Abstract
Purpose:
Our aim was to determine the effect of a surgical technique on biomaterial implant performance, specifically graft retention.
Methods:
Twelve mini pigs were implanted with cell-free, 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC)/N-hydroxysuccinimide (NHS) cross-linked recombinant human collagen type III (RHCIII) hydrogels as substitutes for donor corneal allografts using overlying sutures with or without human amniotic membrane (HAM) versus interrupted sutures with HAM. The effects of the retention method were compared as well as the effects of collagen concentration (13.7% to 15% RHCIII).
Results:
All implanted corneas showed initial haze that cleared with time, resulting in corneas with optical clarity matching those of untreated controls. Biochemical analysis showed that by 12 months post operation, the initial RHCIII implants had been completely remodeled, as type I collagen, was the major collagenous protein detected, whereas no RHCIII could be detected. Histological analysis showed all implanted corneas exhibited regeneration of epithelial and stromal layers as well as nerves, along with touch sensitivity and tear production. Most neovascularization was seen in corneas stabilized by interrupted sutures.
Conclusions:
This showed that the surgical technique used does have a significant effect on the overall performance of corneal implants, overlying sutures caused less vascularization than interrupted sutures.
Translational Relevance:
Understanding the significance of the suturing technique can aid the selection of the most appropriate procedure when implanting artificial corneal substitutes. The same degree of regeneration, despite a higher collagen content indicates that future material development can progress toward stronger, more resistant implants.
Keywords: biosynthetic cornea, corneal regeneration, biomaterials, recombinant human collagen, corneal transplantation
Introduction
The cornea is the transparent, outermost surface of the eye that serves both as a protective covering and the main refractive element in the visual system. From a biomaterials perspective, the cornea can be viewed as a hydrogel composed primarily of collagen and proteoglycans, sandwiched by an outer stratified epithelial layer consisting of five to six layers of cells, and an inner endothelial layer composed of a single layer of cuboidal-shaped cells.1 The hydrogel portion or corneal stroma contains a network of interconnected stromal keratocytes. The healthy cornea is very highly innervated, and the nerves provide trophic support to maintain the epithelial layer and overall health of the cornea.2 The healthy cornea is also avascular and immune privileged. However, these properties, along with the transparency, can be lost due to damage or disease and lead to blindness if irreversible.
Many forms of corneal blindness are treatable by transplantation with donated human corneas to restore sight. However, there is a severe shortage of donated tissues in many countries and in particular, the developing world. In addition, there are a number of conditions such as chemical burns and autoimmune disease where donor allografting is less successful.3 Hence, a range of alternatives to donor corneal tissue from cell-based therapies to biomaterial-based alternatives has been proposed.
In 2010, we reported the successful implantation of 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC)/N-hydroxysuccinimide (NHS) cross-linked recombinant human collagen type III (RHCIII) hydrogels as cell-free scaffolds to induce regeneration of human corneas.4 While these regenerated corneas remain stable without sustained immunosuppression (clinical observations: PF), we found that in several patients, the tight overlying mattress sutures that were used had delayed the epithelialization of the implant which led to the development of epithelial defects resulting in haze formation and thinning of the central graft.4 This suggested that in addition to having an optimized biomaterial implant, the surgical technique, and in particular, graft retention method, also needs to be optimal for best clinical outcome.
There is a range of suturing techniques used in corneal graft retention that appears to be dependent upon surgeon preference. Two of the most commonly used are radially placed interrupted (individual) sutures and continuous sutures.5 Generally, continuous sutures are used but in high risk cases, interrupted sutures are considered preferable as a suture break causes less risk for dehiscence and only one suture has to be replaced. A broken continuous suture is more complicated to repair or replace. It is however debatable, which technique is best from other aspects. Overlying sutures were used in our previous study as the biosynthetic material was not sufficiently robust to tolerate sutures penetrating the implant. These sutures are anchored only in host tissue and lie on top of the implant surface, holding it in place. In transplantation, particularly in high-risk cases, invasion of acute inflammatory cells, polymorphonuclear leukocytes, lymphocytes, and macrophages occurs.4 To limit their adverse effects, human amniotic membrane (HAM) had been previously applied on top of donor grafts to improve the clinical outcome, as it has been documented to have anti-inflammatory, antiscarring, and anti-angiogenic properties.6
The present study was conducted to determine the extent to which clinical outcomes are influenced by the surgical technique and implant composition. We examined the effects of the three surgical retention methods: overlying sutures with and without HAM and interrupted sutures with HAM, on implant-host interactions, and overall regenerative outcomes. Additionally, we compared the effects of implant composition on corneal cell and nerve overgrowth and in-growth, comparing 13.7% hydrogels versus 15% hydrogels (the designation based on the initial concentration of RHCIII used).
Methods
Reagents
Recombinant RHCIII produced in yeast cells (Pichia pastoris) was purchased from FibroGen, Inc. (San Francisco, CA), freeze-dried, and reconstituted. 1-Ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC) was supplied by Sigma-Aldrich (St. Louis, MO). N-hydroxysuccinimide (NHS) was supplied by Fluka (Buchs, Switzerland). Phosphate buffered saline (0.1M PBS, pH 7.4) was prepared from tablets (Calbiochem Corp., Darmstadt, Germany). Milli-Q deionized water (Millipore, Billerica, MD) was used throughout. Sodium dodecyl sulfate–PAGE gels, 4× lithium dodecyl sulphate (LDS) loading buffer and running buffers were from Life Technologies (Grand Island, NY). Dithiothreitol (DTT) and pepsin were from Sigma. Gelcode Blue was from Thermo Scientific (Rockford, IL).
