Abstract
Human γδ T cells display the principal characteristics of professional antigen-presenting cells (APCs), in addition to playing a vital role in immunity through cytokine secretion and their cytotoxic activity. However, it is not clear whether γδ T cells perform APC-like functions under pathological conditions. In this study, we showed that, in contrast to peripheral-derived γδ T cells directly isolated from PBMCs of gastric cancer patients, tumor-activated γδ T cells not only killed tumor cells efficiently but also strongly induced primary CD4+ and CD8+ αβ T cells proliferation and differentiation. More importantly, they abrogated the immunosuppression induced by CD4+CD25+ Treg cells and induced the cytotoxic function of CD8+ αβ T cells from patients with gastric cancer. In conclusion, tumor-activated γδ T cells can induce adaptive immune responses through their APC-like functions, and these cells may be a potentially useful tool in the development of tumor vaccines and immunotherapy.
1. Introduction
γδ T cells are a distinct subset of CD3+ T lymphocytes characterized by the presence of T cell receptors (TCRs), which are encoded by Vγ- and Vδ-gene segments [1]. In human peripheral blood, γδ T cells typically represent only 3–5% of all T lymphocytes and are Vδ2+ γδ T cell subset predominant; however, they are common in the organs and mucosa, and, here, they are Vδ1+ γδ T cell subset predominant, acting as the first defense system against the entry of foreign organisms. In contrast to conventional αβ T cells, γδ T cells express a limited repertoire of TCR V-region genes. Stimulated γδ T cells undergo activation, which results in a plethora of poorly defined changes, including proliferation, proinflammatory cytokine, and chemokine secretion, and altered cell surface phenotypes [1]. γδ T cells participate in the immune response by direct cytolysis, development of memory phenotypes, and modulation of immune cells, and they have been implicated in autoimmune disorders, immune deficiencies, infections, and tumor diseases.
γδ T cells recognize and kill a range of tumor cells with multiple tissue origins [2, 3], and the genetic absence of γδ T cells rendered mice significantly more susceptible to tumor growth in vivo [4–6]. The antitumor properties of γδ T cells have been exploited as a potential target for tumor immunotherapy [2, 7]. It has been reported that the most common subtype of these cells in human blood is Vγ9Vδ2, which recognizes a group of nonpeptide phosphoantigens (PAg)—of which isoprenyl pyrophosphate (IPP) has been well characterized—that are known to be upregulated in infection or cancer [8, 9]. Further, PAgs may be presented to γδ-TCRs via a surface molecule (i.e., F1-ATPase) in a manner somewhat analogous to MHC-mediated antigen presentation [10], suggesting that Vγ9Vδ2 cells can function as professional antigen-presenting cells (APCs) [11–13]. Human γδ T cells exhibit a potent cytotoxicity against various tumor cells as cytotoxic T cells [2, 14–17]. However, the significance of γδ T cells expressing the APC-like phenotype and the mechanisms by which they fight tumor cells remains largely unknown. In this study, we showed that γδ T cells from patients with gastric cancer could not only serve as targets for γδ T-mediated antitumor activity but also display the APC-like phenotype and functions.
2. Materials and Methods
2.1. Patient Subjects
Human peripheral blood and fresh tumor tissue samples were obtained from gastric cancer patients (16 men and 4 women; age: 47–69 years; median age: 58.1 ± 6.4 years) newly diagnosed on the basis of clinical history, gastroscopic examination, and pathological diagnosis. Healthy controls (8 men and 2 women; age: 39–63 years; median age: 54.4 ± 8.7 years) were also enrolled, based on normal results from laboratory and physical examinations. Ethics approval for this study was granted by the Ethics Committee of the Affiliated Hospital of Jiangsu University, and written informed consent was obtained from all patients enrolled.
2.2. Flow Cytometric Assays
Cells (1 × 105) were suspended in PBS containing 2% FBS for 10 min to block nonspecific binding sites and then were incubated at 4°C for 30 min to determine the percentages of subsets of lymphocyte cells with a combination of antibodies as follows: CD3-APC (UCHT1), CD8-PE (B9.11), CD4-FITC (13B8.2), CD80-FITC (MAB104), CD83-PE (HB15a), CD86-PE (HA5.2B7), HLA-DR-PE (IM0464), CD25-PE (B1.49.9), pan γδ-PC5 (IMMU510), and CD45-APC (J33); all were purchased from Beckman Coulter. Cell apoptosis was stained with the Apoptosis Detection Kit I (BD Pharmingen) according to the manufacturer's instructions. For indirect staining, cells were washed twice with PBS and then incubated for 20 min at 4°C with PE-conjugated goat anti-mouse IgG. Compensation was set up with single-stained samples; low forward scatter elements (RBC and debris) were excluded from analysis, and 10,000 events were collected and analyzed by FACSAria cytometer (BD Biosciences).
2.3. Cell Isolation and Purification
Fresh peripheral blood was collected in sodium-heparin vacutainer tubes. Periphery mononuclear cells (PBMCs) were isolated by Ficoll density gradient (Sigma Aldrich) centrifugation. CD8+ T, TCRγδ + T, CD4+CD25− T, and CD4+CD25+ Treg cells were isolated and purified from fresh PBMCs of patients with gastric cancer by magnetic cell separation. In brief, CD8+ T (>95% purity) and TCRγδ + T cells (>95% purity, named as peripheral-derived γδ T cells) were firstly separated by positive selection using human blood TCRγδ + T and CD8+ T Cell Isolation Kits (Miltenyi Biotec) according to the manufacturer's instructions. From the fraction of remaining cells further isolation of CD4+CD25− T cells (>90% purity) and CD4+CD25+ T cells (>95% purity) was accomplished by negative selection and positive selection using a human CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec).
