Abstract
Objectives
To examine the patterns of change in cardiometabolic risk factors associated with the metabolic syndrome in children and adolescents between the ages of 8 to 19 years.
Study Design
Data of children and adolescents who participated in the Fels Longitudinal Study were analyzed. Body mass index, waist circumference, fasting insulin, fasting glucose, triglycerides, high-density lipoprotein cholesterol, systolic blood pressure, and diastolic blood pressure were assessed annually with a standardized protocol.
Results
The proportion of participants having at least 1 change between states of high and normal risk ranged from of 11.0% for body mass index to 30.4% for triglycerides. Youth in the high-risk category at baseline had a higher proportion having changed their status for all risk factors (all P < .05) except waist circumference compared with those in the normal-risk category. There were significant time effects for all risk factors (all P < .01) except fasting glucose and triglyceride levels in metric scores, but insignificant time effects for all risk factors in Z-scores in growth curve analyses.
Conclusions
The cardiometabolic risk factors associated with the MetS were relatively stable among white children and adolescents in the normal risk category. Changes in status were common if the risk factor was elevated.
Cardiometabolic risk factors such as excessive adiposity, abnormal glucose regulation, high triglyceride concentration, low high-density lipoprotein (HDL) cholesterol concentration, and high blood pressure tend to occur together.1 The clustering of 3 or more cardiometabolic risk factors has been referred to as “the metabolic syndrome” (MetS).2 Among adolescents, single risk factors and the MetS have been associated with an increased risk of cardiometabolic disease and metabolic abnormalities.3 Moreover, the occurrence of elevated cardiometabolic risk factors in childhood and adolescence increase the risks of the MetS, type 2 diabetes, coronary heart disease, and stroke in adulthood.4–7
There are many variations in definitions for the MetS in children and adolescents,8 most of which were adapted from the adult definition of the MetS.2 Because different criteria were used to define the MetS components, the prevalence of the MetS in children and adolescents differed tremendously across studies.9 Although the International Federation of Diabetes proposed a standard definition for children and adolescents age 10 years and older,10 there is no consensus regarding the definition of MetS in pediatrics. Regardless of the definition used, the diagnosis of the MetS has been found to be unstable over a 3-year period in adolescents.11
In contrast, there are a number of studies that have examined the tracking or change of the single cardiometabolic risk factors associated with the MetS during childhood and adolescence, including body mass index (BMI),12 blood pressure,13 fasting insulin,14 triglycerides,15 and blood glucose.15 Furthermore, many studies have demonstrated that childhood overweight 16 and blood pressure17 track into adulthood. Data from the Bogalusa Heart Study and the Cardiovascular Risk in Young Finns Study have demonstrated that serum lipids (total cholesterol and triglycerides) and lipoprotein cholesterol levels (low density lipoprotein cholesterol, very low density lipoprotein cholesterol, and HDL cholesterol) also track from childhood to young adulthood.18 Although most previous studies examined the tracking of single cardiometabolic risk factors from childhood and adolescent to young adulthood and adulthood, few studies have examined the tracking of clusters of multiple cardiometabolic risk factors comprising the MetS.19
Most previous studies have examined the tracking or stability of the cardiometabolic risk factors by estimating the intercorrelations between baseline and one or a few follow-up assessments. Changes in risk factor status over a longer period of time remain largely unknown. In addition, advanced methods developed to account for both intraindividual and interindividual variations in longitudinal studies have rarely been used in such studies. Therefore the objective of this study was to examine the patterns of change and to estimate the mean time effects of 8 cardiometabolic risk factors associated with the MetS in children and adolescents with a growth curve analysis approach.
Methods
Data for this study were drawn from the Fels Longitudinal Study.20 In brief, the Fels Longitudinal Study collected data on health history, body composition measures, fasting blood samples, and risk factors of cardiometabolic disease among healthy participants at annual examinations. The Institutional Review Board of Wright State University approved all protocols and procedures. Participants or their guardians signed informed consent statements before they were accepted in the study. Anthropometric and blood pressure data were recorded during regular examinations at 6 and 12 months since 1929. In this study, we only used data at each annual assessment for BMI, waist circumference, systolic blood pressure (SBP), and diastolic blood pressure (DBP). Fasting plasma lipids and lipoproteins were included in the study beginning in 1976 and assessed annually. Because information on fasting insulin and glucose began to be collected annually after 1989, all participants who had no or only 1 fasting insulin measure were excluded from our analyses. To be included in these analyses, participants were (1) aged 8 to 19 years at their first visit, (2) non-Hispanic white, (3) nonpregnant, and (4) had a baseline and at least 1 follow-up visit before age 20.