Corneal Implants
The preparation of RHCIII hydrogels using a syringe mixing system has been described previously.7 Very briefly, aliquots of reconstituted 13.7% or 15% (wt/wt) RHCIII in aqueous solution were loaded into the syringe mixing system. Calculated volumes of EDC and NHS solutions were added and thoroughly mixed at 4°C (ice water). The final solution was intermediately dispensed into curved polypropylene contact lens molds (500-μm thick, 12-mm diameter) and cured at 100% humidity at 21°C for up to 24 hours and then at 37°C for up to 24 hours. The implants were thoroughly washed in fresh 1× PBS and then stored in PBS containing 1% chloroform to maintain sterility. Table 1 gives details of the reagents used in preparation of the 13.7% and 15% RHCIII hydrogels.
Table 1.
Reagents Used in the Preparation of Recombinant Human Collagen Type III (RHCIII) Hydrogels

Surgical Procedure
All animal studies were performed in accordance to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and with ethics approval from the University of Ottawa (protocol EI-5). Twelve female Gottingen mini pigs (Marshall Farms, North Rose, NY), 6-months old were used for testing RHCIII based tissue-engineered corneas. The right cornea of each animal was trephined using a trephine of 6 mm in diameter. The cut was deepened to 500 μm using a diamond knife, and the tissue was removed by manual lamellar dissection using a crescent knife. Recombinant human collagen type III implants, 6.5 mm in diameter and 500-μm thick, were trephined and implanted into the prepared wound bed. Three different retention techniques using a combination of interrupted and overlying sutures with and without HAM were tested. The HAM was placed over the implants prior to suturing (Fig. 1). All unoperated, contralateral (left) corneas served as controls for the implanted corneas. The groups of animals tested were as follows: Group 1, 13.7% RHCIII implants + overlying sutures; Group 2, 15% RHCIII implants + overlying sutures; Group 3, 15% RHCIII implants + overlying sutures + HAM; and Group 4, 15% RHCIII implants + interrupted sutures + HAM. All operated eyes received topical antibiotics (Zymar; Allergan, Irvine, CA) and steroids (Prednisolone; Sandoz, Quebec, Canada) four times daily until suture removal at 4 weeks post operation.
Figure 1. .
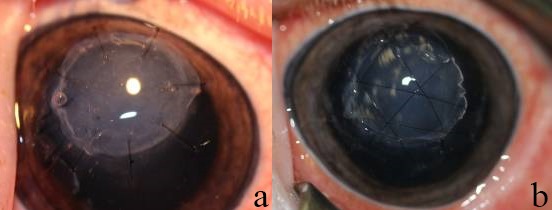
Photos of operated corneas showing the two suturing techniques used: (a) interrupted sutures, and (b) overlying sutures. Both were placed on top of a piece of human amniotic membrane used to minimize postoperative inflammation.
Post Operation Clinical Evaluation
Post operation follow-up included slit-lamp biomicroscopy, IOP measurements, Schrimer's strip test for tear production, aesthesiometry to assess corneal touch sensitivity (Cochet-Bonet aesthesiometer; Handaya Co., Tokyo, Japan), and in vivo confocal microscopy (IVCM), performed as we have previously described.7 The modified MacDonald-Shadduck scoring system was used to evaluate corneal clarity/haze and any inflammation during slit-lamp biomicroscopy.8 All tests were performed at 3, 6, 9, and 12 months post operation. The animals were anesthetized for all these evaluations.
For in vivo confocal microscopy, using the Confoscan3 (Nidek Technologies Srl., Padova, Italy), light scattering at the graft-host tissue interphase marked the implant boundaries. For nerve analyses, 96 IVCM Z-scans sequences were captured, and these videos were separated into still image frames using ImageJ (National Institutes of Health, Bethesda, MD) after importation with Quicktime (Apple Inc., Cupertino, CA). Each z-stack contained 349 frames adding up to a total of 33,504 images. Recurrent image fields were identified and excluded. The criteria used to identify nerves were: bright, straight, branched, and/or thin structures present in the basal subepithelium or anterior stroma.
Light Transmission and Scatter of Neocorneas
Light transmission and back-scattering measurements were carried out at room temperature for white light (supplied by a quartz-halogen lamp source) and for narrow spectral regions (centered at 450, 500, 550, 600, and 650 nm). Briefly, a custom-made instrument measured the percent transmission of samples as compared with open-beam intensity.9 The relative percent of radiation back scattered from the collimated beam by the sample was measured by a circular array of eight photodiodes, 30° off axis.
Collagen Analyses of Regenerated Neocorneas
To determine the extent of remodeling and turnover of the initial cell-free RHCIII implants, the collagen content of the neocorneas, 3-mm diameter samples were trephined out of the center of each operated and contralateral control cornea, snap frozen, and stored at −80°C until analyzed. Each sample was weighed and resuspended in 10 mM HCl containing 1 mg/mL pepsin. The samples were digested with pepsin at 2°C to 8°C for 96 hours and the soluble fraction was recovered by centrifugation. An aliquot was neutralized for ELISA and a separate aliquot was mixed with NuPAGE 4× LDS sample buffer (Life Technologies) denatured at 75°C for 8 minutes and analyzed on 3% to 8% Tris-acetate gels under nonreducing conditions or on 4% to 12% Bis-Tris gels under reducing conditions (20 mM DTT). Proteins were visualized by staining with Gelcode Blue. The insoluble fraction from the pepsin digest was resuspended in a fresh HCl-pepsin solution and extracted for an additional 48 hours (second pepsin extract). The soluble fraction was recovered and analyzed as described above.
