Abstract
Currently the molecular basis for the clinical heterogeneity of X-linked adrenoleukodystrophy (X-ALD) is poorly understood. The genetic bases for all different phenotypic variants of X-ALD are mutations in the gene encoding the peroxisomal ATP-binding cassette (ABC) transporter, ABCD1 (formerly adrenoleukodystrophy protein, ALDP). ABCD1 transports CoA-activated very long-chain fatty acids from the cytosol into the peroxisome for degradation. The phenotypic variability is remarkable ranging from cerebral inflammatory demyelination of childhood onset, leading to death within a few years, to adults remaining pre-symptomatic through more than five decades. There is no general genotype–phenotype correlation in X-ALD. The default manifestation of mutations in ABCD1 is adrenomyeloneuropathy, a slowly progressive dying-back axonopathy affecting both ascending and descending spinal cord tracts as well as in some cases, a peripheral neuropathy. In about 60% of male X-ALD patients, either in childhood (35–40%) or in adulthood (20%), an initial, clinically silent, myelin destabilization results in conversion to a devastating, rapidly progressive form of cerebral inflammatory demyelination. Here, ABCD1 remains a susceptibility gene, necessary but not sufficient for inflammatory demyelination to occur. Although the accumulation of very long-chain fatty acids appears to be essential for the pathomechanism of all phenotypes, the molecular mechanisms underlying these phenotypes are fundamentally different. Cell autonomous processes such as oxidative stress and energy shortage in axons as well as non-cell autonomous processes involving axon–glial interactions seem pertinent to the dying-back axonopathy. Various dynamic mechanisms may underlie the initiation of inflammation, the altered immune reactivity, the propagation of inflammation, as well as the mechanisms leading to the arrest of inflammation after hematopoietic stem cell transplantation. An improved understanding of the molecular mechanisms involved in these events is required for the development of urgently needed therapeutics.
Keywords: ABC transporter, Axonopathy, Demyelination, Inflammation, Leukodystrophy, Peroxisome
Abbreviations: ABCD, ATP-binding cassette transporter subfamily D; AMN, adrenomyeloneuropathy; CALD, cerebral adrenoleukodystrophy; HSCT, hematopoietic stem cell transplantation; VLCFA, very long-chain fatty acids; X-ALD, X-linked adrenoleukodystrophy
Highlights
-
•
Adrenomyeloneuropathy (AMN) is proposed to be the core syndrome of X-ALD.
-
•
The cerebral inflammatory demyelinating form of X-ALD is independent of AMN.
-
•
The same genetic basis but fundamentally different pathomechanisms lead to AMN and cerebral ALD.
-
•
Genetic, epigenetic and environmental factors modulate onset and severity of AMN and cerebral ALD.
1. Introduction and clinical aspects of X-ALD pathophysiology
X-linked adrenoleukodystrophy (X-ALD; OMIM, phenotype MIM number #300100) is the most common inherited peroxisomal disorder. The combined incidence of hemizygotes (all phenotypes) plus heterozygous female carriers is 1:16,800 newborns [1]. One of the key clinical symptoms during aging of X-ALD patients is a slowly progressive axonopathy affecting sensory ascending and motor descending spinal cord tracts with 100% penetrance in men and 65% in heterozygous women by the age of 60 years [2]. Thus, X-ALD represents one of the most common monogenetically inherited neurodegenerative diseases.
The progressive dying-back axonopathy represents the core clinical feature of adrenomyeloneuropathy (AMN) in male patients, with onset usually between 20 and 30 years and in heterozygous females with onset between 40 and 50 years. The initial symptoms include progressive stiffness and weakness of the legs, impaired vibration sense in the lower limbs, sphincter disturbances and impotence, as well as scarce scalp hair (alopecia). About 66% of male AMN patients, but less than 5% of female patients, have adrenocortical insufficiency (Addison disease). Abnormal MRI signals of white matter in the centrum ovale, pyramidal tracts in the brainstem and internal capsules have frequently been observed in AMN, but no gadolinium enhancement is present indicating an intact blood–brain barrier and the absence of an acute inflammatory process [3].
On the other hand, in a total of about 60% of male X-ALD patients, rapidly progressive, inflammatory cerebral demyelination (cerebral X-ALD, CALD) occurs independent of AMN. The onset of inflammation is most common in children (35–40%), before onset of AMN, and less frequent (20%) in adolescents or adults. The inflammatory demyelination starts most often in the midline of the corpus callosum and progresses relentlessly outward as symmetric, confluent lesion in both hemispheres. Clinically, this coincides with a progressive neurologic decline, leading to a vegetative state or death within 3–5 years. Occasionally, a spontaneous arrest of cerebral disease has been observed. Hematopoietic stem cell transplantation or ex vivo gene correction of autologous hematopoietic stem cells can arrest the inflammation in early stages and, thus, provide an efficient treatment for the inflammatory form of X-ALD [4–6]. For pathophysiological considerations, it is important to note that acute inflammation is only observed in the central nervous system (CNS) and not in other tissues of X-ALD patients [3].
In females, cerebral disease is exceedingly rare and has only been verified in a case where both X chromosomes were affected. In an 8-year-old girl with severe CALD, genetic analysis revealed a de novo Xq27-ter deletion on the paternal X-chromosome in addition to the maternally inherited ABCD1 mutation [7]. Approximately 65% of heterozygous females develop an AMN-like syndrome with an average onset later than in the male patients [8]. In addition, female heterozygotes report prominent diffuse pain and are often misdiagnosed with fibromyalgia. Investigations in blood leukocytes reported a “skewed X-inactivation” in 68% of 22 X-ALD carriers with a significant correlation between the extent of the skewing and the severity of neurologic abnormalities [9]. We hypothesize that the ameliorated symptoms in the majority of heterozygous female carriers compared to male patients are due to the X-inactivation patterns in oligodendrocytes and microglia/macrophages. Here the population of cells bearing the normal allele may provide enough functional ABCD1 activity to protect from CALD. When in female heterozygotes symptoms exceed those of AMN, other explanations should be sought [7,10]. The importance of the proportion of cells carrying a healthy allele is emphasized by the observation that in two CALD patients the arrest of inflammation by autologous hematopoietic stem cell gene therapy could be achieved by long-term correction of only about 16% of CD34+-derived cells, as determined by ABCD1-positivity of peripheral granulocytes, monocytes, T and B cells [5,6].
2. Biochemical and genetic aspects of X-ALD pathophysiology
Saturated, unbranched very long-chain fatty acids (VLCFA; fatty acyl-chain length of ≥22 carbons) are degraded in the peroxisomal matrix by the sequential reactions of the enzymes (acyl-CoA oxidase 1, D-bifunctional protein and either peroxisomal β-ketothiolase 1 or sterol carrier protein x) of the β-oxidation pathway. In X-ALD patients, saturated VLCFA, in particular C26:0, accumulate in tissues and body fluids serving as a diagnostic marker for X-ALD [3].
In all X-ALD patients, mutations affecting the ATP-binding cassette (ABC) transporter subfamily D member 1 (ABCD1) gene, located at chromosome Xq28, have been identified [11]. A summary of more than 643 different ABCD1 mutations can be found at the web page http://www.x-ald.nl [12]. The ABCD1 gene encodes the peroxisomal ABC half-transporter ABCD1 (formerly adrenoleukodystrophy protein, ALDP). The ATP-binding domain of ABCD1 is facing the cytosol and substrates are transported from the cytosol into the peroxisome under ATP consumption. It is not clear what determines the substrate specificity of the peroxisomal ABC transporters. For the human ABCD1 protein, CoA-activated VLCFA, such as C26:0-CoA or C24:0-CoA but also C22:0-CoA, are valid substrates; and the degradation of these fatty acids by peroxisomal β-oxidation is strongly reduced in cultured X-ALD fibroblasts [13–15]. In fibroblasts, the peroxisomal import and degradation of C26:0-CoA could be blocked using anti-ABCD1 antibodies [15].
