Abstract
Objective. The aim of this study was to compare efficacy outcomes of initial treatment with adalimumab + MTX vs adalimumab addition following 26 weeks of MTX monotherapy in Japanese early RA patients naive to MTX with high disease activity.
Methods. Patients completing the 26-week, randomized, placebo-controlled trial of adalimumab + MTX were eligible to receive 26 weeks of open-label adalimumab + MTX. Patients were assessed for mean change from baseline in the 28-joint DAS with ESR (DAS28-ESR) and modified total Sharp score (mTSS), and for the proportions of patients achieving clinical, functional or radiographic remission.
Results. Of 333 patients assessed, 278 (137 from the initial adalimumab + MTX and 141 from the initial placebo + MTX groups) completed the 52-week study. Significant differences in clinical and functional parameters observed during the 26-week blinded period were not apparent following the addition of open-label adalimumab to MTX. Open-label adalimumab + MTX slowed radiographic progression through week 52 in both groups, but patients who received adalimumab + MTX throughout the study exhibited less radiographic progression than those who received placebo + MTX during the first 26 weeks (mean ΔmTSS at week 52 = 2.56 vs 3.30, P < 0.001).
Conclusion. Delayed addition of adalimumab in Japanese MTX-naive early RA patients did not impact clinical and functional outcomes at week 52 compared with the earlier addition of adalimumab. However, the accrual of significant structural damage during blinded placebo + MTX therapy contributed to the persistence of differences between the treatment strategies, suggesting that Japanese patients at risk for aggressive disease should benefit from the early inclusion of adalimumab + MTX combination therapy.
Trial registration. ClinicalTrials.gov (http://clinicaltrials.gov/), NCT00870467.
Keywords: adalimumab, rheumatoid arthritis, Japanese patients, MTX naive, safety
Introduction
RA is a debilitating disease associated with inflammation of the synovial tissue in affected joints. Progression of the disease, if not abated, may lead to the erosive loss of bone and cartilage in affected joints and subsequent physical disability. The early inclusion of effective therapies aimed at tight control of disease activity minimizes the risk of irreversible erosive damage [1–4].
International recommendations suggest treatment initiation with MTX administered as monotherapy, which, in the event of an inadequate response, can then be supplemented with or switched to additional synthetic DMARDs or a biologic agent [5, 6]. Patients at risk for aggressive disease (e.g. those with autoantibody positivity, early erosive damage, etc.) may benefit from the early inclusion of a biologic agent, such as a TNF inhibitor, as a biologic combination with MTX suppresses inflammation and halts erosive damage more effectively than the addition of synthetic DMARDs [1, 7]. In fact, high disease activity along with the presence of risk factors may warrant the immediate inclusion of a TNF antagonist in the treatment regimen [5], given the relatively narrow window during which aggressive disease may be halted. Western trials of biologic agents have compared initial combination therapy vs initial MTX monotherapy in such patient populations [8–11], however, studies in Eastern populations are lacking, where environmental, genetic and medical and/or disease management differences may impact drug effectiveness and tolerability.
The combination of adalimumab, a fully human monoclonal antibody against TNF-α, with MTX has been shown in global clinical trials to significantly reduce disease activity, improve physical function and prevent structural damage more effectively than MTX monotherapy in MTX-naive patients with early RA and high disease activity [8, 12]. The HOPEFUL-1 trial (adalimumab, a human anti-TNF monoclonal antibody, outcome study for the persistent efficacy under allocation to treatment strategies in early RA) was conducted to assess the effect of adalimumab in combination with MTX vs MTX alone as a first-line therapy in Japanese patients not previously treated with MTX who had high disease activity and risk factors for aggressive disease. The trial consisted of a 26-week randomized controlled period (adalimumab + MTX vs placebo + MTX) followed by a 26-week open-label (OL) period (OL adalimumab + MTX). Adalimumab in combination with MTX was superior to placebo + MTX during the 26-week blinded period [13]; the current post hoc analysis assessed whether there was continued separation between the treatment strategies through week 52 (i.e. 26 weeks after all patients began receiving combination therapy).