The resistance of the 13.7% and 15% RHCIII implants to collagenase was evaluated as previously described.10 Fifty milligram samples of hydrogels (n = 3 each group) were equilibrated for 1 hour in 5 mL 0.1 M Tris–HCl buffer (pH 7.4) containing 5 mM CaCl2 at 37°C and then transferred to a 5 U/mL (1 mg/mL of a 288 U/mL) collagenase solution (type I Clostridium histolyticum, EC 3.4.24.3; Sigma-Aldrich). The collagenase solution was refreshed every 8 hours to maintain activity. At different time intervals, the samples were removed from the solution, gently blotted on filter paper and weighed. The percent residual mass of the sample was calculated according to the following equation:
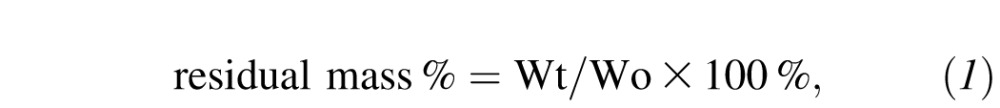 |
where Wo is the initial weight of the hydrogel and Wt is the weight of the hydrogel at each time point.
Immunohistochemistry of Regenerated Neocorneas
Implanted corneas and their respective contralateral controls were fixed in 4% paraformaldehyde and paraffin embedded. Sections 6-μm thick were used for all analyses. Sections were stained with haematoxylin and eosin for histologic examinations. Immunostaining was performed with antibodies against: cytokeratin (CK) 3 + 76 (AE-5, Millipore, Walford, UK), tyrosine kinase B (TrkB) (Santa Cruz Biotechnology Inc., Santa Cruz, CA), smooth muscle actin (SMA; Abcam, Cambridge, UK), von Willebrand factor (vWF; Abcam), F4/80 (AbD Serotec, Oxford, UK), human collagen III (US Biological, Salem, MA). Prior to immunostaining antigen retrieval was performed using with either Tris-EDTA pH 9.0, 90°C, 30 minutes or proteinase K, (Dako, Glostrup, Denmark) 10 minutes, at room temperature. Antibodies used and staining conditions for each are shown in Table 2.
Table 2.
Staining Reagents Used in Immunohistochemistry
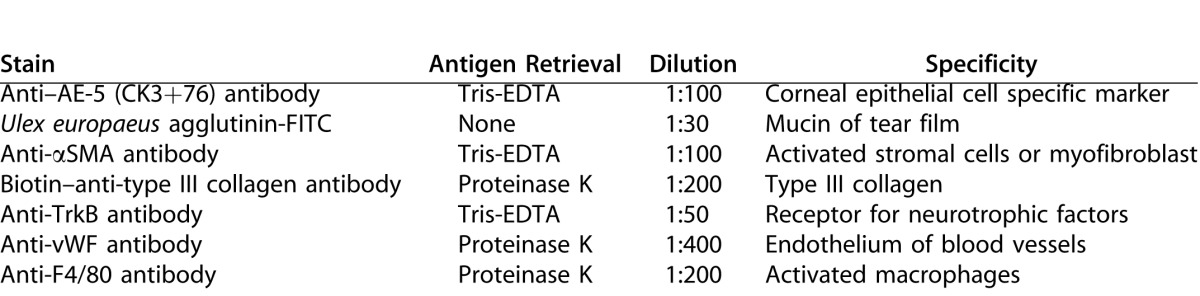
Briefly, all sections were deparaffinized, rehydrated, and blocked with 5% goat serum in PBS with 0,1% Tween 20 (PBS-T). Incubation with the primary antibody was carried out overnight at 4°C. After thorough washing in PBS-T, the sections were incubated with the secondary antibody for 1 hour at room temperature: either goat anti-rabbit Alexa 488 or goat anti-mouse Alexa 488 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), diluted 1:1000 in 5% goat serum in PBS-T. For the biotin-conjugated anti-type III collagen antibody, mouse anti-biotin Alexa 647 antibody (Jackson ImmunoResearch Laboratories, Inc.) diluted 1:50 in 5% goat serum in PBS-T was used. For mucin detection, FITC-conjugated Ulex europaeus agglutinin (UEA; Sigma-Aldrich) was used directly, without prior blocking or antigen retrieval. After staining, the slides were washed in PBS-T, dehydrated, and mounted with Vectashield mounting medium with DAPI (Vector Laboratories, Inc., Burlingame, CA). Fluorescent images were captured using an inverted LSM-700 Zeiss confocal microscope (LSM700; Carl Zeiss Microscopy GmbH, Oberkochen, Germany).
Statistical Analysis
For the majority of our statistical analyses the general linear method (GLM) in Minitab 17 (Minitab Inc., State College, PA) was used, and unlike ANOVA, it can handle unbalanced data and partial nesting. For balanced data GLM generates P values identical to an ANOVA analysis. The critical P value for significance testing was set at 0.05. The critical P value for significance testing was set at 0.05. Tukey test post hoc was used where significant difference was found. Light transmission, light scatter, and quantification of collagen content with ELISA was analyzed using GLM with three factors: treatment (material + surgery technique), subject, and live/control. Subject was nested in treatment and treatment was nested in live/control. Primary vessels, optical clarity, and the number of nerves in the corneas of live animals were compared using the general linear model (GLM) with three factors: treatment, month postoperative, and subject. Treatment and month were crossed and subject was nested in treatment. The Schirmer's test results was analyzed using GLM with four factors: treatment, month, subject, and live/control. Treatment was nested in live/control, subject was nested in treatment and month was crossed with live/control. The number of vessels detected by immunohistochemistry in tissues collected at 12 months was analyzed by GLM with one factor: treatment.
Results
Collagen Hydrogels
The resulting 13.7% and 15% differed primarily in their degradation profile, with the lower RHCIII content implants being degraded more readily by bacterial collagenase (Fig. 2). However, there were no observable differences in their in vivo biocompatibility as we showed above. No rejection episodes were observed for any of the animals.