In addition to ABCD1, two other ABC transporters, ABCD2 and ABCD3, are localized in the peroxisomal membrane [16–18]. The functional unit of the ABCD1 transporter is a dimer; in vivo, in the peroxisomal membrane, apparently predominantly homodimers are formed, although also heterodimers with ABCD2 or ABCD3, the other two peroxisomal members of the ABCD family are possible [19]. In all tissues investigated so far, the β-oxidation of VLCFA was never completely abolished by ABCD1 mutations, not even by those causing a complete loss of function. We could recently show that in primary fibroblasts of X-ALD patients, the residual activity depends largely on the homologous peroxisomal ABC transporter ABCD3. However, ABCD3 was estimated to be about 45 times less efficient than ABCD1 at mediating a direct or indirect transport of C26:0-CoA, across the peroxisomal membrane [15], which provides an explanation why endogenous ABCD3 is unable to rescue the metabolic defect in X-ALD patients. ABCD2, the closest homolog of ABCD1, would be expected to compensate more efficiently than ABCD3 but is not expressed at relevant amounts in fibroblasts and, thus, under normal conditions does not contribute substantially to the residual β-oxidation activity in this cell type. However, because ABCD1, ABCD2 and ABCD3 could all contribute, directly or possibly indirectly (via ω-oxidation), to the transport and, therefore, to the degradation of VLCFA, the extent of the metabolic deficiency in X-ALD probably differs according to the cell type-specific expression profiles of the three peroxisomal ABC transporters (see Chapter 4 of this review).
The lack of a generalized genotype–phenotype correlation in X-ALD becomes clear from three major findings: i) the same ABCD1 mutation can lead to all possible clinical phenotypes within a single kindred [20]; ii) a complete loss of ABCD1 protein (e.g. early frameshift, nonsense mutations or large deletions); as well as iii) the most common mutation (a two-base pair deletion at c.1415_16delAG in exon 5), has been found in patients with the entire clinical spectrum of X-ALD [21]. However, this does not exclude that individual mutations, which lead to a stable and correctly localized protein that can form dimers and mediate residual transporter activity, can be exclusively associated with the AMN phenotype [22,23].
Interestingly, conventional dietary restriction of VLCFA in X-ALD patients did not lower plasma C26:0 levels [3]. Subsequent studies have demonstrated that the accumulated VLCFA in X-ALD are partially absorbed from the diet but predominantly result from endogenous synthesis through elongation of long- and very long-chain fatty acids [24]. Moreover, it was demonstrated that this elongation system is induced, at least in fibroblasts, of X-ALD patients [24]. This would explain why C22:0 does not accumulate, and often is even slightly reduced, in tissues and cells of X-ALD patients, in spite of the fact that C22:0-CoA is an excellent substrate for ABCD1 [15].
3. Lipidomic aspects of the pathophysiology of X-ALD
In X-ALD, the elongated and insufficiently degraded fatty acids lead to abnormally high levels of VLCFA in various tissues and body fluids. The increased intracellular concentration of VLCFA-CoA esters promotes the incorporation of VLCFA into different complex lipids, where they are normally not enriched. The substrate specificity of different lipid metabolizing enzymes determines the amount of incorporated VLCFA. This varies among different cell types and, importantly, between gray and white matter, thereby effecting crucial regional variation in VLCFA distribution. In normal appearing white matter of post mortem brains of CALD cases, a 39fold excess of C26:0 was found in the phosphatidylcholine fraction compared with white matter tissue of controls [25]. Also an excess of C30:0, C32:0 and C34:0 was found in this lipid fraction [26]. In other phospholipids, such as ethanolamines or serines in the cholesterol ester fraction or in triglycerides, C26:0 levels were either normal or less than twofold elevated in unaffected white matter. VLCFA may be selectively enriched in the ganglioside fraction of white but not gray matter of X-ALD patients. This is of particular interest as myelin sheaths contain specific gangliosides, such as GM4, that are essentially absent in the remaining human body. Given that inflammation in X-ALD is localized within the CNS – although VLCFA accumulate in other tissues as well, it is tempting to speculate that a relationship exists between VLCFA in oligodendrocyte-specific lipids and sites vulnerable to inflammation.
The lipid profile of X-ALD brain tissue changes drastically when actively demyelinating regions are investigated. Within such areas, VLCFA are strongly enriched in cholesterol esters [25,27]. It is believed that the cholesterol esters containing VLCFA are predominantly located in invading monocytes/macrophages, entering the CNS after the opening of the blood–brain barrier occurs, and within activated microglia [28]. ABCD1 deficient macrophages/microglia are unable to degrade VLCFA from phagocytosed myelin debris during the inflammatory demyelinating process. The undegraded VLCFA are then stored as cholesterol esters, which can even form crystalline needles inside these cells, contributing to cellular stress.
Another important issue concerning the pathophysiology of X-ALD is the high concentration of VLCFA in lyso-phosphatidylcholines, resulting from the degradation of phosphatidylcholine via hydrolysis of the fatty acid from the sn-2 position by phospholipase 2A. VLCFA-containing lyso-phosphatidylcholine has been demonstrated to induce microglia apoptosis and macrophage recruitment from the periphery [29]. In fact, 1-hexacosanoyl-2-lyso-sn-3-glycero-phosphorylcholine (26:0-lyso-PC) is a diagnostic marker for X-ALD; and this metabolite is now used in prenatal analysis and newborn screening for X-ALD at the Kennedy Krieger Laboratory and Mayo Clinic [30].
4. Functional redundancy of ABC transporters and their role in the pathophysiology of X-ALD
Inherited defects in the peroxisomal transporter ABCD1 is the underlying genetic defect in X-ALD. Thus, the expression level of ABCD1 in different tissues and cell types is of utmost importance for the understanding of the pathology. Interestingly, ABCD1 is rather weakly expressed in the CNS compared to other tissues such as heart, skeletal muscle, liver, kidney or endocrine glands [31,32]. By immunohistochemical analyses, the highest expression level of ABCD1 in the CNS was detected in microglia, astrocytes and epithelial cells, whereas different populations of oligodendrocytes in the subcortical white matter and cerebellum were described with high, low or undetectable levels of ABCD1 [31]. In most neuronal populations, ABCD1 is barely expressed, with the exception of certain neurons in the hypothalamus, the basal nucleus of Meynert, periaqueductal gray matter and locus coeruleus [32]. With regard to the pathomechanism of the spinal cord axonopathy and sensory neuropathy in AMN, it is striking that ABCD1 is highly expressed in the dorsal root ganglion [32]. Interestingly, many cell populations or tissues in the human body that express high levels of proopiomelanocortin (POMC), such as the pituitary gland, dorsal root ganglion, adrenocortical cells, distal tubules of the kidney, liver and skin, also express high levels of ABCD1 [32,33].
In the target tissues of X-ALD, rather complementary expression patterns have been described for the peroxisomal ABC transporters ABCD1 and ABCD2 [34,35]. For example, in the adrenal gland, ABCD1 is strongly expressed in the cortex but is not detectable in the medulla. Conversely, ABCD2 is strongly expressed in the medulla but not in the cortex. Interestingly, the adrenal pathology in X-ALD is restricted to the cortex, where degeneration leads to Addison disease in X-ALD patients.
Overexpression of ABCD2 and, to a lesser extent, ABCD3 can correct the metabolic defect in fibroblasts of X-ALD patients [36,37]. A mouse model for X-ALD was independently generated in three laboratories by targeted disruption of the Abcd1 gene [38–40]. In Abcd1 deficient mice VLCFA accumulate in all tissues analyzed including brain, spinal cord, adrenal gland, skeletal muscle, kidney, and testis [38–41]. A late-onset neurological phenotype develops at about 18–20 months, with moderate axonal degeneration in the long tracts of the spinal cord but without inflammatory cerebral demyelination; thus, this mouse model is reminiscent of AMN [42,43], although adrenal insufficiency could not be demonstrated [44]. By transgenic ubiquitous overexpression of ABCD2, the biochemical defect and the neurological phenotype of the Abcd1-deficient mice could be rescued [45].
Thus, we hypothesize that the expression levels of ABCD2 – and to a lower extent possibly ABCD3 – contribute to the metabolic manifestation of ABCD1 deficiency in different cell types. Yet association studies have demonstrated that allelic variations of ABCD2 or ABCD3 are unlikely to be involved in the clinical heterogeneity of X-ALD [46,47].
