SUMMARY
MicroRNAs of miR-34 family have been originally identified as direct transactivation target of p53 and are putative tumor suppressors. Surprisingly, mice lacking all mir-34 genes show no increase in cancer formation by 18 months of age, hence placing in doubt physiological relevance of previous studies. Here we report that mice with prostate epithelium-specific inactivation of mir-34 and p53 show expansion of prostate stem cell compartment, and develop early invasive adenocarcinomas and high-grade prostatic intraepithelial neoplasia, whereas no such lesions are observed after inactivation of mir-34 or p53 genes alone by 15 months of age. Consistently, combined deficiency for p53 and miR-34 leads to acceleration of MET-dependent growth, self-renewal, and motility of prostate stem/progenitor cells. Our study provides direct genetic evidence that mir-34 genes are bona fide tumor suppressors, and identifies p53/miR-34 joint control of MET expression as a key component of prostate stem cell compartment regulation, aberrations of which may lead to cancer.
Keywords: cancer, MET, miR-34, p53, prostate, stem cells
INTRODUCTION
The microRNA-34 (miR-34) is highly evolutionarily conserved (He et al., 2007). In mammals, the miR-34 family is composed of three processed miRNAs that are encoded by two different genes: miR-34a is encoded by its own transcript, whereas miR-34b and miR-34c share a common primary transcript as a cluster. Due to the high homology among these three members, they have many similar targets and may be functionally redundant (He et al., 2007). miR-34 was the first miRNA reported to be directly transactivated by tumor suppressor p53 (a.k.a Trp53/TP53), and is considered to be an important component of the p53 network (Hermeking, 2012).
In addition to transactivation-dependent decrease in expression levels in p53 deficient tumors, mir-34 is also deleted or epigenetically down-regulated in multiple cancer cell lines and human malignancies (Bader, 2012; Hermeking, 2012). Ectopic expression of miR-34 has been shown to counteract various oncogenic processes by regulating target genes that function in cell cycle, apoptosis, senescence, cell migration, and invasion (Hermeking, 2012). Furthermore, introduction of miR-34 mimics inhibits cancer formation in transplantation experiments (Bader, 2012; Liu et al., 2011).
Contrary to the expectations raised from experiments based on non-physiological approaches, such as exogenous miR-34 introduction and miR-34 knockdown, only minor defects have been reported in studies of mice with targeted inactivating mutations of mir-34 (Concepcion et al., 2012; Wei et al., 2012). Moreover, complete genetic inactivation of miR-34 did not impair the p53 response in a variety of ex vivo and in vivo assays (Concepcion et al., 2012). Most surprisingly, no increase in spontaneous or irradiation-induced carcinogenesis has been observed in mice lacking all mir-34 genes by 18 month of age (Concepcion et al., 2012). Absence of all mir-34 genes also did not accelerate B-cell lymphomagenesis in mice overexpressing c-Myc under the control of Eμ-promoter (Concepcion et al., 2012). These data question the native tumor suppressive function of miR-34. Clarification of miR-34 role as a tumor suppressor is of particular importance because re-introduction of this microRNA into cancer cells has already reached phase 1 clinical trials (Bouchie, 2013).
A number of recent studies have provided evidence of p53-independent expression of miR-34. For example, miR-34a can be up-regulated to repress MYC during oncogene-induced senescence in human TIG3 fibroblasts (Christoffersen et al., 2010), and contributes to megakaryocytic differentiation of K562 cells (Navarro et al., 2009) in a p53-independent fashion. Consistent with these observations, levels of all miR-34 family members remain high in the brains, testes and lungs of mice lacking p53 (Concepcion et al., 2012).
Methylation of miR-34a and miR-34b/c has been found in prostate cancers carrying mutant p53 (Fujita et al., 2008; Kojima et al., 2010; Liu et al., 2011; Lodygin et al., 2008). Furthermore, frequent hypermethylation of miR-34 in cancers with high occurrence of p53 mutations, such as ovarian and mammary carcinomas and soft tissue sarcomas (Corney et al., 2010; Lodygin et al., 2008; Vogt et al., 2011) suggest coexistence of both alterations in the same neoplasms. These findings, together with reports of p53-independent regulation of miR-34, suggest that p53 and miR-34 may cooperate in cancer suppression. This possibility is also supported by our recent observation that p53 and miR-34 may jointly regulate MET receptor tyrosine kinase as a part of coherent feedforward loop in primary ovarian surface epithelium cells (Hwang et al., 2011). However, there is no direct experimental evidence for p53 and miR-34 cooperation in animal models, or if such cooperation regulates MET.
By using newly generated mice carrying conditional alleles of mir-34a and mir-34b/c we show that miR-34 cooperates with p53 in suppression of prostate carcinogenesis by joint MET-mediated control of stem cell compartment.
RESULTS
miR-34 and p53 Deficiency Cooperate in Prostate Carcinogenesis
By using gene targeting of mir-34a and miR-34b/c loci and subsequent crosses of mice we prepared mice with conventional triple knockout (mir-34a−/−mir-34b/c−/−) and conditional (floxed, mir-34aloxP/loxPmir-34b/cloxP/loxP) triple alleles (Figure S1), and designated them as mir-34−/− and mir-34L/L, respectively. Consistent with a previous report (Concepcion et al., 2012), our findings indicate that germ line genetic inactivation of mir-34 has only a minor effect on normal development (Supplemental Results and Discussion, Figure S2). We also have not observed any significant pathological phenotypes, including cancers, in mir-34−/− mice (n=19) between 15 and 18 months of age.
