this age-related increase in pro-inflammatory cytokines that constituted catabolic stimuli (6). Thus, the aging process itself is associated with increased catabolic stimuli, but there is still a lack of evidence for the hypothesis that cytokines predict sarcopenia in prospective studies (59, 154, 155). Nevertheless, sarcopenia is one of the outcomes of cytokine related aging process (63).
Previous reports in elderly populations have demonstrated an association between high levels of IL-6 and poor outcomes (156). Two studies have reported an association between measures of muscle strength and mass and blood levels of TNFα, IL-6 and C-reactive protein (CRP) (157, 158). In the Longitudinal Aging Study of Amsterdam, high levels of cytokine IL-6 and CRP were also associated with an increased risk for loss of muscle strength (154). These cytokines cause an imbalance in muscle tissue synthesis in favor of excess protein breakdown. A chronic elevation of inflammatory cytokines or other pro-inflammatory proteins could result in the predispositoin to sarcopenia (6, 28, 159). High level of cytokines may also result in loss of muscle mass through increased activation of the ubiquitine-protease pathway (160) and lower production of IGF-1. The ubiquitine-proteasome system (UPS) degrades proteins, including the myofibrillar proteins, but its importance in sarcopenia remains to be established. Experimental studies suggest that the UPS is activated through an up-regulation of the genes for ligases (Atrogin and MuRF1) that are highly correlated with muscle proteolysis. These two genes may be controlled by TNFα and IGF-1/Akt (123). However, the role of the cytokines may be more complicated. The effect of high levels of IL-6 is for instance still conflicting. IL-6 may be both a pro-inflammatory and an anti-inflammatory cytokine. Recent experimental studies have also suggested that the blood Il-6 should be differentiated from the muscle-derived form, which is able to inhibit TNFα (161). TNFα stimulates muscle loss through the activation of the apoptosis pathway (122), but the effect of IL-6 depends on its form and localization.
Obesity is linked to inflammation (162, 163) and may have an important role in the process leading the sarcopenia (28). Being both obese and sarcopenic is a condition named “sarcopenic obesity” (11, 46). Sarcopenic obesity has been reported to predict the onset of disability more than sarcopenia or obesity alone. This condition occurs in about 6% of the community-dwelling elderly, and to about 29% of men and 8.4% of women over 80 years of age (88). It has been hypothesized that sarcopenic-obesity is associated with increased fatty infiltration of muscle, but confirmatory data are lacking (46). Fatty infiltration of skeletal muscle is associated with reduced strength (64, 164) and functional status (165), and it is hypothesized that infiltratoin affects muscle function (164). Contractility, motor unit recruitment or muscle metabolism is decreased in the presence of fat infiltration (164), and the excess fatty acids in the muscle fibers interferes with the normal cellular signaling (166). These findings suggest a role of fat mass in the etiology and pathogenesis of sarcopenia, and Roubenoff (167) has suggested a vicious cycle explaining the link between these two body compartment and aging. Loss of muscle mass results in lower physical activity that leads to obesity, that leads to an increase in catabolic over anabolic signals and a further loss of muscle mass. Baumgartner et al. (11, 28), however, noted that the age-related increase in fat mass generally precedes the loss of muscle mass, and that thin people also lose muscle with age. This suggest that sarcopenia occurs regardless of changes in adiposity with age, but that in obese elderly in association with visceral adiposity, the associated low chronic inflammatory state could lead to accelerated muscle loss and thereby sarcopenic-obesity.
Mitochondrial dysfunction
The role of mitochondrial dysfunction in sarcopenia is currently controversial (168). Mitochondrial function may be affected by the cumulative damage to muscle mitochondrial DNA (mtDNA) observed with aging. This may result in a reduction of the metabolic rate of muscle cell protein synthesis, ATP synthesis (169) and finally to the death of the muscle fibers and the loss of muscle mass (22, 170). However, low physical activity could be the primary reason for mitochondrial dysfunction in the elderly. Some investigators report that the decline in mitochondrial functions with aging of can be attenuated by physical activity (171). Others report that mitochondrial impairment is only partially reversed after physical training, but it does not reach the level of improvement observed in young (22, 172, 173).
Apoptosis
Accumulated mutations in muscle tissue mitochondrial DNA are associated with accelerated apoptosis of myocytes, and apoptosis may also be the link between mitochondria dysfunction and loss of muscle mass. Evidence suggests that myocytes apoptosis is a basic mechanism underlying sarcopenia (174), and muscle biopsies of older persons show differences associated with apoptosis (175) compared with younger subjects. Recent reports also suggest that type II fibers (those fibers preferentially affected by the sarcopenia phenomenon), may be more susceptible to death via the apoptotic pathway (176).
Two different pathways have been described, the caspase-dependent and the caspase-independent apoptosis (177). The caspase-dependent pathway is a cascade of factors activated in a sequential order to determine cell death. Aging is also associated with an increased level of several caspases (178), and mitochondria are determinant components for the regulation and induction of apoptosis through the caspase-independent apoptotic pathway. Other mechanisms such as oxidative stress (100), low growth factors or complete immobilization may also result in caspase-dependent and caspase-independent apoptosis in animals studies (177). However, the magnitude of apoptosis compared to the other mechanisms leading to sarcopenia is still unknown. Apotosis may represent a common final mechanism for muscle loss in sarcopenia, but multiple agents and etiologic pathways may also lead to this mechanism.
Genetic influence
Genetic factors are major contributors to variability in muscle strength and likely contribute to susceptibility to sarcopenic agents. Genetic epidemiological studies suggest that between 36 and 65% of an individual muscle strength (179–182), 57% of lower extremity performance (183) and 34% of the ability to perform the activities of daily living (ADL) (184) are explained by heredity. Sarcopenia and poor physical performance in elderly are also associated with birth weight in both men and women independent of adult weight and height, which suggests that exposures very early in life may additionally program risk for sarcopenia in old age in genetic susceptible individuals (185, 186).
Few studies have explored potential candidate genes determining muscle strength. In an analysis of the myostatin pathway, a possible muscle mass regulator, linkage was observed to several areas. The genes growth/differentiation factor 8 (GDF8), cyclin-dependent kinase inhibitor 1A (CDKN1A), and myogenic differentiation antigen 1 (MYOD1) were implicated as positional candidate genes for lower extremity muscle strength (181, 187) also found several other genes in the myostatin pathway (cyclin-dependent kinase 2 (CDK2), retinoblastoma (RB1), and insulin-like growth factor 1 (IGF1)), to be strongly related to muscle strength. Other genes such as the ciliary neurotrophic factor gene variant (CNTF A allele) may be related to loss of muscle power as well as muscle quality during adulthood, according to findings demonstrating that homozygous individuals had lower quadriceps strength values (188), and an association between IGF-2 genotype and arm and leg strength, especially in women, has also been noted (189). The actinin alpha 3 (ACTN3) R577X genotype is also of interest as it has been shown to influence knee extensor peak power in response to strength training as has a polymorphism in the Angiotensin Converting Enzyme (ACE) gene (187, 190). Also, polymorphisms in the Vitamin D receptor (VDR) may be associated with muscle strength because of the relationship between vitamin D and its known effect on both smooth and striated muscle (191). Polymorphisms in the VDR have been associated with sarcopenia in elderly men (192), muscle strength and body composition in premenopausal women (193), and muscle strength in older women (194). Recently, sarcopenia has been identified in the free-living nematode or roundworm Caenorhabditis elegans (195). A Mutation in the daf-2 insulin/IGF-1 signaling or the Age-1 PI-3-kinase in this animal model prevents sarcopenia (196, 197). All of these finding indicate that sarcopenia has a significant heritable component (183) as do other body composition phenotypes.
Low nutritional intake and low protein intake
Muscle protein synthesis rate is reported to be reduced 30% in the elderly, but there is controversy as to the extent to which this reduction is due to nutrition, disease or physical inactivity rather than aging (198, 199). It is recognized by some that protein intake in elders should exceed the 0.8g/kg/j/day recommend intake (200–203). Muscle protein synthesis is also decreased in fasting elderly subjects, especially in specific muscle fractions like mitochondrial proteins (204), and thus, the anorexia of aging and its underlying mechanisms contribute to sarcopenia by reducing protein intake. Several studies suggest that elderly people have an increased risk of impaired energy regulation that disposes them to progressive loss of body weigh including muscle (205, 206). One study reported that resting metabolic rate (RMR) increased less in older subjects compared to younger adults during overfeeding, and decreased less during underfeeding, suggesting some age-related disconnection between changes in energy intake and RMR response (206).
Muscle protein synthesis is directly stimulated by amino acid (207) and essential amino acids intake (208), and protein supplementation has been explored in the prevention of sarcopenia. However, many interventional studies have not reported a significant increase muscle mass or protein synthesis with a high protein diet even when accompanied by resistance training (74, 209, 210). The lack of effect of protein intake on protein synthesis stimulation may have several explanations (112). A higher splanchnic extraction of dietary amino acids has been already reported (141, 142). This could limit the delivery of dietary amino acids to the peripheral skeletal muscle. Then the capacity of amino acids to activate protein synthesis within the muscle may be dose dependent since a lower amount of amino acids is associated with a lower accretion of muscle protein synthesis (211) whereas high dose of amino acids does (140). Another important issue is a possible resistance to the natural stimulatory effect of leucine in aging muscle implying that higher leucine concentrations of leucine may be necessary to stimulate protein synthesis in elderly subjects (212). Carbohydrates added to the protein supplementation may impair the anabolic effect (98, 140, 141). This observation suggests an insulin resistance toward protein metabolism in elderly individuals (103, 213). This resistance to anabolic factors may ultimately be related to a reduced muscle blood flow (214). Elders may also spontaneously reduce their other energy intake in response to supplementation, thus attenuating any potential benefit (74).
Consequences of sarcopenia
Increased clinical and epidemiological interest in sarcopenia is related to the hypothesis that age-related loss of muscle mass and strength results in decreased functional limitation and mobility disability among the elderly (Figure 1). Sarcopenia also plays a predominant role in the etiology and pathogenesis of frailty, which is highly predictive of adverse events such as hospitalization, associated morbidity and disability and mortality (215, 216). Several epidemiological cross-sectional studies have documented associations between low skeletal muscle mass and physical disability (10, 13, 14, 33) or low physical performance (164) with the level of disability 2 to 5 time higher in the sarcopenic groups. Sarcopenia also results in decrease in muscular strength and endurance (217). The (VO2max) declines at the rate of 3 to 8% per decade after the age of 30 years, but adjusted for muscle mass, VO2max no longer declines. This suggests that a loss of muscle mass is a significant contributor to fatigue and decreased endurance (218). Consequently, we can speculate that sarcopenia is a predictor of disability in the elderly, but, very few longitudinal studies (13, 36, 219) have demonstrated that sarcopenia predicts disability and have reported little or no effect of sarcopenia on mobility disability. In the Health Aging Body Composition study, Visser et al. reported that subjects in the lowest quartile of cross sectional thigh area (measured by computed tomography) had higher risk (1.90 for men and 1.68 for women) of developing mobility disability than those in the highest quartile (36), and in the Cardiovascular Health Study, severe sarcopenia (defined using bioelectrical impedance analysis) was a modest independent risk factor for the development of physical disability. During the 8-year follow-up the risk of developing disability was only 1.27 higher for subjects with severe sarcopenia and the risk was not statistically significant in moderate sarcopenia (219). This weak association between sarcopenia and the risk of disability suggests that disability can result in cases of sarcopenia (Figure 1).