5. Pathophysiology of AMN in males and in heterozygous females
AMN represents the core clinical syndrome in X-ALD. The main symptoms are adrenal insufficiency, gait difficulties and bowel and bladder problems [48,49]. The neurological symptoms are related to a myeloneuropathy. Adrenal insufficiency appears independent of the myeloneuropathy in about two-thirds of the AMN patients; there is no correlation between the duration or severity of endocrine dysfunction and the severity of the myeloneuropathy [48]. The age at onset as well as the rate of progression of the dying-back axonopathy can vary dramatically. In male AMN patients, the earliest onset of neurologic symptoms, with 12 years, has been described in an AMN patient collective of the Kennedy Krieger Institute; the mean age of onset in this cohort was 28 years and the latest was 60 years [50]. The molecular basis for this variability is currently unknown but seems to be independent of the nature of the ABCD1 mutation; therefore the time of onset is unpredictable for pre-symptomatic patients. The individual genetic background (modifiers) may contribute to the broad spectrum. Neurotrophic factors such as ciliary neurotrophic factor (CNTF) or brain-derived neurotrophic factor (BDNF) are candidates.
Histological analyses of the dorsal root ganglia from AMN patients did not show apparent neuronal loss, necrosis or apoptosis [49]. Morphometric studies, however, revealed neuronal atrophy with a decrease in the number of large neurons and a corresponding increase in neurons less than 2000 μm² [49]. Many mitochondria in AMN neurons demonstrate lipid inclusions at the ultrastructural level, raising the possibility that, in addition to the peroxisomal defect, impaired mitochondrial function may contribute to the myelopathy through a failure of ATP-dependent axonal transport in AMN spinal tracts with consequent dying-back axonal degeneration [51].
The issue of mitochondrial alterations in X-ALD has been intensely investigated in the Abcd1-deficient mouse model, where impaired oxidative phosphorylation has been observed in the spinal cord [52] but not in brain or skeletal muscle, although the relative increase in accumulation of VLCFA is comparable in these tissues [41,52]. The reasons for mitochondrial dysfunction in some cell types but not in others, in spite of a similar extent of accumulation of VLCFA, could be: i) differences in the lipid species containing VLCFA (as discussed in Chapter 3 of this review); ii) different susceptibility of mitochondria to VLCFA in different cell types; iii) functional characteristics of vulnerable cell types, such as the dorsal root ganglion cell; or iv) a combination thereof. In the Abcd1-deficient mouse model, lipid peroxidation causes oxidative damage, as evidenced by N-malondialdehyde-lysine concentrations. This was observed already at the age of 3.5 months, long before the first neuropathological lesions in the spinal cord are detectable [53]. In addition, an activation of anti-oxidative responses was observed in organotypic spinal cord slices of Abcd1-deficient mice after exposure to exogenous VLCFA [53]. Interestingly, a treatment of Abcd1-deficient mice with a mixture of antioxidants consisting of N-acetyl-cysteine, α-lipoic acid and α-tocopherol reversed the oxidative damage, the axonal degeneration, as well as the locomotor impairment in bar cross and treadmill tests [54,55].
In plasma and blood cells of human X-ALD patients of either AMN or CALD phenotype, signs of oxidative stress have been detected [56,57]. In two independent studies, normal mitochondrial functions were observed in primary human X-ALD fibroblasts under normal culturing conditions [41,52]. However, when the fibroblasts were exposed to 50 μM C26:0, a slight but significant decrease of oligomycin-sensitive respiration, which is indicative of the activity of the mitochondrial H+-ATP synthase, was observed [52]. Also oxidative stress could be increased in human fibroblasts by the application of VLCFA [53]. Lopez-Erauskin and colleagues postulated a model, in which an excess of C26:0 induces mitochondrial production of reactive oxygen species leading to mitochondrial oxidative damage to DNA and proteins, inefficient oxidative phosphorylation and finally to dying-back axonopathy [52]. In vitro, cytotoxicity of VLCFA has been demonstrated for oligodendrocytes, astrocytes and neurons [58]. In these primary cultures, 20 μM C22:0, C24:0 and C26:0, but not C16:0, caused cell death in 24 h, with VLCFA being most toxic to myelin-producing oligodendrocytes.
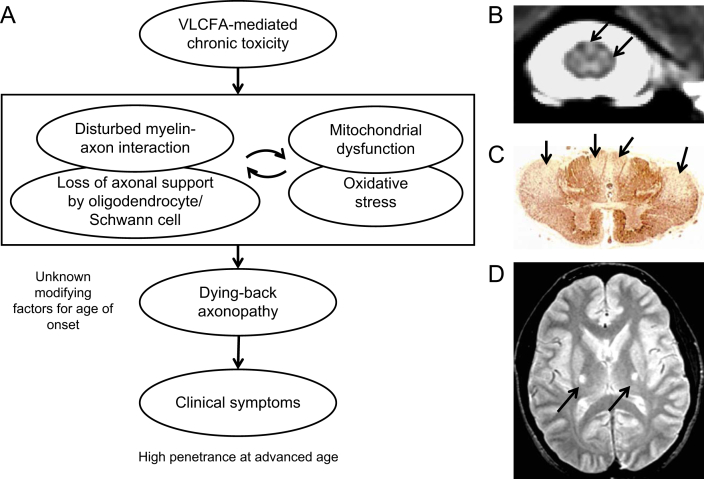
Given the expression pattern of ABCD1 in the nervous system, it seems likely that a non-cell autonomous process is at work. Oligodendrocyte–axonal interaction may be disturbed and constitute a primary trigger for axonal degeneration, oxidative stress and mitochondrial dysfunction in the axons of AMN patients. One argument along this line, is the observation that mice lacking functional peroxisomes only in neurons do not develop axonal degeneration [59]. Also selective deficiency of peroxisomes in oligodendrocytes leads to early axonal loss and neuroinflammation in the absence of demyelination [60]. Recent findings have demonstrated that peroxisomal impairment in Schwann cells of aged mice causes physically unstable paranodal loops that develop swellings filled with vesicles and electron-dense material [61,62]. This novel model of a demyelinating neuropathy demonstrates that peroxisomes serve important functions in the peripheral myelin compartment, required for long-term axonal integrity. A similar mechanism could also contribute to mitochondrial damage and oxidative stress resulting in the dying-back axonopathy seen in AMN. This vicious cycle underlying the pathophysiology of AMN is depicted in Fig. 1A: disturbances in myelin–axon interaction are both the cause and consequence of mitochondrial dysfunction, ultimately resulting in the dying-back axonopathy. The axonopathy in the dorsal and lateral columns can be visualized using magnetization transfer imaging in severely affected patients with AMN (Fig 1B and Ref. [63]). Post mortem the severe atrophy of the lateral columns of the cervical spinal cord of AMN patients can be seen by immunostaining using antibodies against phosphorylated neurofilament (Fig 1C [48]). In some cases, symmetric lesions in the corticospinal tract can be observed on brain MRI of AMN patients (Fig 1D).
Fig. 1.
Hypothetical model and pathology of AMN; (A) Model showing the sequential events leading to the dying-back axonopathy, the main clinical manifestation of AMN. (B) Quantitative magnetization transfer characteristics of the human cervical spinal cord in a severely affected patient with AMN. The arrows indicate the signal hyperintensity in the dorsal and lateral columns (Image was provided by Dr. Ali Fatemi, Department of Neurogenetics, The Kennedy Krieger Institute, Johns Hopkins Medical Institutions, Baltimore, USA). (C) Anti-phosphorylated neurofilament immunostaining of the cervical spinal cord of an AMN patients showing atrophy of the lateral columns (arrows; reproduced with permission from J. Neuropathol. Exp. Neurol.; Powers et al., 2000; 59:89–101). (D) T2-weighted magnetic resonance image of the brain in an AMN patient. The arrows indicate the symmetric lesions in the corticospinal tract.
6. Pathophysiology of inflammation in cerebral X-ALD
New and old concepts suggest that the inability to efficiently degrade VLCFA, as well as the incorporation of VLCFA into different complex lipids, is directly involved in the pathology of the cerebral inflammatory form of X-ALD. However, different mechanisms seem to be involved in: i) the initial cerebral demyelination; ii) the sporadic conversion to rapidly progressive inflammatory demyelination; iii) the environmentally mediated conversion from pure AMN to rapidly progressive inflammatory demyelination; and iv) the inability to arrest inflammation by intrinsic mechanisms or anti-inflammatory therapeutic strategies. No differences in these mechanisms have been observed when comparing childhood and adult cerebral forms of X-ALD. Thus, all inflammatory variants of X-ALD are referred to as CALD within this chapter.