To rule out the possibility that mice somehow physiologically compensate for germ-line mir-34 deficiency, we performed prostate epithelium-specific mir-34 deletion. This was accomplished by using a PB-Cre4 transgene, in which a modified probasin promoter drives postnatal expression of Cre recombinase in the prostate epithelium (Chen et al., 2005; Zhou et al., 2006). Consistent with previous reports and our findings in mir-34−/− mice, mice lacking all mir-34 genes in the prostate epithelium cells (mir-34PE−/− mice) did not show any atypical lesions by 15 months of age (Figures 1A, 1B, and S3A, Table S1).
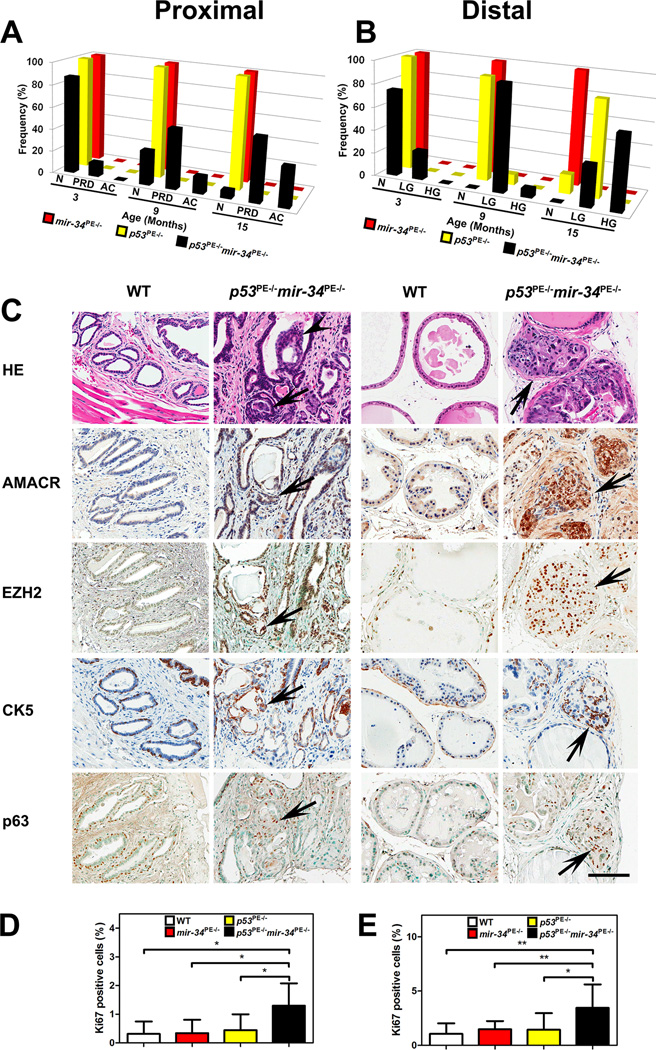
Figure 1. miR-34 and p53 Cooperate in Suppression of Prostate Carcinogenesis.
(A, B) Quantitative analysis of frequency of neoplastic lesions in proximal (A) and distal (B) regions of prostatic ducts. N: normal, PRD: proximal dysplastic lesions, AC: adenocarcinoma, LG: LG PIN, HG: HG PIN. (C) Proximal (left two columns) and distal (right two columns) regions of prostatic ducts in 15-month-old wild-type (WT) and p53PE−/−mir-34PE−/− mice. Adenocarcinomas invading surrounding stroma (arrows) and filling up the lumen (arrowheads) in the proximal regions of prostatic ducts of p53PE−/−mir-34PE−/− mice. PIN4 (arrows) in the distal regions of prostatic ducts of p53PE−/−mir-34PE−/− mice. As compared to the prostate epithelium of WT mice, both adenocarcinomas and PIN4 (arrows) show higher expression levels of AMACR and EZH2 and increased number of CK5 and p63 positive cells. Hematoxylin and eosin (HE images). ABC Elite method with hematoxylin (AMACR, CK5) or methyl green (EZH2, and p63) counterstaining. Scale bar, 100 μm for all images. (D, E) Quantitative analysis of proliferation rate in proximal (D) and distal (E) regions of prostatic ducts. *P<0.05, **P<0.01. Error bars denote SD. See also Figure S3A.
To test if miR-34 may have p53-independent function we determined the expression levels of miR-34 family after p53 deletion in FACS-purified p53L/L prostate epithelium cells exposed to Ad-Cre. Significant levels of miR-34 expression were still detected after p53 inactivation (Figures S3B and S3C).