All measurements were performed by trained data collection staff. Height was measured to 0.1 cm with a Holtain stadiometer, and weight was measured to 0.1 kg on a SECA scale according to standard protocols.21 Participants’ BMI (weight [kg]/height [m]2) was calculated from their measured weight and height; and their BMI percentile was determined on the basis of Centers for Disease Control and Prevention growth charts for 2000.22 Waist circumference was measured to the 0.1 cm at the suprailiac crest. SBP and DBP were measured in the right arm with the participant seated and resting following standard procedures. The mean of 3 blood pressure measurements was calculated and used in all analyses.
Fasting blood samples were drawn after an 8- to 12-hour fast. Serum concentrations of insulin (μU/mL) and glucose (mg/dL), and plasma concentrations of triglycerides (mg/dL) and HDL cholesterol (mg/dL) were assessed with standard assays at the Population Genetics Phenotyping Laboratory, Southwest Foundation for Biomedical Research (San Antonio, Texas), and by the Medical Research Laboratory (Cincinnati, Ohio).
Definition of the High-Risk Status of Cardiometabolic Risk Factors
Threshold levels for the eight cardiovascular risks used to define high risk status were defined as follow: (1) BMI ≥95th percentile for sex and age,22 (2) waist circumference ≥90th percentile by sex, age, and race/ethnicity,23 (3) fasting insulin >20 mU/L (120 pmol/L),24 (4) fasting glucose ≥100 mg/dL (5.6 mmol/L),25 (5) triglycerides ≥100 mg/dL (1.13 mmol/L) for ages 2 to 9 years and ≥130 mg/dL (1.47 mmol/L) for ages 10 to 19 years,26 (6) HDL <35 mg/dL (0.91 mmol/L),26 (7) SBP ≥90th percentile by sex, age, and height percentile,27 and (8) DBP ≥90th percentile by sex, age, and height percentile.27 Status changes in cardiometabolic risk factors were defined as any time the value from 1 examination to the next crossed this threshold (ie, from normal-risk value to a high-risk value or vice versa).
Statistical Analyses
We assessed the patterns of change in risk status for each cardiometabolic risk factor overall, as well as by the high-risk status at baseline. Fisher exact test was used to test the overall differences in the proportion of the number of changes in status between 2 groups. In addition, the continuous metric scores of all 8 cardiometabolic risk factors were standardized (mean of 0 and standard deviation of 1) by sex and age. A linear growth curve analysis was performed to assess the time effects with both metric scores and standardized scores (Z-scores) for each risk factor over time. The metric scores represent the absolute (raw or original) values in metric units; Z-scores represent the relative distance above or below the mean without metric units and account for sex and age. The time since baseline was determined as the difference between the age at each follow-up assessment and the age at baseline.
In the linear growth curve models, intercept and time were modeled as random variables. We assumed that age at baseline may have had an impact on the intercept and time effects, thus it was included in the linear growth curve analysis. The regression coefficients of age at baseline (“age”) represent the difference in the mean levels of cardiometabolic risk factors per 1-year increment in age at baseline; the regression coefficients of “time” represent the mean rates of change (increase or decrease) or linear time effects on cardiometabolic risk factors per 1 year increment in time; the regression coefficients of “time2” represent the quadratic time effects on cardiometabolic risk factors per 1-year increment in time; and the interaction terms between age at baseline and the time variables (“time” and “time2”) represent the difference in the time effects per 1-year increment in age at baseline, respectively. A linear time effect indicates that the mean levels of a cardiometabolic risk factor increase or decrease as a strait line across the baseline and all follow-up assessments. A quadratic time effect indicates that the mean levels increase or decrease as a concave or convex. A combination of both linear and quadratic time effects indicates some nonlinear variations in addition to a linear trend. All analyses were conducted using SAS (version 9.1.3; SPSS, Inc, Chicago, Illinois). A 2-tailed P value ≤ .05 was considered to be statistically significant.
Results
The sample size varied from 209 to 237 depending on the availability of assessments among the 8 risk factor. The participants had a baseline and up to 11 follow-up assessments. The mean number of visits was 7.1 for BMI, SBP, and DBP, 5.6 for waist circumference, 5.5 for triglycerides and HDL cholesterol, 5.0 for fasting glucose, and 4.9 for fasting insulin. The age at baseline ranged from 8 to 18.5 years. The age at last follow-up ranged from 9 to 19.9 years.
The proportion having at least 1 change over time was 13.2% for BMI, 15.3% for waist circumference, 13.5% for fasting insulin, 13.4% for fasting glucose, 30.4% for triglycerides, 11.0% for HDL cholesterol, 11.8% for SBP, and 11.9% for DBP (Table I). The proportion having 1 change in risk status ranged from 4.8% for SBP to 18.1% for triglycerides. The proportion having 2 changes in risk status ranged from 3.3% for fasting glucose to 8.9% for triglycerides and the proportion having changed risk status 3 or more times ranged from 0% for insulin and glucose to 3.4% for triglycerides. Youth in the high-risk category of BMI (P < .0001), insulin (P = .008), glucose (P = .008), triglycerides (P < .0001), HDL cholesterol (P = .021), SBP (P < .0001), or DBP (P = .013) at baseline were more likely to have changed their status over time compared with those in the normal-risk category.