The initiation of cerebral demyelination could well be directly related to the amount of VLCFA in complex lipids, such as phosphatidylcholines, sulfatides or gangliosides. Studies in artificial phospholipid vesicles suggest that the accumulation of VLCFA in myelin could lead to progressive destabilization of myelin sheaths and subsequent demyelination [3,64]. Thus, this initial phase of spontaneous onset of demyelination might be directly related to the level of VLCFA in the myelin sheath. Although VLCFA levels in blood or cultured fibroblasts are indistinguishable in X-ALD patients with different clinical phenotypes, the amounts of VLCFA were found to be higher in normal appearing white matter of CALD patients compared with AMN patients [65]. In addition, oligodendrocytes derived from induced pluripotent stem cells of patients with CALD accumulated more VLCFA than those derived from AMN patients [66].
The demyelination typically begins in the center of the corpus callosum, where the white-matter fiber bundles are the most tightly packed, and spreads outward into the periventricular white matter [67,68]. Genetic segregation analysis provided support for the hypothesis that autosomal genes play a role in the clinical manifestation of X-ALD [50,69,70]. Thus, polymorphisms in genes involved in the different pathways, through which excess levels of VLCFA are redistributed into various lipid species, may play a role in this initial phase of cerebral demyelination. As the majority of VLCFA are derived from endogenous synthesis, the enzymes involved in this pathway such as the elongases of very long-chain fatty acids, ELOVL6 or ELOVL1, are other candidates [71,72]. Thus, it might be a matter of time, the amount of VLCFA in different lipids and/or stochastic factors that determine whether or not and when spontaneous myelin breakdown occurs. In approximately 10–15% of patients that develop cerebral demyelination the demyelinating process halts spontaneously. In these cases contrast enhancement is not seen, and disruption of the blood–brain barrier does not occur [73].
However, in the majority of cases this initial cerebral demyelination converts into a rapidly progressive inflammatory demyelination, opening of the blood–brain barrier and invasion of mononuclear cells predominantly macrophages, many of which contain myelin degradation products [3]. The lesion progresses rapidly in a parieto-occipital distribution in about 80% of cases or in a fronto-parietal distribution in the remaining 20% of cases. The molecular mechanisms responsible for the conversion into full blown inflammation are only poorly understood. It has been shown that decreased brain magnetic resonance perfusion precedes leakage of the blood–brain barrier [74]. This invites speculation on the contribution of blood volume and vascular density to the pathogenesis of demyelination as it has been discussed for decades in the field of multiple sclerosis [75–77]. Another important factor might be that lyso-phosphatidylcholine with incorporated VLCFA can lead to microglial activation and apoptosis [29]. It has been suggested that microglial dysfunction strongly contributes to neuroinflammation and possibly alters the neurovascular unit. Elevated levels of proinflammatory chemokines (IL-8, IL-1ra, MCP-1, MIP-1b) have been observed in the cerebrospinal fluid of CALD patients and correlate with the MRI severity [78]. Areas nearby sites where the blood–brain barrier is disrupted, show infiltration of T cells, mostly CD8 cytotoxic T cells (α/β TCR positive), and, less frequent, B cells into morphologically unaffected white matter [68,79]. This T and B cells invasion beyond the active lesion is strongly suggestive of an immune attack. Cytolysis, rather than apoptosis, appears to be the major mode of oligodendrocytic death [79]. A recent article demonstrated that primary oligodendrocyte death does not elicit anti-CNS immunity [80]. However, these experiments were performed without excess of VLCFA. CD1 molecules, the major MHC-unrestricted lipid antigen presenters, have been observed most prominently within the acute CALD lesion. Interestingly, in particular lipid antigens containing VLCFA, as for example gangliosides, have altered immunological properties [81,82]. As discussed in Chapter 3 of this review, some gangliosides are brain-specific, which would explain why there is no inflammatory reaction in other affected tissues in X-ALD patients, such as peripheral nerve, adrenocortical cells or Leydig cells in the testis. However, also proteolipid protein, a highly abundant protein of myelin, contains VLCFA and might be a candidate antigen for eliciting auto immunity following myelin breakdown in CALD.
There are several reports that a moderate or severe head trauma can initiate the conversion to rapidly progressive inflammatory demyelination [83–85]. In some of these cases, demyelination started at the site of the original contusion [83–85]. This again emphasizes the importance of blood–brain barrier integrity in X-ALD. It is possible that an increased permeability of the blood–brain barrier enhances the recognition of VLCFA-containing antigens such as proteolipid protein or gangliosides, possibly involving CD1 lipid presentation. Head trauma represents only one of several potential environmental factors that can trigger the cerebral phenotype in patients genetically at risk for CALD. The observation that monogenetic twins can present with discordant phenotypes underlines the importance of epigenetic, environmental and stochastic factors in the process of CALD [86].
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only therapeutic approach that can arrest inflammatory cerebral demyelination – when performed at an early stage of disease [6]. It is important to mention that in contrast to HSCT performed for lysosomal CNS diseases, there is no cross-correction of other cell types after HSCT in X-ALD because ABCD1, as a peroxisomal membrane protein cannot be released. The mechanism of HSCT-mediated arrest of the brain inflammation in X-ALD is currently not clear. However, the success of HSC gene therapy indicates that only partial correction of the HSC progeny is necessary to halt cerebral disease [5,6]. It is also important to note that after the transplantation procedure, demyelinating lesions continue to expand, usually for 12–18 months, before the progression is arrested [6].
Within the active lesion, oxidative stress has been observed in activated astrocytes and macrophages [87]. The immunoreactivity of oxidative stress markers (manganese-superoxide dismutase, 4-hydroxynonenal, malondialdehyde) tended to vary directly with the degree of inflammation and myelin breakdown [87]. Invading macrophages and activated microglia cells accumulate VLCFA probably originating from the phagocytosed myelin. As already shown by Schaumburg and coworkers in 1975, this leads to an accumulation of ultrastructural cytoplasmic needle-like crystalline inclusions, later described to consist of VLCFA-containing cholesterol esters [67]. It is suggestive that these intracellular aggregates influence immune functions, such as cytokine secretion, and might be incompatible with the generation of an anti-inflammatory milieu. It may thus not be surprising that traditional anti-inflammatory therapy has failed. The apparent cytotoxicity of lyso-phosphatidylcholine containing VLCFA for macrophages and microglia may additionally be of critical importance [29]. The invasion of macrophages and possibly cross-trafficking of monocytes may be the reason for success of HSCT. Inflammatory demyelination and microglial cell death provides an opportunity for long-term repopulation of the brain parenchyma with residential macrophages/microglia derived from peripheral stem cell progenitors [6,88,89], although other non-cell mediated mechanisms may also be at play. A hypothetical model showing the sequential events leading to the inflammatory demyelination in CALD is depicted in Fig. 2.
Fig. 2.
Hypothetical model showing the sequential events leading to the inflammatory demyelination in CALD. The inset images are characteristic MRI features of a boy afflicted by CALD. The left panel shows a T2-weighted image with a symmetric and confluent demyelinating lesion within the parieto-occipital lobes. The right panel shows a T1-weighted image post gadolinium administration. Gadolinium enhancement indicates active inflammation and disruption of the blood brain barrier.
7. Pathophysiology of adrenals, testis and hair in X-ALD
ABCD1 protein is present in the adrenal cortex but not in the adrenal medulla [32,33], while ABCD2 shows the opposite distribution [34]. This is in good agreement with the pathological findings of lamellae and lamellar–lipid profiles, which were shown to contain VLCFA esterified to cholesterol, in adrenocortical cells [90]. These saturated fatty acids were proposed to be toxic to the adrenal cortex resulting in apoptotic cell death [90]. There is no evidence for a reversal of adrenal failure after hematopoietic cell transplantation in X-ALD [91]. However, hormone replacement therapy successfully manages the adrenal insufficiency.
In the testis of X-ALD patients, lamellae and lamellar–lipid profiles of VLCFA-cholesterol esters are present in interstitial cells of Leydig and their precursors and can be seen at an ultrastructural level. In addition there can be some Leydig cell loss. Degenerative changes in seminiferous tubules in AMN appear indistinguishable from those of adult cerebral ALD [90,92]. In a study of 49 AMN patients an impairment of sexual functions was suffered by 53.8% of the patients [93]. The majority (81.6%) showed testicular dysfunctions reflected by a subnormal testosterone/LH ratio and/or elevated gonadotropins [93].
Hair of AMN patients is typically thin and sparse (alopecia). The scant scalp hair may be related to the fact that ABCD1 is normally well expressed in hair follicles [32]. The thinning of scalp hair can occur as early as in adolescence and does not appear to be predictive for the neurologic phenotype [94].