To test if p53 and miR-34 may cooperate in suppressing prostate carcinogenesis we generated p53PE−/− and p53PE−/−mir-34PE−/− mice by crossing p53L/L mice with mir-34L/L and PB-Cre4 mice. Consistent with previous reports on lack or low frequency of neoplastic lesions in mice with prostate epithelium-specific p53 inactivation (Chen et al., 2005; Zhou et al., 2006), only 1 out of 11 p53PE−/− mice (9%) showed PIN1 by 9 months of age in the distal regions of prostatic ducts. By 15 months of age more of p53PE−/− mice developed PINs. However all of them were of low-grade (PIN1 or PIN2; Figures 1A, 1B, and S3A, Table S1). No significant changes were observed in the proximal regions of prostatic ducts, which are known to encompass a prostate epithelium stem cell compartment (Leong et al., 2008; Tsujimura et al., 2002).
In contrast, beginning at 3 months of age p53PE−/−mir-34PE−/− mice showed dysplastic lesions characterized by varying degree of nuclear atypia and loss of normal cellular arrangement in the proximal regions of prostatic ducts (Figures 1A and S3A, Table S1). From 9 months of age mice majority of mice had advanced dysplastic lesions which frequently filled up expanded ducts, and 15% and 36% of mice developed early invasive adenocarcinomas at 9 and 15 months, respectively (Figures 1A and S3A, Table S1). In the distal regions of prostatic ducts, the first PIN1 lesions were detected already by 3 months of age (Figures 1B and S3A, Table S1). High-grade PIN lesions (PIN3 and 4) have been observed by 9 months of age and 64% (9 out of 14) of mice had such lesions at 15 months of age. Consistent with these findings, adenocarcinomas and high-grade PIN lesions of the proximal and distal regions of prostatic ducts, respectively, characterized by elevated expression of markers of early prostate cancer, such as AMACR and EZH2, and increased number of K5 and p63 positive cells (Figure 1C). Similarly, higher proliferative activity has been observed in both proximal and distal regions of prostatic ducts of p53PE−/−mir-34PE−/− mice (Figures 1D and 1E). In summary, these results show that miR-34 and p53 cooperate in suppression of prostate carcinogenesis.
p53 and miR-34 Cooperate in Control of Prostate Stem Cell Activity
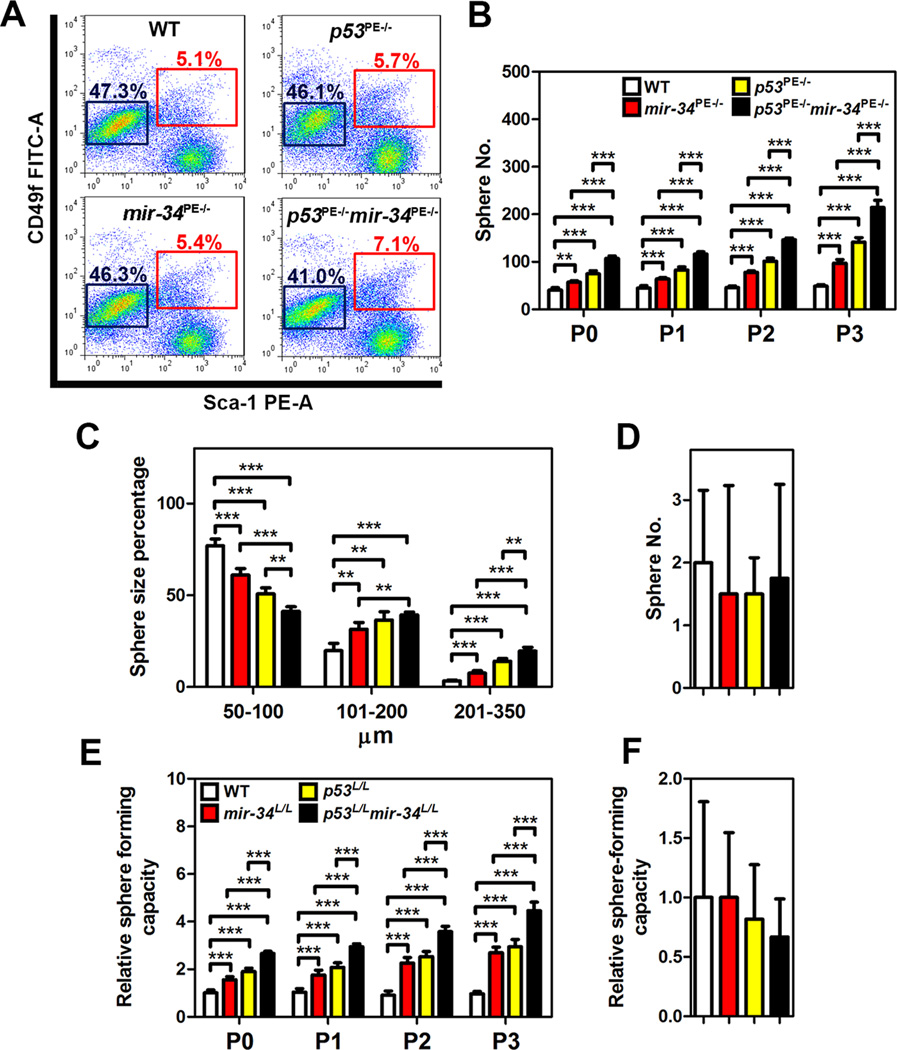
According to our pathological evaluation, the stem/progenitor cell-enriched proximal regions of prostatic ducts were specifically affected in p53PE−/−mir-34PE−/− mice. To test if combined p53 and miR-34 deficiency affects functional properties of prostate stem/progenitor cells, we isolated such cells by FACS as CD49fhi/Sca-1+ fraction. Mice with prostate-specific deletions of either mir-34 or p53 had slightly more stem cells than background matched wild-type mice (Figure 2A). However, the pool of CD49fhi/Sca-1+ cells deficient for both miR-34 and p53 increased by 39% and constituted 7.1% of the prostate epithelium, vs 5.1% in WT. Notably, the CD49fhi/Sca-1+ fraction isolated from prostates of p53PE−/−mir-34PE−/− mice formed prostaspheres far more efficiently and of larger size (Figures 2B and 2C). Both higher frequency and size of spheres formed by p53 and miR-34 deficient CD49fhi/Sca-1+ stem cells were maintained over multiple passages (dissociation and clonal formation), suggesting role of these genes in the control of self-renewal. At the same time, no difference among genotypes was observed in CD49flo/Sca-1− luminal cells (Figure 2D). These cells formed very few spheres after first plating and no spheres were observed after first passage. Thus, miR-34 and/or p53 deficiency are unlikely to reprogram differentiated cells towards stem cell state.