2.4. Generation of Tumor-Activated γδ T Cells
Gastric cancer tissues were minced and digested with a triple enzyme mixture comprising collagenase type IV, hyaluronidase, and deoxyribonuclease for 2 h at room temperature. After digestion, the cells were washed twice in RPMI 1640 and then irradiated (30 Gy) and preserved. Peripheral-derived γδ T cells (6 × 105 cells/mL) were then cocultured with the irradiated tumor tissue cells (3 : 1 ratio) in RPMI 1640 containing 10% human serum supplemented with l-glutamine, 2-mercaptoethanol, IL-2 (200 U/mL; R&D Systems), and IL-15 (20 ng/mL; R&D Systems) for generation and expansion of tumor-activated γδ T cells.
2.5. Proliferation Assay of γδ T Cells
Irradiated (30 Gy) PBMCs or tumor tissue cells (2 × 104 cells/well) seeded in 96-well plates with 200 μL RPMI 1640 medium containing 10% FBS and IL-2 (100 U/mL) were added to purified autologous γδ T cells (6 × 104 cells/well) and incubated at 37°C 5% CO2 for 3 days. Cells were pulsed with 1 μCi/well of [3H]TdR and harvested after 12 h. The incorporation of [3H]TdR was determined using a liquid scintillation counter (LS6500; Beckman Coulter, Brea, CA, USA).
2.6. In Vitro Functional Assay
To determine the functional effect of tumor-activated γδ T cells on adaptive immune T cells, an in vitro functional assay was performed as previously described [18]. In brief, autologous CD4+CD25− T cells or CD8+ T cells (1 × 106 cells/mL) were labeled for 15 min with 4.5 μM carboxyfluorescein succinimidyl ester (CFSE; Sigma Aldrich). Labeled CD4+ or CD8+ T cells (2 × 105 cells/mL) were cocultured with γδ T cells alone or together in the indicated ratios in 24-well plates containing 10% FBS-RPMI 1640 medium at 37°C in 5% CO2. To determine the functional effect of the tumor-activated γδ T cells on CD4+CD25+ Treg cells, autologous CD4+ T cells (2 × 105 cells/mL) were cocultured with CD4+CD25+ Treg cells (2 × 105 cells/mL) in the absence or presence of γδ T cells, anti-CD3 (OKT3; eBioscience), and anti-CD28 (CD28.2; eBioscience). Proliferation of CD4+ or CD8+ T cells was determined by fluorescence correlation microscopy (FCM) to assess CFSE dilution on day 3. Transwell experiments were also performed with 24-well plates with a pore size of 0.4 μm (Corning Costar, Cambridge, MA, USA). To determine whether the effect of tumor-activated γδ T cells could be blocked by specific antibodies, T cell activity was assessed in the absence or presence of various antibodies such as those against IL-1β (AF-201-NA; R&D Systems), IL-6 (AF-206-NA; R&D Systems), IL-12 (MAB1510; R&D Systems), CD80 (MAB140; R&D Systems), CD86 (MAB141; R&D Systems), and IFN-γ (AB-285-NA; R&D Systems).
2.7. Cytotoxic Assay
The cytotoxic activity of effector T cells was determined by measuring the amount of lactate dehydrogenase (LDH) released from target cells. The commercial LDH Cytotoxicity kit (Beyotime, China) was used according to the manufacturer's instructions. Maximum LDH release of target cells was determined by lysing target cells for 45 min (lysis buffer provided within the assay) and subsequently measuring the LDH from the culture medium. Absorbance values after the colorimetric reaction were measured at 490 nm with a reference wavelength of 655 nm, using a Bio-Rad Model 550 microplate reader (Bio-Rad, Hercules, CA, USA).
2.8. Measurement of Cytokines by ELISA
The supernatants from γδ T cells culture were collected and stored at −80°C until analysis. Cytokines of IFN-γ, IL-1, IL-4, IL-6, IL-10, and IL-12 concentrations were measured using commercial enzyme-linked immunosorbent assay (R&D systems) according to the manufacturer's instructions.
2.9. Confocal Microscopy
Tumor cells were labeled with CFSE and incubated with tumor-activated γδ T cells stained with mouse antihuman HLA-DR-PE-Cy5 before analysis. Images were captured using confocal microscopy (Leica TCS SP5; Leica Microsystems, Bannockburn, IL, USA) using the LAS AF confocal software. Protein antigen capture between cell-cell interactions was visualized using time-lapse confocal microscopy as described previously [19].
2.10. Statistical Analysis
Unless indicated otherwise, data are expressed as mean ± SD. Comparison between two groups was performed by Student's t-tests. Data from more than two groups were compared using one-way ANOVA with the Tukey-Kramer multiple comparison test. P < 0.05 was considered statistically significant.
3. Results
3.1. Tumor-Activated γδ T Cells Display Characteristics of APCs
γδ T cells were directly isolated from the peripheral blood of gastric cancer patients (defined as peripheral-derived γδ T cells) and cultured with irradiated autologous PBMCs in the presence of IL-2 and IL-15. The results showed no obvious proliferation of the peripheral-derived γδ T cells. In contrast, when the peripheral-derived γδ T cells were cultured with irradiated autologous tumor cells in the presence of IL-2 and IL-15, they showed markedly increased proliferation (defined as tumor-activated γδ T cells) (Figure 1(a); n = 9, P < 0.01).
Figure 1.