8. Concluding remarks
X-linked adrenoleukodystrophy is a genetically and metabolically well-defined disease that poses challenges in explaining the clinical heterogeneity of phenotypes. While the presentation of AMN is the default manifestation of mutations in ABCD1, the cerebral disease cannot be explained by mutations in ABCD1 alone. Here, ABCD1 remains a susceptibility gene, necessary but not sufficient for inflammatory demyelination to occur. The systematic nature of the inflammatory disease raises questions as to the role of the vascular supply and blood–brain barrier integrity. We conclude that in both AMN and CALD, the pathophysiology is a dynamic cell autonomous and non-cell autonomous process during which, despite the Mendelian nature of the disease, environmental and epigenetic factors are crucial. It is important to consider the fundamentally different molecular mechanisms and cell interactions underlying AMN and CALD while developing novel therapeutic strategies urgently needed for these phenotypes.
Acknowledgments
This work was supported by the Austrian Science Fund (FWF): P26112-B19. The authors thank Dr. Ali Fatemi for providing the image for Fig. 1B and Dr. Wolfgang Köhler for his discussion and contribution to Fig. 1A.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Bezman L., Moser H.W. Incidence of X-linked adrenoleukodystrophy and the relative frequency of its phenotypes. Am. J. Med. Genet. 1998;76:415–419. [PubMed] [Google Scholar]
- 2.Kemp S., Berger J., Aubourg P. X-linked adrenoleukodystrophy: clinical, metabolic, genetic and pathophysiological aspects. Biochim. Biophys. Acta. 2012;1822:1465–1474. doi: 10.1016/j.bbadis.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Moser H.W., Smith K.D., Watkins P.A., Powers J., Moser A.B. X-linked Adrenoleukodystrophy. In: Scriver R., Beaudet A.L., Sly W.S., Valle D., editors. The Metabolic & Molecular Bases of Inherited Disease. eighth ed. McGraw-Hill Book Co.; New York: 2001. pp. 3257–3301. [Google Scholar]
- 4.Peters C., Charnas L.R., Tan Y., Ziegler R.S., Shapiro E.G., DeFor T., Grewal S.S., Orchard P.J., Abel S.L., Goldman A.I., Ramsay N.K., Dusenbery K.E., Loes D.J., Lockman L.A., Kato S., Aubourg P.R., Moser H.W., Krivit W. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004;104:881–888. doi: 10.1182/blood-2003-10-3402. [DOI] [PubMed] [Google Scholar]
- 5.Cartier N., Hacein-Bey-Abina S., Bartholomae C.C., Veres G., Schmidt M., Kutschera I., Vidaud M., Abel U., Dal-Cortivo L., Caccavelli L., Mahlaoui N., Kiermer V., Mittelstaedt D., Bellesme C., Lahlou N., Lefrere F., Blanche S., Audit M., Payen E., Leboulch P., l'Homme B., Bougneres P., Von Kalle C., Fischer A., Cavazzana-Calvo M., Aubourg P. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 6.Cartier N., Aubourg P. Hematopoietic stem cell transplantation and hematopoietic stem cell gene therapy in X-linked adrenoleukodystrophy. Brain Pathol. 2010;20:857–862. doi: 10.1111/j.1750-3639.2010.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hershkovitz E., Narkis G., Shorer Z., Moser A.B., Watkins P.A., Moser H.W., Manor E. Cerebral X-linked adrenoleukodystrophy in a girl with Xq27-Ter deletion. Ann. Neurol. 2002;52:234–237. doi: 10.1002/ana.10248. [DOI] [PubMed] [Google Scholar]
- 8.Moser H.W., Mahmood A., Raymond G.V. X-linked adrenoleukodystrophy. Nat. Clin. Pract. Neurol. 2007;3:140–151. doi: 10.1038/ncpneuro0421. [DOI] [PubMed] [Google Scholar]
- 9.Maier E.M., Kammerer S., Muntau A.C., Wichers M., Braun A., Roscher A.A. Symptoms in carriers of adrenoleukodystrophy relate to skewed X inactivation. Ann. Neurol. 2002;52:683–688. doi: 10.1002/ana.10376. [DOI] [PubMed] [Google Scholar]
- 10.Thibert R., Hyland K., Chiles J., Steinberg S., Eichler F. Levodopa response reveals sepiapterin reductase deficiency in a female heterozygote with adrenoleukodystrophy. JIMD Rep. 2012;3:79–82. doi: 10.1007/8904_2011_65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosser J., Douar A.M., Sarde C.O., Kioschis P., Feil R., Moser H., Poustka A.M., Mandel J.L., Aubourg P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361:726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- 12.Kemp S., Pujol A., Waterham H.R., van Geel B.M., Boehm C.D., Raymond G.V., Cutting G.R., Wanders R.J., Moser H.W. ABCD1 mutations and the X-linked adrenoleukodystrophy mutation database: role in diagnosis and clinical correlations. Hum. Mutat. 2001;18:499–515. doi: 10.1002/humu.1227. [DOI] [PubMed] [Google Scholar]
- 13.van Roermund C.W., Visser W.F., Ijlst L., Waterham H.R., Wanders R.J. Differential substrate specificities of human ABCD1 and ABCD2 in peroxisomal fatty acid beta-oxidation. Biochim. Biophys. Acta. 2011;1811:148–152. doi: 10.1016/j.bbalip.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 14.van Roermund C.W., Visser W.F., Ijlst L., van Cruchten A., Boek M., Kulik W., Waterham H.R., Wanders R.J. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 2008;22:4201–4208. doi: 10.1096/fj.08-110866. [DOI] [PubMed] [Google Scholar]
- 15.Wiesinger C., Kunze M., Regelsberger G., Forss-Petter S., Berger J. Impaired very long-chain acyl-CoA beta-oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dysfunction. J. Biol. Chem. 2013;288:19269–19279. doi: 10.1074/jbc.M112.445445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamijo K., Taketani S., Yokota S., Osumi T., Hashimoto T. The 70-kDa peroxisomal membrane protein is a member of the Mdr (P-glycoprotein)-related ATP-binding protein superfamily. J. Biol. Chem. 1990;265:4534–4540. [PubMed] [Google Scholar]
- 17.Lombard-Platet G., Savary S., Sarde C.O., Mandel J.L., Chimini G. A close relative of the adrenoleukodystrophy (ALD) gene codes for a peroxisomal protein with a specific expression pattern. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1265–1269. doi: 10.1073/pnas.93.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holzinger A., Kammerer S., Berger J., Roscher A.A. cDNA cloning and mRNA expression of the human adrenoleukodystrophy related protein (ALDRP), a peroxisomal ABC transporter. Biochem. Biophys. Res. Commun. 1997;239:261–264. doi: 10.1006/bbrc.1997.7391. [DOI] [PubMed] [Google Scholar]
- 19.Berger J., Gartner J. X-linked adrenoleukodystrophy: clinical, biochemical and pathogenetic aspects. Biochim. Biophys. Acta. 2006;1763:1721–1732. doi: 10.1016/j.bbamcr.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Berger J., Molzer B., Fae I., Bernheimer H. X-linked adrenoleukodystrophy (ALD): a novel mutation of the ALD gene in 6 members of a family presenting with 5 different phenotypes. Biochem. Biophys. Res. Commun. 1994;205:1638–1643. doi: 10.1006/bbrc.1994.2855. [DOI] [PubMed] [Google Scholar]
- 21.Smith K.D., Kemp S., Braiterman L.T., Lu J.F., Wei H.M., Geraghty M., Stetten G., Bergin J.S., Pevsner J., Watkins P.A. X-linked adrenoleukodystrophy: genes, mutations, and phenotypes. Neurochem. Res. 1999;24:521–535. doi: 10.1023/a:1022535930009. [DOI] [PubMed] [Google Scholar]