Table I.
Total number of changes in 8 cardiometabolic risk factors between 8 to 19 years of age in the Fels Longitudinal Study
| Cardiometabolic Risk factor | Number of changes | Total | High-risk status at baseline*
|
||
|---|---|---|---|---|---|
| Normal | High-risk | P value† | |||
| BMI (n = 228) | <.0001 | ||||
| 0 | 198 (86.8)‡ | 194 (89.8) | 4 (33.3) | ||
| 1 | 17 (7.5) | 14 (6.5) | 3 (25.0) | ||
| 2 | 9 (4.0) | 4 (1.9) | 5 (41.7) | ||
| 3+ | 4 (1.8) | 4 (1.9) | 0 (0.0) | ||
| Waist circumference (n = 209) | .09 | ||||
| 0 | 177 (84.7) | 164 (86.3) | 13 (68.4) | ||
| 1 | 16 (7.6) | 13 (6.8) | 3 (15.8) | ||
| 2 | 10 (4.8) | 8 (4.2) | 2 (10.5) | ||
| 3+ | 6 (2.9) | 5 (2.6) | 1 (5.3) | ||
| Fasting insulin (n = 237) | .008 | ||||
| 0 | 205 (86.5) | 194 (88.2) | 11 (64.7) | ||
| 1 | 24 (10.1) | 18 (8.2) | 6 (35.3) | ||
| 2 | 8 (3.4) | 8 (3.6) | 0 (0.0) | ||
| 3+ | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Fasting glucose (n = 237) | .008 | ||||
| 0 | 206 (86.6) | 195 (88.7) | 11 (61.1) | ||
| 1 | 24 (10.1) | 17 (7.7) | 7 (38.9) | ||
| 2 | 8 (3.3) | 8 (3.6) | 0 (0.0) | ||
| 3+ | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Triglycerides (n = 237) | <.0001 | ||||
| 0 | 165 (69.6) | 145 (74.0) | 20 (48.8) | ||
| 1 | 43 (18.1) | 28 (14.3) | 15 (36.6) | ||
| 2 | 21 (8.9) | 16 (8.2) | 5 (8.9) | ||
| 3+ | 8 (3.4) | 7 (3.6) | 1 (2.4) | ||
| HDL cholesterol (n = 237) | .021 | ||||
| 0 | 211 (89.0) | 208 (90.0) | 3 (50.0) | ||
| 1 | 12 (5.1) | 10 (4.3) | 2 (33.3) | ||
| 2 | 10 (4.2) | 9 (3.9) | 1 (16.7) | ||
| 3+ | 4 (1.7) | 4 (1.7) | 0 (0.0) | ||
| SBP (n = 228) | <.0001 | ||||
| 0 | 201 (88.2) | 200 (90.1) | 1 (16.7) | ||
| 1 | 11 (4.8) | 8 (3.6) | 3 (50.0) | ||
| 2 | 10 (4.4) | 10 (4.5) | 0 (0.0) | ||
| 3+ | 6 (2.6) | 4 (1.8) | 2 (33.3) | ||
| DBP (n = 227) | .013 | ||||
| 0 | 200 (88.1) | 198 (89.2) | 2 (40.0) | ||
| 1 | 11 (4.9) | 10 (4.5) | 1 (20.0) | ||
| 2 | 9 (4.0) | 8 (3.6) | 1 (20.0) | ||
| 3+ | 7 (3.1) | 6 (2.7) | 1 (20.0) | ||
The elevated level for each cardiometabolic risk factor was defined with the following cutoff points: BMI: ≥95th percentile for sex and age according to the growth charts from the Centers for Disease Control and Prevention. Waist circumference: ≥90th percentile by sex, age, and race/ethnicity. Fasting insulin: >20 mIU/mL. Fasting glucose: ≥100 mg/dL. Triglyceride levels: ≥100 mg/dL (ages 2 to 9 years) or 130 mg/dL (ages 10 to 19 years). HDL cholesterol: <35 mg/dL. Systolic blood pressure and diastolic blood pressure: ≥90th percentile by sex, age, and height percentile.
Fisher exact test for the overall differences in the proportion of the number of changes for each cardiometabolic risk factor between 2 groups.
The numbers in the table represent n (%).