Figure 1.
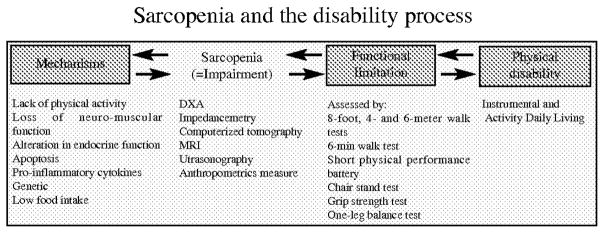
Sarcopenia and the disability process
Low physical activity and being sedentary, an important cause of sarcopenia, are also hallmarks of mobility disability. In the New Mexico Aging Process study, sarcopenia (measured by DXA) was not a risk factor for developing disability in the absence of obesity (46), but those with sarcopenic-obesity had a 2.6 higher risk to develop disability (46). Sarcopenia results in disability in the obese only because fat mass is a risk factor for disability more than lean mass, and growing evidence from cross-sectional and longitudinal studies suggests that obesity impairs physical function (220–229). In the Cardiovascular Health study, fat mass not muscle mass was a predictor of disability over 3-years of follow-up (224), and during a 4-years follow-up, obesity predicted self-reported limitation in older women (222) in the Study of Osteoporotic Fractures, but the age-related decrease of muscle appears to precipitate into disability more so in the presence of obesity, but this tends to be the case in sedentary obese not in the active obese. In the EPIDOS study, muscle strength adjusted for muscle mass (muscle quality) was not significantly different between sedentary obese and not obese, but muscle strength adjusted for muscle mass was higher in active participants and especially in the lower limbs of obese subjects. The effect of weight resistance training is greater in obese than in lean subjects and may explain these results (230).
Half the population of American elderly are obese or overweight (231) and most have a higher muscle mass and muscle strength compared to those who are not obese (232), but the higher lean mass in the obese is low compared to their total body weight and predicts functional limitation. This high muscle mass may also act as a nutritional reserve during a medical event, and an absolute high muscle mass may, in part, explain the lower rate of death in obese elderly (233–235). In elderly, the benefit of a higher muscle mass in obesity offset the associated cardiovascular risk factors, but a low muscle mass is associated with decreased survival rates following acute illness (236) and with a doubled risk of nosocomial infection in care of the elderly (237). Muscle strength is also associated with lower mortality in the Health ABC study (238).
Relationship between sarcopenia and physical performance
Part of the theoritical model for sarcopenia potentially involves the positive association between muscle mass and strength and in improved functional performance and reduced disability. The relationship between muscle mass and strength is linear (239), but the relationship between physical performance (such as walking speed) and muscle mass is curvilinear (240, 241) (Figure 2). Thus a threshold defining the amount of muscle mass under which muscle mass predicts poorer physical performance and physical disability should be detectable, but a specific threshold may exist for each physical task. The relationships among strength, muscle mass and function have important implications regarding the selection of therapeutic approaches. An increase in muscle mass and strength in the healthy elderly could have little effect on a specific physical performance, but a small increase in muscle mass among sarcopenic elderly could result in a significant increase in physical performance despite a relatively small increase in muscle strength. An increase in muscle mass may have no effect on walking speed in the healthy elderly but a significant impact in very frail. However, differences in functional outcomes and population characteristics are major determinants in the success of interventional studies on sarcopenia, and these differences are attributable, in part, to these methodological considerations.
Figure 2.

Relationship between muscle mass, muscle strength and physical performances
Other factors and associations should also be considered. Janssen et al. recently reported that a loss of muscle mass was greater in the legs than the arms (242), but the loss of muscle quality in elderly seems more significant in arms for men compared to women whereas loss of muscle quality in the leg seems similar in men and women (243). Sex hormones are probably one of the underlying mechanisms for these differences; however, tt is also possible that women maintain more activity on upper limbs than men because of their trend to remain active by performing housework and gardening, while the men lose some of their upper limb activity with work retirement. Because of the functional importance of leg muscle groups, discrepancies between upper or lower limbs muscle have important implications for mobility and disability prevention. Intervention should focus on increasing muscle mass and strength of functionally important muscle groups and to identify among the elderly, those individuals for whom intervention will be most relevant. In addition, the ability to perform activities of daily living such as walking, climbing stairs, standing-up from a chair relies on dynamic movements in which power in addition to strength is needed. Improving low-power capacity is important to maintain physical function, but muscle power is correlated to muscle strength and muscle mass, it changes independently of muscle strength and muscle mass.
Muscle strength is related to muscle mass, but the ability to perform activities of daily living also relies on other physiological characteristics (such as flexibility, coordination, praxis, and balance). Increasing muscle strength is a potential therapeutic approach against sarcopenia, but each individual should develop his/her own strategy to perform physical tasks. Elderly women recruit mainly their coordination ability, while men rely on their muscle strength to perform the same physical task (244). Moreover, co-deficiencies (sarcopenia plus poor balance; sarcopenia plus poor flexibility; sarcopenia plus poor endurance, etc.) can produce a greater than additive effect for poor outcomes.
Treatment and future perspectives
Sarcopenia is treated currently with pharmacological treatment and lifestyle interventions. Conisiderable evidence suggests that sarcopenia is a reversible cause of disability and could benefit from intervention, especially at the early stage of sarcopenia (8, 245, 246). However, the effects and ability of these interventions to improve function and prevent disability and reduce the age-related skeletal muscle decline in elderly are unknown.
Physical activity
No pharmacological or behavioral intervention to reverse sarcopenia has proven to be as efficacious as resistance training. The American College of Sport Medicine and the American Heart Association suggested that training at a 70–90% of 1-RM (maximal repetition) on two or more nonconsecutive days per week was the appropriate training intensity to produce gains in muscle size and strength, even in frail elderly (135, 247). Resistance training in elderly increases strength that is low in absolute term but similar relative to muscle mass, but the increased muscle size is relatively moderate (between 5 and 10%) compared to the increase in muscle strength. Most of the increase in strength is in neural adaptation of the motor unit pathway (5), but disuse results in a rapid detraining (248). Several reports suggest that maintaining the benefits from resistance training is possible with as little as one exercise program per week (249).
Older subjects participate in physical activity programs in the long term (250), but organizing resistance training sessions and programs are challenging in frail elderly subjects and some practitioners are reluctant to prescribe high intensity exercise in elderly patients. Currently, only 12% of the United-States elderly population performs strength training at least twice a week (251). Thus, new exercise programs could be relevant in frail elderly population such as whole body vibration exercise, a safe, simple and effective way to exercise musculoskeletal structures (252–254). Beneficial effects on joint pain and cardiovascular system have also been reported (252). However, more studies are needed to ascertain beneficial effects and safety of whole body vibration exercise on sarcopenia (252).
Nutrition
In elderly populations, any form of weight loss in thin, normal, overweight and obese elderly results in loss of muscle mass and increased rate of death (255). Weight loss should be avoided after the seventh decade of life (256, 257) especially if it results in a reduction of the BMI and no corresponding reduction in waist circumference because waist circumference is related to cardiovascular disease while BMI is related to total body mass and lean mass. In malnourished elderly, poor protein intake is a barrier to gains in muscle tissue and strength from interventions such as resistance training. Increasing protein intake in elderly and especially in frail elderly (higher than the recommended 0.8g/kg body weight per day) can minimize the sarcopenic process (258). Higher protein intake is associated with a significant decrease in lean mass in the elderly (259), but it is not clear if protein supplementation in the absence of malnutrition enhances muscle mass and muscle strength, as protein supplementation alone or in association with physical training has proved unsuccessful (see (135) for references). New approaches, based on specific nutriments, including essential amino acids (leucine) (260) suggested an anabolic effect (261). It has been recently reported that essential amino acids stimulate protein anabolism in elderly whereas nonessential amino acids add no effect in association to essential amino acids (208, 262). The acute muscle protein synthesis in response to resistance training and essential amino acids ingestion is similar in old and young subjects but delayed in older subjects (262). In supraphysiologic concentration, leucine stimulates muscle protein synthesis (260), which may be related to a direct effect of leucine on the initiation of mRNA translation, and amino acids supplements are ineffective for muscle protein synthesis if they do not contain sufficient leucine (211). The quantity and quality of amino acids in the diet are important factors for stimulating protein synthesis, and nutritional supplementation with whey proteins, a rich source of leucine, is a possible safe strategy to prevent sarcopenia (263, 264). However, caloric restriction can prevent the loss of muscle mass in animal and supposedly some human studies (265, 266).
The schedule of the protein supplementation is relevant to improve muscle protein synthesis. A large amount of amino acid supplementation in one meal per day is more efficient in increasing the anabolic effect than intermittent protein intake (267). The anabolic effect of protein supplementation may be maximized with a large amount of a highly efficient nutritional supplement (such as essential amino acid and especially leucine) once a day. Another way to optimize postprandial protein anabolism is to administer “fast” protein (i.e. fastly digested protein by analogy with “fast” carbohydrate concept) which is an interesting nutritional strategy (268, 269). However, no randomized clinical trial actually supports the benefits of this specific approach on muscle mass synthesis. In association with strength training, the timing of the protein supplementation may also affect muscle tissue anabolism. Compared to protein supplementation taken between resistance training sessions, protein supplementation taken immediately after the resistance training produces a 25% increase in quadriceps muscle cross-sectional area. No increase occurs if the supplementation happens at some passing of time after the training session (270).
Prevention of sarcopenia should occur throughout life. The possible influence of specifics exposures at critical development periods may have a major impact on the risk of sarcopenia in old age (271, 185). An adequate diet in childhood and young adulthood affect bone development and calcium maintenance is required throughout life, thus the same appears to be a reasonable lifestyle and treatment regime for sarcopenia. A well balanced diet, along with adequate amounts of essential minerals, fatty acids and amino acids, together and an active and healthy lifestyle with regular periods of aerobic and resistance training would go a long way toward reducing the prevalence of sarcopenia and other chronic diseases in future elderly generations.
Testosterone
About 20% of men older than 60 years and 50% of men older than 80 years are considered hypogonadic defined as a total testosterone concentration 2 SDs below the mean of healthy young men (272). There are conflicting and inconclusive results of the effectiveness of testosterone therapy on muscle mass and muscle strength in elderly. Testosterone increases muscle mass and strength at supraphysiological doses in young subjects under resistance training (273), but such dose levels are not administered in the elderly. Some interventional studies report a modest increase in lean mass and most report no increase in strength (see (135) for references). For the few studies that report an increase in strength, the magnitude was lower than through resistance training. Moreover, the anabolic effect of testosterone on lean mass and strength seems weaker in the elderly than in young (135). A recent meta-analysis indicated that there is a moderate increase in muscle strength among men participating in 11 randomized studies (with one study influencing the mean effect size) (274). Studies of DHEA have also reported no change in muscle strength (135).
Testosterone is currently not recommended for the treatment of sarcopenia, and side-effects associated with other androgens limit their use also. The potential risks associated with testosterone therapy (e.g. increased of prostate-specific antigen, hematocrit and cardiovascular risks) compared to the low level of evidence concerning the benefits on physical performances and function explain the actual recommendations (275). High doses of testosterone have not be given in RCT for fear of prostate cancer (276), and sense data from the Baltimore Longitudinal Study on Aging report a positive correlation between free testosterone blood level and prostate cancer (277).
