Abstract
Intrauterine growth restriction and premature delivery decrease circulating levels of the neurotrophic hormone leptin and increase the risk of adult psychiatric disease. In mouse models, neonatal leptin replacement normalizes brain growth and improves the neurodevelopmental outcomes of growth restricted mice, but leptin supplementation of well-grown mice decreases adult locomotor activity. We hypothesized isolated neonatal leptin deficiency is sufficient to reduce adult brain volumes and program behavioral outcomes, including hyperactivity. C57Bl/6 pups were randomized to daily injections of saline or PEG-leptin antagonist (LX, 12.5 mg/kg) from postnatal day 4 to 14. After 4 months, fear conditioning and open field testing were performed followed by carotid radiotelemetry for the measurement of baseline activity and blood pressure. Neonatal LX did not significantly increase cue-based fear or blood pressure, but increased adult locomotor activity during assessment in both the open field (beam breaks: control 930±40, LX 1099±42, P<0.01) and the home cage (radiotelemetry counts: control 4.5±0.3, LX 5.6±0.3, P=0.02). Follow-up MRI revealed significant reductions in adult frontal cortex volumes following neonatal LX administration (control 45.1±0.4 mm3, LX 43.8±0.4 mm3, P=0.04). This was associated with a significant increase in cerebral cortex leptin receptor mRNA expression. In conclusion, isolated neonatal leptin deficiency increases cerebral cortex leptin receptor expression and reduces frontal cortex volumes in association with increased adult locomotor activity. We speculate neonatal leptin deficiency may contribute to the adverse neurodevelopmental outcomes associated with perinatal growth restriction, and postnatal leptin therapy may be protective.
Keywords: leptin, growth restriction, hyperactivity, developmental origins
1. INTRODUCTION
Individuals with a history of low birth weight as a result of intrauterine growth restriction (IUGR) or prematurity are at increased risks of adult psychiatric disease [1–3]. While the origins of adult disease are multifactorial, IUGR and prematurity are both associated with suboptimal nutritional delivery and impaired brain growth during the formative phases of development [2, 4, 5]. Global nutritional interventions can improve neurodevelopmental outcomes, but accelerated neonatal growth may increase adiposity and obesity-related morbidities [6–8]. Targeted interventions that support brain growth without excessive somatic growth may thus optimize long-term neurologic and metabolic outcomes.
Leptin, a critical component of adult metabolic and cardiovascular regulation, is neurotrophic factor during perinatal development [9, 10]. As a product of the mature adipocyte that readily crosses the placenta, circulating leptin levels correlate with adiposity and are significantly reduced in both IUGR and premature infants [11, 12]. In mice and humans with mutations in the leptin gene, exogenous leptin administration improves brain growth in newborns as well as in adults[10, 13].
To further clarify the programming effects of leptin, a number of animal models have been employed. In IUGR piglets, neonatal leptin administration elicits neurotrophic effects [14]. Because of their delayed developmental trajectory, neonatal rats and mice are utilized to further model the critical third trimester of human development. In these studies, neonatal growth restriction decreases circulating leptin, impairs neurodevelopment, and elicits adult hypertension [15, 17], while neonatal leptin supplementation normalizes blood pressure and brain growth [17]. Likewise, leptin null mice have decreased adult brain weights and impaired hypothalamic projections that improve with neonatal leptin therapy [9, 10]. The fact that neonatal leptin administration elicits neurotrophic effects in leptin deficient mice does not provide a causal link between neonatal leptin deficiency and the programmed adult phenotypes. To clarify the importance of neonatal leptin exposure, we tested the hypothesis that isolated neonatal leptin deficiency is sufficient to reduce brain growth and program adult behavior in the absence of neonatal growth restriction.
2. METHODS
2.1. Animal Model
All procedures were approved by the University of Iowa Animal Care and Use Committee and comply with the guidelines of the Animal Welfare Act, National Institutes of Health guidelines on the care and use of laboratory animals. C57BL/6J mice delivered naturally and offspring were weighed within 24h of delivery. To avoid the confounding effects of perinatal growth restriction, mice with birth weights below the 10th percentile were excluded, and all litters were culled to 6 pups to facilitate postnatal growth [17]. To assess the programming effects of neonatal pegylated super murine leptin antagonist (LX) exposure, pups within each litter were randomized to daily intraperitoneal injections of either LX (12.5 mg/kg from day 4 to 14) or vehicle alone (10 ml/kg of normal saline) [18, 19]. Pup weights were obtained daily from day 4 to 14 and again on weaning day 21. Adult phenotypes, including body weight and feed intake, were evaluated beginning at an attained age of 4 to 6 months. To verify LX’s biologic activity, feed intake and body weight were recorded over a 5 day baseline period for 6 adult control mice. Those 6 mice were then randomized to 5 daily injections of either LX (12.5 mg/kg/d) or saline alone (10 ml/kg/d) and feed intake / body weight were again recorded.