Figure 2. Deletions of both p53 and mir-34 Promote Prostate Stem Cell Expansion and Sphere-forming Capacity.
(A) Quantitative analysis of distribution of CD49fhi/Sca-1+ stem cells and CD49flo/Sca-1− luminal cells from 3-month-old wild-type (WT), mir-34PE−/−, p53PE−/−, and p53PE−/−mir-34PE−/− mice (n=3). Red and blue frames represent stem cell and luminal cell populations, respectively. (B-D) Frequency (B, D) and size (C) of spheres formed by CD49fhi/Sca-1+ stem cells (B, C) and CD49flo/Sca-1− (D) luminal cells from 3-month-old WT, mir-34PE−/−, p53PE−/−, and p53PE−/−mir-34PE−/− mice (n=3). (E, F) Relative frequency of sphere formation by CD49fhi/Sca-1+ stem cells (E) and CD49flo/Sca-1− luminal cells (F) isolated from WT, mir-34L/L, p53L/L, and p53L/Lmir-34L/L mice followed by Ad-Cre infection (n=3). Spheres counts were normalized to the Ad-blank-infected spheres of each passage. **P<0.01, ***P<0.001. Error bars denote SD.
To test whether observed properties represent direct effects of p53 and/or miR-34 on prostate stem cells we have isolated CD49fhi/Sca-1+ stem/progenitor cells and CD49flo/Sca-1− luminal cells from prostates of wild-type, mir-34L/L, p53L/L and p53L/Lmir-34L/L mice, and, followed by infection with Ad-Cre or Ad-blank, subjected them to the prostasphere formation experiments (Figures 2E and 2F). Consistently, lack of both p53 and miR-34 had the most pronounced effect on frequency of stem cells in consecutive passages.
p53 and miR-34 Regulation of Stem/progenitor Cells Depends on MET
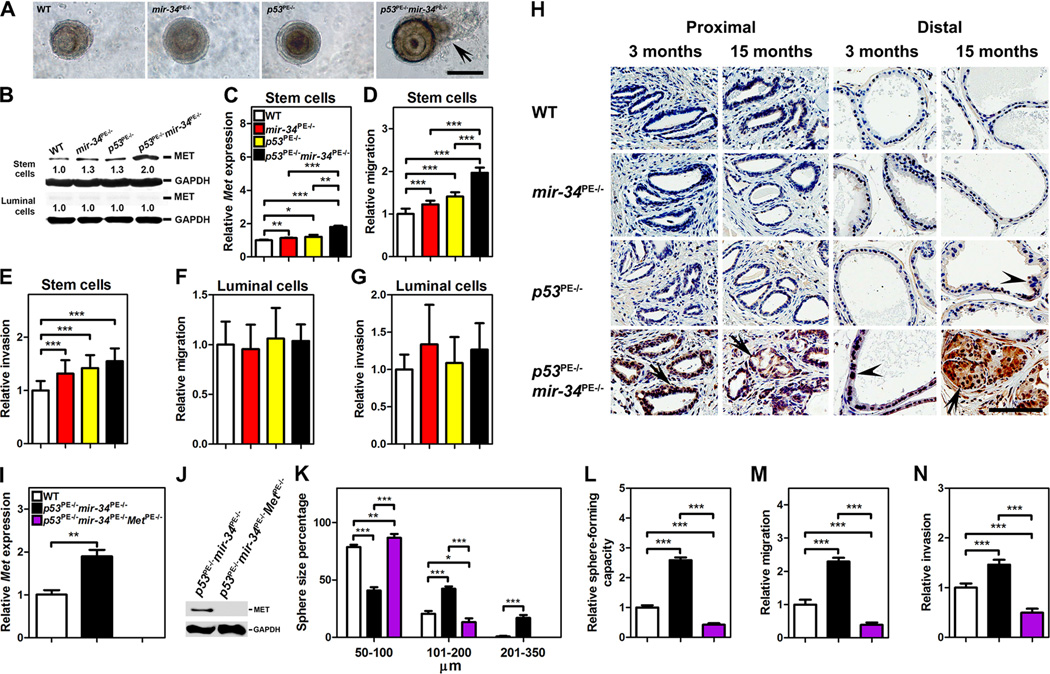
In addition to invasive growth of cells in the prostate stem cell compartment of p53PE−/− mir-34PE−/− mice, we have noted that some of the cells from the p53 and miR-34 deficient prostaspheres were spreading into surrounding matrix (Figure 3A). Since MET plays a crucial role in regulation of cell motility and invasion (Trusolino et al., 2010) and is known target of p53 (Hwang et al., 2011) and miR-34 (He et al., 2007; Hwang et al., 2011), we have tested its expression in FACS sorted populations of the prostate epithelium. CD49fhi/Sca-1+ prostate stem/progenitor cells had far higher levels of expression as compared to CD49flo/Sca-1− luminal cells (Figure 3B). Deficiency for either miR-34 or p53 slightly increased MET levels in stem/progenitor cells, while such cells from p53PE−/−mir-34PE−/− showed the highest MET expression (Figures 3B and 3C). Consistently, CD49fhi/Sca-1+ prostate stem cells deficient for both miR-34 and p53 had the highest motility in migration assay (Figure 3D) and some trend, albeit not statistically significant, towards increased invasive activity (Figure 3E). To the contrary, luminal cells deficient for p53 and/or miR-34 had no significant differences in their motility (Figure 3F) or invasion (Figure 3G).