New synthetic androgen modulators such as the 7 α-methyl-19-nortesterone (or MENT or trestolone) are potential alternatives to testosterone, but randomized trials have not been conducted. MENT has an anabolic effect on bone and muscle in rats (278) and may have small negative effects on the prostate. Another therapeutic perspective is the Selective Androgen Receptor Modulators (SARMs) that has the same anabolic effect on muscle tissue as testosterone but without the side-effects (279). These new drugs may expand the clinical application of androgens in sarcopenia as they enter the clinical phase of research.
Growth hormone
GH increases muscle strength and mass in young subjects with hypopituitarism, but in the elderly, who are frequently GH-deficient, most studies report that GH supplementation does not increase muscle mass or strength (135) even in association with resistance training (122, 135). GH increases mortality in ill malnourished persons (280), and potential serious and frequent side effects such as arthralgia, edema, cardiovascular side-effect and insulin resistance occur with GH supplementation (117). To date, there is little clinical research support for the use of GH supplementation in the treatment of sarcopenia. GH is able to induce IGF-I mRNA production and increased suppression of cytokine signaling-2 (SOC-2) in myoblast cell experimental studies (281). SOC-2 has been reported to be a major modulator of GH action (282). SOC-2 is a cytokine-inducible protein that inhibits cytokines production through a negative feedback mechanism. SOC-2 system may be used in future studies on sarcopenia and other keys anabolic signaling proteins are potentially involved (283). Previous observations examining the association of IGF-I with muscle strength and physical performance in older populations provide conflicting results (284–286). Interestingly, in a study among obese postmenopausal women, the administration of GH alone or in combination with IGF-I caused a greater increase in fat-free mass and a greater reduction in fat mass than those achieved by diet and exercise alone (287). However, the clinical applications of these findings are limited by safety issues. Recent studies have found that IGF-I correlates with risk of prostate cancer in men, premenopausal breast cancer in women, and lung cancer and colorectal cancer in both men and women (288). Several IGF binding protein (IGFBP) have been describe. Most of their physiological and pathological effects are unknown. However, IGFBP-5 is reported to be an important modulator of myogenesis (289).
Myostatin
Myostatin is a recently discovered natural inhibitor of muscle growth (290), and mutations in the myostatin gene result in muscle hypertrophy in animals and in humans (291, 292). Antagonism of myostatin enhanced muscle tissue regeneration in aged mice (292) by increasing satellite cell proliferation. Smoking impairs muscle protein synthesis and increases the expression of myostatin in human (293). Antagonist of myostatin drug (such as follistatin or caveolin-3) have a potential therapeutic impact in future studies on sarcopenia (294–296). Recombinant human antibodies to myostatin (myo-029) are actually tested in RCT in muscular dystrophy human, and a soluble activin type IIB receptor that reduces myostatin effect is actually in development. A single gene myostatin inhibitor enhances muscle mass and strength in mouse (297). All these new approaches may be relevant to the treatment of sarcopenia in the future.
Estrogens and Tibolone
A recent review on the effect of estrogen and tibolone on muscle strength and body composition (109) report an increase muscle strength but only tibolone appears to increase lean body mass and decrease total fat mass. Tibolone is a synthetic steroid with estrogenic, androgenic and progestogenic activity. HRT and tibolone may both react with the intra-nuclear receptor in the muscle fibers (298, 299), and tibolone may also act by binding androgen receptors in the muscle fibers and increase free testosterone and GH. However, further research is needed to confirm these findings and the long term safety of these drugs in elderly population. In fact, no study has currently confirmed the positive findings in older persons.
Vitamin D
Vitamin D supplementation between 700 and 800 iu per day reduces the risk of hip fracture (and any non vertebral fracture) in community-dwelling and nursing home elderly (300) and the risk of falls (143). The underlying mechanism may be the increased muscle strength. Janssen et al. reported an histological muscle atrophy, predominantly type II fibers, in vitamin D deficiency (301). Whether vitamin D prevents sarcopenia remains to be proven, but the relationship of vitamin D and calcium on muscle mass and function in the elderly is another important area for research.
Creatine
Creatine supplementation is supposed to increase muscle mass synthesis (see (302) for review) by increasing intra muscular creatine and phosphocreatine (303) which allows increased resistance training to stimulate muscle mass synthesis. Several mechanisms for this action are hypothesized such as an increased expression of myogenic transcription factors (304), but few clinical trials in elderly samples (in addition to physical training or not) report conflicting results (303, 305–311). It is unknown whether, creatine supplementation prevents or reduces sarcopenia and its associated disability and morbidity (302, 312).
Angiotensin II Converting Enzyme inhibitors (ACE inhibitors)
Growing evidence suggests that ACE inhibitors may prevent sarcopenia ((313, 314) and see (258) for review). Activation of the renine-angiotensin-aldostrone system may be involved in the progress of sarcopenia. Angiotensin II infused in rats results in muscle atrophy (294), and several mechanisms such as influences on oxidative stress, metabolic and inflammation pathway have been suggested through epidemiological and experimental studies. ACE inhibitor reduces the level of angiotensine II in vascular muscle cell, and angiotensin II may be a risk factor for sarcopenia through the related increase in pro-inflammatory cytokines production (315). ACE inhibitors may also improve exercise tolerance via changes in skeletal muscle myosin heavy chain composition (316). This decrease in inflammatory markers via ACE inhibitors may improve the microvascular endothelium and the blood flow, consequently slowing muscle loss (317). The ACE gene polymorphism also affects the muscle anabolic response and muscular efficiency after physical training (318).
Cytokine inhibitors
The age-related inflammation process is supposed to be an important factor in the development of sarcopenia, and antiinflammatory drugs may delay its onset and progression. Cytokine inhibitors, such as thalidomide, increase weight and lean tissue anabolism in AIDS patients (319). TNFα produces muscle tissue atrophy in vitro. Anti-TNFα antibodies, a treatment provided to rheumatoid arthritis patients, may also be an alternative therapeutic opportunity for sarcopenia (320). However, the benefit/risk balance of these drugs is a major limitation that has not yet been tested in sarcopenic patients. Epidemiological data also suggest that fatty fish consumption rich in the anti inflammatory actions of omega-3 fatty acid may prevent sarcopenia (321).
Genes
Many genetic factors contribute to muscle mass and strength (322, 323). Treatment based on the basic physiopathology of sarcopenia can be expected in the future (195). Understanding the fundamental pathways leading to sarcopenia, such as the expression pattern of genes and proteomics will probably determine future treatment strategies. Many genes that have been reported to be expressed differentially in young or old muscle tissue may have a significant role in the pathogenesis of sarcopenia.
Apoptosis
Although evidence of the role of apoptosis on sarcopenia is lacking in human studies, recent experimental studies suggest that apoptosis may be a determinant factor leading to muscle loss. Our understanding of the mechanisms of apoptosis suggests that caspase inhibitors may represent a possible future therapy (177). Apoptosis may be reversible. For instance, exercise training reverses the skeletal muscle apoptosis (324) and caloric restriction reduce apoptosis pathway stimulated by TNFα (266, 325). Redox modulators such as carotenoids (326) seem to be important factors in influencing loss of muscle strength, functional limitation and disability. Interest in all these molecules is actually suggested by basic research but may be studied in future clinical researches.
Conclusion
Improved understanding and treatment of sarcopenia would have a dramatic impact on improving the health and quality of life for the elderly, reducing the associated comorbidity and disability and stabilizing rising health care costs. However, continued research is needed to fleshout a consensus operational clinical definition of sarcopenia applicable in clinical management and clinical and epidemiological research across populations. Sarcopenia is a complex multifactorial condition, the inter-related underpinnings and onset of which are difficult to detect and poorly understood. Thus, a comprehensive approach to sarcopenia requires a multi-modal approach. Reducing the loss of muscle mass and muscle strength is relevant if the decrease in physical performances and increase in disability are affected. Defining target elderly populations for specific treatments in clinical trials is an important issue if the findings and their interpretation are to be inferred to other groups and populations of elderly individuals. An important clinical endpoint should be the prevention of mobility disability along with the reducing, stopping or reversing the loss of muscle mass, muscle strength or muscle quality.
Currently, resistance strength training is the only treatment that affects the muscle aspects of sarcopenia. There are no pharmacological approaches that provide definitive evidence in the ability to prevent the decline in physical function and sarcopenia. Current and future pharmacological and clinical trials and epidemiological studies could radically change our therapeutic approach to understanding and treating mobility disability in elderly. However, this change requires the concerted effort to develop a clear and applicable operational definition of sarcopenia that at the least works well within populations.
Footnotes
Financial disclosure statement: Yves Rolland, Stefan Czerwinski, Gabor Abellan van Kan, Matteo Cesari, Graziano Onder, Jean Woo, Richard Baumgartner, Fabien Pillard, Yves Boirie, Cameron Chumlea, and Bruno Vellas have reported no financial or other conflicts of interest that might bias their work. John E. Morley consults for M&P Pharmaceuticals, Nestle and Amgen.