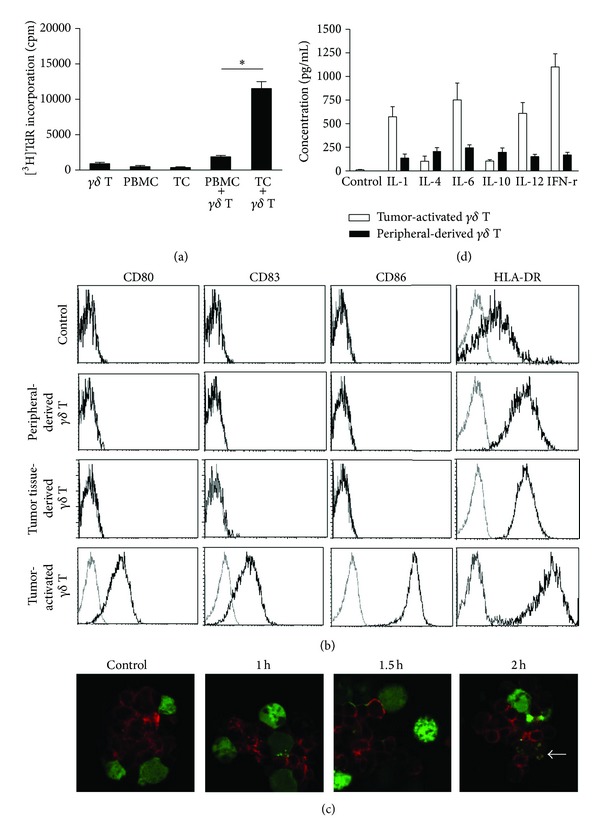
APC-like characteristics of tumor-activated γδ T cells. (a) The proliferation of γδ T cells was analyzed from [3H] thymine deoxyriboside (TdR) incorporation. Irradiated (30 Gy) PBMCs or tumor tissue cells (2 × 104 cells/well) were cocultured with peripheral-derived γδ T cells (6 × 104 cells/well) from patients with gastric cancer in 96-well plates for 3 days in the presence of IL-2 (200 U/mL) and IL-15 (20 ng/mL). [3H]TdR incorporation was measured during the last 12 h of the incubation. Results are expressed as means ± SD (cpm) of three wells. The data are representative of at least nine independent experiments. (b) FCM was used to analyze gating for the CD3+TCRγδ + cell population; phenotypes including CD80, CD83, CD86, and HLA-DR on the γδ T cells from PBMCs of healthy donors (control), peripheral-derived, tumor tissue-derived, and tumor-activated γδ T cells. Results are representative of nine independent experiments. (c) Uptake and sorting of soluble proteins in tumor-activated γδ T cells. γδ T cells were cocultured with CFSE-prelabeled tumor cells (green fluorescence) for the indicated time. The cells were then collected, cytocentrifuged, and stained with TCRγδ-PECY5 mAb (red fluorescence). The interaction between γδ T cells and tumor cells was observed using confocal microscopy. The arrow shows the colocalization of the TCR of γδ T cells with some components of tumor cells. Results are representative of three independent experiments. (d) Tumor-activated or peripheral-derived γδ T cells (1 × 106 cells/mL) were cultured in RPMI 1640 containing IL-2 (200 U/mL) and IL-15 (20 ng/mL) for 12 h, and the culture supernatants were collected and assayed for IFN-γ, IL-1, IL-4, IL-6, IL-10, and IL-12 using ELISA. Data are representative of five independent experiments. *P < 0.01.
To evaluate the potential functions of tumor-activated γδ T cells, we assessed the pattern of surface expression of immune-costimulatory molecules and the profile of cytokines secreted. The results showed no detectable expression of CD80, CD83, or CD86 on the surface of γδ T cells from PBMCs of normal donors (control), peripheral-derived γδ T cells, and γδ T cells directly isolated from tumor tissues (defined as tumor tissue-derived γδ T cells). In addition, the antigen-presenting molecule HLA-DR was strongly expressed on γδ T cells, and the expression in cells from patients was higher than that in cells from normal donors. Interestingly, we found that the T cell costimulatory molecules CD80 and CD86 and the mature molecule CD83 were substantially upregulated on the tumor-activated γδ T cells, along with the obvious increased expression of HLA-DR (Figure 1(b); n = 9). To explore the phenomenon that the factors from tumor tissue alone were particular to induce APC-like phenotypes in autogenetic γδ T cells, a group by using normal γδ T cells activated by tumor tissue was set up. We found that the γδ T cells proliferated but lacked changes of APC-like phenotypes (similar to control), suggesting that the γδ T cell proliferation was caused by an allogeneic response. Taking together, the factors from tumor tissue alone could contribute to induce APC-like phenotypes in autogenetic γδ T cells.
Confocal microscopy results showed that, when γδ T cells from patients were cocultured with autologous tumor cells for 2 h, the antigen component from the tumor cells was colocalized on the γδ T cells (Figure 1(c); n = 3), suggesting that γδ T cells could capture some antigens from the tumor cells. Analysis of the profiles of cytokines in the culture supernatants showed that the tumor-activated γδ T cells secreted substantially more Th1-prone cytokines such as IFN-γ, IL-1, IL-6, and IL-12, but not IL-4 and IL-10, than peripheral-derived γδ T cells did (Figure 1(d); n = 5).