Figure 3. MET Expression is Essential for Increased Growth, Sphere-forming Capacity, Motility, and Invasion of p53 and miR-34-Deficient Prostate Stem Cells.
(A–H) Prostasphere formation (A), Western blot (B) and qRT-PCR (C) of Met expression, migration (D, F), and invasion (E, G) by CD49fhi/Sca-1+ stem cells (A-E) and CD49flo/Sca-1− luminal cells (C, F, G) of 3-month-old wild-type (WT), mir-34PE−/−, p53PE−/−, and p53PE−/−mir-34PE−/− mice (n=3). (A) Note outgrowth of cells from prostaspheres prepared from p53PE−/−mir-34PE−/− mice (arrow). A–D, Scale bar, 100 μm. (H) MET expression in cells of proximal and distal regions of prostatic ducts of 3- and 15-month-old WT, mir-34PE−/−, p53PE−/−, and p53PE−/−mir-34PE−/− mice. MET expression (arrows) is detected in the proximal regions of prostatic ducts of 3- and 15-month-old p53PE−/−mir-34PE−/− mice and in PIN4 of distal region in 15-month-old p53PE−/−mir-34PE−/− mice. PIN1 (arrowheads) in distal regions of prostatic ducts lack MET expression in both p53PE−/− and p53PE−/−mir-34PE−/− mice. ABC Elite method with hematoxylin counterstaining, H, Scale bar, 100 μm. (I-N) qRT-PCR (I) and Western blot (J) of Met expression, prostasphere size (K), sphere-forming capacity (L), migration (M), and invasion (N) of CD49fhi/Sca-1+ stem cells isolated from 3-month-old WT, p53PE−/−mir-34PE−/− and p53PE−/− mir-34PE−/−MetPE−/− mice (n=3). *P<0.05, **P<0.01, ***P<0.001. Error bars denote SD.
Consistent with ex vivo results, we also observed elevated levels of MET expression in cells of the proximal regions of prostatic ducts of p53PE−/−mir-34PE−/− mice, as compared to wild-type mice and mice with inactivation of either mir-34 or p53 (Figure 3H). MET expression was below detectable levels in the epithelium of the distal regions of prostatic ducts in all strains. The only exception was elevated MET expression in high-grade PINs in p53PE−/−mir-34PE−/− mice, suggesting additional mechanisms of MET regulation, such as a possible increase in number of stem cell-like cells, in such lesions.