References
- 1.Landi F, et al. Body mass index and mortality among older people living in the community. J Am Geriatr Soc. 1999;47(9):1072–6. doi: 10.1111/j.1532-5415.1999.tb05229.x. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg Summary comments. Am J Clin Nutr. 1989;(50):1231–3. [Google Scholar]
- 3.Schwartz RS. Sarcopenia and physical performance in old age: introduction. Muscle Nerve Suppl. 1997;5:S10–2. [PubMed] [Google Scholar]
- 4.Hughes VA, et al. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr. 2002;76(2):473–81. doi: 10.1093/ajcn/76.2.473. [DOI] [PubMed] [Google Scholar]
- 5.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25(1):17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 6.Roubenoff R, V, Hughes A. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55(12):M716–24. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- 7.Morley JE, et al. Sarcopenia. J Lab Clin Med. 2001;137(4):231–43. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 8.Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127(5 Suppl):998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher D, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83(1):229–39. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner RN, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner R. In vivo body composition studies. In: Yasumura WJS, Pierson RN Jr, editors. Ann NY Acad Sci ed Body composition in healthy aging. Vol. 904. 2000. pp. 437–448. [DOI] [PubMed] [Google Scholar]
- 12.Rolland Y, et al. Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc. 2003;51(8):1120–4. doi: 10.1046/j.1532-5415.2003.51362.x. [DOI] [PubMed] [Google Scholar]
- 13.Janssen I, et al. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413–21. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 14.Melton LJ, 3rd, et al. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48(6):625–30. [PubMed] [Google Scholar]
- 15.Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol. 1990;45(3):M82–8. doi: 10.1093/geronj/45.3.m82. [DOI] [PubMed] [Google Scholar]
- 16.Janssen I, et al. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52(1):80–5. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 17.Thomas DR. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26(4):389–99. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Morley JE. Weight loss in the nursing home. J Am Med Dir Assoc. 2007;8(4):201–4. doi: 10.1016/j.jamda.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83(4):735–43. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 20.Friedman PJ, Campbell AJ, Caradoc-Davies TH. Prospective trial of a new diagnostic criterion for severe wasting malnutrition in the elderly. Age Ageing. 1985;14(3):149–54. doi: 10.1093/ageing/14.3.149. [DOI] [PubMed] [Google Scholar]
- 21.Girasole G, et al. Oestrogens prevent the increase of human serum soluble interleukin-6 receptor induced by ovariectomy in vivo and decrease its release in human osteoblastic cells in vitro. Clin Endocrinol (Oxf) 1999;51(6):801–7. doi: 10.1046/j.1365-2265.1999.00896.x. [DOI] [PubMed] [Google Scholar]
- 22.Waters DL, et al. Skeletal muscle mitochondrial function and lean body mass in healthy exercising elderly. Mech Ageing Dev. 2003;124(3):301–9. doi: 10.1016/s0047-6374(02)00197-5. [DOI] [PubMed] [Google Scholar]
- 23.van Kan GA, et al. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9(2):71–2. doi: 10.1016/j.jamda.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Abellan Van Kan A, et al. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12(1):29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 25.Chumlea WC, et al. Techniques of assessing muscle mass and function (sarcopenia) for epidemiological studies of the elderly. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):45–51. doi: 10.1093/gerona/50a.special_issue.45. [DOI] [PubMed] [Google Scholar]
- 26.Chumlea WC, Roche AF, Webb P. Body size, subcutaneous fatness and total body fat in older adults. Int J Obes. 1984;8(4):311–7. [PubMed] [Google Scholar]
- 27.Chumlea WC, Baumgartner RN. Status of anthropometry and body composition data in elderly subjects. Am J Clin Nutr. 1989;50(5 Suppl):1158–66. doi: 10.1093/ajcn/50.5.1158. discussion 1231–5. [DOI] [PubMed] [Google Scholar]
- 28.Baumgartner RN, WD . Sarcopenia and sarcopenic-obesity. In: Pathy MSJ, editor. Principles and Practice of Geriatric Medicine. Vol. 2. United Kingdom: John Wiley and Sons Ltd; 2006. pp. 909–933. [Google Scholar]
- 29.Heymsfield SB, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52(2):214–8. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, et al. Dual-energy X-ray absorptiometry is a valid tool for assessing skeletal muscle mass in older women. J Nutr. 2007;137(12):2775–80. doi: 10.1093/jn/137.12.2775. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, et al. Intermuscular adipose tissue-free skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in adults. J Appl Physiol. 2004;97(2):655–60. doi: 10.1152/japplphysiol.00260.2004. [DOI] [PubMed] [Google Scholar]
- 32.Newman AB, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51(11):1602–9. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 33.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 34.Delmonico MJ, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55(5):769–74. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 35.Song MY, et al. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79(5):874–80. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 36.Visser M, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 37.Chumlea WC, Roche AF. Ultrasonic and skinfold caliper measures of subcutaneous adipose tissue thickness in elderly men and women. Am J Phys Anthropol. 1986;71(3):351–7. doi: 10.1002/ajpa.1330710310. [DOI] [PubMed] [Google Scholar]
- 38.Reeves ND, Maganaris CN, Narici MV. Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol. 2004;91(1):116–8. doi: 10.1007/s00421-003-0961-9. [DOI] [PubMed] [Google Scholar]
- 39.Chumlea WC, Baumgartner RN, Roche AF. Specific resistivity used to estimate fat-free mass from segmental body measures of bioelectric impedance. Am J Clin Nutr. 1988;48(1):7–15. doi: 10.1093/ajcn/48.1.7. [DOI] [PubMed] [Google Scholar]
- 40.Chumlea WC, et al. Bioelectric and anthropometric assessments and reference data in the elderly. J Nutr. 1993;123(2 Suppl):449–53. doi: 10.1093/jn/123.suppl_2.449. [DOI] [PubMed] [Google Scholar]
- 41.Baumgartner RN, Chumlea WC, Roche AF. Estimation of body composition from bioelectric impedance of body segments. Am J Clin Nutr. 1989;50(2):221–6. doi: 10.1093/ajcn/50.2.221. [DOI] [PubMed] [Google Scholar]
- 42.Janssen I, et al. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89(2):465–71. doi: 10.1152/jappl.2000.89.2.465. [DOI] [PubMed] [Google Scholar]
- 43.Narici M, Cerretelli P. Changes in human muscle architecture in disuse-atrophy evaluated by ultrasound imaging. J Gravit Physiol. 1998;5(1):73–4. [PubMed] [Google Scholar]
- 44.Martin AD, et al. Anthropometric estimation of muscle mass in men. Med Sci Sports Exerc. 1990;22(5):729–33. doi: 10.1249/00005768-199010000-00027. [DOI] [PubMed] [Google Scholar]
- 45.Heymsfield SB, et al. Anthropometric measurement of muscle mass: revised equations for calculating bone-free arm muscle area. Am J Clin Nutr. 1982;36(4):680–90. doi: 10.1093/ajcn/36.4.680. [DOI] [PubMed] [Google Scholar]
- 46.Baumgartner RN, et al. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12(12):1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 47.Heymsfield SB, et al. A radiographic method of quantifying protein-calorie undernutrition. Am J Clin Nutr. 1979;32(3):693–702. doi: 10.1093/ajcn/32.3.693. [DOI] [PubMed] [Google Scholar]
- 48.Rantanen T, et al. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51(5):636–41. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 49.Overend TJ, et al. Knee extensor and knee flexor strength: cross-sectional area ratios in young and elderly men. J Gerontol. 1992;47(6):M204–10. doi: 10.1093/geronj/47.6.m204. [DOI] [PubMed] [Google Scholar]
- 50.Visser M, et al. Reexamining the sarcopenia hypothesis. Muscle mass versus muscle strength. Health, Aging, and Body Composition Study Research Group. Ann N Y Acad Sci. 2000;904:456–61. [PubMed] [Google Scholar]
- 51.Bassey EJ, et al. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 1992;82(3):321–7. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- 52.Lauretani F, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95(5):1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 53.Callahan D, et al. Assessment of lower extremity muscle power in functionally-limited elders. Aging Clin Exp Res. 2007;19(3):194–9. doi: 10.1007/BF03324689. [DOI] [PubMed] [Google Scholar]
- 54.Cunningham DA, et al. Ageing and isokinetic plantar flexion. Eur J Appl Physiol Occup Physiol. 1987;56(1):24–9. doi: 10.1007/BF00696371. [DOI] [PubMed] [Google Scholar]
- 55.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46(3):451–6. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- 56.Poulin MJ, et al. Eccentric and concentric torques of knee and elbow extension in young and older men. Can J Sport Sci. 1992;17(1):3–7. [PubMed] [Google Scholar]
- 57.Vandervoort AA, Kramer JF, Wharram ER. Eccentric knee strength of elderly females. J Gerontol. 1990;45(4):B125–8. doi: 10.1093/geronj/45.4.b125. [DOI] [PubMed] [Google Scholar]
- 58.Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58(11):1012–7. doi: 10.1093/gerona/58.11.m1012. [DOI] [PubMed] [Google Scholar]
- 59.Rolland YM, et al. Loss of appendicular muscle mass and loss of muscle strength in young postmenopausal women. J Gerontol A Biol Sci Med Sci. 2007;62(3):330–5. doi: 10.1093/gerona/62.3.330. [DOI] [PubMed] [Google Scholar]
- 60.Baumgartner RN, et al. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107(2):123–36. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 61.Malbut KE, Dinan S, Young A. Aerobic training in the ‘oldest old’: the effect of 24 weeks of training. Age Ageing. 2002;31(4):255–60. doi: 10.1093/ageing/31.4.255. [DOI] [PubMed] [Google Scholar]
- 62.Zachwieja JJ, Yarasheski KE. Does growth hormone therapy in conjunction with resistance exercise increase muscle force production and muscle mass in men and women aged 60 years or older? Phys Ther. 1999;79(1):76–82. [PubMed] [Google Scholar]
- 63.Morley JE, Baumgartner RN. Cytokine-related aging process. J Gerontol A Biol Sci Med Sci. 2004;59(9):M924–9. doi: 10.1093/gerona/59.9.m924. [DOI] [PubMed] [Google Scholar]
- 64.Goodpaster BH, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90(6):2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 65.Kortebein P, et al. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. Jama. 2007;297(16):1772–4. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 66.Lee JS, et al. Associated Factors and Health Impact of Sarcopenia in Older Chinese Men and Women: A Cross-Sectional Study. Gerontology. 2007;53(6):166–172. doi: 10.1159/000107355. [DOI] [PubMed] [Google Scholar]
- 67.Sheffield-Moore M, et al. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab. 2004;287(3):E513–22. doi: 10.1152/ajpendo.00334.2003. [DOI] [PubMed] [Google Scholar]
- 68.Coggan AR, et al. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72(5):1780–6. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- 69.Charifi N, et al. Effects of endurance training on satellite cell frequency in skeletal muscle of old men. Muscle Nerve. 2003;28(1):87–92. doi: 10.1002/mus.10394. [DOI] [PubMed] [Google Scholar]
- 70.Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol. 1993;265(2 Pt 1):E210–4. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]
- 71.Hasten DL, et al. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab. 2000;278(4):E620–6. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- 72.Jozsi AC, et al. Changes in power with resistance training in older and younger men and women. J Gerontol A Biol Sci Med Sci. 1999;54(11):M591–6. doi: 10.1093/gerona/54.11.m591. [DOI] [PubMed] [Google Scholar]
- 73.Welle S, Thornton C, Statt M. Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am J Physiol. 1995;268(3 Pt 1):E422–7. doi: 10.1152/ajpendo.1995.268.3.E422. [DOI] [PubMed] [Google Scholar]