2.2 Behavioral Phenotypes
Behavioral phenotypes were first assessed during a fear conditioning protocol, as previously described [17]. On the first day, mice were trained to associate a tone (80 dB, 20 sec) with a co-terminated electric foot-shock (0.5 mA, 1 sec) a total of 5 times at 2 minute intervals. The time spent in a characteristic “freezing” posture was digitally captured and recorded. The following day, cue conditioned fear was assessed by recording freezing during 3 minutes of baseline, 3 minutes of representation of the learned auditory cue, and 4 minutes of recovery. The following week, open field testing was performed [17]. Mice were placed into a 40.6 cm by 40.6 cm brightly illuminated field containing an invisible laser beam grid. The frequency of beam breaks was utilized to measure the duration and intensity of locomotor activity.
2.3 Tail cuff blood pressure
Following the behavioral testing, blood pressures and heart rates were recorded by indirect tail-cuff apparatus (BP 2000; Visitech Systems, Apex, NC). Thirty cycles were completed daily for 5 days, as previously detailed [17].
2.4 Brain Morphology
Prior to the planned radiotelemetry studies, MRI was completed with a 4.7 Tesla Varian Unity/INOVA system (Varian Inc., Palo Alto, CA) [17]. Anesthesia was provided by isoflurane with titration based upon respiratory status and pedal reflexes. T1 and T2-weighted images were acquired in axial, sagittal, and coronal planes. MRI acquisition and processing were completed by investigators blinded to group assignment.
2.5 Radiotelemetry
At 7–8 months of age, radiotelemetry catheters (PA-C10; Data Sciences International) were implanted in the left carotid artery of male mice [20]. All telemetry implants were performed using isoflurane titrated based upon respiratory status and pedal reflexes. Local anesthesia was provided with 0.5% bupivacaine application along the surgical incision and analgesia was provided with subcutaneous flunixin (2.5 mg/kg once or twice daily for 24 h). After a 7 d recovery period, arterial pressures, heart rate and relative locomotor activity were sampled for 10 sec every 5 min for 60 h (encompassing 3 dark cycles and 2 light cycles). During the subsequent light cycle, blood pressure and heart rate were recorded before and after mice were transferred to a metabolic cage (Hatteras Instruments) that restricts movement and evokes a psychological stress response [21]. Following the telemetry studies, mice were euthanized by removal of the heart during isoflurane anesthesia.
2.6 Leptin Receptor Expression
Given the effects of estrus cycling on blood pressure, female mice were utilized for gene expression analysis, rather than radiotelemetry. Under isoflurane anesthesia titrated to respiratory status, the brain was excised, then segmented into cerebral cortex and midbrain segments, as previously described [22]. The “midbrain” segment included the thalamus and hypothalamus. Quantitative real-time RT-PCR (qPCR) utilized the TaqMan reagent and instrumentation systems (Applied Biosystems, Foster City, CA). Taqman gene expression assay primer/probe sets for mouse leptin receptor (Lepr; assay ID = Mm00440181_m1) and GAPDH were purchased from Applied Biosystems (Foster City, CA).
2.7 Data Analysis
All values are presented as mean ± standard error of the mean. Each group was comprised of mice from at least 8 separate litters. Whenever phenotypes were obtained from a single sex (radiotelemetry and RT-PCR), data were compared by unpaired two-tailed Student’s t test. All other data were compared by 2-way ANOVA, factoring for sex and LX administration. Post hoc analysis (Holm-Sidak method) was performed if statistically significant differences were detected. A value of P<0.05 was considered significant. All analyses were performed using SigmaPlot 12.0 (Systat Software Inc.).