- 22.Guimaraes C.P., Lemos M., Menezes I., Coelho T., Sa-Miranda C., Azevedo J.E. Characterisation of two mutations in the ABCD1 gene leading to low levels of normal ALDP. Hum. Genet. 2001;109:616–622. doi: 10.1007/s00439-001-0632-z. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill G.N., Aoki M., Brown R.H., Jr. ABCD1 translation-initiator mutation demonstrates genotype-phenotype correlation for AMN. Neurology. 2001;57:1956–1962. doi: 10.1212/wnl.57.11.1956. [DOI] [PubMed] [Google Scholar]
- 24.Kemp S., Valianpour F., Denis S., Ofman R., Sanders R.J., Mooyer P., Barth P.G., Wanders R.J. Elongation of very long-chain fatty acids is enhanced in X-linked adrenoleukodystrophy. Mol. Genet. Metab. 2005;84:144–151. doi: 10.1016/j.ymgme.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Theda C., Moser A.B., Powers J.M., Moser H.W. Phospholipids in X-linked adrenoleukodystrophy white matter: fatty acid abnormalities before the onset of demyelination. J. Eurol. Sci. 1992;110:195–204. doi: 10.1016/0022-510x(92)90028-j. [DOI] [PubMed] [Google Scholar]
- 26.Sharp P., Johnson D., Poulos A. Molecular species of phosphatidylcholine containing very long chain fatty acids in human brain: enrichment in X-linked adrenoleukodystrophy brain and diseases of peroxisome biogenesis brain. J. Neurochem. 1991;56:30–37. doi: 10.1111/j.1471-4159.1991.tb02558.x. [DOI] [PubMed] [Google Scholar]
- 27.Khan M., Pahan K., Singh A.K., Singh I. Cytokine-induced accumulation of very long-chain fatty acids in rat C6 glial cells: implication for X-adrenoleukodystrophy. J. Neurochem. 1998;71:78–87. doi: 10.1046/j.1471-4159.1998.71010078.x. [DOI] [PubMed] [Google Scholar]
- 28.Schaumburg H.H., Powers J.M., Suzuki K., Raine C.S. Adreno-leukodystrophy (sex-linked Schilder disease). Ultrastructural demonstration of specific cytoplasmic inclusions in the central nervous system. Arch. Neurol. 1974;31:210–213. doi: 10.1001/archneur.1974.00490390092013. [DOI] [PubMed] [Google Scholar]
- 29.Eichler F.S., Ren J.Q., Cossoy M., Rietsch A.M., Nagpal S., Moser A.B., Frosch M.P., Ransohoff R.M. Is microglial apoptosis an early pathogenic change in cerebral X-linked adrenoleukodystrophy? Ann. Neurol. 2008;63:729–742. doi: 10.1002/ana.21391. [DOI] [PubMed] [Google Scholar]
- 30.Hubbard W.C., Moser A.B., Liu A.C., Jones R.O., Steinberg S.J., Lorey F., Panny S.R., Vogt R.F., Jr., Macaya D., Turgeon C.T., Tortorelli S., Raymond G.V. Newborn screening for X-linked adrenoleukodystrophy (X-ALD): validation of a combined liquid chromatography-tandem mass spectrometric (LC-MS/MS) method. Mol. Genet. Metab. 2009;97:212–220. doi: 10.1016/j.ymgme.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Fouquet F., Zhou J.M., Ralston E., Murray K., Troalen F., Magal E., Robain O., Dubois-Dalcq M., Aubourg P. Expression of the adrenoleukodystrophy protein in the human and mouse central nervous system. Neurobiol. Dis. 1997;3:271–285. doi: 10.1006/nbdi.1997.0127. [DOI] [PubMed] [Google Scholar]
- 32.Hoftberger R., Kunze M., Weinhofer I., Aboul-Enein F., Voigtlander T., Oezen I., Amann G., Bernheimer H., Budka H., Berger J. Distribution and cellular localization of adrenoleukodystrophy protein in human tissues: implications for X-linked adrenoleukodystrophy. Neurobiol. Dis. 2007;28:165–174. doi: 10.1016/j.nbd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Hoftberger R., Kunze M., Voigtlander T., Unterberger U., Regelsberger G., Bauer J., Aboul-Enein F., Garzuly F., Forss-Petter S., Bernheimer H., Berger J., Budka H. Peroxisomal localization of the proopiomelanocortin-derived peptides beta-lipotropin and beta-endorphin. Endocrinology. 2010;151:4801–4810. doi: 10.1210/en.2010-0249. [DOI] [PubMed] [Google Scholar]
- 34.Troffer-Charlier N., Doerflinger N., Metzger E., Fouquet F., Mandel J.L., Aubourg P. Mirror expression of adrenoleukodystrophy and adrenoleukodystrophy related genes in mouse tissues and human cell lines. Eur. J. Cell Biol. 1998;75:254–264. doi: 10.1016/S0171-9335(98)80121-0. [DOI] [PubMed] [Google Scholar]
- 35.Berger J., Albet S., Bentejac M., Netik A., Holzinger A., Roscher A.A., Bugaut M., Forss-Petter S. The four murine peroxisomal ABC-transporter genes differ in constitutive, inducible and developmental expression. Eur. J. Biochem. 1999;265:719–727. doi: 10.1046/j.1432-1327.1999.00772.x. [DOI] [PubMed] [Google Scholar]
- 36.Netik A., Forss-Petter S., Holzinger A., Molzer B., Unterrainer G., Berger J. Adrenoleukodystrophy-related protein can compensate functionally for adrenoleukodystrophy protein deficiency (X-ALD): implications for therapy. Hum. Mol. Genet. 1999;8:907–913. doi: 10.1093/hmg/8.5.907. [DOI] [PubMed] [Google Scholar]
- 37.Kemp S., Wei H.M., Lu J.F., Braiterman L.T., McGuinness M.C., Moser A.B., Watkins P.A., Smith K.D. Gene redundancy and pharmacological gene therapy: implications for X-linked adrenoleukodystrophy. Nat. Med. 1998;4:1261–1268. doi: 10.1038/3242. [DOI] [PubMed] [Google Scholar]
- 38.Forss-Petter S., Werner H., Berger J., Lassmann H., Molzer B., Schwab M.H., Bernheimer H., Zimmermann F., Nave K.A. Targeted inactivation of the X-linked adrenoleukodystrophy gene in mice. J. Neurosci. Res. 1997;50:829–843. doi: 10.1002/(SICI)1097-4547(19971201)50:5<829::AID-JNR19>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 39.Lu J.F., Lawler A.M., Watkins P.A., Powers J.M., Moser A.B., Moser H.W., Smith K.D. A mouse model for X-linked adrenoleukodystrophy. Proc. Natl. Acad. Sci. U. S. A. 1997;94:9366–9371. doi: 10.1073/pnas.94.17.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi T., Shinnoh N., Kondo A., Yamada T. Adrenoleukodystrophy protein-deficient mice represent abnormality of very long chain fatty acid metabolism. Biochem. Biophys. Res. Commun. 1997;232:631–636. doi: 10.1006/bbrc.1997.6340. [DOI] [PubMed] [Google Scholar]
- 41.Oezen I., Rossmanith W., Forss-Petter S., Kemp S., Voigtlander T., Moser-Thier K., Wanders R.J., Bittner R.E., Berger J. Accumulation of very long-chain fatty acids does not affect mitochondrial function in adrenoleukodystrophy protein deficiency. Hum. Mol. Genet. 2005;14:1127–1137. doi: 10.1093/hmg/ddi125. [DOI] [PubMed] [Google Scholar]
- 42.Pujol A., Hindelang C., Callizot N., Bartsch U., Schachner M., Mandel J.L. Late onset neurological phenotype of the X-ALD gene inactivation in mice: a mouse model for adrenomyeloneuropathy. Hum. Mol. Genet. 2002;11:499–505. doi: 10.1093/hmg/11.5.499. [DOI] [PubMed] [Google Scholar]
- 43.Dumser M., Bauer J., Lassmann H., Berger J., Forss-Petter S. Lack of adrenoleukodystrophy protein enhances oligodendrocyte disturbance and microglia activation in mice with combined Abcd1/Mag deficiency. Acta Neuropathol. 2007;114:573–586. doi: 10.1007/s00401-007-0288-4. [DOI] [PubMed] [Google Scholar]
- 44.Lu J.F., Barron-Casella E., Deering R., Heinzer A.K., Moser A.B., deMesy K.L., Bentley G.S., Wand C.M.M., Pei Z., Watkins P.A., Pujol A., Smith K.D., Powers J.M. The role of peroxisomal ABC transporters in the mouse adrenal gland: the loss of Abcd2 (ALDR), Not Abcd1 (ALD), causes oxidative damage. Lab. Invest. 2007;87:261–272. doi: 10.1038/labinvest.3700512. [DOI] [PubMed] [Google Scholar]