- 74.Fiatarone MA, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330(25):1769–75. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 75.Yarasheski KE, et al. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men >/=76 yr old. Am J Physiol. 1999;277(1 Pt 1):E118–25. doi: 10.1152/ajpendo.1999.277.1.E118. [DOI] [PubMed] [Google Scholar]
- 76.Ivey FM, et al. Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J Gerontol A Biol Sci Med Sci. 2000;55(11):M641–8. doi: 10.1093/gerona/55.11.m641. [DOI] [PubMed] [Google Scholar]
- 77.Cress ME, et al. Exercise: effects on physical functional performance in independent older adults. J Gerontol A Biol Sci Med Sci. 1999;54(5):M242–8. doi: 10.1093/gerona/54.5.m242. [DOI] [PubMed] [Google Scholar]
- 78.Hikida RS, et al. Effects of high-intensity resistance training on untrained older men. II. Muscle fiber characteristics and nucleo-cytoplasmic relationships. J Gerontol A Biol Sci Med Sci. 2000;55(7):B347–54. doi: 10.1093/gerona/55.7.b347. [DOI] [PubMed] [Google Scholar]
- 79.Hagerman FC, et al. Effects of high-intensity resistance training on untrained older men. I. Strength, cardiovascular, and metabolic responses. J Gerontol A Biol Sci Med Sci. 2000;55(7):B336–46. doi: 10.1093/gerona/55.7.b336. [DOI] [PubMed] [Google Scholar]
- 80.Hameed M, Harridge SD, Goldspink G. Sarcopenia and hypertrophy: a role for insulin-like growth factor-1 in aged muscle? Exerc Sport Sci Rev. 2002;30(1):15–9. doi: 10.1097/00003677-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 81.Trappe S. Master athletes. Int J Sport Nutr Exerc Metab. 2001;11(Suppl):S196–207. doi: 10.1123/ijsnem.11.s1.s196. [DOI] [PubMed] [Google Scholar]
- 82.Raguso CA, et al. A 3-year longitudinal study on body composition changes in the elderly: role of physical exercise. Clin Nutr. 2006;25(4):573–80. doi: 10.1016/j.clnu.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 83.Edstrom E, et al. Factors contributing to neuromuscular impairment and sarcopenia during aging. Physiol Behav. 2007;92(1–2):129–35. doi: 10.1016/j.physbeh.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 84.Lauretani F, et al. Axonal degeneration affects muscle density in older men and women. Neurobiol Aging. 2006;27(8):1145–54. doi: 10.1016/j.neurobiolaging.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Belanger AY, McComas AJ. Extent of motor unit activation during effort. J Appl Physiol. 1981;51(5):1131–5. doi: 10.1152/jappl.1981.51.5.1131. [DOI] [PubMed] [Google Scholar]
- 86.Andersen JL, Terzis G, Kryger A. Increase in the degree of coexpression of myosin heavy chain isoforms in skeletal muscle fibers of the very old. Muscle Nerve. 1999;22(4):449–54. doi: 10.1002/(sici)1097-4598(199904)22:4<449::aid-mus4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 87.Essen-Gustavsson B, Borges O. Histochemical and metabolic characteristics of human skeletal muscle in relation to age. Acta Physiol Scand. 1986;126(1):107–14. doi: 10.1111/j.1748-1716.1986.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 88.Oertel G. Changes in human skeletal muscles due to ageing. Histological and histochemical observations on autopsy material. Acta Neuropathol (Berl) 1986;69(3–4):309–13. doi: 10.1007/BF00688309. [DOI] [PubMed] [Google Scholar]
- 89.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):11–6. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 90.McComas AJ. 1998 ISEK Congress Keynote Lecture: Motor units: how many, how large, what kind? International Society of Electrophysiology and Kinesiology. J Electromyogr Kinesiol. 1998;8(6):391–402. doi: 10.1016/s1050-6411(98)00020-0. [DOI] [PubMed] [Google Scholar]
- 91.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–27. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 92.Doherty TJ, et al. Effects of motor unit losses on strength in older men and women. J Appl Physiol. 1993;74(2):868–74. doi: 10.1152/jappl.1993.74.2.868. [DOI] [PubMed] [Google Scholar]
- 93.Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103(1):31–9. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 94.Lee CE, McArdle A, Griffiths RD. The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr. 2007 doi: 10.1016/j.clnu.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 95.Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5(3):129–42. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 96.Fargnoli J, et al. Decreased expression of heat shock protein 70 mRNA and protein after heat treatment in cells of aged rats. Proc Natl Acad Sci U S A. 1990;87(2):846–50. doi: 10.1073/pnas.87.2.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Edstrom E, et al. Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61(7):663–74. doi: 10.1093/gerona/61.7.663. [DOI] [PubMed] [Google Scholar]
- 98.Volpi E, et al. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85(12):4481–90. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boirie Y, et al. Differential insulin sensitivities of glucose, amino acid, and albumin metabolism in elderly men and women. J Clin Endocrinol Metab. 2001;86(2):638–44. doi: 10.1210/jcem.86.2.7193. [DOI] [PubMed] [Google Scholar]
- 100.Goulet ED, et al. No difference in insulin sensitivity between healthy postmenopausal women with or without sarcopenia: a pilot study. Appl Physiol Nutr Metab. 2007;32(3):426–33. doi: 10.1139/H07-005. [DOI] [PubMed] [Google Scholar]
- 101.Boirie Y, et al. Tissue-specific regulation of mitochondrial and cytoplasmic protein synthesis rates by insulin. Diabetes. 2001;50(12):2652–8. doi: 10.2337/diabetes.50.12.2652. [DOI] [PubMed] [Google Scholar]
- 102.Guillet C, Boirie Y. Insulin resistance: a contributing factor to age-related muscle mass loss? Diabetes Metab. 2005;31(Spec No 2):5S20–5S26. doi: 10.1016/s1262-3636(05)73648-x. [DOI] [PubMed] [Google Scholar]
- 103.Guillet C, et al. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. Faseb J. 2004;18(13):1586–7. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 104.Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care. 2003;6(3):295–9. doi: 10.1097/01.mco.0000068965.34812.62. [DOI] [PubMed] [Google Scholar]
- 105.Rasmussen BB, Phillips SM. Contractile and nutritional regulation of human muscle growth. Exerc Sport Sci Rev. 2003;31(3):127–31. doi: 10.1097/00003677-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 106.Dionne IJ, Kinaman KA, Poehlman ET. Sarcopenia and muscle function during menopause and hormone-replacement therapy. J Nutr Health Aging. 2000;4(3):156–61. [PubMed] [Google Scholar]
- 107.Phillips SK, et al. The weakness of old age is not due to failure of muscle activation. J Gerontol. 1992;47(2):M45–9. doi: 10.1093/geronj/47.2.m45. [DOI] [PubMed] [Google Scholar]
- 108.Kramer PR, Kramer SF, Guan G. 17 beta-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis Rheum. 2004;50(6):1967–75. doi: 10.1002/art.20309. [DOI] [PubMed] [Google Scholar]
- 109.Jacobsen DE, et al. Postmenopausal HRT and tibolone in relation to muscle strength and body composition. Maturitas. 2007;58(1):7–18. doi: 10.1016/j.maturitas.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 110.Taaffe DR, et al. Estrogen replacement, muscle composition, and physical function: The Health ABC Study. Med Sci Sports Exerc. 2005;37(10):1741–7. doi: 10.1249/01.mss.0000181678.28092.31. [DOI] [PubMed] [Google Scholar]
- 111.Gower BA, Nyman L. Associations among oral estrogen use, free testosterone concentration, and lean body mass among postmenopausal women. J Clin Endocrinol Metab. 2000;85(12):4476–80. doi: 10.1210/jcem.85.12.7009. [DOI] [PubMed] [Google Scholar]
- 112.Volpi E, Nazemi R, Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. 2004;7(4):405–10. doi: 10.1097/01.mco.0000134362.76653.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brown M, Birge SJ, Kohrt WM. Hormone replacement therapy does not augment gains in muscle strength or fat-free mass in response to weight-bearing exercise. J Gerontol A Biol Sci Med Sci. 1997;52(3):B166–70. doi: 10.1093/gerona/52a.3.b166. [DOI] [PubMed] [Google Scholar]
- 114.Morley JE. Growth hormone: fountain of youth or death hormone? J Am Geriatr Soc. 1999;47(12):1475–6. doi: 10.1111/j.1532-5415.1999.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 115.Musaro A, et al. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400(6744):581–5. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- 116.Chen Y, Zajac JD, MacLean HE. Androgen regulation of satellite cell function. J Endocrinol. 2005;186(1):21–31. doi: 10.1677/joe.1.05976. [DOI] [PubMed] [Google Scholar]
- 117.Blackman MR, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. Jama. 2002;288(18):2282–92. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 118.Lange KH, et al. GH administration and discontinuation in healthy elderly men: effects on body composition, GH-related serum markers, resting heart rate and resting oxygen uptake. Clin Endocrinol (Oxf) 2001;55(1):77–86. doi: 10.1046/j.1365-2265.2001.01344.x. [DOI] [PubMed] [Google Scholar]
- 119.Papadakis MA, et al. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124(8):708–16. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 120.Thompson JL, et al. The effects of recombinant human insulin-like growth factor-I and growth hormone on body composition in elderly women. J Clin Endocrinol Metab. 1995;80(6):1845–52. doi: 10.1210/jcem.80.6.7539817. [DOI] [PubMed] [Google Scholar]
- 121.Rudman D, et al. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323(1):1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- 122.Yarasheski KE, et al. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol. 1995;268(2 Pt 1):E268–76. doi: 10.1152/ajpendo.1995.268.2.E268. [DOI] [PubMed] [Google Scholar]
- 123.Clavel S, et al. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat Tibialis Anterior muscle. Mech Ageing Dev. 2006;127(10):794–801. doi: 10.1016/j.mad.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 124.Morley JE, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410–3. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- 125.Morley JE, et al. Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: progressive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin-like growth factor 1 to growth hormone. Proc Natl Acad Sci U S A. 1997;94(14):7537–42. doi: 10.1073/pnas.94.14.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Galvao DA, et al. Exercise can prevent and even reverse adverse effects of androgen suppression treatment in men with prostate cancer. Prostate Cancer Prostatic Dis. 2007 doi: 10.1038/sj.pcan.4500975. [DOI] [PubMed] [Google Scholar]
- 127.Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab. 1992;75(4):1092–8. doi: 10.1210/jcem.75.4.1400877. [DOI] [PubMed] [Google Scholar]
- 128.Morley JE, et al. Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc. 1993;41(2):149–52. doi: 10.1111/j.1532-5415.1993.tb02049.x. [DOI] [PubMed] [Google Scholar]
- 129.Katznelson L, et al. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81(12):4358–65. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- 130.Sih R, et al. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661–7. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- 131.Ly LP, et al. A double-blind, placebo-controlled, randomized clinical trial of transdermal dihydrotestosterone gel on muscular strength, mobility, and quality of life in older men with partial androgen deficiency. J Clin Endocrinol Metab. 2001;86(9):4078–88. doi: 10.1210/jcem.86.9.7821. [DOI] [PubMed] [Google Scholar]
- 132.Kenny AM, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56(5):M266–72. doi: 10.1093/gerona/56.5.m266. [DOI] [PubMed] [Google Scholar]
- 133.Wittert GA, et al. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci. 2003;58(7):618–25. doi: 10.1093/gerona/58.7.m618. [DOI] [PubMed] [Google Scholar]
- 134.Emmelot-Vonk MH, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. Jama. 2008;299(1):39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 135.Borst SE. Interventions for sarcopenia and muscle weakness in older people. Age Ageing. 2004;33(6):548–55. doi: 10.1093/ageing/afh201. [DOI] [PubMed] [Google Scholar]