Figure 2.
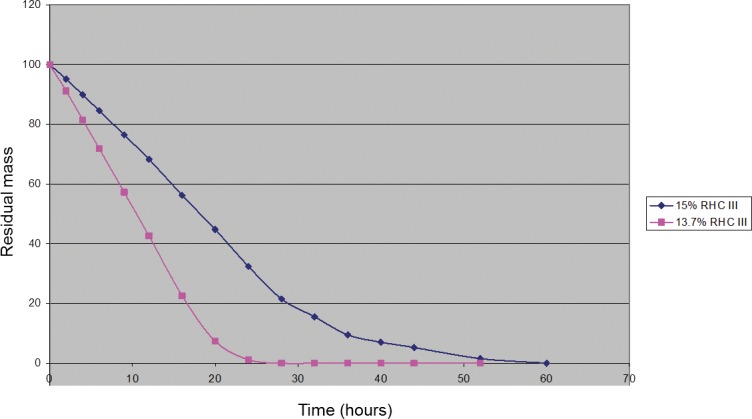
The collagenase degradation profile for 15% RHCIII hydrogels as compared with 13.7%.
Clinical Evaluation
Postoperative corneal haze was observed at 3 months after the surgery, but this decreased over the 12 month follow-up period (Table 3A). This was confirmed by the comparison of light transmission and scatter of the operated and nonoperated corneas at 12 months post implantation (Table 3B). The values for light transmission, optical clarity, and light scatter did not significantly differ between any of the treatment groups or any of the controls.
Table 3A.
Optical Clarity of Implanted Corneas Over Time After Graft Retention Using Different Suturing Methods (Modified Macdonald-Shadduck Scoring System 0–6)
Some neovascularization was noted in all operated eyes. The greatest number of in-growing vessels was found in the 15% RHCIII implants that were covered with HAM and secured with interrupted sutures (Table 4). This group showed a significantly higher vessel number (P = 0.045) compared with the other three groups. No vascularization was noted in any of the unoperated corneas. The vessels remained throughout the entire study although they decreased in diameter and became extremely fine by 12 months post operation (PF, clinical observation).
Table 3B.
Actual Measurements of Light Transmission and Scatter Through the Different Groups of Regenerated Neocorneas at 12 Months After Operation (n = 3 Corneas per Group)
No significant differences in IOP, tear production, or corneal sensitivity were observed among the different groups. Nor were there any differences in the control, unoperated corneas.
In vivo confocal microscopy analysis showed complete regeneration of corneal epithelium and stromal layers by 3 months post operation in all the experimental groups. The presence of nerves was noted in all groups by 3 months post operation (Fig. 3). Nerve regeneration during the 12 month post operation study was evaluated (data not shown). There was no difference between the group averages but there was a significant increase in nerve numbers as time progressed (P = 0.001). The rate of regrowth could not be shown to be significantly different between the groups (P = 0.391).
Figure 3.
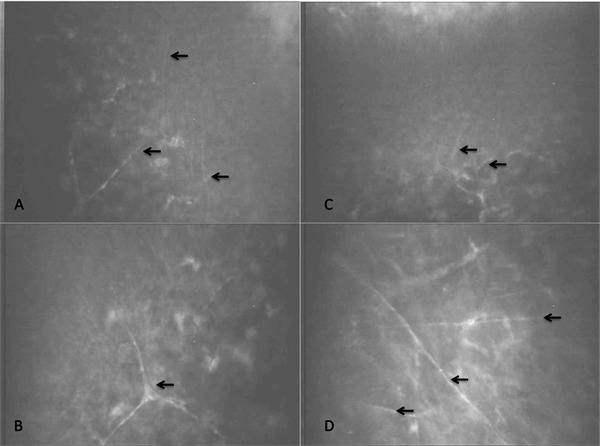
Nerve regeneration into the central cornea of a typical cornea, as observed by in vivo confocal microscopy. (A, B) Unoperated control cornea showing the subepithelial zone and anterior stroma, respectively, at 9 months postoperative. (C, D) Corresponding zones from the contralateral implanted cornea. Nerves are indicated by the arrows.
Immunohistochemistry
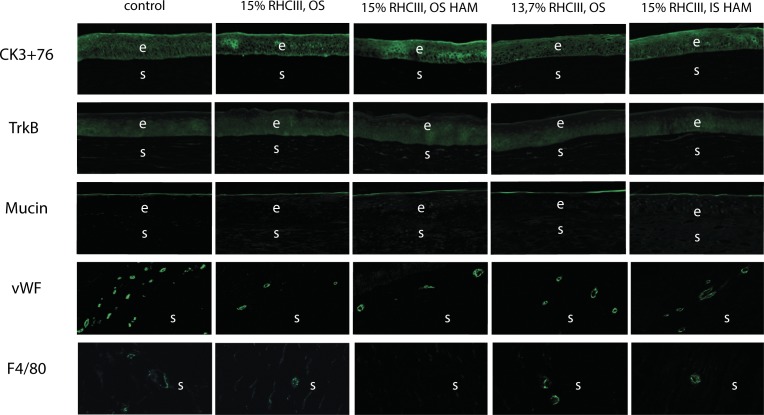
Hematoxylin and eosin staining showed that at 12 months post implantation, all implanted corneas had regenerated a stratified epithelium and underlying stroma that was morphologically similar to their corresponding contralateral corneas (Fig. 4). However, a region of hyperepithelization corresponding to the site of implantation was present in all operated eyes (Fig. 4).
Figure 4.