- 45.Pujol A., Ferrer I., Camps C., Metzger E., Hindelang C., Callizot N., Ruiz M., Pampols T., Giros M., Mandel J.L. Functional overlap between ABCD1 (ALD) and ABCD2 (ALDR) transporters: a therapeutic target for X-adrenoleukodystrophy. Hum. Mol. Genet. 2004;13:2997–3006. doi: 10.1093/hmg/ddh323. [DOI] [PubMed] [Google Scholar]
- 46.Maier E.M., Mayerhofer P.U., Asheuer M., Kohler W., Rothe M., Muntau A.C., Roscher A.A., Holzinger A., Aubourg P., Berger J. X-linked adrenoleukodystrophy phenotype is independent of ABCD2 genotype. Biochem. Biophys. Res. Commun. 2008;377:176–180. doi: 10.1016/j.bbrc.2008.09.092. [DOI] [PubMed] [Google Scholar]
- 47.Matsukawa T., Asheuer M., Takahashi Y., Goto J., Suzuki Y., Shimozawa N., Takano H., Onodera O., Nishizawa M., Aubourg P., Tsuji S. Identification of novel SNPs of ABCD1, ABCD2, ABCD3, and ABCD4 genes in patients with X-linked adrenoleukodystrophy (ALD) based on comprehensive resequencing and association studies with ALD phenotypes. Neurogenetics. 2011;12:41–50. doi: 10.1007/s10048-010-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powers J.M., DeCiero D.P., Ito M., Moser A.B., Moser H.W. Adrenomyeloneuropathy: a neuropathologic review featuring its noninflammatory myelopathy. J. Neuropathol. Exp. Neurol. 2000;59:89–102. doi: 10.1093/jnen/59.2.89. [DOI] [PubMed] [Google Scholar]
- 49.Powers J.M., DeCiero D.P., Cox C., Richfield E.K., Ito M., Moser A.B., Moser H.W. The dorsal root ganglia in adrenomyeloneuropathy: neuronal atrophy and abnormal mitochondria. J. Neuropathol. Exp. Neurol. 2001;60:493–501. doi: 10.1093/jnen/60.5.493. [DOI] [PubMed] [Google Scholar]
- 50.Moser H.W., Moser A.B., Smith K.D., Bergin A., Borel J., Shankroff J., Stine O.C., Merette C., Ott J., Krivit W. Adrenoleukodystrophy: phenotypic variability and implications for therapy. J. Inherit. Metab. Dis. 1992;15:645–664. doi: 10.1007/BF01799621. [DOI] [PubMed] [Google Scholar]
- 51.Powers J.M. Adreno-leukodystrophy: a personal historical note. Acta Neuropathol. 2005;109:124–127. doi: 10.1007/s00401-004-0961-9. [DOI] [PubMed] [Google Scholar]
- 52.Lopez-Erauskin J., Galino J., Ruiz M., Cuezva J.M., Fabregat I., Cacabelos D., Boada J., Martinez J., Ferrer I., Pamplona R., Villarroya F., Portero-Otin M., Fourcade S., Pujol A. Impaired mitochondrial oxidative phosphorylation in the peroxisomal disease X-linked adrenoleukodystrophy. Hum. Mol. Genet. 2013;22:3296–3305. doi: 10.1093/hmg/ddt186. [DOI] [PubMed] [Google Scholar]
- 53.Fourcade S., Lopez-Erauskin J., Galino J., Duval C., Naudi A., Jove M., Kemp S., Villarroya F., Ferrer I., Pamplona R., Portero-Otin M., Pujol A. Early oxidative damage underlying neurodegeneration in X-adrenoleukodystrophy. Hum. Mol. Genet. 2008;17:1762–1773. doi: 10.1093/hmg/ddn085. [DOI] [PubMed] [Google Scholar]
- 54.Lopez-Erauskin J., Fourcade S., Galino J., Ruiz M., Schluter A., Naudi A., Jove M., Portero-Otin M., Pamplona R., Ferrer I., Pujol A. Antioxidants halt axonal degeneration in a mouse model of X-adrenoleukodystrophy. Ann. Neurol. 2011;70:84–92. doi: 10.1002/ana.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galea E., Launay N., Portero-Otin M., Ruiz M., Pamplona R., Aubourg P., Ferrer I., Pujol A. Oxidative stress underlying axonal degeneration in adrenoleukodystrophy: a paradigm for multifactorial neurodegenerative diseases? Biochim. Biophys. Acta. 2012;1822:1475–1488. doi: 10.1016/j.bbadis.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Vargas C.R., Wajner M., Sirtori L.R., Goulart L., Chiochetta M., Coelho D., Latini A., Llesuy S., Bello-Klein A., Giugliani R., Deon M., Mello C.F. Evidence that oxidative stress is increased in patients with X-linked adrenoleukodystrophy. Biochim. Biophys. Acta. 2004;1688:26–32. doi: 10.1016/j.bbadis.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Petrillo S., Piemonte F., Pastore A., Tozzi G., Aiello C., Pujol A., Cappa M., Bertini E. Glutathione imbalance in patients with X-linked adrenoleukodystrophy. Mol. Genet. Metab. 2013;109:366–370. doi: 10.1016/j.ymgme.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hein S., Schonfeld P., Kahlert S., Reiser G. Toxic effects of X-linked adrenoleukodystrophy-associated, very long chain fatty acids on glial cells and neurons from rat hippocampus in culture. Hum. Mol. Genet. 2008;17:1750–1761. doi: 10.1093/hmg/ddn066. [DOI] [PubMed] [Google Scholar]
- 59.Bottelbergs A., Verheijden S., Hulshagen L., Gutmann D.H., Goebbels S., Nave K.A., Kassmann C., Baes M. Axonal integrity in the absence of functional peroxisomes from projection neurons and astrocytes. Glia. 2010;58:1532–1543. doi: 10.1002/glia.21027. [DOI] [PubMed] [Google Scholar]
- 60.Kassmann C.M., Lappe-Siefke C., Baes M., Brugger B., Mildner A., Werner H.B., Natt O., Michaelis T., Prinz M., Frahm J., Nave K.A. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 2007;39:969–976. doi: 10.1038/ng2070. [DOI] [PubMed] [Google Scholar]
- 61.Kassmann C.M., Nave K.A. Oligodendroglial impact on axonal function and survival – a hypothesis. Curr. Opin. Neurol. 2008;21:235–241. doi: 10.1097/WCO.0b013e328300c71f. [DOI] [PubMed] [Google Scholar]
- 62.Kassmann C.M., Quintes S., Rietdorf J., Mobius W., Sereda M.W., Nientiedt T., Saher G., Baes M., Nave K.A. A role for myelin-associated peroxisomes in maintaining paranodal loops and axonal integrity. FEBS Lett. 2011;585:2205–2211. doi: 10.1016/j.febslet.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 63.Fatemi A., Smith S.A., Dubey P., Zackowski K.M., Bastian A.J., van Zijl P.C., Moser H.W., Raymond G.V., Golay X. Magnetization transfer MRI demonstrates spinal cord abnormalities in adrenomyeloneuropathy. Neurology. 2005;64:1739–1745. doi: 10.1212/01.WNL.0000164458.02141.06. [DOI] [PubMed] [Google Scholar]
- 64.Ho J.K., Moser H., Kishimoto Y., Hamilton J.A. Interactions of a very long chain fatty acid with model membranes and serum albumin. Implications for the pathogenesis of adrenoleukodystrophy. J. Clin. Invest. 1995;96:1455–1463. doi: 10.1172/JCI118182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asheuer M., Bieche I., Laurendeau I., Moser A., Hainque B., Vidaud M., Aubourg P. Decreased expression of ABCD4 and BG1 genes early in the pathogenesis of X-linked adrenoleukodystrophy. Hum. Mol. Genet. 2005;14:1293–1303. doi: 10.1093/hmg/ddi140. [DOI] [PubMed] [Google Scholar]
- 66.Jang J., Kang H.C., Kim H.S., Kim J.Y., Huh Y.J., Kim D.S., Yoo J.E., Lee J.A., Lim B., Lee J., Yoon T.M., Park I.H., Hwang D.Y., Daley G.Q., Kim D.W. Induced pluripotent stem cell models from X-linked adrenoleukodystrophy patients. Ann. Neurol. 2011;70:402–409. doi: 10.1002/ana.22486. [DOI] [PubMed] [Google Scholar]
- 67.Schaumburg H.H., Powers J.M., Raine C.S., Suzuki K., Richardson E.P., Jr. Adrenoleukodystrophy. A clinical and pathological study of 17 cases. Arch. Neurol. 1975;32:577–591. doi: 10.1001/archneur.1975.00490510033001. [DOI] [PubMed] [Google Scholar]