3. RESULTS
3.1 Feed Intake
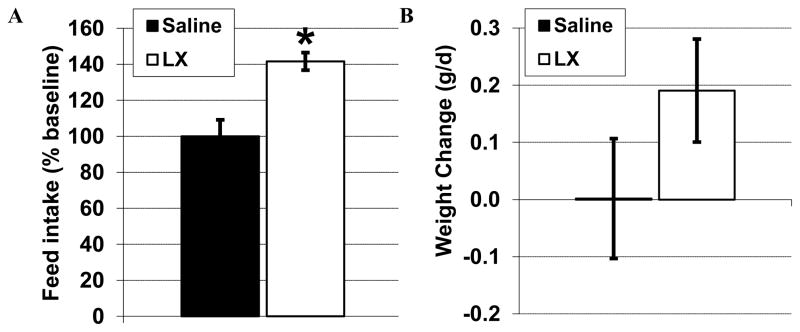
To verify biologic activity, LX was administered to a cohort of adult control mice. Consistent with antagonism of leptin-mediated anorexia, LX administration acutely increased the feed intake of the adult mice whether compared to baseline feed intake or the effect seen when littermate controls received only saline (both P=0.02, Figure 1A). While saline administration had no effect on body weight, LX induced a modest weight gain of 0.19+/−0.09 g/d (P=0.09 versus baseline and P=0.20 versus saline, Figure 1B). Although it was not possible to measure feed intake of the breastfed newborn pups, daily LX administration from day 4 to day 14 did not significantly alter pup weight. Likewise, neonatal LX administration did not significantly influence adult weight or feed intake (Table 1).
Figure 1.
Food intake was recorded for control adult mice at baseline, then again after 5 daily injections of either LX (open bar, 12.5 mg/kg ip, N=3) or vehicle alone (solid bar, 10 ml/kg normal saline, N=3). As an inhibitor of the anorexigenic response to leptin, LX increased food intake (A) without statistically significantly effects on body weight (B). *P<0.05 versus baseline and versus saline.
Table 1.
Neonatal LX administration did not alter longitudinal body weights or adult food intake.
| N | Control, M | LX, M | Control, F | LX, F |
|---|---|---|---|---|
| 18 | 17 | 21 | 20 | |
| Weight, 4d (g) | 2.4+/−0.3 | 2.8+/−0.4 | 3.0+/−0.5 | 3.0+/−0.4 |
| Weight, 14d (g) | 6.6+/−0.2 | 6.9+/−0.3 | 5.6+/−0.4 | 5.8+/−0.3 |
| Weight, adult (g) | 31.3+/−0.5 | 31.3+/−0.8 | 23.1+/−0.6 | 24.3+/−0.8 |
| Food Intake (g/kg/d) | 112+/−4 | 116+/−5 | 126+/−5 | 122+/−4 |
3.2 Adult Phenotypes
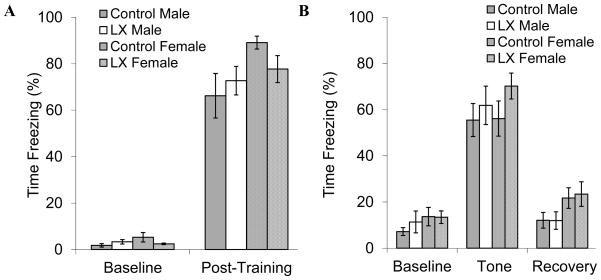
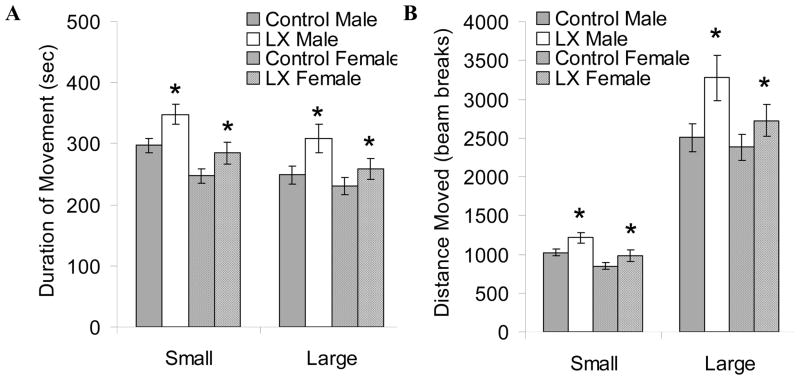
Figure 2 summarizes the sequence of the adult investigations. LX-exposed mice did not have significant alterations in fear-related freezing during training to associate the auditory cue and aversive stimulus (Figure 3A). Unpaired cue-elicit freezing was readily apparent the following day, especially among LX-exposed mice (ANOVA P=0.17 vs. control mice, Figure 3B). To further assess the effects of neonatal leptin deficiency on anxiety and locomotor activity, open field testing was performed. Both adult male and female LX-exposed mice had significantly increased locomotor activity whether measured as duration or distance of movement (Figure 4). Since the increase in open field activity may reflect a hyperactivity response to psychological stress, we proceeded to investigate locomotor activity and blood pressure by radiotelemetry.
Figure 2.