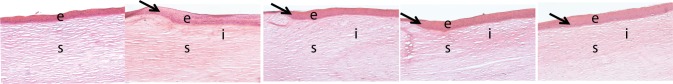
Haematoxylin and eosin staining of an unoperated (control) cornea and operated corneas from each experimental group. The arrows show the region where hyperepithelialization begins. OS, overlying sutures; IS, interrupted sutures; e, epithelium; s, stroma, i, implant location.
Epithelium
All neocorneas and unoperated, control corneas stained positively for both CK 3 + 76 and the TrkB neurotrophin receptor (Fig. 5). No intergroup differences were observed for either antibody.
Figure 5.
Immunostaining of an unoperated (control) cornea and operated corneas from each experimental group. For vWF and F4/80 controls, the images show the scleral, vascularized part of the sample, which served as an internal control; the corneal part was negative for vWF and F4/80 in unoperated eyes.
Tear Film Formation
All implanted corneas, like unoperated controls, stained positively for mucin, a major component of the tear film, showing that tear film production by corneal epithelial cells had been restored (Fig. 5). No observable differences in the staining intensity or the thickness of the mucin layer were present.
Effect of Implantation on Induction of Immune Response and Neovascularization
The F4/80 antibody, which specifically detects mature macrophages, stained a few cells (1–4) in the corneal stromas of operated animals from all groups except for the 15%-RHCIII implanted group where implants were covered with HAM and stabilized with overlying sutures (Fig. 5).
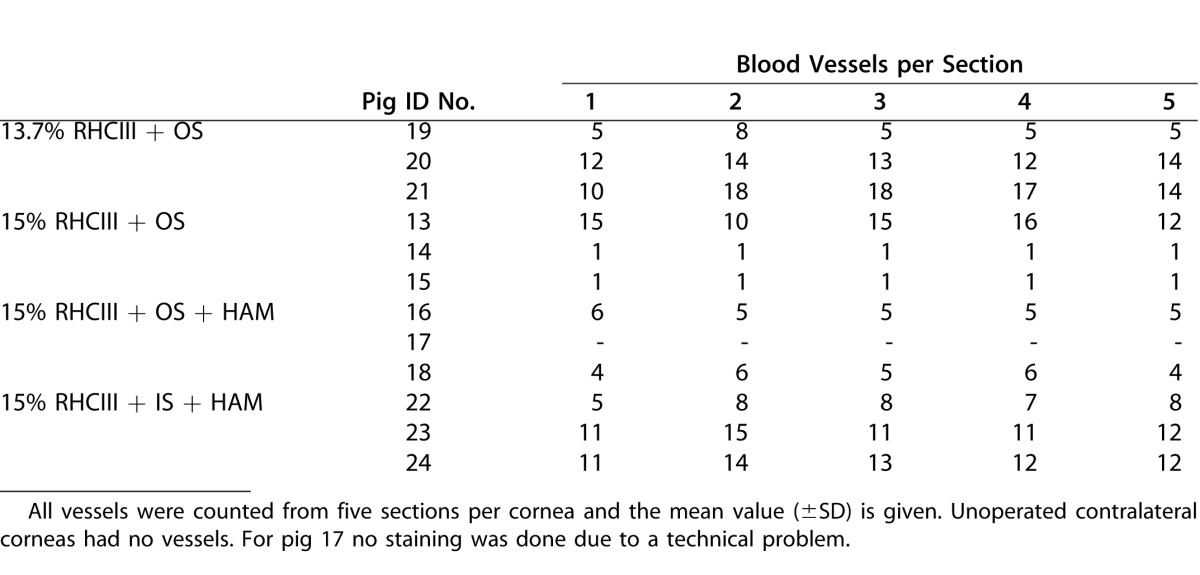
All implanted corneas were positive for vWF present on endothelial cells of blood vessels (Fig. 5). All observed blood vessels (mostly capillaries), were located in the mid or upper-mid stroma. Mean vessel numbers for the various groups are shown in Table 5. No significant difference between treatments was found (P = 0.369). All corneas were negative for SMA, found in myofibroblasts, the cell type thought to be the major cause of hazing (data not shown).
Table 4.
Numbers of Blood Vessels Growing Into Implanted Hydrogels at Various Times Post Implantation, as Visualized by Slit-Lamp Biomicroscopy
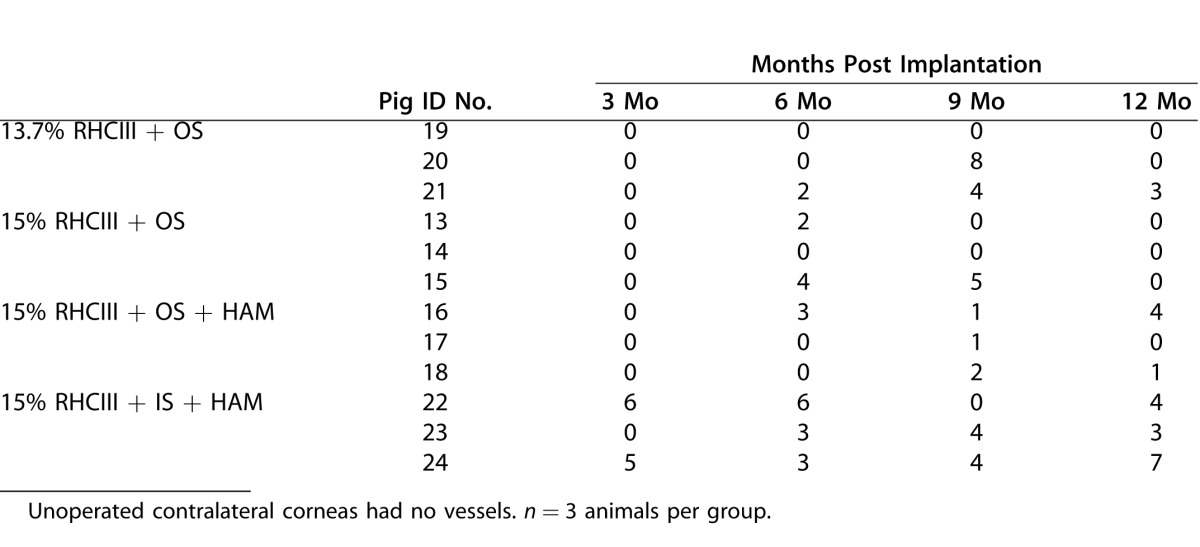
Table 5.