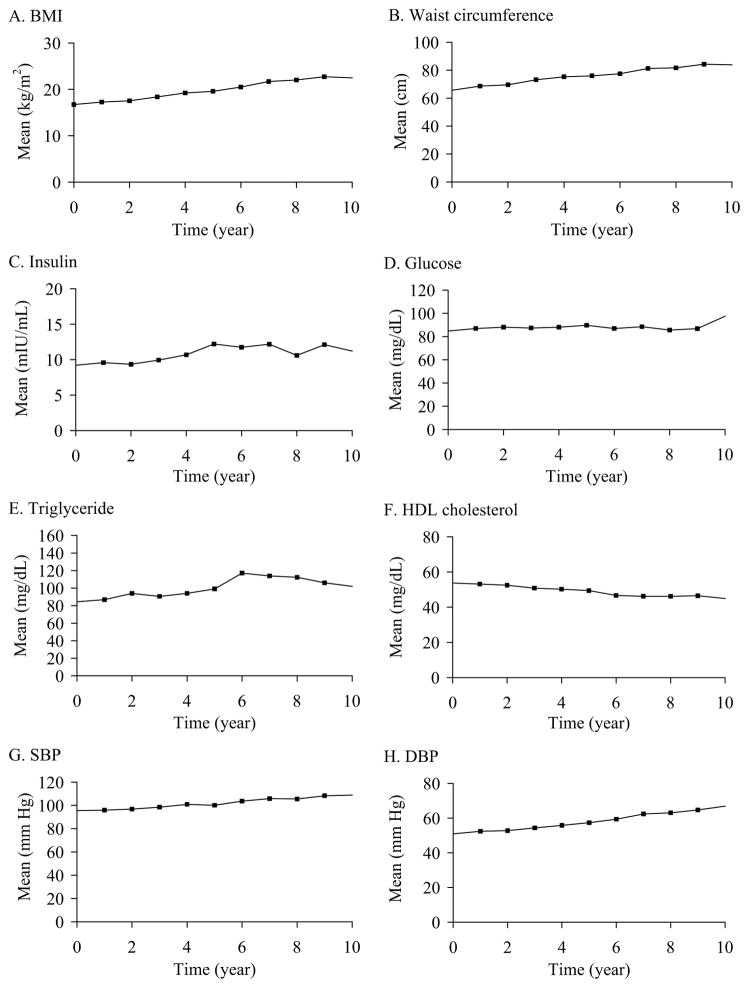
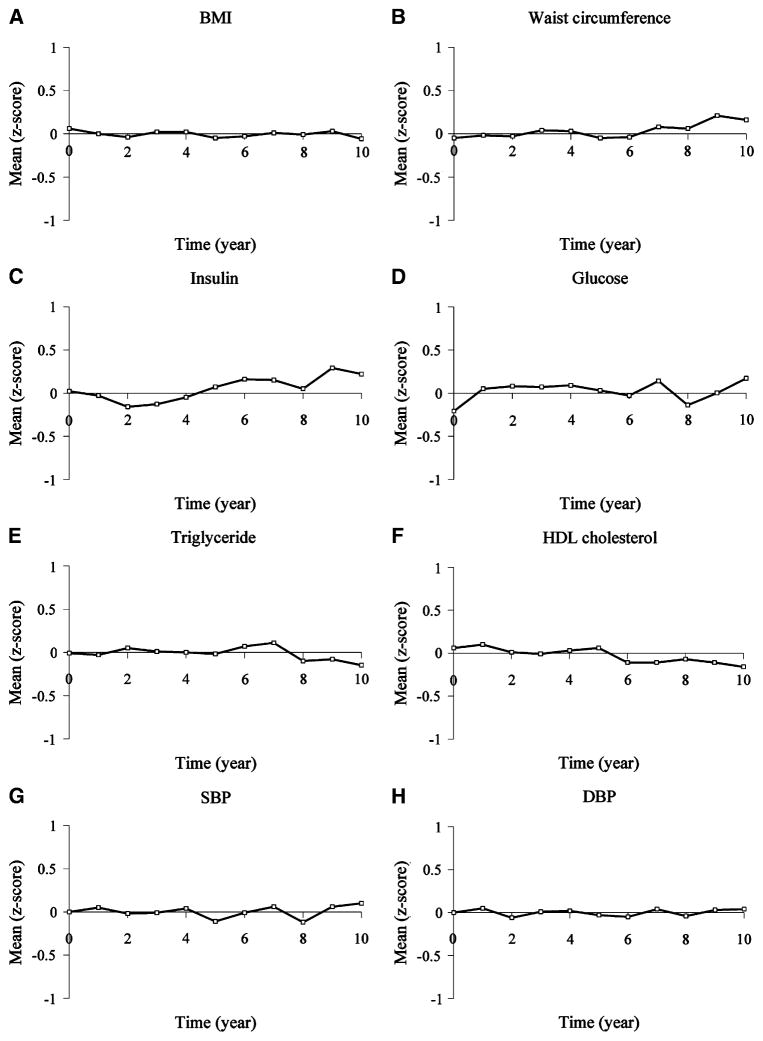
The means of metric- and Z-scores for the 8 cardiometabolic risk factor are illustrated in Figure 1 and Figure 2, respectively, and the results of linear growth curve analyses for these variables are shown in Table II. In growth curve analyses with metric scores, there were significant linear and quadratic time effects for waist circumference and fasting insulin (all P < .0001), significant linear time effects only for BMI, SBP, DBP (all P < .001), HDL cholesterol (P = .002), and insignificant time effects for fasting glucose and triglycerides. There were no in significant time effects on all 8 risk factors in growth curve analyses with Z-scores. In addition, age at baseline had significant impact on the linear time effects for fasting insulin (P < .0001) and on the quadratic time effects for waist circumference (P < .001) and HDL (P = .009) in growth curve analyses with metric scores, and on both linear and quadratic time effects for fasting glucose (all P = .001) in growth curve analyses with Z-scores.