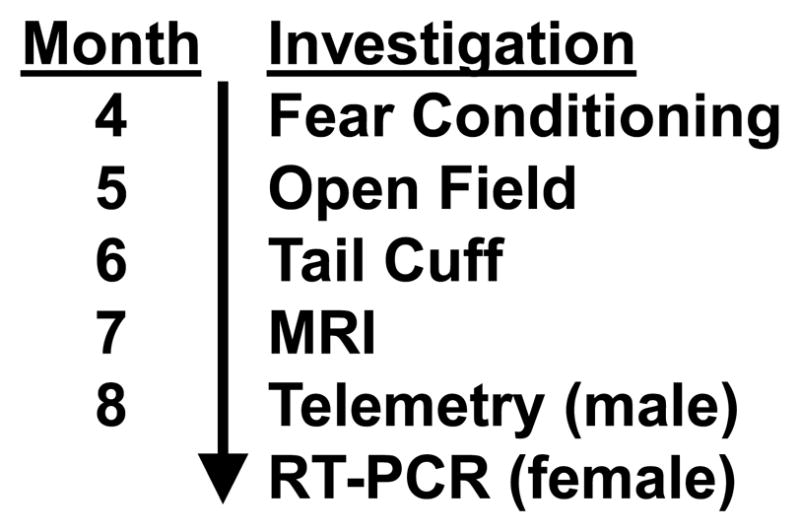
After receiving LX or saline injections from postnatal days 4 to 14, the mice underwent a series of investigations, beginning with fear conditioning and open field testing at 4–6 months and culminating in carotid radiotelemetry (males) or gene expression analysis (females). While the sequence of investigations was consistent, not all studies were performed in all mice.
Figure 3.
Control male (gray bars, N=12) and LX-exposed male (white bars, N=12) as well as control female (cross-hatched gray bars, N=17) and LX-exposed female mice (cross-hatched white bars, N=18) underwent fear conditioning. On the first day of the protocol, mice were trained to associate a cue (tone) and context (conditioning chamber) with a stressor (foot shock), and fear-related freezing was recorded (A). The following day, the same mice were placed in a different context and cue-based fear was assessed as a test of hippocampal and amygdala function (B).
Figure 4.
Open field testing was completed for adult control male (gray bars, N=11) and LX-exposed male (white bars, N=12) as well as control female (cross-hatched gray bars, N=16) and LX-exposed female mice (cross-hatched white bars, N=15). Beam breaks were recorded while the mice explored a 40.6 cm × 40.6 cm open field for 30 minutes. Based on the frequency of beam breaks, movements were automatically subdivided into “small” and “large” velocity. Both male and female LX mice were significantly more active than their control counterparts as measured by both duration of movement (A) and distance moved (B). *P<0.05 versus control
Consistent with the results of open field testing, neonatal LX exposure increased the locomotor activity of adult male mice allowed to roam freely in their home cage (LX-exposed: 5.6±0.3, control: 4.5±0.3, P=0.02, Figure 5A). LX exposure did not significantly alter adult blood pressures or heart rates whether the mice remained in their home cage or were placed within a stress-inducing metabolic chamber (Figure 5). Likewise, LX did not significantly alter blood pressure or heart rate when male and female mice were placed within the stress-evoking tail cuff apparatus (Table 2). On volumetric MRI, neonatal LX administrations led to selective reductions in adult frontal cortex volumes and male hippocampal volumes (Table 3). This was associated with a significant LX-induced increase in leptin receptor expression within the cerebral cortex (Figure 6).
Figure 5.
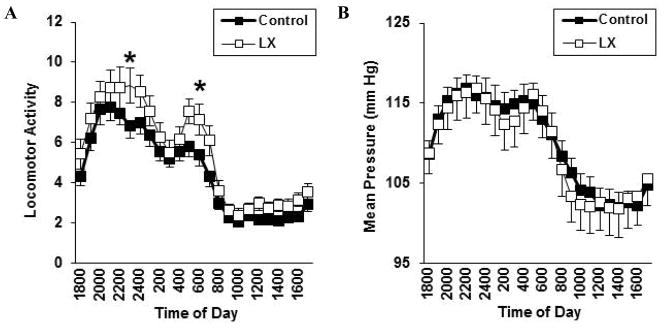
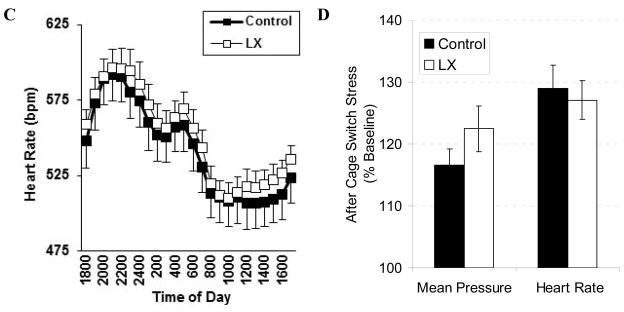
For adult male mice only, carotid telemeters were placed following the behavioral studies. Baseline telemetry was performed followed by cage switch to elicit psychological stress. Compared to control mice (solid symbols, N=16), LX-exposed mice (open symbols, N=15) again had increased levels of activity (A) without alteration in mean blood pressure (B) or heart rate (C) even when placed in the stress-evoking environment (D).