Numbers of Blood Vessels Found in Regenerated Neocorneas as Visualized by Immunostaining
Implant Remodeling
The cross-linked RHCIII implants prepared for this study stained positively with a biotinylated anti-type III collagen prior to implantation. However, after 12-months grafting into pigs, no type III collagen could be detected in the regenerated neocorneas, as was the case with the unoperated, healthy corneas (not shown).
Collagen Composition of Neocorneas
Analysis of the pepsin resistant proteins extracted from regenerated neocorneas and control eyes by SDS-PAGE revealed significant amounts of type I collagen in all the eyes (Fig. 6). The gels demonstrated the presence of α, β, and γ components indicating that the secreted collagen molecules formed fibrils that were covalently cross-linked. The amount of type I collagen present in the extracts from the operated and unoperated eyes were similar across all groups. Analysis of a the second pepsin extract yielded a fraction enriched in high molecular weight aggregates of collagen relative to α chains (Fig. 7). In addition to bands comigrating with the type I collagen standard, a pepsin-resistant band that migrated above the α1 chain of type I collagen was detected. This band was shown to be sensitive to digestion by purified bacterial collagenase (data not shown) in addition to being pepsin resistant, confirming it is a collagenous peptide. The relative mobility of this collagenous peptide is consistent with that of the α1(V) chain of type V collagen, one of the major collagenous proteins present in cornea.11–14 Reducing SDS-PAGE of the second pepsin extract from pigs in the 15% RHCII + OS group contained bands consistent in size with type VI collagen chains migrating at approximately 60 and 37 kd (Fig. 7). Extracts from other groups were analyzed and showed a similar banding pattern (data not shown). These bands were not seen on nonreduced gels, consistent with the disulfide cross-linked nature of type VI collagen, another collagen species reported to be present in significant quantities in normal cornea.15–18 Quantitation of type I collagen content of the two pepsin extracts was performed by ELISA using polyclonal antibodies specific for porcine type I collagen. Comparison of the collagen content estimated by SDS-PAGE and by ELISA from the two pepsin extracts indicated the ELISA assay was biased toward the higher molecular weight aggregates since the ELISA values were much higher in the second pepsin extract, which was enriched in β and γ components of type I collagen. To quantitate the amount of type I collagen present in the operated and nonoperated eye extracts the ELISA values from both pepsin extracts were combined and normalized to the original wet weight of the tissue sample. There was no significant difference between the collagen I content of the operated groups, between the control groups or any of the treatments paired to their controls. We found no type III collagen in any of the samples analyzed.
Figure 6.
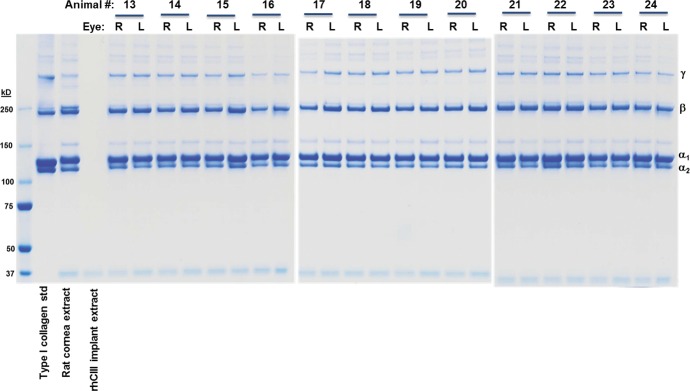
Sodium dodecyl sulfate–PAGE analysis of pepsin soluble proteins extracted from porcine corneal samples. Aliquots of the first pepsin extract were mixed with LDS sample buffer, denatured and fractionated on 3% to 8% Tris-acetate gels using Tris-acetate running buffer under nonreducing conditions. On the right side of the gel a porcine type I collagen standard is shown as well as a pepsin extract from a normal rat cornea and an implant prepared from 15% RHCIII. R, right eyes with implants; L, left eyes, controls.
Figure 7.
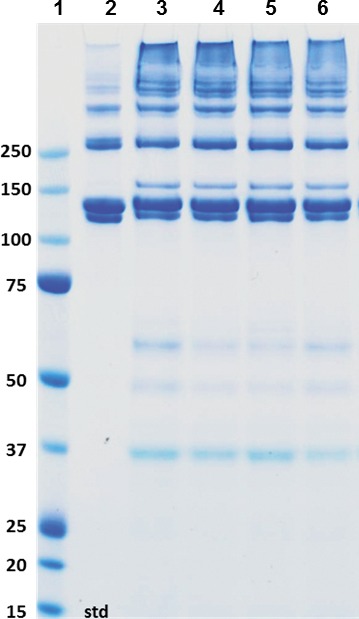
Sodium dodecyl sulfate–PAGE analysis of pepsin soluble proteins extracted from porcine corneal samples. Aliquots of the second pepsin extract were mixed with LDS sample buffer, denatured, reduced with 20 mM DTT and fractionated on 4% to 12% Bis-Tris gels using 3-(N-morpholino)propanesulfonic acid running buffer. Lane 1, porcine type I collagen standard, lanes 3 to 6, extracts from two different pigs from the 15% RHCIII + OS, odd numbered lanes are extracts from implanted eyes and even numbered lanes are from control eyes.