Methods
Patients
Adult patients ≥20 years of age with active RA, as defined by the 1987 revised ACR criteria [14], of <2 years duration and not previously treated with MTX were eligible for enrolment in this study. In addition, patients were required to have at least 10 tender joints (of 68 assessed), 8 swollen joints (of 66 assessed), CRP ≥1.5 mg/dl or ESR ≥28 mm/hour and at least one joint erosion (JE) or RF positivity. Exclusion criteria included prior exposure to more than two DMARDs, previous treatment with CYC, ciclosporin, AZA, tacrolimus or biologic DMARDs, and patients with a chronic infection, interstitial pneumonia or a history of tuberculosis or malignancy. The study was conducted with the approval of the study site ethical review boards and in accordance with the ethical principles of the Declaration of Helsinki; all patients provided written informed consent.
Study design
This phase 3 trial (clinicaltrials.gov identifier NCT00870467 [13]) was conducted at 94 centres in Japan from 11 April 2009 through 1 August 2011 and consisted of two periods. During the first period (blinded period), patients were randomized 1:1 to receive 40 mg adalimumab every other week + weekly MTX (initiated at 6 mg/week) or placebo every other week + weekly MTX for the first 26 weeks. The dose of MTX could be increased to 8 mg/week at week 8 if a ≥20% improvement in the tender or swollen joint count from baseline was not achieved or at the discretion of the investigator, except in the case of a safety concern. Reduction of MTX to 4 mg/week was also permitted and at the discretion of the investigator. For ethical reasons, patients were eligible to be rescued with OL adalimumab + MTX if they experienced a ≥20% increase from baseline in tender and swollen joint counts at week 12, 16 or 20 (rescue period). Patients completing 26 weeks of study drug, either during the blinded or rescue period, were eligible to receive OL adalimumab + MTX for an additional 26 weeks (OL period).
The primary endpoint of the study was the change in modified total Sharp score (mTSS) from baseline to week 26. Details of the scoring of radiographs as well as the results of the primary endpoint have been described [13]. Briefly, 22 and 20 bilateral joints of the hands, wrists and feet were scored for JE and joint space narrowing, respectively, the sum representing the mTSS [15]. Radiographs were read by two independent radiologists blinded to time, treatment and sequence at baseline, rescue (if necessary), week 26, and week 52, or at early termination. Clinical assessments included the 28-joint DAS with ESR (DAS28-ESR), the simplified disease activity index (SDAI) and the clinical disease activity index (CDAI). These assessments are composite measures of disease activity and may include tender and swollen joint counts, acute phase reactants (CRP or ESR), patient’s global health on a visual analogue scale (VAS), patient’s global assessment on a VAS and/or physician’s global assessment on a VAS. Physical function was assessed through the disability index of the HAQ (HAQ-DI). Effectiveness measures for this post hoc analysis included the change from baseline to week 52 in DAS28-ESR and mTSS, the proportions of patients in DAS28-ESR remission (<2.6), with low disease activity (≥2.6 to <3.2), with moderate disease activity (≥3.2 to ≤5.1) or with high disease activity (>5.1), the proportions of patients achieving various definitions of clinical remission [SDAI ≤3.3, CDAI ≤2.8, Boolean (TJC ≤1, SJC ≤1, CRP ≤1 and patient’s global assessment ≤10 on a 100-mm VAS)] and functional (HAQ-DI <0.5) remission, the proportions of patients without radiographic progression (defined as ΔmTSS ≤0.5) from baseline to week 52, as well as the proportions of patients experiencing clinically relevant radiographic progression (ΔmTSS >3).