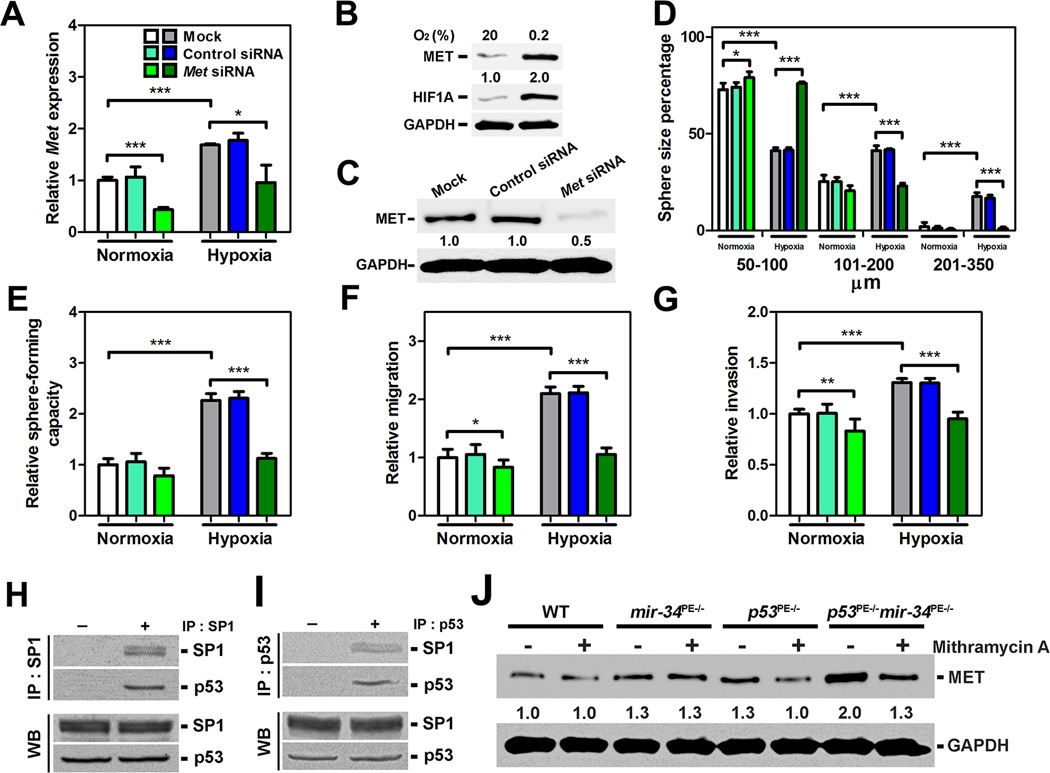
To test if MET overexpression is essential for the observed phenotypes, Met was inactivated using a conditional MetL/L allele. Met inactivation abrogated growth, sphereforming capacity, cell motility, and invasion of p53 and miR-34 deficient CD49fhi/Sca-1+ prostate stem cells (Figures 3I–3N). Effects of MET downregulation on growth, sphere-forming capacity, cell motility, and invasion of wild-type prostate stem cells were less prominent (Figures 4A–4G), consistent with lower levels of MET expression in such cells. However, two-fold induction of MET expression by hypoxia resulted in comparable increase of all the above parameters (Figures 4A–4G). Similar to p53/mir-34 inactivation experiments, this phenotype was reversed after MET knock-down, indicating a critical role of MET in prostate stem cell regulation.
Figure 4. MET is Essential for Growth, Sphere-forming Capacity, Motility, and Invasion of Wild-Type Prostate Stem Cells, and is Partially Regulated by SP1 Interacting with p53.
(A–G) qRT-PCR (A) and Western blot (B, C) of Met expression, prostasphere size (D), sphere-forming capacity (E), migration (F), and invasion (G) of CD49fhi/Sca-1+ stem cells isolated from 3-month-old wild-type (WT) mice (n=3) and cultured under normoxic (20% O2, A, B, D–G) and hypoxic (0.2% O2, A–G) conditions. *P<0.05, **P<0.01, ***P<0.001. Error bars denote SD. Very similar results were obtained in separate experiments with two different Met siRNAs. (H, I) Co-immunoprecipitation of cell lysates with SP1 (H) or p53 (I) antibodies followed by Western blot with p53 or SP1 antibodies, respectively, in CD49fhi/Sca-1+ stem cells isolated from 3-month-old WT mice (n=3; Upper panels, IP). Samples of the same lysates were used for Western blot with p53 or SP1 antibodies before precipitation (Lower panels, WB). (J) Effect of mithramycin A (100 nM) on MET expression of CD49fhi/Sca-1+ stem cells isolated from 3-month-old WT, mir-34PE−/−, p53PE−/−, and p53PE−/−mir-34PE−/− mice (n=3).
Previously it has been shown that p53 may negatively regulate MET expression by miR-34-mediated targeting of MET (Corney et al., 2007; He et al., 2007; Hwang et al., 2011). Supporting these observations, we have observed presence of preserved 3'UTR carrying two binding sites for miR-34 in prostate stem cells (Figure S4). We have also reported that p53 also represses MET expression by miR-34-independent inhibition of SP1 binding to Met promoter in the ovarian surface epithelium cells (Hwang et al., 2011). Consistent with this mechanism, MET reciprocal co-immunoprecipitation experiments have shown that p53 physically interacts with endogenous SP1 in the prostate stem cells (Figures 4H and 4I). Furthermore, SP1 inhibition results in reduction of MET expression in prostate stem cells deficient for either p53 or both miR-34 and p53, but not for miR-34 alone (Figure 4J).
DISCUSSION
Our study provides a direct genetic proof that microRNAs of miR-34 family may act as tumor suppressors in concert with other genes, such as p53. These findings offer a solid physiological basis for rational design of diagnostic and therapeutic approaches. Since lack of mir-34 genes alone is insufficient for cancer initiation, their downregulation is likely to occur at some point during tumor progression. However, preexistence of mir-34 methylation in some normal cells cannot be excluded. Further genomic studies in conjunction with animal modeling should be able to address this question. Although our current studies have been focused on prostate cancer, tissue-specific inactivation of mir-34 and p53 in other tissues will address likely interactions of these genes in other cell lineages.
Our observations confirm the earlier findings that p53 may negatively regulate MET expression by miR-34-mediated targeting of MET and by miR-34-independent inhibition of SP1 binding to MET promoter. Notably, according to our previous ex vivo studies inactivation of both mechanisms is required to achieve the highest MET overexpression, cell motility, and invasion (Hwang et al., 2011). Our present study supports this possibility in autochthonous model of cancer. Importantly, our findings show that convergence of p53 and miR-34 effects on MET regulation occurs both in p53-dependent and independent manner. Specific mechanisms for p53-independent miR-34 regulation remain to be determined.