- 68.Powers J.M., Liu Y., Moser A.B., Moser H.W. The inflammatory myelinopathy of adreno-leukodystrophy: cells, effector molecules, and pathogenetic implications. J. Neuropathol. Exp. Neurol. 1992;51:630–643. doi: 10.1097/00005072-199211000-00007. [DOI] [PubMed] [Google Scholar]
- 69.Maestri N.E., Beaty T.H. Predictions of a 2-locus model for disease heterogeneity: application to adrenoleukodystrophy. Am. J. Med. Genet. 1992;44:576–582. doi: 10.1002/ajmg.1320440509. [DOI] [PubMed] [Google Scholar]
- 70.Smith K.D., Sack G., Beaty T., Bergin A., Naidu S., Moser A., Moser H. A genetic-basis for the multiple phenotypes of X-linked adrenoleukodystrophy. Am. J. Hum. Genet. 1991;49 165–165. [Google Scholar]
- 71.Ofman R., Dijkstra I.M., van Roermund C.W., Burger N., Turkenburg M., van Cruchten A., van Engen C.E., Wanders R.J., Kemp S. The role of ELOVL1 in very long-chain fatty acid homeostasis and X-linked adrenoleukodystrophy. EMBO Mol. Med. 2010;2:90–97. doi: 10.1002/emmm.201000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kemp S., Wanders R. Biochemical aspects of X-linked adrenoleukodystrophy. Brain Pathol. 2010;20:831–837. doi: 10.1111/j.1750-3639.2010.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Korenke G.C., Pouwels P.J., Frahm J., Hunneman D.H., Stoeckler S., Krasemann E., Jost W., Hanefeld F. Arrested cerebral adrenoleukodystrophy: a clinical and proton magnetic resonance spectroscopy study in three patients. Pediatr. Neurol. 1996;15:103–107. doi: 10.1016/0887-8994(95)00156-5. [DOI] [PubMed] [Google Scholar]
- 74.Musolino P.L., Rapalino O., Caruso P., Caviness V.S., Eichler F.S. Hypoperfusion predicts lesion progression in cerebral X-linked adrenoleukodystrophy. Brain. 2012;135:2676–2683. doi: 10.1093/brain/aws206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Putnam T.J., Wilcox H.B. A reducing substance found in chromophilic adenomas and in the normal anterior pituitary. Am. J. Pathol. 1933;9:649–650. [PMC free article] [PubMed] [Google Scholar]
- 76.Arnold A.C., Pepose J.S., Hepler R.S., Foos R.Y. Retinal periphlebitis and retinitis in multiple sclerosis. I. Pathologic characteristics. Ophthalmology. 1984;91:255–262. doi: 10.1016/s0161-6420(84)34296-8. [DOI] [PubMed] [Google Scholar]
- 77.Lightman S., McDonald W.I., Bird A.C., Francis D.A., Hoskins A., Batchelor J.R., Halliday A.M. Retinal venous sheathing in optic neuritis. Its significance for the pathogenesis of multiple sclerosis. Brain. 1987;110:405–414. doi: 10.1093/brain/110.2.405. [DOI] [PubMed] [Google Scholar]
- 78.Lund T.C., Stadem P.S., Panoskaltsis-Mortari A., Raymond G., Miller W.P., Tolar J., Orchard P.J. Elevated cerebral spinal fluid cytokine levels in boys with cerebral adrenoleukodystrophy correlates with MRI severity. PLoS One. 2012;7:e32218. doi: 10.1371/journal.pone.0032218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ito M., Blumberg B.M., Mock D.J., Goodman A.D., Moser A.B., Moser H.W., Smith K.D., Powers J.M. Potential environmental and host participants in the early white matter lesion of adreno-leukodystrophy: morphologic evidence for CD8 cytotoxic T cells, cytolysis of oligodendrocytes, and CD1-mediated lipid antigen presentation. J. Neuropathol. Exp. Neurol. 2001;60:1004–1019. doi: 10.1093/jnen/60.10.1004. [DOI] [PubMed] [Google Scholar]
- 80.Locatelli G., Wortge S., Buch T., Ingold B., Frommer F., Sobottka B., Kruger M., Karram K., Buhlmann C., Bechmann I., Heppner F.L., Waisman A., Becher B. Primary oligodendrocyte death does not elicit anti-CNS immunity. Nat. Neurosci. 2012;15:543–550. doi: 10.1038/nn.3062. [DOI] [PubMed] [Google Scholar]
- 81.Kannagi R., Nudelman E., Hakomori S. Possible role of ceramide in defining structure and function of membrane glycolipids. Proc. Natl. Acad. Sci. U. S. A. 1982;79:3470–3474. doi: 10.1073/pnas.79.11.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tagawa Y., Laroy W., Nimrichter L., Fromholt S.E., Moser A.B., Moser H.W., Schnaar R.L. Anti-ganglioside antibodies bind with enhanced affinity to gangliosides containing very long chain fatty acids. Neurochem. Res. 2002;27:847–855. doi: 10.1023/a:1020221410895. [DOI] [PubMed] [Google Scholar]
- 83.Raymond G.V., Seidman R., Monteith T.S., Kolodny E., Sathe S., Mahmood A., Powers J.M. Head trauma can initiate the onset of adreno-leukodystrophy. J. Neurol. Sci. 2010;290:70–74. doi: 10.1016/j.jns.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 84.Weller M., Liedtke W., Petersen D., Opitz H., Poremba M. Very-late-onset adrenoleukodystrophy: possible precipitation of demyelination by cerebral contusion. Neurology. 1992;42:367–370. doi: 10.1212/wnl.42.2.367. [DOI] [PubMed] [Google Scholar]
- 85.Berger J., Pujol A., Aubourg P., Forss-Petter S. Current and future pharmacological treatment strategies in X-linked adrenoleukodystrophy. Brain Pathol. 2010;20:845–856. doi: 10.1111/j.1750-3639.2010.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferrer I., Aubourg P., Pujol A. General aspects and neuropathology of X-linked adrenoleukodystrophy. Brain Pathol. 2010;20:817–830. doi: 10.1111/j.1750-3639.2010.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Powers J.M., Pei Z., Heinzer A.K., Deering R., Moser A.B., Moser H.W., Watkins P.A., Smith K.D. Adreno-leukodystrophy: oxidative stress of mice and men. J. Neuropathol. Exp. Neurol. 2005;64:1067–1079. doi: 10.1097/01.jnen.0000190064.28559.a4. [DOI] [PubMed] [Google Scholar]
- 88.Varvel N.H., Grathwohl S.A., Baumann F., Liebig C., Bosch A., Brawek B., Thal D.R., Charo I.F., Heppner F.L., Aguzzi A., Garaschuk O., Ransohoff R.M., Jucker M. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc. Natl. Acad. Sci. U. S. A. 2012;109:18150–18155. doi: 10.1073/pnas.1210150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davoust N., Vuaillat C., Androdias G., Nataf S. From bone marrow to microglia: barriers and avenues. Trends Immunol. 2008;29:227–234. doi: 10.1016/j.it.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Powers J.M., Moser H.W., Moser A.B., Schaumburg H.H. Fetal adrenoleukodystrophy: the significance of pathologic lesions in adrenal gland and testis. Hum. Pathol. 1982;13:1013–1019. doi: 10.1016/s0046-8177(82)80093-2. [DOI] [PubMed] [Google Scholar]
- 91.Petryk A., Polgreen L.E., Chahla S., Miller W., Orchard P.J. No evidence for the reversal of adrenal failure after hematopoietic cell transplantation in X-linked adrenoleukodystrophy. Bone Marrow Transplant. 2012;47:1377–1378. doi: 10.1038/bmt.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Powers J.M., Schaumburg H.H. The testis in adreno-leukodystrophy. Am. J. Pathol. 1981;102:90–98. [PMC free article] [PubMed] [Google Scholar]
- 93.Brennemann W., Kohler W., Zierz S., Klingmuller D. Testicular dysfunction in adrenomyeloneuropathy. Eur. J. Endocrinol. 1997;137:34–39. doi: 10.1530/eje.0.1370034. [DOI] [PubMed] [Google Scholar]
- 94.Harris-Jones J.N., Nixon P.G. Familial Addison's disease with spastic paraplegia. J. Clin. Endocrinol. Metab. 1955;15:739–744. doi: 10.1210/jcem-15-6-739. [DOI] [PubMed] [Google Scholar]