- 136.Genazzani AD, Lanzoni C, Genazzani AR. Might DHEA be considered a beneficial replacement therapy in the elderly? Drugs Aging. 2007;24(3):173–85. doi: 10.2165/00002512-200724030-00001. [DOI] [PubMed] [Google Scholar]
- 137.Percheron G, et al. Effect of 1-year oral administration of dehydroepiandrosterone to 60- to 80-year-old individuals on muscle function and cross-sectional area: a double-blind placebo-controlled trial. Arch Intern Med. 2003;163(6):720–7. doi: 10.1001/archinte.163.6.720. [DOI] [PubMed] [Google Scholar]
- 138.Perry HM, 3rd, et al. Longitudinal changes in serum 25-hydroxyvitamin D in older people. Metabolism. 1999;48(8):1028–32. doi: 10.1016/s0026-0495(99)90201-9. [DOI] [PubMed] [Google Scholar]
- 139.Szulc P, et al. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr. 2004;80(2):496–503. doi: 10.1093/ajcn/80.2.496. [DOI] [PubMed] [Google Scholar]
- 140.Volpi E, et al. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101(9):2000–7. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Volpi E, et al. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277(3 Pt 1):E513–20. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 142.Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr. 1997;65(2):489–95. doi: 10.1093/ajcn/65.2.489. [DOI] [PubMed] [Google Scholar]
- 143.Bischoff-Ferrari HA, et al. Effect of Vitamin D on falls: a meta-analysis. Jama. 2004;291(16):1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 144.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88(12):5766–72. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 145.Bischoff HA, et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33(1):19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- 146.Boland R. Role of vitamin D in skeletal muscle function. Endocr Rev. 1986;7(4):434–48. doi: 10.1210/edrv-7-4-434. [DOI] [PubMed] [Google Scholar]
- 147.Wassner SJ, et al. Vitamin D Deficiency, hypocalcemia, and increased skeletal muscle degradation in rats. J Clin Invest. 1983;72(1):102–12. doi: 10.1172/JCI110947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jacques PF, et al. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr. 1997;66(4):929–36. doi: 10.1093/ajcn/66.4.929. [DOI] [PubMed] [Google Scholar]
- 149.Stein MS, et al. Falls relate to vitamin D and parathyroid hormone in an Australian nursing home and hostel. J Am Geriatr Soc. 1999;47(10):1195–201. doi: 10.1111/j.1532-5415.1999.tb05199.x. [DOI] [PubMed] [Google Scholar]
- 150.Drinka PJ, et al. Determinants of vitamin D levels in nursing home residents. J Am Med Dir Assoc. 2007;8(2):76–9. doi: 10.1016/j.jamda.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 151.Hamid Z, et al. Vitamin D deficiency in residents of academic long-term care facilities despite having been prescribed vitamin D. J Am Med Dir Assoc. 2007;8(2):71–5. doi: 10.1016/j.jamda.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 152.Morley JE. Should all long-term care residents receive vitamin D? J Am Med Dir Assoc. 2007;8(2):69–70. doi: 10.1016/j.jamda.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 153.Roubenoff R, et al. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998;53(1):M20–6. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- 154.Schaap LA, et al. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119(6):526, e9–17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 155.Payette H, et al. Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham Heart Study. J Am Geriatr Soc. 2003;51(9):1237–43. doi: 10.1046/j.1532-5415.2003.51407.x. [DOI] [PubMed] [Google Scholar]
- 156.Morey MC, et al. Evaluation of a supervised exercise program in a geriatric population. J Am Geriatr Soc. 1989;37(4):348–54. doi: 10.1111/j.1532-5415.1989.tb05503.x. [DOI] [PubMed] [Google Scholar]
- 157.Visser M, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 158.Cesari M, et al. Sarcopenia, obesity, and inflammation--results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82(2):428–34. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- 159.Ferrucci L, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–46. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 160.Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335(25):1897–905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- 161.Vincent KR, et al. Resistance exercise and physical performance in adults aged 60 to 83. J Am Geriatr Soc. 2002;50(6):1100–7. doi: 10.1046/j.1532-5415.2002.50267.x. [DOI] [PubMed] [Google Scholar]
- 162.Yudkin JS, et al. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 163.Ryan AS, Nicklas BJ. Reductions in plasma cytokine levels with weight loss improve insulin sensitivity in overweight and obese postmenopausal women. Diabetes Care. 2004;27(7):1699–705. doi: 10.2337/diacare.27.7.1699. [DOI] [PubMed] [Google Scholar]
- 164.Visser M, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50(5):897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 165.Sipila S, Suominen H. Knee extension strength and walking speed in relation to quadriceps muscle composition and training in elderly women. Clin Physiol. 1994;14(4):433–42. doi: 10.1111/j.1475-097x.1994.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 166.Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr. 2007;85(3):662–77. doi: 10.1093/ajcn/85.3.662. [DOI] [PubMed] [Google Scholar]
- 167.Roubenoff R. Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci. 2000;904:553–7. doi: 10.1111/j.1749-6632.2000.tb06515.x. [DOI] [PubMed] [Google Scholar]
- 168.Dirks AJ, et al. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev. 2006;5(2):179–95. doi: 10.1016/j.arr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 169.Stump CS, et al. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci U S A. 2003;100(13):7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bua EA, et al. Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J Appl Physiol. 2002;92(6):2617–24. doi: 10.1152/japplphysiol.01102.2001. [DOI] [PubMed] [Google Scholar]
- 171.Kent-Braun JA, Ng AV, Young K. Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol. 2000;88(2):662–8. doi: 10.1152/jappl.2000.88.2.662. [DOI] [PubMed] [Google Scholar]
- 172.Jubrias SA, et al. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol. 2001;90(5):1663–70. doi: 10.1152/jappl.2001.90.5.1663. [DOI] [PubMed] [Google Scholar]
- 173.Melov S, et al. Resistance exercise reverses aging in human skeletal muscle. PLoS ONE. 2007;2(5):e465. doi: 10.1371/journal.pone.0000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dupont-Versteegden EE. Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol. 2005;40(6):473–81. doi: 10.1016/j.exger.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 175.Giresi PG, et al. Identification of a molecular signature of sarcopenia. Physiol Genomics. 2005;21(2):253–63. doi: 10.1152/physiolgenomics.00249.2004. [DOI] [PubMed] [Google Scholar]
- 176.Solomon A, Bouloux P. Endocrine therapies for sarcopenia in older men. Br J Hosp Med (Lond) 2006;67(9):477–81. doi: 10.12968/hmed.2006.67.9.22000. [DOI] [PubMed] [Google Scholar]
- 177.Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41(12):1234–8. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 178.Leeuwenburgh C. Role of apoptosis in sarcopenia. J Gerontol A Biol Sci Med Sci. 2003;58(11):999–1001. doi: 10.1093/gerona/58.11.m999. [DOI] [PubMed] [Google Scholar]
- 179.Reed T, et al. Genetic influences and grip strength norms in the NHLBI twin study males aged 59–69. Ann Hum Biol. 1991;18(5):425–32. doi: 10.1080/03014469100001722. [DOI] [PubMed] [Google Scholar]
- 180.Arden NK, Spector TD. Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J Bone Miner Res. 1997;12(12):2076–81. doi: 10.1359/jbmr.1997.12.12.2076. [DOI] [PubMed] [Google Scholar]
- 181.Huygens W, et al. Linkage of myostatin pathway genes with knee strength in humans. Physiol Genomics. 2004;17(3):264–70. doi: 10.1152/physiolgenomics.00224.2003. [DOI] [PubMed] [Google Scholar]
- 182.Frederiksen H, et al. Hand grip strength: a phenotype suitable for identifying genetic variants affecting mid- and late-life physical functioning. Genet Epidemiol. 2002;23(2):110–22. doi: 10.1002/gepi.1127. [DOI] [PubMed] [Google Scholar]
- 183.Carmelli D, Reed T. Stability and change in genetic and environmental influences on hand-grip strength in older male twins. J Appl Physiol. 2000;89(5):1879–83. doi: 10.1152/jappl.2000.89.5.1879. [DOI] [PubMed] [Google Scholar]
- 184.Christensen K, et al. Genetic and environmental influences on functional abilities in Danish twins aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2000;55(8):M446–52. doi: 10.1093/gerona/55.8.m446. [DOI] [PubMed] [Google Scholar]
- 185.Sayer AA, et al. Does sarcopenia originate in early life? Findings from the Hertfordshire cohort study. J Gerontol A Biol Sci Med Sci. 2004;59(9):M930–4. doi: 10.1093/gerona/59.9.m930. [DOI] [PubMed] [Google Scholar]
- 186.Yliharsila H, et al. Birth size, adult body composition and muscle strength in later life. Int J Obes (Lond) 2007;31(9):1392–9. doi: 10.1038/sj.ijo.0803612. [DOI] [PubMed] [Google Scholar]
- 187.Huygens W, et al. Quantitative trait loci for human muscle strength: linkage analysis of myostatin pathway genes. Physiol Genomics. 2005;22(3):390–7. doi: 10.1152/physiolgenomics.00010.2005. [DOI] [PubMed] [Google Scholar]
- 188.Roth SM, et al. CNTF genotype is associated with muscular strength and quality in humans across the adult age span. J Appl Physiol. 2001;90(4):1205–10. doi: 10.1152/jappl.2001.90.4.1205. [DOI] [PubMed] [Google Scholar]
- 189.Schrager MA, et al. Insulin-like growth factor-2 genotype, fat-free mass, and muscle performance across the adult life span. J Appl Physiol. 2004;97(6):2176–83. doi: 10.1152/japplphysiol.00985.2003. [DOI] [PubMed] [Google Scholar]
- 190.Delmonico MJ, et al. Alpha-actinin-3 (ACTN3) R577X polymorphism influences knee extensor peak power response to strength training in older men and women. J Gerontol A Biol Sci Med Sci. 2007;62(2):206–12. doi: 10.1093/gerona/62.2.206. [DOI] [PubMed] [Google Scholar]
- 191.Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13(3):187–94. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]
- 192.Roth SM, et al. Vitamin D receptor genotype is associated with fat-free mass and sarcopenia in elderly men. J Gerontol A Biol Sci Med Sci. 2004;59(1):10–5. doi: 10.1093/gerona/59.1.b10. [DOI] [PubMed] [Google Scholar]
- 193.Grundberg E, et al. Genetic variation in the human vitamin D receptor is associated with muscle strength, fat mass and body weight in Swedish women. Eur J Endocrinol. 2004;150(3):323–8. doi: 10.1530/eje.0.1500323. [DOI] [PubMed] [Google Scholar]
- 194.Geusens P, et al. Quadriceps and grip strength are related to vitamin D receptor genotype in elderly nonobese women. J Bone Miner Res. 1997;12(12):2082–8. doi: 10.1359/jbmr.1997.12.12.2082. [DOI] [PubMed] [Google Scholar]
- 195.Fisher AL. Of worms and women: sarcopenia and its role in disability and mortality. J Am Geriatr Soc. 2004;52(7):1185–90. doi: 10.1111/j.1532-5415.2004.52320.x. [DOI] [PubMed] [Google Scholar]
- 196.Herndon LA, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419(6909):808–14. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- 197.Kenyon C, et al. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366(6454):461–4. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 198.Chaput JP, et al. Relationship between antioxidant intakes and class I sarcopenia in elderly men and women. J Nutr Health Aging. 2007;11(4):363–9. [PubMed] [Google Scholar]
- 199.Lord C, et al. Dietary animal protein intake: association with muscle mass index in older women. J Nutr Health Aging. 2007;11(5):383–7. [PubMed] [Google Scholar]
- 200.Campbell WW, et al. Effects of an omnivorous diet compared with a lactoovovegetarian diet on resistance-training-induced changes in body composition and skeletal muscle in older men. Am J Clin Nutr. 1999;70(6):1032–9. doi: 10.1093/ajcn/70.6.1032. [DOI] [PubMed] [Google Scholar]
- 201.Campbell WW, et al. Increased energy requirements and changes in body composition with resistance training in older adults. Am J Clin Nutr. 1994;60(2):167–75. doi: 10.1093/ajcn/60.2.167. [DOI] [PubMed] [Google Scholar]