Discussion
Following surgical implantation is pigs, RHCIII corneal implants cross-linked with EDC/NHS resulted in full regeneration of the cornea, regardless of their collagen content. No differences in the quality of tissue regeneration could be shown in 13.7% vs. 15% RHCIII hydrogels. Furthermore, cell and nerve in-growth observed were similar to what we previously reported for 10% (starting concentration) collagen hydrogels in pigs,19 and in human patients.4 The importance of this observation is that relatively small (<5%) changes in collagen concentration do not affect the regeneration process, and hence, future formulations of RHCIII-based corneal implants will not require a long, 12-month animal study, but possibly just establishment of compatibility and timing of cell and nerve ingrowth (e.g., by in vivo confocal microscopy) over a shorter time span. Higher collagen concentrations have the potent to yield implants that are more robust and easier to handle. In general, the overlying sutures resulted in a smaller amount of neovascularization than the interrupted sutures, in agreement with reports that the individual knots in the interrupted sutures have a tendency to attract vessel ingrowth.20 Although clinical examination showed a significantly larger number of blood vessels in implants stabilized by the interrupted sutures (P = 0.045), immunostaining for vascular endothelial cells showed no statistically significant difference in capillary counts among the groups (P = 0.369). This is not surprising, as the immunostaining represents only five random sections, usually in close proximity to each other, whereas the macroscopically visible primary vessels are given as total counts. The vessels persisted throughout the study but tended to regress. It should also be noted that the pigs could not be fitted with bandage lenses, which are used in humans after overlying sutures and following HAM application to further reduce inflammation.4 This may account for the persistent vessels observed in the animals but not in our human patients. The application of HAM appeared to have no effect on the outcome, as no significant differences could be shown for any of the tested parameters in groups where HAM was applied compared with no HAM. However, at 12 months post implantation, there was very little if any inflammatory responses visible in any of the groups, indicating that RHCIII implants most likely exhibit a low immunogenicity and are well tolerated, despite the lack of immunosuppressive therapy. The 15% RHCIII + OS + HAM group exhibited no staining for macrophages, whereas all the other groups had such small numbers of these cells (1–4) that their presence is most likely negligible for the clinical outcome. No adverse effects, such as delayed epithelialization or ulcer formation were observed in any of the groups. The significance of HAM might be higher in the case of allografts where a clear immune response always accompanies transplantation.4 The epithelia of all groups showed the cytokeratin differentiation marker and presence of receptors for neurotrophins, which convey signals from nerve cells, crucial for epithelial survival and necessary for maintenance of overall ocular surface health.21 Although hyperepithelialization was observed in all operated eyes, clearly demarking the implantation region, this did not affect light transmission. The function of all the regenerated neocorneas as a refractive element appeared to be normal, as light transmission of greater than 85% and scatter of 3% or less were well within the values for healthy, functional corneas. These results are also consistent with the lack of myofibroblasts within the stromal compartment, as this is the cell type thought to be the major cause of corneal hazing.22
The presence of tears and corneal sensitivity in all the operated eyes is indicative of nerve in-growth in the corneas, as we had previously shown in implants composed of medical grade porcine or recombinant human collagen,4,7,19 a finding that is not common in transplanted allografts.23,24 Nerve regeneration occurred in all groups, as shown by IVCM and the nerve numbers were increasing significantly during the follow-up period. The two groups with 15% RHCIII and overlying sutures had the highest number of nerves, but these differences were not statistically significant.
Analysis of the collagen extracted from 12-month samples indicated that type I collagen was synthesized, secreted, and assembled into fibrils that were covalently cross-linked. Our analysis showed the type I collagen content in the operated and nonoperated eyes was similar suggesting significant remodeling of the implant, which originally consisted of only recombinant type III collagen, had occurred during the course of the study. This conclusion is supported by the lack of staining for type III collagen in the neocorneas at 12 months post implantation. These findings indicate that the RHCIII used to make the implants was turned over during the course of the study and replaced with primarily type I collagen. In addition to type I collagen, collagenous peptides consistent in size with type V and VI collagens were seen. The possible presence of these two collagen types is further evidence of a remodeling process that resulted in the deposition of collagen types found in the normal cornea.
In conclusion, RHCIII implants performed equally well, regardless of their collagen content. All groups showed very little inflammatory response indicating that the implants were well tolerated. The suturing technique influenced the clinical outcome, with interrupted sutures being less favorable, whereas HAM had no effect. It should be noted that young, healthy mini pigs were used in this study. The differences in surgical technique and HAM significance could be more pronounced in elderly patients with less than perfect health, who may not be able to compensate for any problems caused by the surgery. Overall, our results showed that the surgical technique used has a small but significant effect on the performance of corneal implants.
Acknowledgments
The authors thank the Canadian Stem Cell Network, Swedish Research Council, Linköping University, and the County of Östergötland for funding of this project (MG, PF). They also thank Rejean Munger and Christopher Noel for their help with the pig surgeries and follow-ups, Jae-Il Ahn for performing the corneal transmission examinations, and Emilia Wiechec for her valuable advice with the immunohistochemistry.