Table 2.
Systolic blood pressure (SBP) and heart rate (HR) were measured while mice were restrained in a tail cuff apparatus.
| N | Control, M | LX, M | Control, F | LX, F |
|---|---|---|---|---|
| 14 | 14 | 24 | 23 | |
| SBP, mmHg | 114+/−3 | 112+/−1 | 114+/−2 | 114+/−2 |
| HR, bpm | 641+/−15 | 629+/−10 | 644+/−11 | 654+/−13 |
Table 3.
Brain and brainstem volumetric MRI was performed on adult mice. LX mice had significant reductions in the frontal cortex with additional reduction seen in male hippocampal volumes.
| N | Control, M | LX, M | Control, F | LX, F |
|---|---|---|---|---|
| 9 | 10 | 12 | 12 | |
| Total Volume | 431+/−3 | 423+/−2 | 451+/−7 | 456+/−5 |
| Frontal Cortex | 46.7+/−0.4 | 45.7+/−0.3* | 43.5+/−0.6 | 41.9+/−0.8* |
| Cerebral Cortex, NOS | 99.1+/−0.8 | 97.0+/−0.5* | 101.8+/−1.4 | 103.7+/−1.2 |
| Brainstem | 78.2+/−0.6 | 77.4+/−0.7 | 95.8+/−1.7 | 97.7+/−1.2 |
| Cerebellum | 52.2+/−0.4 | 51.7+/−0.3 | 50.7+/−1.1 | 52.2+/−0.8 |
| Basal Ganglia | 34.8+/−0.3 | 34.2+/−0.2 | 33.2+/−0.8 | 34.5+/−0.6 |
| Olfactory System | 32.1+/−0.2 | 31.7+/−0.3 | 28.0+/−1.4 | 24.1+/−1.7 |
| White Matter Tracts | 28.9+/−0.2 | 28.4+/−0.2 | 30.1+/−0.8 | 32.3+/−0.8 |
| Hippocampus | 25.0+/−0.2 | 24.3+/−0.1** | 27.1+/−0.5 | 28.1+/−0.6 |
| Thalamus | 18.8+/−0.1 | 18.5+/−0.1 | 19.4+/−0.3 | 19.9+/−0.3 |
| Hypothalamus | 9.41+/−0.09 | 9.23+/−0.11 | 15.06+/−0.35 | 14.85+/−0.36 |
| Ventricles | 2.75+/−0.03 | 2.72+/−0.03 | 3.25+/−0.12 | 3.59+/−0.13 |
| Pituitary | 1.64+/−0.03 | 1.58+/−0.03 | 2.04+/−0.07 | 2.05+/−0.08 |
| Amygdala | 0.95+/−0.03 | 0.91+/−0.02 | 1.05+/−0.05 | 1.11+/−0.03 |
P<0.05 and
P<0.01 versus control;
NOS: not otherwise specified; volumes are expressed in mm3
Figure 6.
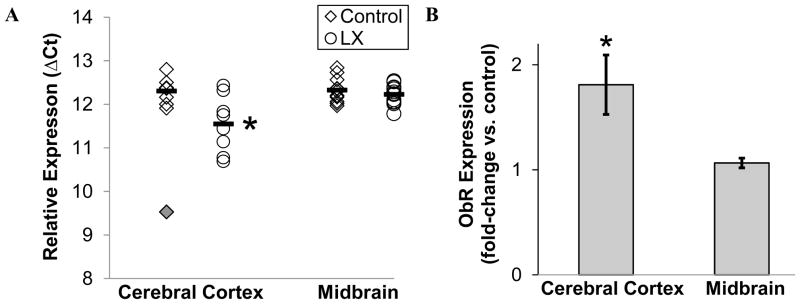
Leptin receptor mRNA expression was assessed in samples of adult female cerebral cortex and midbrain, including hypothalamus. Results were compared for control (open rhombus, N=7) and LX-exposed mice (round symbols, N= 9) after normalization by sample-specific GAPDH expression. LX mice had a significant increase in cerebral cortex leptin receptor mRNA, as shown by a lower dCT (A) and higher relative expression versus saline-treated controls (B). Cerebral cortex leptin receptor expression for one mouse (solid rhombus) was a statistical outlier. The horizontal bars denote the group’s mean value. *P<0.05
4. DISCUSSION
The sequelae of perinatal growth restriction include a higher incidence of psychiatric illnesses, including attention-deficit/hyperactivity disorder [1, 2, 23]. Among the nutritional deficiencies that may mechanistically link undernutrition and adult disease, leptin has substantial theoretical support. Our current study is the first to show that isolated neonatal leptin deficiency increases murine locomotor activity in association with a loss of frontal lobe volume and an increase in cerebral cortex leptin receptor expression. Our studies thus complement corollary investigations showing exogenous leptin administration reduces adult locomotor activity, increases regional brain volumes and decreases leptin receptor expression within the brain [17, 24–26], solidifying the importance of perinatal leptin signaling in neurodevelopment and behavioral regulation.
