Abstract
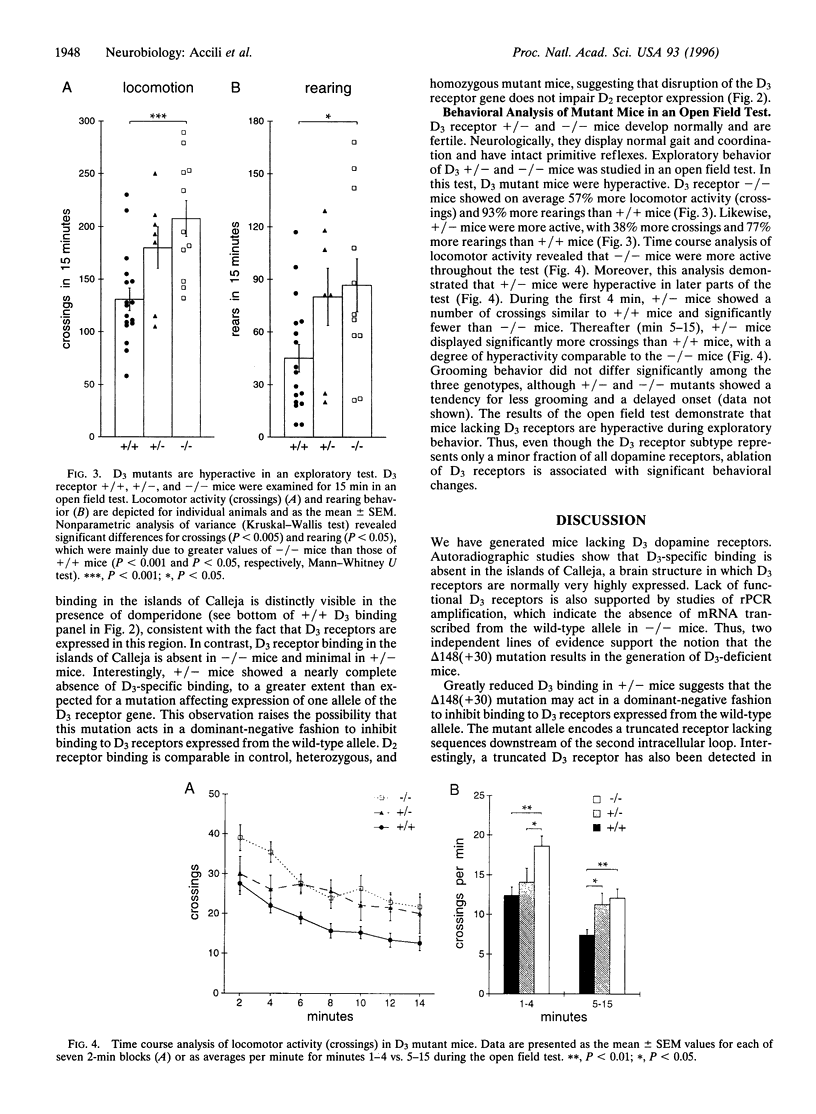
While most effects of dopamine in the brain are mediated by the D1 and D2 receptor subtypes, other members of this G protein-coupled receptor family have potentially important functions. D3 receptors belong to the D2-like subclass of dopamine receptors, activation of which inhibits adenylyl cyclase. Using targeted mutagenesis in mouse embryonic stem cells, we have generated mice lacking functional D3 receptors. A premature chain-termination mutation was introduced in the D3 receptor gene after residue Arg-148 in the second intracellular loop of the predicted protein sequence. Binding of the dopamine antagonist [125I]iodosulpride to D3 receptors was absent in mice homozygous for the mutation and greatly reduced in heterozygous mice. Behavioral analysis of mutant mice showed that this mutation is associated with hyperactivity in an exploratory test. Homozygous mice lacking D3 receptors display increased locomotor activity and rearing behavior. Mice heterozygous for the D3 receptor mutation show similar, albeit less pronounced, behavioral alterations. Our findings indicate that D3 receptors play an inhibitory role in the control of certain behaviors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accili D., Drago J., Lee E. J., Johnson M. D., Cool M. H., Salvatore P., Asico L. D., José P. A., Taylor S. I., Westphal H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996 Jan;12(1):106–109. doi: 10.1038/ng0196-106. [DOI] [PubMed] [Google Scholar]
- Baik J. H., Picetti R., Saiardi A., Thiriet G., Dierich A., Depaulis A., Le Meur M., Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995 Oct 5;377(6548):424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- Bouthenet M. L., Souil E., Martres M. P., Sokoloff P., Giros B., Schwartz J. C. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991 Nov 15;564(2):203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Caine S. B., Koob G. F. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993 Jun 18;260(5115):1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Daly S. A., Waddington J. L. Behavioural effects of the putative D-3 dopamine receptor agonist 7-OH-DPAT in relation to other "D-2-like" agonists. Neuropharmacology. 1993 May;32(5):509–510. doi: 10.1016/0028-3908(93)90177-5. [DOI] [PubMed] [Google Scholar]
- Diaz J., Lévesque D., Lammers C. H., Griffon N., Martres M. P., Schwartz J. C., Sokoloff P. Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience. 1995 Apr;65(3):731–745. doi: 10.1016/0306-4522(94)00527-c. [DOI] [PubMed] [Google Scholar]
- Drago J., Gerfen C. R., Lachowicz J. E., Steiner H., Hollon T. R., Love P. E., Ooi G. T., Grinberg A., Lee E. J., Huang S. P. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P. Doyne Lecture. Rhodopsin and autosomal dominant retinitis pigmentosa. Eye (Lond) 1992;6(Pt 1):1–10. doi: 10.1038/eye.1992.2. [DOI] [PubMed] [Google Scholar]
- Fishburn C. S., Belleli D., David C., Carmon S., Fuchs S. A novel short isoform of the D3 dopamine receptor generated by alternative splicing in the third cytoplasmic loop. J Biol Chem. 1993 Mar 15;268(8):5872–5878. [PubMed] [Google Scholar]
- Freedman J. E., Waszczak B. L., Cox R. F., Liu J. C., Greif G. J. The dopamine D3 receptor and 7-OH-DPAT. Trends Pharmacol Sci. 1994 Jun;15(6):173–174. doi: 10.1016/0165-6147(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Gingrich J. A., Caron M. G. Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci. 1993;16:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- Guo N., Klitenick M. A., Tham C. S., Fibiger H. C. Receptor mechanisms mediating clozapine-induced c-fos expression in the forebrain. Neuroscience. 1995 Apr;65(3):747–756. doi: 10.1016/0306-4522(94)00552-g. [DOI] [PubMed] [Google Scholar]