Disclosure: M. Kozak Ljunggren, None; R.A. Elizondo, None; E. Edin, None; D. Olsen, FibroGen, Inc. (E); K. Merrett, None; C.-J. Lee, None; G. Salerud, None; J. Polarek, FibroGen, Inc. (E); P. Fagerholm, Canadian Stem Cell Network (F), Swedish Research Council (F), Linköping University (F), County of Östergötland (F); M. Griffith, Canadian Stem Cell Network (F), Swedish Research Council (F), Linköping University (F), County of Östergötland (F)
References
- 1.Griffith M, Jackson WB, Lagali N, et al. Artificial corneas: a regenerative medicine approach. Eye (Lond) 2009;23:1985–1989. doi: 10.1038/eye.2008.409. [DOI] [PubMed] [Google Scholar]
- 2.Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye (Lond) 2003;17:989–995. doi: 10.1038/sj.eye.6700616. [DOI] [PubMed] [Google Scholar]
- 3.Williams KA, Esterman AJ, Bartlett C, et al. How effective is penetrating corneal transplantation? Factors influencing long-term outcome in multivariate analysis. Transplantation. 2006;81:896–901. doi: 10.1097/01.tp.0000185197.37824.35. [DOI] [PubMed] [Google Scholar]
- 4.Fagerholm P, Lagali NS, Merrett K, et al. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med. 2010;2:46–61. doi: 10.1126/scitranslmed.3001022. [DOI] [PubMed] [Google Scholar]
- 5.Lee RM, Lam FC, Georgiou T, et al. Suturing techniques and postoperative management in penetrating keratoplasty in the United Kingdom. Clin Ophthalmol. 2012;6:1335–1340. doi: 10.2147/OPTH.S35460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin CR, Fagerholm P, Muzakare L, et al. Regeneration of corneal cells and nerves in an implanted collagen corneal substitute. Cornea. 2008;27:580–589. doi: 10.1097/ICO.0b013e3181658408. [DOI] [PubMed] [Google Scholar]
- 7.Merrett K, Fagerholm P, McLaughlin CR, et al. Tissue-engineered recombinant human collagen-based corneal substitutes for implantation: performance of type I versus type III collagen. Invest Ophthalmol Vis Sci. 2008;49:3887–3894. doi: 10.1167/iovs.07-1348. [DOI] [PubMed] [Google Scholar]
- 8.Altmann S, Emanuel A, Toomey M, et al. A quantitative rabbit model of vaccinia keratitis. Invest Ophthalmol Vis Sci. 2010;51:4531–4540. doi: 10.1167/iovs.09-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Priest D, Munger R. A new instrument for monitoring the optical properties of corneas. Invest Ophthalmol Vis Sci. 1998;39(s352) suppl. [Google Scholar]
- 10.Deng C, Li F, Hackett JM, et al. Collagen and glycopolymer based hydrogel for potential corneal application. Acta Biomater. 2010;6:187–194. doi: 10.1016/j.actbio.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Cintron C, Hong BS, Covington HI, Macarak EJ. Heterogeneity of collagens in rabbit cornea: type III collagen. Invest Ophthalmol Vis Sci. 1988;29:767–775. [PubMed] [Google Scholar]
- 12.Kern P, Menasche M, Robert L. Relative rates of biosynthesis of collagen type I, type V and type VI in calf cornea. Biochem J. 1991;274(pt 2):615–617. doi: 10.1042/bj2740615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee RE, Davison PF. The collagens of the developing bovine cornea. Exp Eye Res. 1984;39:639–652. doi: 10.1016/0014-4835(84)90063-0. [DOI] [PubMed] [Google Scholar]
- 14.Doane KJ, Babiarz JP, Fitch JM, Linsenmayer TF, Birk DE. Collagen fibril assembly by corneal fibroblasts in three-dimensional collagen gel cultures: small-diameter heterotypic fibrils are deposited in the absence of keratan sulfate proteoglycan. Exp Cell Res. 1992;202:113–124. doi: 10.1016/0014-4827(92)90410-a. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann DR, Trueb B, Winterhalter KH, Witmer R, Fischer RW. Type VI collagen is a major component of the human cornea. FEBS Lett. 1986;197:55–58. doi: 10.1016/0014-5793(86)80297-6. [DOI] [PubMed] [Google Scholar]
- 16.Cintron C, Hong BS. Heterogeneity of collagens in rabbit cornea: type VI collagen. Invest Ophthalmol Vis Sci. 1988;29:760–766. [PubMed] [Google Scholar]
- 17.Jander R, Rauterberg J, Glanville RW. Further characterization of the three polypeptide chains of bovine and human short-chain collagen (intima collagen) Eur J Biochem. 1983;133:39–46. doi: 10.1111/j.1432-1033.1983.tb07427.x. [DOI] [PubMed] [Google Scholar]
- 18.Chu ML, Conway D, Pan TC, et al. Amino acid sequence of the triple-helical domain of human collagen type VI. J Biol Chem. 1988;263:18601–18606. [PubMed] [Google Scholar]
- 19.Liu Y, Gan L, Carlsson DJ, et al. A simple, cross-linked collagen tissue substitute for corneal implantation. Invest Ophthalmol Vis Sci. 2006;47:1869–1875. doi: 10.1167/iovs.05-1339. [DOI] [PubMed] [Google Scholar]
- 20.Dana MR, Schaumberg DA, Kowal VO, et al. Corneal neovascularization after penetrating keratoplasty. Cornea. 1995;14:604–609. [PubMed] [Google Scholar]
- 21.You L, Kruse FE, Volcker HE. Neurotrophic factors in the human cornea. Invest Ophthalmol Vis Sci. 2000;41:692–702. [PubMed] [Google Scholar]
- 22.Wilson SE. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Exp Eye Res. 2012;99:78–88. doi: 10.1016/j.exer.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter A, Slowik C, Somodi S, Vick HP, Guthoff R. Corneal reinnervation following penetrating keratoplasty–correlation of esthesiometry and confocal microscopy. Ger J Ophthalmol. 1996;5:513–517. [PubMed] [Google Scholar]
- 24.Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25:171–177. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]