- 202.Campbell WW, Evans WJ. Protein requirements of elderly people. Eur J Clin Nutr. 1996;50(Suppl 1):S180–3. discussion S183–5. [PubMed] [Google Scholar]
- 203.Campbell WW, et al. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56(6):M373–80. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- 204.Rooyackers OE, et al. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93(26):15364–9. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Roberts SB, et al. Effects of age on energy expenditure and substrate oxidation during experimental underfeeding in healthy men. J Gerontol A Biol Sci Med Sci. 1996;51(2):B158–66. doi: 10.1093/gerona/51a.2.b158. [DOI] [PubMed] [Google Scholar]
- 206.Wilson MM, Morley JE. Invited review: Aging and energy balance. J Appl Physiol. 2003;95(4):1728–36. doi: 10.1152/japplphysiol.00313.2003. [DOI] [PubMed] [Google Scholar]
- 207.Bennet WM, et al. The effect of amino acid infusion on leg protein turnover assessed by L-[15N]phenylalanine and L-[1-13C]leucine exchange. Eur J Clin Invest. 1990;20(1):41–50. doi: 10.1111/j.1365-2362.1990.tb01789.x. [DOI] [PubMed] [Google Scholar]
- 208.Volpi E, et al. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–8. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Campbell WW, et al. Effects of resistance training and dietary protein intake on protein metabolism in older adults. Am J Physiol. 1995;268(6 Pt 1):E1143–53. doi: 10.1152/ajpendo.1995.268.6.E1143. [DOI] [PubMed] [Google Scholar]
- 210.Welle S, Thornton CA. High-protein meals do not enhance myofibrillar synthesis after resistance exercise in 62- to 75-yr-old men and women. Am J Physiol. 1998;274(4 Pt 1):E677–83. doi: 10.1152/ajpendo.1998.274.4.E677. [DOI] [PubMed] [Google Scholar]
- 211.Katsanos CS, et al. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82(5):1065–73. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 212.Dardevet D, et al. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr. 2000;130(11):2630–5. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- 213.Rasmussen BB, et al. Insulin resistance of muscle protein metabolism in aging. Faseb J. 2006;20(6):768–9. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fujita S, et al. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56(6):1615–22. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ferrucci L, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52(4):625–34. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 216.Rantanen T. Muscle strength, disability and mortality. Scand J Med Sci Sports. 2003;13(1):3–8. doi: 10.1034/j.1600-0838.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- 217.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 218.Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in VO2 max. J Appl Physiol. 1988;65(3):1147–51. doi: 10.1152/jappl.1988.65.3.1147. [DOI] [PubMed] [Google Scholar]
- 219.Janssen I. Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54(1):56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 220.Jensen GL. Obesity and functional decline: epidemiology and geriatric consequences. Clin Geriatr Med. 2005;21(4):677–87. v. doi: 10.1016/j.cger.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 221.Ensrud KE, et al. Correlates of impaired function in older women. J Am Geriatr Soc. 1994;42(5):481–9. doi: 10.1111/j.1532-5415.1994.tb04968.x. [DOI] [PubMed] [Google Scholar]
- 222.Sarkisian CA, et al. Modifiable risk factors predict functional decline among older women: a prospectively validated clinical prediction tool. The Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48(2):170–8. doi: 10.1111/j.1532-5415.2000.tb03908.x. [DOI] [PubMed] [Google Scholar]
- 223.Friedmann JM, Elasy T, Jensen GL. The relationship between body mass index and self-reported functional limitation among older adults: a gender difference. J Am Geriatr Soc. 2001;49(4):398–403. doi: 10.1046/j.1532-5415.2001.49082.x. [DOI] [PubMed] [Google Scholar]
- 224.Visser M, et al. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr. 1998;68(3):584–90. doi: 10.1093/ajcn/68.3.584. [DOI] [PubMed] [Google Scholar]
- 225.Zamboni M, et al. The relationship between body composition and physical performance in older women. J Am Geriatr Soc. 1999;47(12):1403–8. doi: 10.1111/j.1532-5415.1999.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 226.Davison KK, et al. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. 2002;50(11):1802–9. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- 227.Sternfeld B, et al. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156(2):110–21. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- 228.Zoico E, et al. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes Relat Metab Disord. 2004;28(2):234–41. doi: 10.1038/sj.ijo.0802552. [DOI] [PubMed] [Google Scholar]
- 229.Villareal DT, et al. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12(6):913–20. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 230.Rolland Y, et al. Muscle strength in obese elderly women: effect of recreational physical activity in a cross-sectional study. Am J Clin Nutr. 2004;79(4):552–7. doi: 10.1093/ajcn/79.4.552. [DOI] [PubMed] [Google Scholar]
- 231.Ford ES, Mokdad AH, Giles WH. Trends in waist circumference among U.S. adults. Obes Res. 2003;11(10):1223–31. doi: 10.1038/oby.2003.168. [DOI] [PubMed] [Google Scholar]
- 232.Lebrun CE, et al. Fat mass rather than muscle strength is the major determinant of physical function and disability in postmenopausal women younger than 75 years of age. Menopause. 2006;13(3):474–81. doi: 10.1097/01.gme.0000222331.23478.ec. [DOI] [PubMed] [Google Scholar]
- 233.Janssen I, Katzmarzyk PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. J Am Geriatr Soc. 2005;53(12):2112–8. doi: 10.1111/j.1532-5415.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 234.Bigaard J, et al. Waist circumference, BMI, smoking, and mortality in middle-aged men and women. Obes Res. 2003;11(7):895–903. doi: 10.1038/oby.2003.123. [DOI] [PubMed] [Google Scholar]
- 235.Kanaya AM, et al. Association of total and central obesity with mortality in postmenopausal women with coronary heart disease. Am J Epidemiol. 2003;158(12):1161–70. doi: 10.1093/aje/kwg271. [DOI] [PubMed] [Google Scholar]
- 236.Griffiths RD. Muscle mass, survival, and the elderly ICU patient. Nutrition. 1996;12(6):456–8. doi: 10.1016/s0899-9007(96)00141-4. [DOI] [PubMed] [Google Scholar]
- 237.Cosqueric G, et al. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr. 2006;96(5):895–901. doi: 10.1017/bjn20061943. [DOI] [PubMed] [Google Scholar]
- 238.Newman AB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–7. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 239.Newman AB, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51(3):323–30. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 240.Buchner DM, et al. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing. 1996;25(5):386–91. doi: 10.1093/ageing/25.5.386. [DOI] [PubMed] [Google Scholar]
- 241.Rantanen T, et al. Coimpairments as predictors of severe walking disability in older women. J Am Geriatr Soc. 2001;49(1):21–7. doi: 10.1046/j.1532-5415.2001.49005.x. [DOI] [PubMed] [Google Scholar]
- 242.Janssen I, et al. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89(1):81–8. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 243.Lynch NA, et al. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol. 1999;86(1):188–94. doi: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- 244.Singh AS, et al. Cross-sectional relationship between physical fitness components and functional performance in older persons living in long-term care facilities. BMC Geriatr. 2006;6:4. doi: 10.1186/1471-2318-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Guralnik JM, et al. Progressive versus catastrophic loss of the ability to walk: implications for the prevention of mobility loss. J Am Geriatr Soc. 2001;49(11):1463–70. doi: 10.1046/j.1532-5415.2001.4911238.x. [DOI] [PubMed] [Google Scholar]
- 246.Roth SM, Ferrell RF, Hurley BF. Strength training for the prevention and treatment of sarcopenia. J Nutr Health Aging. 2000;4(3):143–55. [PubMed] [Google Scholar]
- 247.Nelson ME, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1094–105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 248.Hakkinen K, et al. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol. 1998;84(4):1341–9. doi: 10.1152/jappl.1998.84.4.1341. [DOI] [PubMed] [Google Scholar]
- 249.Taaffe DR, et al. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc. 1999;47(10):1208–14. doi: 10.1111/j.1532-5415.1999.tb05201.x. [DOI] [PubMed] [Google Scholar]
- 250.Fielding RA, et al. Activity adherence and physical function in older adults with functional limitations. Med Sci Sports Exerc. 2007;39(11):1997–2004. doi: 10.1249/mss.0b013e318145348d. [DOI] [PubMed] [Google Scholar]
- 251.MMWR. US department of health and human services; 2004. Strength training among adults aged >65 years - United States 2001; pp. 25–28. [Google Scholar]
- 252.Cardinale M, et al. Whole-body vibration can reduce calciuria induced by high protein intakes and may counteract bone resorption: A preliminary study. J Sports Sci. 2007;25(1):111–9. doi: 10.1080/02640410600717816. [DOI] [PubMed] [Google Scholar]
- 253.Cardinale M, Rittweger J. Vibration exercise makes your muscles and bones stronger: fact or fiction? J Br Menopause Soc. 2006;12(1):12–8. doi: 10.1258/136218006775997261. [DOI] [PubMed] [Google Scholar]
- 254.Bogaerts A, et al. Impact of whole-body vibration training versus fitness training on muscle strength and muscle mass in older men: a 1-year randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2007;62(6):630–5. doi: 10.1093/gerona/62.6.630. [DOI] [PubMed] [Google Scholar]
- 255.Morley JE. Anorexia and weight loss in older persons. J Gerontol A Biol Sci Med Sci. 2003;58(2):131–7. doi: 10.1093/gerona/58.2.m131. [DOI] [PubMed] [Google Scholar]
- 256.Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med. 2001;161(9):1194–203. doi: 10.1001/archinte.161.9.1194. [DOI] [PubMed] [Google Scholar]
- 257.Elia M. Obesity in the elderly. Obes Res. 2001;9(Suppl 4):244S–248S. doi: 10.1038/oby.2001.126. [DOI] [PubMed] [Google Scholar]
- 258.Morais JA, Chevalier S, Gougeon R. Protein turnover and requirements in the healthy and frail elderly. J Nutr Health Aging. 2006;10(4):272–83. [PubMed] [Google Scholar]
- 259.Houston DK, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87(1):150–5. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 260.Fujita S, Volpi E. Amino acids and muscle loss with aging. J Nutr. 2006;136(1 Suppl):277S–80S. doi: 10.1093/jn/136.1.277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Timmerman KL, Volpi E. Amino acid metabolism and regulatory effects in aging. Curr Opin Clin Nutr Metab Care. 2008;11(1):45–9. doi: 10.1097/MCO.0b013e3282f2a592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Drummond MJ, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008 doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hayes A, Cribb PJ. Effect of whey protein isolate on strength, body composition and muscle hypertrophy during resistance training. Curr Opin Clin Nutr Metab Care. 2008;11(1):40–4. doi: 10.1097/MCO.0b013e3282f2a57d. [DOI] [PubMed] [Google Scholar]
- 264.Rieu I, et al. Increased availability of leucine with leucine-rich whey proteins improves postprandial muscle protein synthesis in aging rats. Nutrition. 2007;23(4):323–31. doi: 10.1016/j.nut.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 265.Kim JH, et al. Lifelong exercise and mild (8%) caloric restriction attenuate age-induced alterations in plantaris muscle morphology, oxidative stress and IGF-1 in the Fischer-344 rat. Exp Gerontol. 2008;43(4):317–29. doi: 10.1016/j.exger.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Dirks Naylor AJ, Leeuwenburgh C. Sarcopenia: the role of apoptosis and modulation by caloric restriction. Exerc Sport Sci Rev. 2008;36(1):19–24. doi: 10.1097/jes.0b013e31815ddd9d. [DOI] [PubMed] [Google Scholar]