Figure 1.
The mean levels in the metric scores of the 8 cardiometabolic risk factors in participants of the Fels Longitudinal Study between 8 to 19 years of age.
Figure 2.
The mean levels in the Z-scores of the 8 cardiometabolic risk factors in participants of the Fels Longitudinal Study between 8 to 19 years of age.
Table II.
Effects of time and age at baseline for the 8 cardiometabolic risk factors in participants of the Fels Longitudinal Study between 8 to 19 years of age
| Cardiometabolic risk factor | Predictor | Metric score
|
Z-score
|
||||
|---|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | ||
| BMI (kg/m2, n = 228) | |||||||
| Age* | 0.826 | 0.114 | <.0001 | 0.035 | 0.040 | .388 | |
| Time† | 0.784 | 0.042 | <.0001 | 0.016 | 0.012 | .172 | |
| Time2 | −0.005 | 0.004 | .206 | −0.002 | 0.001 | .070 | |
| Age × Time | −0.028 | 0.032 | .379 | −0.010 | 0.009 | .264 | |
| Age × Time2 | −0.002 | 0.005 | .686 | 0.000 | 0.002 | .929 | |
| Waist circumference (cm, n = 209) | |||||||
| Age | 2.399 | 0.243 | <.0001 | −0.034 | 0.026 | .194 | |
| Time | 3.558 | 0.176 | <.0001 | 0.010 | 0.018 | .585 | |
| Time2 | −0.096 | 0.017 | <.0001 | −0.001 | 0.002 | .663 | |
| Age × Time | 0.005 | 0.068 | .939 | 0.013 | 0.007 | .065 | |
| Age × Time2 | −0.046 | 0.012 | <.001 | −0.002 | 0.001 | .072 | |
| Fasting insulin (mIU/mL, n = 237) | |||||||
| Age | 0.552 | 0.131 | <.0001 | −0.011 | 0.020 | .573 | |
| Time | 1.880 | 0.258 | <.0001 | −0.007 | 0.039 | .862 | |
| Time2 | −0.118 | 0.028 | <.0001 | 0.004 | 0.004 | .323 | |
| Age × Time | −0.337 | 0.079 | <.0001 | −0.006 | 0.012 | .627 | |
| Age × Time2 | 0.008 | 0.013 | .527 | 0.000 | 0.002 | .829 | |
| Fasting glucose (mg/dL, n = 237) | |||||||
| Age | −0.813 | 0.391 | .039 | −0.065 | 0.019 | .001 | |
| Time | 0.955 | 0.512 | .063 | 0.012 | 0.040 | .773 | |
| Time2 | −0.049 | 0.053 | .350 | 0.002 | 0.004 | .660 | |
| Age × Time | 0.219 | 0.154 | .155 | 0.044 | 0.012 | .001 | |
| Age × Time2 | −0.030 | 0.026 | .243 | −0.007 | 0.002 | .001 | |
| Triglyceride (mg/dL, n = 237) | |||||||
| Age | 1.940 | 1.194 | .106 | −0.008 | 0.025 | .756 | |
| Time | 3.465 | 2.658 | .193 | −0.001 | 0.030 | .984 | |
| Time2 | −0.164 | 0.270 | .545 | −0.002 | 0.003 | .641 | |
| Age × Time | −0.705 | 0.973 | .469 | 0.008 | 0.011 | .486 | |
| Age × Time2 | 0.177 | 0.141 | .210 | 0.000 | 0.002 | .995 | |
| HDL cholesterol (mg/dL, n = 237) | |||||||
| Age | −0.458 | 0.295 | 0.123 | 0.038 | 0.027 | 0.171 | |
| Time | −0.832 | 0.262 | 0.002 | 0.008 | 0.025 | 0.765 | |
| Time2 | −0.036 | 0.028 | 0.204 | −0.001 | 0.003 | 0.750 | |
| Age × Time | −0.145 | 0.098 | 0.139 | 0.001 | 0.009 | 0.953 | |
| Age × Time2 | 0.038 | 0.015 | 0.009 | 0.000 | 0.001 | 0.766 | |
| SBP (mm Hg, n = 228) | |||||||
| Age | 1.584 | 0.319 | <0.0001 | 0.013 | 0.037 | 0.720 | |
| Time | 1.251 | 0.216 | <0.0001 | −0.009 | 0.023 | 0.712 | |
| Time2 | 0.035 | 0.022 | 0.118 | 0.002 | 0.002 | 0.511 | |
| Age × Time | 0.120 | 0.173 | 0.486 | 0.006 | 0.019 | 0.765 | |
| Age × Time2 | −0.025 | 0.028 | 0.376 | −0.002 | 0.003 | 0.574 | |
| DBP (mm Hg, n = 227) | |||||||
| Age | 1.751 | 0.419 | <0.0001 | 0.023 | 0.035 | 0.508 | |
| Time | 1.245 | 0.322 | 0.0001 | 0.006 | 0.027 | 0.823 | |
| Time2 | 0.053 | 0.034 | 0.115 | 0.000 | 0.003 | 0.975 | |
| Age × Time | 0.065 | 0.247 | 0.791 | −0.003 | 0.021 | 0.898 | |
| Age × Time2 | −0.010 | 0.038 | 0.784 | −0.001 | 0.003 | 0.832 | |
Age (years) at baseline, centered at 8.
Time since baseline, calculated as the difference between age at each follow-up assessment and age at baseline, centered at baseline assessment.
Discussion
With a longitudinal design, we demonstrated that 8 cardiometabolic risk factors associated with the MetS were relatively stable among white children and adolescents when the levels of these risks were in the normal risk category. Changes in status were common if the risk factor was elevated. The proportion of change in participants’ high-risk status determined with proposed cutoff values ranged between 11% and 14% for all risk factors except waist circumference (15.3%) and triglycerides (30.4%).