Safety
Adverse events (AEs) and clinical laboratory parameters were assessed throughout exposure to adalimumab + MTX. AEs of interest were summarized on the basis of initial treatment assignment (adalimumab + MTX or placebo + MTX) as both the number of events and events per 100 patient-years (E/100 PY). AEs were coded using Standardized MedDRA Queries (SMQs) version 13.1. Treatment-emergent AEs were defined as any event with an onset date on or after the first dose of adalimumab + MTX and up to 70 days after the last dose.
Statistical analyses
This post hoc analysis included data from the per protocol set (PPS), which excluded all patients with a major protocol violation. All analyses are based on the initial treatment assignment (adalimumab + MTX or placebo + MTX) and included patients entering into the OL period following completion of the blinded or rescue period. Fisher’s exact test and Wilcoxon rank sum test were used for discrete and continuous variables, respectively. Last observation carried forward (LOCF) was used to impute missing data. LOCF was used for the analysis of radiographic progression to avoid the overestimation of mTSS in the control group. The last value during the blinded period was carried forward for those patients who entered into the rescue period but did not enter into the OL period. The safety analysis set included all patients receiving at least one dose of adalimumab + MTX.
Results
Patients
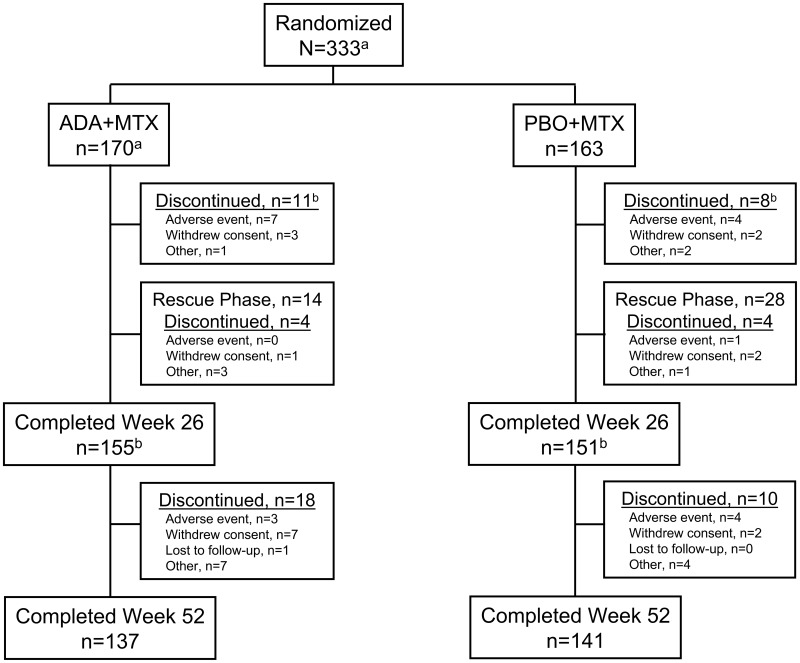
Of the 333 patients initially randomized, 155 and 151 completed 26 weeks of therapy from the initial adalimumab + MTX and placebo + MTX groups, respectively (Fig. 1). Of these, 10 patients from the adalimumab + MTX group and 24 patients from the placebo + MTX group completed the 26-week study following receipt of OL adalimumab + MTX rescue therapy. A total of 152 patients from the initial adalimumab + MTX and 150 patients from the initial placebo + MTX group entered into the OL period, with 137 and 141, respectively, completing the 52-week study. Withdrawal of consent appeared to be the primary reason for discontinuation in the OL period.
Fig. 1.
Patient disposition through week 52.
aPPS. One patient randomized to ADA + MTX received two doses of study drug at baseline and was excluded from this analysis. bThree patients in the ADA + MTX group and one in the PBO + MTX group discontinued from the study at week 26. ADA: adalimumab; PBO: placebo.
Baseline demographics and disease characteristics were well matched between treatment groups (Table 1). Patients tended to have aggressive RA, evidenced by the presence of multiple risk factors for rapid disease progression (e.g. anti-CCP positivity, RF positivity, early erosive damage and elevated CRP). Consistent with aggressive RA, baseline disease activity (mean DAS28 = 6.6) and functional disability were high (mean HAQ-DI = 1.2).