- Landwehrmeyer B., Mengod G., Palacios J. M. Differential visualization of dopamine D2 and D3 receptor sites in rat brain. A comparative study using in situ hybridization histochemistry and ligand binding autoradiography. Eur J Neurosci. 1993 Feb 1;5(2):145–153. doi: 10.1111/j.1460-9568.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Landwehrmeyer B., Mengod G., Palacios J. M. Dopamine D3 receptor mRNA and binding sites in human brain. Brain Res Mol Brain Res. 1993 Apr;18(1-2):187–192. doi: 10.1016/0169-328x(93)90188-u. [DOI] [PubMed] [Google Scholar]
- Large C. H., Stubbs C. M. The dopamine D3 receptor: Chinese hamsters or Chinese whispers? Trends Pharmacol Sci. 1994 Feb;15(2):46–47. doi: 10.1016/0165-6147(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Park B. H., Fishburn C. S., Carmon S., Accili D., Fuchs S. Structural organization of the murine D3 dopamine receptor gene. J Neurochem. 1995 Feb;64(2):482–486. doi: 10.1046/j.1471-4159.1995.64020482.x. [DOI] [PubMed] [Google Scholar]
- Pilon C., Lévesque D., Dimitriadou V., Griffon N., Martres M. P., Schwartz J. C., Sokoloff P. Functional coupling of the human dopamine D3 receptor in a transfected NG 108-15 neuroblastoma-glioma hybrid cell line. Eur J Pharmacol. 1994 Jul 15;268(2):129–139. doi: 10.1016/0922-4106(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Schmauss C., Haroutunian V., Davis K. L., Davidson M. Selective loss of dopamine D3-type receptor mRNA expression in parietal and motor cortices of patients with chronic schizophrenia. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8942–8946. doi: 10.1073/pnas.90.19.8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scibilia R. J., Lachowicz J. E., Kilts C. D. Topographic nonoverlapping distribution of D1 and D2 dopamine receptors in the amygdaloid nuclear complex of the rat brain. Synapse. 1992 Jun;11(2):146–154. doi: 10.1002/syn.890110208. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Monsma F. J., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992 Feb;13(2):61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Sokoloff P., Andrieux M., Besançon R., Pilon C., Martres M. P., Giros B., Schwartz J. C. Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: comparison with D2 receptor. Eur J Pharmacol. 1992 Apr 10;225(4):331–337. doi: 10.1016/0922-4106(92)90107-7. [DOI] [PubMed] [Google Scholar]
- Sokoloff P., Giros B., Martres M. P., Bouthenet M. L., Schwartz J. C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990 Sep 13;347(6289):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Sokoloff P., Martres M. P., Giros B., Bouthenet M. L., Schwartz J. C. The third dopamine receptor (D3) as a novel target for antipsychotics. Biochem Pharmacol. 1992 Feb 18;43(4):659–666. doi: 10.1016/0006-2952(92)90227-a. [DOI] [PubMed] [Google Scholar]
- Sokoloff P., Schwartz J. C. Novel dopamine receptors half a decade later. Trends Pharmacol Sci. 1995 Aug;16(8):270–275. doi: 10.1016/s0165-6147(00)89044-6. [DOI] [PubMed] [Google Scholar]
- Sunahara R. K., Seeman P., Van Tol H. H., Niznik H. B. Dopamine receptors and antipsychotic drug response. Br J Psychiatry Suppl. 1993 Dec;(22):31–38. [PubMed] [Google Scholar]
- Svensson K., Carlsson A., Huff R. M., Kling-Petersen T., Waters N. Behavioral and neurochemical data suggest functional differences between dopamine D2 and D3 receptors. Eur J Pharmacol. 1994 Oct 3;263(3):235–243. doi: 10.1016/0014-2999(94)90718-8. [DOI] [PubMed] [Google Scholar]
- Svensson K., Carlsson A., Waters N. Locomotor inhibition by the D3 ligand R-(+)-7-OH-DPAT is independent of changes in dopamine release. J Neural Transm Gen Sect. 1994;95(1):71–74. doi: 10.1007/BF01283032. [DOI] [PubMed] [Google Scholar]
- Waters N., Svensson K., Haadsma-Svensson S. R., Smith M. W., Carlsson A. The dopamine D3-receptor: a postsynaptic receptor inhibitory on rat locomotor activity. J Neural Transm Gen Sect. 1993;94(1):11–19. doi: 10.1007/BF01244979. [DOI] [PubMed] [Google Scholar]
- Xu M., Moratalla R., Gold L. H., Hiroi N., Koob G. F., Graybiel A. M., Tonegawa S. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994 Nov 18;79(4):729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]