Previous studies have shown that p53 and miR-34 affect iPS cell reprograming (Choi et al., 2011; Krizhanovsky and Lowe, 2009). p53 mediates the onset of senescence of endothelial progenitor cells (Rosso et al., 2006) and negatively regulates proliferation and survival of neural stem cells (Meletis et al., 2006). Constitutive p53 activation results in depletion of adult stem cells in bone marrow, brain and testes (Liu et al., 2010). It has been shown that ectopic expression of miR-34a may inhibit prostate cancer stem cells and metastasis by directly repressing CD44 (Liu et al., 2011). However, role of miR-34 in regulation of normal adult stem cells has been unclear. Our study fills this gap by showing that miR-34 regulates prostate stem/progenitor cells in cooperation with p53. It will be of interest to see if similar cooperation of miR-34 and p53 may play role in stem cell compartments of other cell lineages.
It has been previously reported that prostate cancer propagating cells (aka cancer stem cells or cancer initiation cells) express MET and depletion of MET results in decrease in prostasphere formation (Rajasekhar et al., 2011). However, direct role of MET in regulation of normal prostate stem/progenitor cells, and mechanisms controlling its expression have been uncertain. Our studies on prostate cells collected either in the early stages of carcinogenesis or immediately after mir-34 and p53 inactivation provide a missing link between normal MET-dependent biological functions of these genes and promotion of aberrant expansion of stem/progenitor cell pool, which may eventually lead to cancer. Considering that MET is particularly overexpressed in stem/progenitor cells lacking both p53 and miR-34, its therapeutic targeting may be especially effective in p53 and miR-34 deficient cancer cases.
Some cancers arise from stem/progenitor cells (Flesken-Nikitin et al., 2013; Schepers et al., 2012), while others may originate from more differentiated cells (Friedmann-Morvinski et al., 2012). In our study we have observed that neoplastic lesions in the distal regions of prostatic ducts, which are mainly populated by transit-amplifying and differentiated cells, never progress to frank invasive adenocarcinomas. These findings support observations in other models that cancers arising from stem cell compartments are more aggressive (Flesken-Nikitin et al., 2013). Our autochthonous mouse model of prostate cancer based on prostate epithelium-specific inactivation of p53 and mir-34 should provide a valuable tool for further elucidation of the role of individual cell subpopulations in prostate cancer pathogenesis.
EXPERIMENTAL PROCEDURES
Generation of Mice with mir-34 Conventional and Conditional Targeted Mutations
All animal experiments were carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Laboratory Animal Use and Care Committee at Cornell University. mir-34a and mir-34b/c gene-targeting vectors were introduced into embryonic stem cells and homologous recombinants were identified by positive/negative selection and by a quantitative approach, respectively (Figure S1A). After germ line transmission of the targeted allele, FRT-flanked Neo cassette was excised by crosses with FLPeR transgenic mice. The resulting mice carrying conditional (floxed) alleles were crossed to EIIa-Cre mice to obtain conventional null alleles (Figures S1A and S1B). Lack of individual miR-34 family members was confirmed by qRT-PCR of the brain and the prostate of mir-34−/− mice (Figure S1C).
Animal Phenotyping, Cell Culture and Molecular Biological Experiments
Pathological assessment, Immunohistochemistry and quantitative image analysis were performed as described earlier (Hwang et al., 2011; Zhou et al., 2006) and in Supplemental Information. MicroRNA in situ hybridization, cell culture, quantitative reverse transcription PCR, western blot analysis and co-immunoprecipitation were performed according to earlier established protocols (Corney et al., 2010; Hwang et al., 2011) and described in details in Supplemental Information.
Statistical Analysis
Statistical analyses were performed with InStat 3.10 and Prism 6 software. (GraphPad, Inc., San Diego, CA). Two-tailed unpaired t-test, direct Fisher's tests and log-rank Mantel-Haenszel test were used as appropriate.
Supplementary Material
Highlights.