Table 1.
Baseline demographics and disease characteristics
| Parametera | Adalimumab + MTX (n = 170) | Placebo + MTX (n = 163) |
|---|---|---|
| Age, years | 54.0 (13.2) | 54.0 (13.2) |
| Female, n (%) | 143 (84.1) | 128 (78.5) |
| RA duration, years | 0.3 (0.4) | 0.3 (0.4) |
| Weight, kg | 54.4 (9.7) | 56.1 (12.3) |
| Previous DMARD use, n (%) | 74 (43.5) | 87 (53.4) |
| 1 DMARD | 57 (33.5) | 69 (42.3) |
| 2 DMARDs | 17 (10.0) | 18 (11.0) |
| Baseline corticosteroid use, n (%) | 58 (34.1) | 49 (30.1) |
| RF positive, n (%) | 145 (85.3) | 136 (83.4) |
| Mean titre (s.d.), IU/ml | 154.6 (202.9) | 163.7 (362.8) |
| Anti-CCP positive, n (%) | 144 (84.7) | 136 (83.4) |
| Mean titre (s.d.), U/ml | 388.3 (695.7) | 241.3 (367.2) |
| ESR, mm/h | 59.8 (30.2) | 61.8 (29.0) |
| CRP, mg/dl | 2.9 (3.0) | 3.1 (3.3) |
| Swollen joint count | ||
| 0–28 | 11.6 (4.7) | 11.8 (5.3) |
| 0–66 | 16.5 (6.2) | 17.3 (7.7) |
| Tender joint count | ||
| 0–28 | 13.2 (5.9) | 13.2 (6.1) |
| 0–66 | 20.7 (9.3) | 21.1 (10.2) |
| mTSS | 13.7 (22.3) | 13.6 (17.4) |
| Erosion score | 7.5 (11.7) | 7.3 (9.2) |
| Joint space narrowing score | 6.2 (11.4) | 6.2 (9.4) |
| DAS28-ESR | 6.6 (0.9) | 6.6 (1.0) |
| HAQ-DI score | 1.1 (0.7) | 1.3 (0.7) |
| SDAI score | 40.7 (12.0) | 41.4 (13.8) |
| CDAI score | 37.8 (10.9) | 38.3(12.4) |
| Physician’s global assessment of disease activity, mm | 65.9 (18.4) | 66.2 (18.8) |
| Patient’s global assessment of disease activity, mm | 64.3 (24.8) | 66.4 (23.7) |
aAll values are given as mean (s.d.), unless otherwise indicated.
Clinical, functional and radiographic outcomes
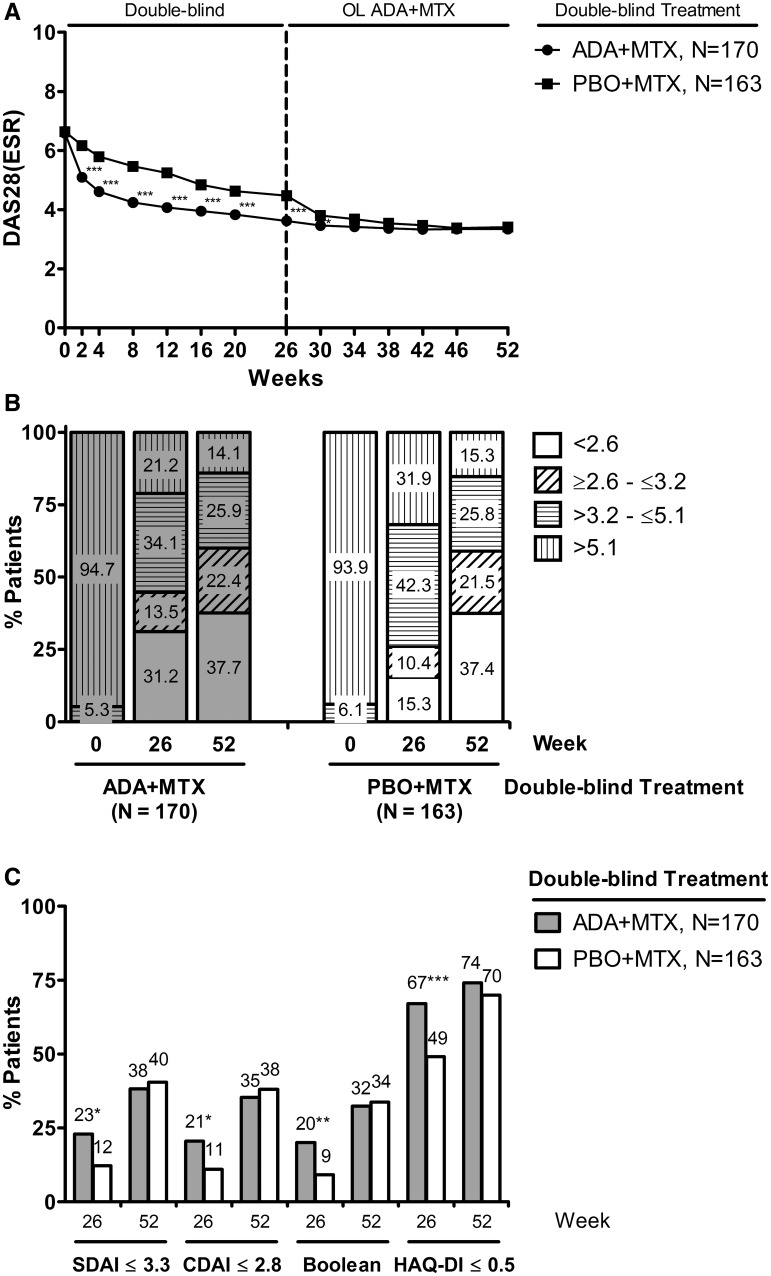