We utilized a battery of tests to assess both baseline activity and anxiety or fear-related behavior. Intriguingly, LX increased overall open field activity and locomotor activity within the home cage, but did not significantly alter the expression of either fear or anxiety. The regulation of locomotor activity is complex with important contributions of the dopaminergic, noradrenergic and serotonergic systems and prominent involvement of the frontal cortex [27]. In this context, the reduction in adult frontal cortex volume following neonatal LX exposure is intriguing. While we did not attempt to assay additional aspects of the human attention-deficit hyperactivity disorder, the association between attention deficit disorder and a loss of the frontal lobe volume is noteworthy, albeit controversial [28, 29]. Of concern, preterm infants not only have reduced brain volumes, they also have a significant reduction in frontal lobe white matter growth during childhood [30].
Leptin’s role in fetal and infant neuronal development makes it an attractive candidate to reverse the deleterious neurocognitive and behavioral phenotypes associated with prematurity and intrauterine growth restriction. A recent post-hoc evaluation of data from a nutritional intervention trial showed strong correlations between breast milk intake, brain volumes and intelligence [31]. Breast milk contains a number of trophic factors, including leptin, which are not present in infant formulas. In our prior studies, neonatal leptin administration had robust effects on adult brain volumes, while complementary investigations have shown effects of adult leptin administration on frontal lobe development [13, 17]. Further linking leptin deficiency and locomotor activity are studies by Walsh and colleagues showing an association between leptin or leptin receptor genetic variants and physical activity [32]. The pegylated leptin antagonist utilized in the current investigations allowed potent, sustained central leptin deficiency that, just as importantly, is reversible within days of LX discontinuation [19], implicating postnatal days 4–14 as a critical window of leptin-modulated developmental susceptibility.
Our study also identified some pertinent negatives, including a lack of neonatal LX-induced alterations in body weight, adult feed intake or adult blood pressure. The contrast between the lack of neonatal LX effect on weight gain and the prominent adult LX effect on acute feed intake suggests that the neonatal mouse has a developmentally attenuated anorexigenic response to leptin receptor stimulation. This is consistent with a number of investigations showing neonatal leptin or neonatal LX do not acutely alter pup growth despite an induction in hypothalamic activation [17, 33], presumably because leptin exposure during that phase of neurodevelopment is acting as trophic factor stimulating development of the pathway that will eventually become leptin-responsive [9]. The lack of interaction between neonatal LX and adult feed intake contrasts with studies performed in rats, but in those studies, the predisposition of neonatal LX mice to relative hyperphagia was only seen after the rats were advanced to a hypercaloric diet [34], suggesting neonatal LX inhibits the modulation of feed intake that should occur during provision of a hypercaloric diet. While we noted that adult LX administration led to a significant increase in feed intake, verifying the biologic potency of the preparation, there was not a significant effect of adult LX administration on daily weight gain. While the lack a statistically significant effect of acute LX administration on adult weight may be attributable to our limited sample size and lack of long-term follow-up, it could also be attributable to unmeasured changes in resting energy expenditure or voluntary locomotor activity.
The absence of an adult metabolic phenotype in our study is consistent with our data showing no significant effect of neonatal LX on midbrain leptin receptor expression. In contrast, we identified an increase in leptin receptor expression within the cerebral cortex samples. With reports of leptin-induced improvements in frontal lobe volume even among adults [13], this raises the possibility of therapeutic leptin administration in select patient populations, including those with a history of low birth weight. While we did not detect a hemodynamic phenotype in LX-exposed mice, individuals with a history of growth restriction are at heightened risk of cardiovascular disease [35], and this could be exacerbated by leptin either at times of increased adiposity or during trials of exogenous leptin administration.
Our results support and extend earlier observations demonstrating a critical impact of neonatal leptin on the behavioral and structural phenotypes of mice. This model translates most directly to the premature population who must undergo critical phases of neurodevelopment without the benefit of transplacental leptin and IUGR infants that may lack sufficient endogenous leptin production. Future studies are necessary to determine if postnatal leptin supplementation can improve the behavioral outcomes of premature or intrauterine growth restriction infants.