To investigate the immunostimulatory properties of the tumor-activated γδ T cells, the ability to induce expansion of effector CD4+ and CD8+ αβ T cells was examined using in vitro functional assay. The results showed that, in contrast to peripheral-derived γδ T cells, tumor-activated γδ T cells induced significantly higher proliferation of CD4+ T cells (Figures 2(a) and 2(b); n = 3, P < 0.01) and CD8+ T cells (Figures 2(c) and 2(d); n = 3, P < 0.01) in a number-dependent pattern, as assessed by the reduction in CFSE signals. Further, the proliferation of CD4+ or CD8+ T cells induced by the tumor-activated γδ T cells was blocked in a transwell experiment and anti-CD80/CD86 antibody blocking experiment (Figure 2(e); n = 3, all P < 0.01), suggesting that the effect of tumor-activated γδ T cells on CD4+ or CD8+ T cells is dependent on cell-cell contact and costimulatory molecule CD80/CD86.
Figure 2.
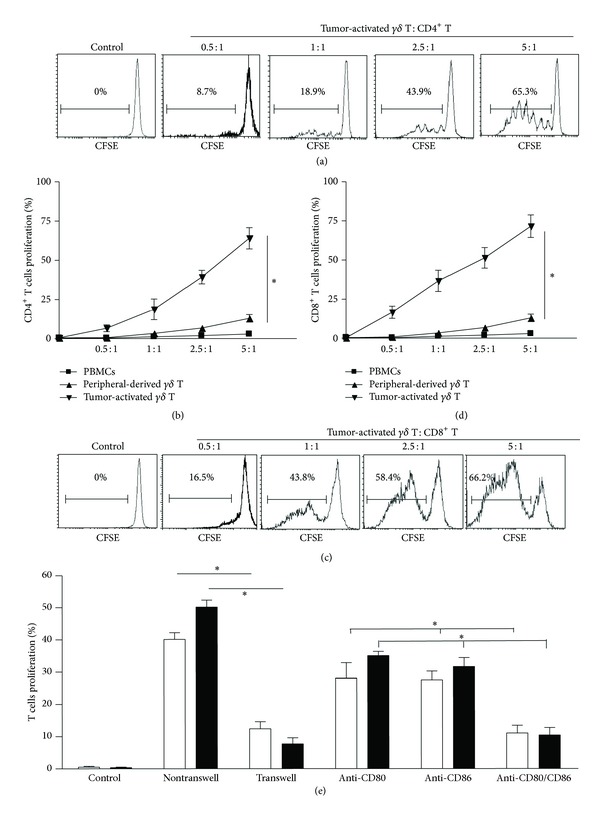
Stimulatory effect of tumor-activated γδ T cells on primary CD4+ and CD8+ T cells. For this test, 2 × 105 cells/mL CFSE-prelabeled primary CD4+ T cells ((a) and (b)) or CD8+ T cells ((c) and (d)) as responders were incubated with tumor-activated or peripheral-derived γδ T cells at the indicated ratios in 24-well plates with or without blocking antibodies (2 μg/mL) and in transwell plates (e). On day 3, the proliferation of CD4+ or CD8+ T cells was determined by FCM, wherein CFSE dilution was assessed. Results are representative of 3 independent experiments. *P < 0.01.
3.2. Tumor-Activated γδ T Cells Abrogate Immunosuppression Induced by CD4+CD25+ Treg Cells
Our previous study demonstrated that CD4+CD25+ Treg cells isolated from tumor patients inhibited the proliferation of autologous effector CD4+ T cells [20]. To determine the effect of tumor-activated γδ T cells on CD4+CD25+ Treg cells, the peripheral-derived γδ T cells or tumor-activated γδ T cells were cocultured with CD4+CD25− T cells and CD4+CD25+ Treg cells, respectively. As shown in Figures 3(a) and 3(b), the proliferation of CD4+CD25− T cells was inhibited in the presence of CD4+CD25+ Treg cells isolated from gastric cancer patients, and it did not change when peripheral-derived γδ T cells were added to the culture system. However, when tumor-activated γδ T cells were added, the proliferation of CD4+CD25− T cells was significantly increased (n = 3, P < 0.01), suggesting that tumor-activated γδ T cells, but not peripheral-derived γδ T cells, abrogate the immunosuppressive effect induced by CD4+CD25+ Treg cells. Further, the above-mentioned effects were blocked in a transwell experiment (Figure 3(c); n = 3, P < 0.01) but not by specific antibodies against cytokines such as IFN-γ, IL-1, IL-6, and IL-12. Further experimental results showed that tumor-activated γδ T cells neither induced the proliferation of CD4+CD25+ Treg cells (Figure 3(d); n = 3) nor enhanced the apoptosis of CD4+CD25+ Treg cells (Figure 3(e); n = 3), when cocultured with CD4+CD25+ Treg cells.
Figure 3.