Our finding that the frequency of changes in risk status for overweight was similar to the findings of previous studies, which showed that overall mean change in BMI Z-scores over time was not statistically significant.12 However, our results suggested that greater than 60% of children and adolescents who had a high-risk category at baseline tended to change their status in the follow-up, indicating a considerable fluctuation or regression of overweight status over time. Few previous studies assessed the stability of waist circumference during childhood and adolescence. One study that tracked the waist circumference of adolescents as they progressed into adulthood showed that the tracking coefficient (ie, correlation coefficient) was moderate (0.79).19 The overall proportion of at least 1 status change for waist circumference was similar to that of BMI in our study; however, the insignificant difference in the proportion of change between those with and without a high-risk category at baseline for waist circumference indicated that abdominal obesity as measured by waist circumference seemed to be more stable than general overweight as defined by BMI from childhood to adolescence.
Similar to results from previous studies,14 our results indicated that there was a significant time effects on the metric scores of fasting insulin concentration over time. This was not the case when Z-scores were used in growth curve modeling, suggesting that the relative ranks of fasting insulin standardized according to sex and age may be stable over time. However, the fact that about 35% of children and adolescent with a high-risk category had changed their risk status at least once in the follow-up assessments indicates instability in their risk status. In addition, fasting glucose appeared to be relatively stable in both metric- and Z-scores over time. Our results were in contrast to the findings of the Cittadella study18 in which postprandial blood glucose was found to be instable with increasing age. The use of fasting glucose in our study versus postprandial glucose in the Cittadella study may contribute to the inconsistent results, because fasting glucose and postprandial glucose have distinct metabolic mechanisms.28 Nevertheless, the relative stability of fasting glucose in its metric values had clinical implications because the diagnosis of diabetes and prediabetes is made according to its metric values rather than its percentile values or rank orders.25
Triglycerides was the cardiometabolic risk factor with the most changes in status, indicating that children and adolescents may frequently fluctuate between their normal or high-risk status with increasing age.26 The results of insignificant time effects on mean levels in both metric- and Z-scores for triglyceride levels in growth curve analyses seemed to be contradictory to the high variability in the dichotomized risk status. Perhaps the use of relatively low threshold values to define high triglyceride levels in children and adolescents could be attributable to these inconsistent results. In fact, we replicated our analyses using the threshold value of 130 mg/dL to define high triglyceride levels regardless of age and obtained a slightly decreased proportion of change in high-risk status at least once (27.8%). Furthermore, we used the threshold value of 150 mg/dL as suggested by the International Federation of Diabetes10 and obtained the lowest proportion of change in high-risk status (19.4%). The contradiction in findings between the threshold analyses and growth curve models also highlights the problems of translating a continuous variable into a dichotomy. The relative stability of triglycerides in both metric- and Z-scores over time adds support for the use of a single threshold value regardless of age to define the elevated concentration of triglycerides in children and adolescents.