Highlights.
Perinatal growth restriction increases the risk of adult psychiatric disease
Growth restriction decreases circulating leptin, a neurotrophic hormone
Neonatal leptin deficiency decreases frontal lobe volume and programs hyperactivity
Increased leptin receptor expression suggests a potential therapeutic intervention
Acknowledgments
This study was funded by the National Institutes of Health (HD050359)
Abbreviations
- LX
pegylated super murine leptin antagonist
- IUGR
intrauterine growth restricted
Footnotes
COMPETING INTERESTS
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hack M, Youngstrom ER, Cartar L, Schluchter M, Gerry Taylor H, Flannery D, et al. Behavioral outcomes and evidence of psychopathology among very low birth weight infants at age 20 years. Pediatrics. 2004;114:932–40. doi: 10.1542/peds.2003-1017-L. [DOI] [PubMed] [Google Scholar]
- 2.Heinonen K, Räikkönen K, Pesonen AK, Andersson S, Kajantie E, Eriksson JG, et al. Trajectories of growth and symptoms of attention-deficit/hyperactivity disorder in children: a longitudinal study. BMC Pediatr. 2011;11:84. doi: 10.1186/1471-2431-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schothorst PF, Swaab-Barneveld H, van Engeland H. Psychiatric disorders and MND in non-handicapped preterm children. Prevalence and stability from school age into adolescence. Eur Child Adolesc Psychiatry. 2007;16:439–48. doi: 10.1007/s00787-007-0617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Semin Perinatol. 2003;27:302–10. doi: 10.1016/s0146-0005(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 5.Kesler SR, Reiss AL, Vohr B, Watson C, Schneider KC, Katz KH, et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008;152:513–20. doi: 10.1016/j.jpeds.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beardsall K, Ong KK, Murphy N, Ahmed ML, Zhao JH, Peeters MW, et al. Heritability of childhood weight gain from birth and risk markers for adult metabolic disease in prepubertal twins. J Clin Endocrinol Metab. 2009;94:3708–13. doi: 10.1210/jc.2009-0757. [DOI] [PubMed] [Google Scholar]
- 7.Hermann GM, Miller RL, Erkonen GE, Dallas LM, Hsu E, Zhu V, et al. Neonatal catch up growth increases diabetes susceptibility but improves behavioral and cardiovascular outcomes of low birth weight male mice. Pediatr Res. 2009;66:53–8. doi: 10.1203/PDR.0b013e3181a7c5fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rotteveel J, van Weissenbruch MM, Twisk JW, Delemarre-Van de Waal HA. Infant and childhood growth patterns, insulin sensitivity, and blood pressure in prematurely born young adults. Pediatrics. 2008;122:313–21. doi: 10.1542/peds.2007-2012. [DOI] [PubMed] [Google Scholar]
- 9.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–10. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 10.Steppan CM, Swick AG. A role for leptin in brain development. Biochem Biophys Res Commun. 1999;256:600–2. doi: 10.1006/bbrc.1999.0382. [DOI] [PubMed] [Google Scholar]
- 11.Pighetti M, Tommaselli GA, D’Elia A, Di Carlo C, Mariano A, Di Carlo A, et al. Maternal serum and umbilical cord blood leptin concentrations with fetal growth restriction. Obstet Gynecol. 2003;102:535–43. doi: 10.1016/s0029-7844(03)00668-9. [DOI] [PubMed] [Google Scholar]
- 12.Val niene M, Verkauskiene R, Boguszewski M, Dahlgren J, Lasiene D, Lasas L, et al. Leptin levels at birth and in early postnatal life in small- and appropriate-for-gestational-age infants. Medicina (Kaunas) 2007;43:784–91. [PubMed] [Google Scholar]
- 13.Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, et al. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab. 2005;90:2851–4. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- 14.Attig L, Djiane J, Gertler A, Rampin O, Larcher T, Boukthir S, et al. Study of hypothalamic leptin receptor expression in low-birth-weight piglets and effects of leptin supplementation on neonatal growth and development. Am J Physiol Endocrinol Metab. 2008;295:E1117–25. doi: 10.1152/ajpendo.90542.2008. [DOI] [PubMed] [Google Scholar]
- 15.Coupé B, Amarger V, Grit I, Benani A, Parnet P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology. 2010;151:702–13. doi: 10.1210/en.2009-0893. [DOI] [PubMed] [Google Scholar]