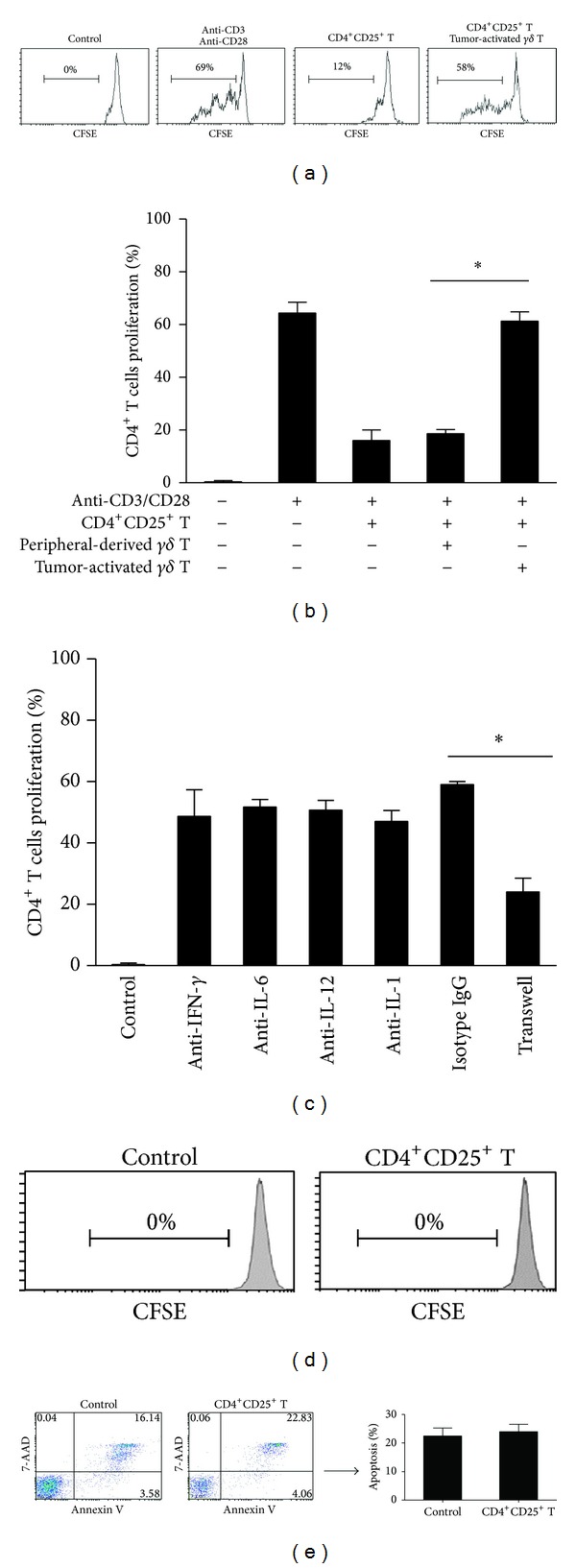
Abrogation of immunosuppressive effect of CD4+CD25+ Treg cells by tumor-activated γδ T cells. ((a) and (b)) Primary CD4+ T cells were prelabeled with CFSE as responders, and 2 × 105 cells/mL CFSE-prelabeled primary CD4+ T cells were incubated alone or coincubated with autologous CD4+CD25+ Treg cells at a ratio of 1 : 1 and/or tumor-activated γδ T cells at a ratio of 1 : 1 : 3 in the presence of anti-CD3 mAb (1 μg/mL) and anti-CD28 mAb (1 μg/mL) with or without blocking antibodies. The same combinations were also incubated in transwell plates (c) as indicated. The proliferation of CD4+ T cells was checked on day 3 by FCM used to assess CFSE dilution. (d) 2 × 105 cells/mL CFSE-prelabeled CD4+CD25+ Treg cells were incubated alone (control) or coincubated with tumor-activated γδ T cells at a ratio of 1 : 1. The proliferation of CD4+CD25+ Treg cells was checked on day 3 by FCM used to assess CFSE dilution. (e) 2 × 105 cells/mL tumor-activated γδ T cells were incubated alone (control) or coincubated with CD4+CD25+ Treg cells at a ratio of 1 : 1. The apoptosis of CD4+CD25+ Treg cells was checked on day 2 by FCM. Results are representative of three independent experiments. *P < 0.01.
3.3. Tumor-Activated γδ T Cells Not Only Directly Kill Tumor Cells but Also Activate the Cytotoxic Effects of Autologous CD8+ T Cells
To determine the ability of tumor-activated γδ T cells to kill tumor cells and its role in the cytotoxicity of effector CD8+ T cells, we examined the cytolysis of tumor-activated γδ T cells and primary autologous CD8+ T cells incubated with tumor-activated γδ T cells. The results showed that tumor cells were not lysed by peripheral-derived γδ T cells, CD8+ T cells alone, or CD8+ T cells cocultured with peripheral-derived γδ T cells. Conversely, tumor-activated γδ T cells could lyse tumor cells (Figure 4; n = 3, P < 0.01), and cytotoxicity for the tumor cells was increased in the presence of CD8+ T cells (P < 0.05), suggesting that tumor-activated γδ T cells not only directly kill tumor cells but also activate the cytotoxicity of CD8+ T cells.
Figure 4.
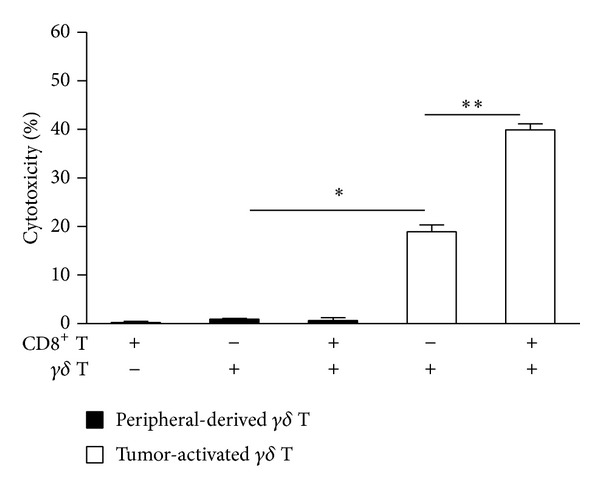
Tumor-activated γδ T cells trigger the cytotoxic effect of autologous CD8+ T cells. Autologous CD8+ T cells (2 × 105 cells/mL) were cocultured with tumor cells in a 24-well plate for 6 h in the absence or presence of peripheral-derived γδ T cells or tumor-activated γδ T cells at a ratio of 1 : 1 : 1. The culture supernatants were collected and the cytotoxic activity of effector cells was measured using an LDH assay. Results are expressed as means ± SD and are representative of three independent experiments. *P < 0.01, **P < 0.05.