The findings of most previous studies on the tracking of blood pressure have shown that the correlation coefficients between baseline and follow-up ranged from 0.16 to 0.36 for SBP and 0.15 to 0.24 for DBP, indicating weak stability during childhood and adolescence.13 In addition, 1 previous study found that there was only 28% agreement in high blood pressure status between age 7 and the 2 subsequent examinations at age 9, 11, and 13 years.29 Our results, in contrast to previous findings, suggested that both SBP and DBP were relatively stable over time and that the overall change in the status of elevated SBP was very similar to that of DBP. The low prevalence of elevated blood pressure among white children and adolescents in this study may be attributable to the relative stability.
The change or instability of status in cardiometabolic risk factors from childhood to adolescence could be resulted from (1) natural development such as growth and physiological maturation across pubertal stages, (2) behavioral modifications such as change in the frequency and intensity of physical activity or dietary habits, (3) medical treatment or public health intervention for those with an elevated level of risk factors, and (4) measurement errors such as variations in equipments, assays, examiners, or quality control methods. Future research on identifying specific predictors associated with change in the status of these cardiometabolic risk factors, particularly change in the clusters of these variables, would be helpful to understand the dynamics of growth during childhood and adolescence.
There were 2 strengths in our study. First, the follow-up period for many of the subjects was long, and each of 8 cardiometabolic risk factors was measured at least annually, resulting in up to a total of 10 follow-up assessments. Use of multiple follow-up measures enabled us to assess the patterns of change more adequately than with just 2 time points. Second, we examined the patterns of change in the 8 cardiometabolic risk factors associated with the MetS with growth curve analyses simultaneously, which enabled us to compare the differences and similarities among these factors in a single study. However, our results were subject to 2 limitations. First, because 98% of participants of the Fels Longitudinal Study were non-Hispanic whites and we only analyzed data of white children and adolescents, our results may be generalized to non-Hispanic white populations. Caution may be needed when applying our results to other racial/ethnic populations. Second, because of relatively small sample size in our study, particularly among children with a very high Z-score (ie, Z-score ≥2) at baseline, we were unable to conduct separate growth curve analyses to assess the patterns of change in cardiometabolic risk factors over time in this clinically meaningful subgroup of children.
In sum, the MetS, clustering of multiple cardiometabolic risk factors, has been well recognized; however, its precise definition including the optimal threshold values of its elements and its utility are still not established in children and adolescents.8,30 In this study, we provided comprehensive information about the dynamic changes in the 8 cardiometabolic risk factors in a long period of time during childhood and adolescence. Our results suggest that these risk factors appear to be relatively stable on average in the total sample, albeit considerable changes in risk status may occur commonly in the subsample of youth with a high-risk category at baseline. The fact that cardiometabolic risk factors are variable over time is important because cross-sectional assessment of these risk cardiometabolic risk factors can provide challenges in predicting disease risk later on in life. Long-term monitoring is critical to identify the dynamics of change in risk status during childhood and adolescence and, in turn, the prediction of disease risks in adulthood.
Acknowledgments
We thank Dr. Roger M. Siervogel, PhD, at Lifespan Health Research Center, Department of Community Health, Wright State University School of Medicine, Dayton, Ohio, for his support and assistance in data collection and the children and adolescents who participated in the Fels Longitudinal Study.
Glossary
- BMI
Body mass index
- DBP
Diastolic blood pressure
- HDL
High-density lipoprotein
- MetS
Metabolic syndrome
- SBP
Systolic blood pressure
Footnotes
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Contents of this paper do not necessarily represent the views or policies of the National Institutes of Health.
Author Disclosures
The following authors have no financial arrangement or affiliation with a corporate organization or a manufacturer of a product discussed in this supplement: Chaoyang Li, MD, PhD, Earl S. Ford, MD, MPH, Terry T.-K. Huang, PhD, MPH, Shumei Sun, PhD, and Elizabeth Goodman, MD.