- 16.Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, et al. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149:470–5. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- 17.Erkonen GE, Hermann GM, Miller RL, Thedens DL, Nopoulos PC, Wemmie JA, et al. Neonatal leptin administration alters regional brain volumes and blocks neonatal growth restriction-induced behavioral and cardiovascular dysfunction in male mice. Pediatr Res. 2011;69:406–12. doi: 10.1203/PDR.0b013e3182110c7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beltrand J, Sloboda DM, Connor KL, Truong M, Vickers MH. The Effect of Neonatal Leptin Antagonism in Male Rat Offspring Is Dependent upon the Interaction between Prior Maternal Nutritional Status and Post-Weaning Diet. J Nutr Metab. 2012;2012:296935. doi: 10.1155/2012/296935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shpilman M, Niv-Spector L, Katz M, Varol C, Solomon G, Ayalon-Soffer M, et al. Development and characterization of high affinity leptins and leptin antagonists. J Biol Chem. 2011;286:4429–42. doi: 10.1074/jbc.M110.196402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roghair RD, Segar JL, Volk KA, Chapleau MW, Dallas LM, Sorenson AR, et al. Vascular nitric oxide and superoxide anion contribute to sex-specific programmed cardiovascular physiology in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R651–62. doi: 10.1152/ajpregu.90756.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roghair RD, Wemmie JA, Volk KA, Scholz TD, Lamb FS, Segar JL. Maternal antioxidant blocks programmed cardiovascular and behavioural stress responses in adult mice. Clin Sci (Lond) 2011;121:427–36. doi: 10.1042/CS20110153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kummet GJ, Haskell SE, Hermann GM, Ni C, Volk KA, Younes AK, et al. Neonatal SSRI Exposure Programs a Hypermetabolic State in Adult Mice. J Nutr Metab. 2012;2012:431574. doi: 10.1155/2012/431574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conrad AL, Richman L, Lindgren S, Nopoulos P. Biological and environmental predictors of behavioral sequelae in children born preterm. Pediatrics. 2010;125:e83–9. doi: 10.1542/peds.2009-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toste FP, de Moura EG, Lisboa PC, Fagundes AT, de Oliveira E, Passos MC. Neonatal leptin treatment programmes leptin hypothalamic resistance and intermediary metabolic parameters in adult rats. Br J Nutr. 2006;95:830–7. doi: 10.1079/bjn20061726. [DOI] [PubMed] [Google Scholar]
- 25.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–6. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 26.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, et al. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology. 2008;149:1906–13. doi: 10.1210/en.2007-0981. [DOI] [PubMed] [Google Scholar]
- 27.Bubser M, Schmidt WJ. 6-Hydroxydopamine lesion of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterrupted tasks in the radial maze. Behav Brain Res. 1990;37:157–68. doi: 10.1016/0166-4328(90)90091-r. [DOI] [PubMed] [Google Scholar]
- 28.Almeida LG, Ricardo-Garcell J, Prado H, Barajas L, Fernández-Bouzas A, Avila D, et al. Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: a cross-sectional study. J Psychiatr Res. 2010;44:1214–23. doi: 10.1016/j.jpsychires.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2002;52:785–94. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- 30.Ment LR, Kesler S, Vohr B, Katz KH, Baumgartner H, Schneider KC, et al. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics. 2009;123:503–11. doi: 10.1542/peds.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gadian DG, Lucas A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res. 2010;67:357–62. doi: 10.1203/PDR.0b013e3181d026da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh S, Haddad CJ, Kostek MA, Angelopoulos TJ, Clarkson PM, Gordon PM, et al. Leptin and leptin receptor genetic variants associate with habitual physical activity and the arm body composition response to resistance training. Gene. 2012;510:66–70. doi: 10.1016/j.gene.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdennebi-Najar L, Desai M, Han G, Casillas E, Jean D, Arieh G, et al. Basal, endogenous leptin is metabolically active in newborn rat pups. J Matern Fetal Neonatal Med. 2011;24:1486–91. doi: 10.3109/14767058.2010.547638. [DOI] [PubMed] [Google Scholar]
- 34.Attig L, Solomon G, Ferezou J, Abdennebi-Najar L, Taouis M, Gertler A, et al. Early postnatal leptin blockage leads to a long-term leptin resistance and susceptibility to diet-induced obesity in rats. Int J Obes (Lond) 2008;32:1153–60. doi: 10.1038/ijo.2008.39. [DOI] [PubMed] [Google Scholar]
- 35.Eriksson JG, Forsén TJ, Kajantie E, Osmond C, Barker DJ. Childhood growth and hypertension in later life. Hypertension. 2007;49:1415–21. doi: 10.1161/HYPERTENSIONAHA.106.085597. [DOI] [PubMed] [Google Scholar]