4. Discussion
In general, APCs such as dendritic cells (DCs) sample Ags from target cells or pathogens by phagocytosis and then present or cross-present processed Ags to MHC class II I molecules, to trigger adaptive immune responses by the release of cytokines, expression of costimulatory molecules, and Ag stimulation [12]. On the one hand, γδ T cells display characteristics of adaptive immunity, wherein they express a rearranged TCR receptor, generate immunologic memory, and transform into cytotoxic T lymphocytes. On the other, they may also be considered part of the innate immune system, since they respond rapidly to antigenic stimuli, have limited TCR gene usage, and express pattern-recognition receptors [13]. To our knowledge, the present study is the first to identify an APC-like function for γδ T cells in tumor patients.
Previous studies demonstrated that human γδ T cells from tonsillar tissue have APC functions, efficiently cross-presenting soluble proteins to effector CD8+ αβ T cells and inducing effector cells differentiation and activation [1, 11, 21]. However, these results were acquired using γδ T cells stimulated by the isoprenoid metabolite isopentenyl pyrophosphate (IPP) in vitro, and whether γδ T cells function like APCs under different disease circumstances, particularly tumors remained largely unknown. In the present study, peripheral-derived γδ T cells from gastric cancer patients were used. Our findings showed that these peripheral-derived γδ T cells were activated and proliferated when stimulated by autologous tumor cells in vitro, suggesting that tumor cells were equipped with the signals to activate peripheral-derived γδ T cells. The underlying mechanism is not clear at present. We previously found that that autologous tumor cells selectively expanded γδ T cells among CD4−CD8− PBMCs from cancer patients and this phenomenon was related to TCR and NKG2D signals.
Accumulating experimental and clinical data indicate that γδ T cells can recognize aminobisphosphonates and phosphorylated intermediates of the bacterial nonmevalonate isoprenoid pathway, known as phosphoantigens. In addition, high concentrations of IPP, possibly generated because of a dysregulated mevalonate pathway as well as ectopically expressed mitochondrial F1-ATPase/apolipoprotein I complex on malignant cells, can selectively induce γδ T cell expansion through the TCR pathway [3, 8, 22]. NKG2D recognizes the stress-inducible MHC class I-related chains A and B (MIC A/B) and glycophosphatidylinositol-linked proteins UL16-binding proteins (ULBPs), which are expressed by many tumor cells [23–26]. The engagement of NKG2D provides a costimulatory signal for γδ T cell activation, allowing for the amplification of TCR-mediated priming upon recognition of ligand(s) on tumor cells.
The tumor-activated γδ T cells showed APC-like characteristics in terms of phenotype, cytokine profile, and functions. (i) They strongly expressed HLA-DR and the costimulator molecular CD80/CD86 and secreted the proinflammatory cytokines IL-1, IL-6, IL-12, and IFN-γ. (ii) They triggered the proliferation and differentiation of primary CD4+ or CD8+ αβ T cells and inhibited the function of CD4+CD25+ Treg cells. (iii) Most importantly, in addition to their direct cytotoxicity, they activated CD8+ αβ T-mediated cytotoxicity to tumor cells. These phenotypic and functional features are consistent with those found previously [11, 19, 27]. However, our understanding of the APC effects of γδ T cells on αβ T cell differentiation is currently rudimentary. Our results showed that peripheral-derived γδ T cells have no APC-like functions, which exclude their involvement in the control of αβ T cell responses in patients with gastric cancer. In contrast, tumor-activated γδ T cells behave like APCs by the rapid acquisition of APC characteristics in the activation of peripheral-derived γδ T cells, in a manner reminiscent of mature dendritic cells (DCs). The responses induced by the γδ T cells were potent, and they induced robust proliferation responses among primary autologous CD4+ and CD8+ αβ T cells, possibly through an unknown antigen-presenting pathway, proinflammatory cytokines, and costimulator molecules. These functions are highly beneficial, and γδ T cells are a unique and conserved population of lymphocytes that have been the subject of a recent explosion of interest owing to their essential contributions to many types of immune responses and immunopathology [28]. The functional difference between peripheral-derived and tumor-activated γδ T cells is ascribed to the suppressive state of the immune system in cancer patients.
Considerable attention in immunotherapy research is currently focused on human γδ T cells because of their functional uniformity [29]. The role of γδ T cells in oncology is concentrated around their potential applications in cancer treatment, for direct cytolysis. In terms of cellular immunotherapy, it may be important to emphasize that αβ T cell differentiation induced by γδ T cells leads to CD4+ T helper cell and effector CD8+ T cell responses. Further, experimental and clinical data indicate that γδ T cells exhibit a potent HLA-unrestricted lytic activity against various tumor cell lines and display antitumor effects [17, 30, 31]. In addition to TCR-dependent recognition, activation of the killer receptor NKG2D is involved in the cytotoxic activity of γδ T cells. In general, the cytotoxicity of γδ T cells to tumor cells involves the TCR and NKG2D receptors and depends on the perforin/granzyme pathway.