References
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Goran MI, Ball GD, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab. 2003;88:1417–27. doi: 10.1210/jc.2002-021442. [DOI] [PubMed] [Google Scholar]
- 4.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–37. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawlor DA, Martin RM, Gunnell D, Galobardes B, Ebrahim S, Sandhu J, et al. Association of body mass index measured in childhood, adolescence, and young adulthood with risk of ischemic heart disease and stroke: findings from 3 historical cohort studies. Am J Clin Nutr. 2006;83:767–73. doi: 10.1093/ajcn/83.4.767. [DOI] [PubMed] [Google Scholar]
- 6.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–6. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Sun SS, Liang R, Huang TT, Daniels SR, Arslanian S, Liu K, et al. Childhood obesity predicts adult metabolic syndrome: the Fels Longitudinal Study. J Pediatr. 2008;152:191–200. doi: 10.1016/j.jpeds.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford ES, Li C. Defining the metabolic syndrome in children and adolescents: will the real definition please stand up? J Pediatr. 2008;152:160–4. doi: 10.1016/j.jpeds.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 9.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–7. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 10.Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–61. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 11.Goodman E, Daniels SR, Meigs JB, Dolan LM. Instability in the diagnosis of metabolic syndrome in adolescents. Circulation. 2007;115:2316–22. doi: 10.1161/CIRCULATIONAHA.106.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crimmins NA, Dolan LM, Martin LJ, Bean JA, Daniels SR, Lawson ML, et al. Stability of adolescent body mass index during three years of follow-up. J Pediatr. 2007;151:383–7. doi: 10.1016/j.jpeds.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Fuentes RM, Notkola IL, Shemeikka S, Tuomilehto J, Nissinen A. Tracking of systolic blood pressure during childhood: a 15-year follow-up population-based family study in eastern Finland. J Hypertens. 2002;20:195–202. doi: 10.1097/00004872-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Huang TT, Johnson MS, Gower BA, Goran MI. Effect of changes in fat distribution on the rates of change of insulin response in children. Obes Res. 2002;10:978–84. doi: 10.1038/oby.2002.133. [DOI] [PubMed] [Google Scholar]
- 15.Pagnan A, Ambrosio GB, Vincenzi M, Mormino P, Maiolino P, Gerin L, et al. Precursors of atherosclerosis in children: the Cittadella study. Follow-up and tracking of total serum cholesterol, triglycerides, and blood glucose. Prev Med. 1982;11:381–90. doi: 10.1016/0091-7435(82)90042-1. [DOI] [PubMed] [Google Scholar]
- 16.Singh AS, Mulder C, Twisk JW, van MW, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9:474–88. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood. a systematic review and meta-regression analysis. Circulation. 2008 Jun 24;117(25):3171–80. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webber LS, Srinivasan SR, Wattigney WA, Berenson GS. Tracking of serum lipids and lipoproteins from childhood to adulthood. The Bogalusa Heart Study. Am J Epidemiol. 1991;133:884–99. doi: 10.1093/oxfordjournals.aje.a115968. [DOI] [PubMed] [Google Scholar]
- 19.Eisenmann JC, Welk GJ, Wickel EE, Blair SN. Stability of variables associated with the metabolic syndrome from adolescence to adulthood: the Aerobics Center Longitudinal Study. Am J Hum Biol. 2004;16:690–6. doi: 10.1002/ajhb.20079. [DOI] [PubMed] [Google Scholar]
- 20.Roche AF. Growth, Maturation and Body Composition: The Fels Longitudinal Study 1929–1991. Cambridge, United Kingdom: Cambridge University Press; 1992. [Google Scholar]
- 21.Lohman T, Martorell R, Roche AF. Anthropometric Standardization Manual. Springfield, IL: Human Kinetics; 1988. [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Guo SS, Grummer Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 23.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–44. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 24.Williams CL, Hayman LL, Daniels SR, Robinson TN, Steinberger J, Paridon S, et al. Cardiovascular health in childhood: A statement for health professionals from the Committee on Atherosclerosis, Hypertension, and Obesity in the Young (AHOY) of the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2002;106:143–60. doi: 10.1161/01.cir.0000019555.61092.9e. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31(Suppl 1):S55–60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 26.Jolliffe CJ, Janssen I. Distribution of lipoproteins by age and gender in adolescents. Circulation. 2006;114:1056–62. doi: 10.1161/CIRCULATIONAHA.106.620864. [DOI] [PubMed] [Google Scholar]
- 27.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 28.Meyer C, Pimenta W, Woerle HJ, Van HT, Szoke E, Mitrakou A, et al. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care. 2006;29:1909–14. doi: 10.2337/dc06-0438. [DOI] [PubMed] [Google Scholar]
- 29.St George IM, Williams SM, Silva PA. The stability of high blood pressure in Dunedin children: an eight year longitudinal study. N Z Med J. 1990;103:115–7. [PubMed] [Google Scholar]
- 30.Goodman E. Metabolic syndrome and the mismeasure of risk. J Adolesc Health. 2008;42:538–40. doi: 10.1016/j.jadohealth.2008.03.011. [DOI] [PubMed] [Google Scholar]