miR-34 is a tumor suppressor in autochthonous mouse model of prostate cancer
miR-34 has p53-independent regulation and cooperates with p53 in cancer suppression
miR-34 and p53 jointly regulate prostate stem/progenitor cell activity
MET is a joint p53/miR-34 downstream target key to control of stem cell compartment
ACKNOWLEDGEMENTS
We would like to thank all members of the Nikitin Lab for their advice and support, Lavanya Sayam (NYSTEM supported FACS Core) for her help with fluorescence activated cell sorting and Dr. Thomas A. Cleland, Department of Psychology, Cornell for his advice on loco-motor tests and Drs. Anton Berns and Snorri S. Thorgeirsson for their generous gifts of p53loxP/loxP and MetloxP/loxP mice, respectively. This work has been supported by NIH (CA096823) and NYSTEM (C023050, C028125) grants to AYN, NYSTEM Cornell mammalian cell reprogramming core grant (N08S-004) to JCS, fellowship funding from the Cornell Comparative Cancer Biology Training Program to CYC and CIH, and Cornell Vertebrate Genomics Scholarship to DCC and postdoctoral fellowship (NIH NICHD T32HD052471) to AFN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet. 2012;3:120. doi: 10.3389/fgene.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchie A. First microRNA mimic enters clinic. Nat Biotechnol. 2013;31:577. doi: 10.1038/nbt0713-577. [DOI] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Lin CP, Ho JJ, He X, Okada N, Bu P, Zhong Y, Kim SY, Bennett MJ, Chen C, et al. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat Cell Biol. 2011;13:1353–1360. doi: 10.1038/ncb2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen NR, Shalgi R, Frankel LB, Leucci E, Lees M, Klausen M, Pilpel Y, Nielsen FC, Oren M, Lund AH. p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC. Cell Death Differ. 2010;17:236–245. doi: 10.1038/cdd.2009.109. [DOI] [PubMed] [Google Scholar]
- Concepcion CP, Han YC, Mu P, Bonetti C, Yao E, D'Andrea A, Vidigal JA, Maughan WP, Ogrodowski P, Ventura A. Intact p53-dependent responses in miR-34-deficient mice. PLoS Genet. 2012;8:e1002797. doi: 10.1371/journal.pgen.1002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007;67:8433–8438. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- Corney DC, Hwang CI, Matoso A, Vogt M, Flesken-Nikitin A, Godwin AK, Kamat AA, Sood AK, Ellenson LH, Hermeking H, et al. Frequent downregulation of miR-34 family in human ovarian cancers. Clin Cancer Res. 2010;16:1119–1128. doi: 10.1158/1078-0432.CCR-09-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesken-Nikitin A, Hwang CI, Cheng CY, Michurina TV, Enikolopov G, Nikitin AY. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495:241–245. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann-Morvinski D, Bushong EA, Ke E, Soda Y, Marumoto T, Singer O, Ellisman MH, Verma IM. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science. 2012;338:1080–1084. doi: 10.1126/science.1226929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Kojima K, Hamada N, Ohhashi R, Akao Y, Nozawa Y, Deguchi T, Ito M. Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochem Biophys Res Commun. 2008;377:114–119. doi: 10.1016/j.bbrc.2008.09.086. [DOI] [PubMed] [Google Scholar]
- He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermeking H. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer. 2012;12:613–626. doi: 10.1038/nrc3318. [DOI] [PubMed] [Google Scholar]
- Hwang CI, Matoso A, Corney DC, Flesken-Nikitin A, Korner S, Wang W, Boccaccio C, Thorgeirsson SS, Comoglio PM, Hermeking H, et al. Wild-type p53 controls cell motility and invasion by dual regulation of MET expression. Proc Natl Acad Sci U S A. 2011;108:14240–14245. doi: 10.1073/pnas.1017536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Fujita Y, Nozawa Y, Deguchi T, Ito M. MiR-34a attenuates paclitaxel-resistance of hormone-refractory prostate cancer PC3 cells through direct and indirect mechanisms. Prostate. 2010;70:1501–1512. doi: 10.1002/pros.21185. [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V, Lowe SW. Stem cells: The promises and perils of p53. Nature. 2009;460:1085–1086. doi: 10.1038/4601085a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–808. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Ou L, Clemenson GD, Jr, Chao C, Lutske ME, Zambetti GP, Gage FH, Xu Y. Puma is required for p53-induced depletion of adult stem cells. Nat Cell Biol. 2010;12:993–998. doi: 10.1038/ncb2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Korner H, Knyazev P, Diebold J, Hermeking H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7:2591–2600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133:363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- Navarro F, Gutman D, Meire E, Caceres M, Rigoutsos I, Bentwich Z, Lieberman J. miR-34a contributes to megakaryocytic differentiation of K562 cells independently of p53. Blood. 2009;114:2181–2192. doi: 10.1182/blood-2009-02-205062. [DOI] [PubMed] [Google Scholar]
- Rajasekhar VK, Studer L, Gerald W, Socci ND, Scher HI. Tumour-initiating stem-like cells in human prostate cancer exhibit increased NF-kappaB signalling. Nat Commun. 2011;2:162. doi: 10.1038/ncomms1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso A, Balsamo A, Gambino R, Dentelli P, Falcioni R, Cassader M, Pegoraro L, Pagano G, Brizzi MF. p53 Mediates the accelerated onset of senescence of endothelial progenitor cells in diabetes. J Biol Chem. 2006;281:4339–4347. doi: 10.1074/jbc.M509293200. [DOI] [PubMed] [Google Scholar]
- Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, Shapiro E, Lepor H, Sun TT, Wilson EL. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J Cell Biol. 2002;157:1257–1265. doi: 10.1083/jcb.200202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M, Munding J, Gruner M, Liffers ST, Verdoodt B, Hauk J, Steinstraesser L, Tannapfel A, Hermeking H. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458:313–322. doi: 10.1007/s00428-010-1030-5. [DOI] [PubMed] [Google Scholar]
- Wei J, Shi Y, Zheng L, Zhou B, Inose H, Wang J, Guo XE, Grosschedl R, Karsenty G. miR-34s inhibit osteoblast proliferation and differentiation in the mouse by targeting SATB2. J Cell Biol. 2012;197:509–521. doi: 10.1083/jcb.201201057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Flesken-Nikitin A, Corney DC, Wang W, Goodrich DW, Roy-Burman P, Nikitin AY. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 2006;66:7889–7898. doi: 10.1158/0008-5472.CAN-06-0486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.