Our results also suggested that tumor-activated γδ T cells directly lyse the target tumor cells and trigger the activation and functioning of CD4+ and CD8+ T cells. Of note, the molecular mechanisms underlying γδ T cell-mediated activation of conventional CD4+ or CD8+ αβ T cells are similar to those employed by professional APCs, which involve TCR signals and the costimulatory molecules CD80/CD86. Therefore, a possible mechanism for the APC-like behavior of tumor-activated γδ T cells is that these cells are loaded with tumor components for a brief period, during which they process and express costimulatory molecules CD80/CD86. The recognition of tumor cells by γδ T cells does not depend on MHC-mediated Ag presentation, which may represent a key advantage in immunotherapy during advanced stages of cancer [32].
It is well established that the aggregation of CD4+CD25+ Treg cells in the tumor milieu is involved in the immune escape of tumors and is detrimental for immunotherapy among tumor patients [20, 33, 34]. Further, an interaction between CD4+CD25+ Treg cells and γδ T cells has been reported [35]. Recent data have further shown that γδ T cells from tumor tissues are positively correlated with Foxp3+ suppressive T cells in advanced breast tumors and inversely correlated with relapse-free and overall survival of breast cancer patients [36]. These findings raise interesting questions on whether tumor-activated γδ T cells are capable of weakening CD4+CD25+ Treg cell-mediated immunosuppression. Our results demonstrated an additional value to the proposed immunotherapy with γδ T cells, wherein the immune suppression by CD4+CD25+ Treg cells can be overcome since it requires cell-cell contact. The underlying mechanism could be that tumor-activated γδ T cells show enhanced stimulation of CD4+ T cell proliferation, rather than direct suppression by CD4+CD25+ Treg cells, since our results indicated that tumor-activated γδ T cells failed to induce proliferation or apoptosis of CD4+CD25+ Treg cells.
From the results, we speculated that tumor-activated γδ T cells kill tumor cells and simultaneously present certain Ag(s) from tumor cells. Tumor-antigen signals, together with costimulatory molecules and cytokines, could effectively trigger the activation and functioning of CD4+ and CD8+ T cells. Further investigation of this hypothesis may show that γδ T cells were compared favorably with professional APCs such as DCs with respect to their advantage of expansion in vitro and direct activation by signals preferentially expressed on tumor cells, such as NKG2D ligands MIC A/B and ULBPs [22, 37, 38], and, thus, these cells may become a continual and renewable source of functional APCs. They may be used to produce tumor vaccines to induce an adaptive immune response against tumors. We believe that γδ T cells will play a key role in developing cancer immunotherapy strategies.
Acknowledgments
The authors thank Dr. Yichuan Xiao for critical reading. This work was supported by grants from the National Natural Science Foundation of China (81370889, 81370965), the Natural Science Foundation of Jiangsu Province (BK20131248), and Jiangsu University Science Funding for Outstanding Professional (11JDG127).
Conflict of Interests
The authors declare that they have no conflict of interests.
Authors' Contribution
Chaoming Mao, Xiao Mou, and Yuepeng Zhou contributed equally to this work.
References
- 1.Ribot JC, DeBarros A, Silva-Santos B. Searching for “signal 2”: costimulation requirements of γδ T cells. Cellular and Molecular Life Sciences. 2011;68(14):2345–2355. doi: 10.1007/s00018-011-0698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes AQ, Martins DS, Silva-Santos B. Targeting γδ T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Research. 2010;70(24):10024–10027. doi: 10.1158/0008-5472.CAN-10-3236. [DOI] [PubMed] [Google Scholar]
- 3.Kabelitz D, Wesch D, He W. Perspectives of γδ T cells in tumor immunology. Cancer Research. 2007;67(1):5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Eltoum IE, Guo B, Beck BH, Cloud GA, Lopez RD. Protective immunosurveillance and therapeutic antitumor activity of γδ T cells demonstrated in a mouse model of prostate cancer. Journal of Immunology. 2008;180(9):6044–6053. doi: 10.4049/jimmunol.180.9.6044. [DOI] [PubMed] [Google Scholar]
- 5.Street SE, Hayakawa Y, Zhan Y, et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and γδ T cells. Journal of Experimental Medicine. 2004;199(6):879–884. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girardi M, Oppenheim DE, Steele CR, et al. Regulation of cutaneous malignancy by γδ T cells. Science. 2001;294(5542):605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 7.Hannani D, Ma Y, Yamazaki T, Déchanet-Merville J, Kroemer G, Zitvogel L. Harnessing γδ T cells in anticancer immunotherapy. Trends in Immunology. 2012;33(5):199–206. doi: 10.1016/j.it.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Gober HJ, Kistowska M, Angman L, Jenö P, Mori L, de Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. Journal of Experimental Medicine. 2003;197(2):163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunological Reviews. 2007;215(1):59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 10.Anderson J, Gustafsson K, Himoudi N. Licensing of killer dendritic cells in mouse and humans: functional similarities between IKDC and human blood gammadelta T-lymphocytes. Journal of Immunotoxicology. 2012;9(3):259–266. doi: 10.3109/1547691X.2012.685528. [DOI] [PubMed] [Google Scholar]
- 11.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human γδ cells. Science. 2005;309(5732):264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 12.Himoudi N, Morgenstern DA, Yan M, et al. Human γδ T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. Journal of Immunology. 2012;188(4):1708–1716. doi: 10.4049/jimmunol.1102654. [DOI] [PubMed] [Google Scholar]