- 267.Arnal MA, et al. Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr. 1999;69(6):1202–8. doi: 10.1093/ajcn/69.6.1202. [DOI] [PubMed] [Google Scholar]
- 268.Boirie Y, et al. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94(26):14930–5. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Dangin M, et al. Influence of the protein digestion rate on protein turnover in young and elderly subjects. J Nutr. 2002;132(10):3228S–33S. doi: 10.1093/jn/131.10.3228S. [DOI] [PubMed] [Google Scholar]
- 270.Wolfson L, et al. Training balance and strength in the elderly to improve function. J Am Geriatr Soc. 1993;41(3):341–3. doi: 10.1111/j.1532-5415.1993.tb06716.x. [DOI] [PubMed] [Google Scholar]
- 271.Sayer AA, et al. Falls, sarcopenia, and growth in early life: findings from the Hertfordshire cohort study. Am J Epidemiol. 2006;164(7):665–71. doi: 10.1093/aje/kwj255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Szulc P, et al. Increased bone resorption in moderate smokers with low body weight: the Minos study. J Clin Endocrinol Metab. 2002;87(2):666–74. doi: 10.1210/jcem.87.2.8232. [DOI] [PubMed] [Google Scholar]
- 273.Bhasin S, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335(1):1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 274.Ottenbacher KJ, et al. Androgen treatment and muscle strength in elderly men: A meta-analysis. J Am Geriatr Soc. 2006;54(11):1666–73. doi: 10.1111/j.1532-5415.2006.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bhasin S, Buckwalter JG. Testosterone supplementation in older men: a rational idea whose time has not yet come. J Androl. 2001;22(5):718–31. [PubMed] [Google Scholar]
- 276.Borst SE, et al. Anabolic effects of testosterone are preserved during inhibition of 5alpha-reductase. Am J Physiol Endocrinol Metab. 2007;293(2):E507–14. doi: 10.1152/ajpendo.00130.2007. [DOI] [PubMed] [Google Scholar]
- 277.Parsons JK, et al. Serum testosterone and the risk of prostate cancer: potential implications for testosterone therapy. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2257–60. doi: 10.1158/1055-9965.EPI-04-0715. [DOI] [PubMed] [Google Scholar]
- 278.Venken K, et al. Bone and muscle protective potential of the prostate-sparing synthetic androgen 7alpha-methyl-19-nortestosterone: evidence from the aged orchidectomized male rat model. Bone. 2005;36(4):663–70. doi: 10.1016/j.bone.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 279.Li JJ, et al. Discovery of Potent and Muscle Selective Androgen Receptor Modulators through Scaffold Modifications. J Med Chem. 2007;50(13):3015–3025. doi: 10.1021/jm070312d. [DOI] [PubMed] [Google Scholar]
- 280.Takala J, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341(11):785–92. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- 281.Frost RA, Nystrom GJ, Lang CH. Regulation of IGF-I mRNA and signal transducers and activators of transcription-3 and -5 (Stat-3 and -5) by GH in C2C12 myoblasts. Endocrinology. 2002;143(2):492–503. doi: 10.1210/endo.143.2.8641. [DOI] [PubMed] [Google Scholar]
- 282.Leroith D, Nissley P. Knock your SOCS off! J Clin Invest. 2005;115(2):233–6. doi: 10.1172/JCI24228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Leger B, et al. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008;11(1):163–175B. doi: 10.1089/rej.2007.0588. [DOI] [PubMed] [Google Scholar]
- 284.Barbieri M, et al. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. 2003;284(3):E481–7. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- 285.Cappola AR, et al. Association of IGF-I levels with muscle strength and mobility in older women. J Clin Endocrinol Metab. 2001;86(9):4139–46. doi: 10.1210/jcem.86.9.7868. [DOI] [PubMed] [Google Scholar]
- 286.Onder G, et al. Body mass index, free insulin-like growth factor I, and physical function among older adults: results from the ilSIRENTE study. Am J Physiol Endocrinol Metab. 2006;291(4):E829–34. doi: 10.1152/ajpendo.00138.2006. [DOI] [PubMed] [Google Scholar]
- 287.Thompson JL, et al. Effects of human growth hormone, insulin-like growth factor I, and diet and exercise on body composition of obese postmenopausal women. J Clin Endocrinol Metab. 1998;83(5):1477–84. doi: 10.1210/jcem.83.5.4826. [DOI] [PubMed] [Google Scholar]
- 288.Pollak M. Insulin-like growth factor physiology and cancer risk. Eur J Cancer. 2000;36(10):1224–8. doi: 10.1016/s0959-8049(00)00102-7. [DOI] [PubMed] [Google Scholar]
- 289.Cobb LJ, et al. Partitioning of IGFBP-5 actions in myogenesis: IGF-independent anti-apoptotic function. J Cell Sci. 2004;117(Pt 9):1737–46. doi: 10.1242/jcs.01028. [DOI] [PubMed] [Google Scholar]
- 290.Artaza JN, et al. Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology. 2005;146(8):3547–57. doi: 10.1210/en.2005-0362. [DOI] [PubMed] [Google Scholar]
- 291.Schuelke M, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350(26):2682–8. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 292.Siriett V, et al. Antagonism of myostatin enhances muscle regeneration during sarcopenia. Mol Ther. 2007;15(8):1463–70. doi: 10.1038/sj.mt.6300182. [DOI] [PubMed] [Google Scholar]
- 293.Petersen AM, et al. Smoking impairs muscle protein synthesis and increases the expression of myostatin and MAFbx in muscle. Am J Physiol Endocrinol Metab. 2007;293(3):E843–8. doi: 10.1152/ajpendo.00301.2007. [DOI] [PubMed] [Google Scholar]
- 294.Solomon AM, Bouloux PM. Modifying muscle mass - the endocrine perspective. J Endocrinol. 2006;191(2):349–60. doi: 10.1677/joe.1.06837. [DOI] [PubMed] [Google Scholar]
- 295.Nakatani M, et al. Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. Faseb J. 2008;22(2):477–87. doi: 10.1096/fj.07-8673com. [DOI] [PubMed] [Google Scholar]
- 296.Ohsawa Y, et al. Muscular atrophy of caveolin-3-deficient mice is rescued by myostatin inhibition. J Clin Invest. 2006;116(11):2924–34. doi: 10.1172/JCI28520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Haidet AM, et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci U S A. 2008;105(11):4318–22. doi: 10.1073/pnas.0709144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lemoine S, et al. Estrogen receptor alpha mRNA in human skeletal muscles. Med Sci Sports Exerc. 2003;35(3):439–43. doi: 10.1249/01.MSS.0000053654.14410.78. [DOI] [PubMed] [Google Scholar]
- 299.Wiik A, et al. Oestrogen receptor beta is present in both muscle fibres and endothelial cells within human skeletal muscle tissue. Histochem Cell Biol. 2005;124(2):161–5. doi: 10.1007/s00418-005-0030-z. [DOI] [PubMed] [Google Scholar]
- 300.Bischoff-Ferrari HA, et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. Jama. 2005;293(18):2257–64. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 301.Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75(4):611–5. doi: 10.1093/ajcn/75.4.611. [DOI] [PubMed] [Google Scholar]
- 302.Candow DG, Chilibeck PD. Effect of creatine supplementation during resistance training on muscle accretion in the elderly. J Nutr Health Aging. 2007;11(2):185–8. [PubMed] [Google Scholar]
- 303.Brose A, Parise G, Tarnopolsky MA. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J Gerontol A Biol Sci Med Sci. 2003;58(1):11–9. doi: 10.1093/gerona/58.1.b11. [DOI] [PubMed] [Google Scholar]
- 304.Willoughby DS, Rosene JM. Effects of oral creatine and resistance training on myogenic regulatory factor expression. Med Sci Sports Exerc. 2003;35(6):923–9. doi: 10.1249/01.MSS.0000069746.05241.F0. [DOI] [PubMed] [Google Scholar]
- 305.Bermon S, et al. Effects of creatine monohydrate ingestion in sedentary and weight-trained older adults. Acta Physiol Scand. 1998;164(2):147–55. doi: 10.1046/j.1365-201X.1998.00427.x. [DOI] [PubMed] [Google Scholar]
- 306.Chrusch MJ, et al. Creatine supplementation combined with resistance training in older men. Med Sci Sports Exerc. 2001;33(12):2111–7. doi: 10.1097/00005768-200112000-00021. [DOI] [PubMed] [Google Scholar]
- 307.Eijnde BO, et al. Effects of creatine supplementation and exercise training on fitness in men 55–75 yr old. J Appl Physiol. 2003;95(2):818–28. doi: 10.1152/japplphysiol.00891.2002. [DOI] [PubMed] [Google Scholar]
- 308.Gotshalk LA, et al. Creatine supplementation improves muscular performance in older men. Med Sci Sports Exerc. 2002;34(3):537–43. doi: 10.1097/00005768-200203000-00023. [DOI] [PubMed] [Google Scholar]
- 309.Jakobi JM, et al. Neuromuscular properties and fatigue in older men following acute creatine supplementation. Eur J Appl Physiol. 2001;84(4):321–8. doi: 10.1007/s004210000373. [DOI] [PubMed] [Google Scholar]
- 310.Rawson ES, Wehnert ML, Clarkson PM. Effects of 30 days of creatine ingestion in older men. Eur J Appl Physiol Occup Physiol. 1999;80(2):139–44. doi: 10.1007/s004210050570. [DOI] [PubMed] [Google Scholar]
- 311.Rawson ES, Clarkson PM. Acute creatine supplementation in older men. Int J Sports Med. 2000;21(1):71–5. doi: 10.1055/s-2000-8859. [DOI] [PubMed] [Google Scholar]
- 312.Tarnopolsky MA, Safdar A. The potential benefits of creatine and conjugated linoleic acid as adjuncts to resistance training in older adults. Appl Physiol Nutr Metab. 2008;33(1):213–27. doi: 10.1139/H07-142. [DOI] [PubMed] [Google Scholar]
- 313.Carter CS, et al. Angiotensin-converting enzyme inhibition intervention in elderly persons: effects on body composition and physical performance. J Gerontol A Biol Sci Med Sci. 2005;60(11):1437–46. doi: 10.1093/gerona/60.11.1437. [DOI] [PubMed] [Google Scholar]
- 314.Onder G, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359(9310):926–30. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- 315.Han Y, Runge MS, Brasier AR. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kappa B transcription factors. Circ Res. 1999;84(6):695–703. doi: 10.1161/01.res.84.6.695. [DOI] [PubMed] [Google Scholar]
- 316.Vescovo G, et al. Improved exercise tolerance after losartan and enalapril in heart failure: correlation with changes in skeletal muscle myosin heavy chain composition. Circulation. 1998;98(17):1742–9. doi: 10.1161/01.cir.98.17.1742. [DOI] [PubMed] [Google Scholar]
- 317.Payne GW. Effect of inflammation on the aging microcirculation: impact on skeletal muscle blood flow control. Microcirculation. 2006;13(4):343–52. doi: 10.1080/10739680600618918. [DOI] [PubMed] [Google Scholar]
- 318.Onder G, Vedova CD, Pahor M. Effects of ACE inhibitors on skeletal muscle. Curr Pharm Des. 2006;12(16):2057–64. doi: 10.2174/138161206777442137. [DOI] [PubMed] [Google Scholar]
- 319.Haslett P, et al. The metabolic and immunologic effects of short-term thalidomide treatment of patients infected with the human immunodeficiency virus. AIDS Res Hum Retroviruses. 1997;13(12):1047–54. doi: 10.1089/aid.1997.13.1047. [DOI] [PubMed] [Google Scholar]
- 320.Calabrese LH, Zein N, Vassilopoulos D. Safety of antitumour necrosis factor (anti-TNF) therapy in patients with chronic viral infections: hepatitis C, hepatitis B, and HIV infection. Ann Rheum Dis. 2004;63(Suppl 2):ii18–ii24. doi: 10.1136/ard.2004.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Robinson SM, et al. Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire cohort study. J Am Geriatr Soc. 2008;56(1):84–90. doi: 10.1111/j.1532-5415.2007.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Silventoinen K, et al. Heritability of body size and muscle strength in young adulthood: a study of one million Swedish men. Genet Epidemiol. 2008;32(4):341–9. doi: 10.1002/gepi.20308. [DOI] [PubMed] [Google Scholar]
- 323.Mascher H, et al. Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294(1):E43–51. doi: 10.1152/ajpendo.00504.2007. [DOI] [PubMed] [Google Scholar]
- 324.Marzetti E, et al. Effects of short-term GH supplementation and treadmill exercise training on physical performance and skeletal muscle apoptosis in old rats. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R558–67. doi: 10.1152/ajpregu.00620.2007. [DOI] [PubMed] [Google Scholar]
- 325.Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. Faseb J. 2005;19(6):668–70. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- 326.Semba RD, Lauretani F, Ferrucci L. Carotenoids as protection against sarcopenia in older adults. Arch Biochem Biophys. 2007;458(2):141–5. doi: 10.1016/j.abb.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