- 13.Modlin RL, Sieling PA. Now presenting: γδ T cells. Science. 2005;309(5732):252–253. doi: 10.1126/science.1115264. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima J, Murakawa T, Fukami T, et al. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous γδ T cells. European Journal of Cardio-Thoracic Surgery. 2010;37(5):1191–1197. doi: 10.1016/j.ejcts.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 15.Dokouhaki P, Han M, Joe B, et al. Adoptive immunotherapy of cancer using ex vivo expanded human gammadelta T cells: a new approach. Cancer Letters. 2010;297(1):126–136. doi: 10.1016/j.canlet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Corvaisier M, Moreau-Aubry A, Diez E, et al. V γ 9V δ 2 T cell response to colon carcinoma cells. Journal of Immunology. 2005;175(8):5481–5488. doi: 10.4049/jimmunol.175.8.5481. [DOI] [PubMed] [Google Scholar]
- 17.Todaro M, D’Asaro M, Caccamo N, et al. Efficient killing of human colon cancer stem cells by γδ T lymphocytes. Journal of Immunology. 2009;182(11):7287–7296. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 18.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang R. Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27(2):334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Gertner J, Wiedemann A, Poupot M, Fournié JJ. Human γδ T lymphocytes strip and kill tumor cells simultaneously. Immunology Letters. 2007;110(1):42–53. doi: 10.1016/j.imlet.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Mao C, Wang S, Jiang Q, et al. Increased CD4+CD25+FOXP3+ regulatory T cells in cancer patients from conversion of CD4+CD25- T cells through tumor-derived factors. Onkologie. 2008;31(5):243–248. doi: 10.1159/000121360. [DOI] [PubMed] [Google Scholar]
- 21.Brandes M, Willimann K, Bioley G, et al. Cross-presenting human γδ T cells induce robust CD8+ αβ T cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(7):2307–2312. doi: 10.1073/pnas.0810059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shojaei H, Oberg HH, Juricke M, et al. Toll-like receptors 3 and 7 agonists enhance tumor cell lysis by human γσ T cells. Cancer Research. 2009;69(22):8710–8717. doi: 10.1158/0008-5472.CAN-09-1602. [DOI] [PubMed] [Google Scholar]
- 23.Li K, Mandai M, Hamanishi J, et al. Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: high expression of ULBP2 is an indicator of poor prognosis. Cancer Immunology, Immunotherapy. 2009;58(5):641–652. doi: 10.1007/s00262-008-0585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen XM, Xu XQ, Sun K, Hallett WHD, Zhao JD, Zhang DL. NKG2D ligands expression and NKG2D-mediated cytotoxicity in human laryngeal squamous carcinoma cells. Scandinavian Journal of Immunology. 2008;67(5):441–447. doi: 10.1111/j.1365-3083.2008.02086.x. [DOI] [PubMed] [Google Scholar]
- 25.Cosman D, Müllberg J, Sutherland CL, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14(2):123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 26.Eleme K, Taner SB, Önfelt B, et al. Cell surface organization of stress-inducible proteins ULBP and MICA that stimulate human NK cells and T cells via NKG2D. Journal of Experimental Medicine. 2004;199(7):1005–1010. doi: 10.1084/jem.20032194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meuter S, Eberl M, Moser B. Prolonged antigen survival and cytosolic export in cross-presenting human γδ T cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(19):8730–8735. doi: 10.1073/pnas.1002769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nature Reviews Immunology. 2013;13(2):88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nature Reviews Immunology. 2009;9(10):704–716. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrarini M, Ferrero E, Dagna L, Poggi A, Zocchi MR. Human γδ T cells: a nonredundant system in the immune-surveillance against cancer. Trends in Immunology. 2002;23(1):14–18. doi: 10.1016/s1471-4906(01)02110-x. [DOI] [PubMed] [Google Scholar]
- 31.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Research. 2007;67(15):7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanca T, Costa MF, Goncalves-Sousa N, et al. Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic γδ T lymphocytes to tumor beds. Journal of Immunology. 2013;190(12):6673–6680. doi: 10.4049/jimmunol.1300434. [DOI] [PubMed] [Google Scholar]
- 33.Green VL, Michno A, Stafford ND, Greenman J. Increased prevalence of tumour infiltrating immune cells in oropharyngeal tumours in comparison to other subsites: relationship to peripheral immunity. Cancer Immunology, Immunotherapy. 2013;62(5):863–873. doi: 10.1007/s00262-013-1395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi Y, He HW, Wang JX, et al. The functional impairment of HCC-infiltrating γδ T cells, partially mediated by regulatory T cells in a TGFβ- and IL-10-dependent manner. Journal of Hepatology. 2013;58:977–983. doi: 10.1016/j.jhep.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Wu C. CD4+CD25+ Treg cells inhibit human memory γδ T cells to produce IFN-γ in response to M tuberculosis antigen ES AT-6. Blood. 2008;111(12):5629–5636. doi: 10.1182/blood-2008-02-139899. [DOI] [PubMed] [Google Scholar]
- 36.Ma C, Zhang Q, Ye J, et al. Tumor-infiltrating gammadelta T lymphocytes predict clinical outcome in human breast cancer. Journal of Immunology. 2012;189(10):5029–5036. doi: 10.4049/jimmunol.1201892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pende D, Rivera P, Marcenaro S, et al. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Research. 2002;62(21):6178–6186. [PubMed] [Google Scholar]
- 38.Qi J, Zhang J, Zhang S, Cui L, He W. Immobilized MICA could expand human Vδ1 γδ T cells in vitro that displayed major histocompatibility complex class I chain-related A-dependent cytotoxicity to human epithelial carcinomas. Scandinavian Journal of Immunology. 2003;58(2):211–220. doi: 10.1046/j.1365-3083.2003.01288.x. [DOI] [PubMed] [Google Scholar]