Treatment with adalimumab + MTX during the blinded period led to significant reductions in disease activity vs placebo + MTX (Fig. 2A) [13]. Patients who continued adalimumab + MTX throughout the study demonstrated a steady decline in mean DAS28-ESR levels through week 30, which then stabilized through week 52. The switch in placebo + MTX patients to OL adalimumab + MTX at week 26 resulted in an abrupt decline in mean DAS28-ESR levels. As a result, the differences in mean DAS28-ESR values observed during the first 26 weeks subsided within 8 weeks of adding OL adalimumab to the initial placebo + MTX population (Fig. 2A). Additionally, 26 weeks of OL adalimumab + MTX therapy in the initial placebo + MTX group led to a shift in the distribution of patients in the varying levels of disease activity (remission, low, moderate or high disease activity) such that the balance at week 52 was comparable with those who received adalimumab + MTX throughout the study (Fig. 2B). Furthermore, differences that were apparent between treatment groups at week 26 in the proportions of patients achieving additional composite measures of clinical or functional remission were less striking following an additional 26 weeks of OL adalimumab + MTX treatment (Fig. 2B and C).
Fig. 2.
Clinical and functional responses following up to 52 weeks of treatment with adalimumab (ADA) + MTX.
(A) Mean DAS28-ESR values by visit. (B) The percentages of patients in remission (DAS28-ESR <2.6), low disease activity (DAS28-ESR ≥2.6 to ≤3.2), moderate disease activity (DAS28-ESR >3.2 to ≤5.1) or high disease activity (DAS28-ESR >5.1) at the indicated time points. (C) The percentages of patients satisfying the indicated definitions of clinical (SDAI, CDAI, Boolean) or functional (HAQ-DI) remission at weeks 26 and 52. ***P < 0.001, **P < 0.01 and *P < 0.05.
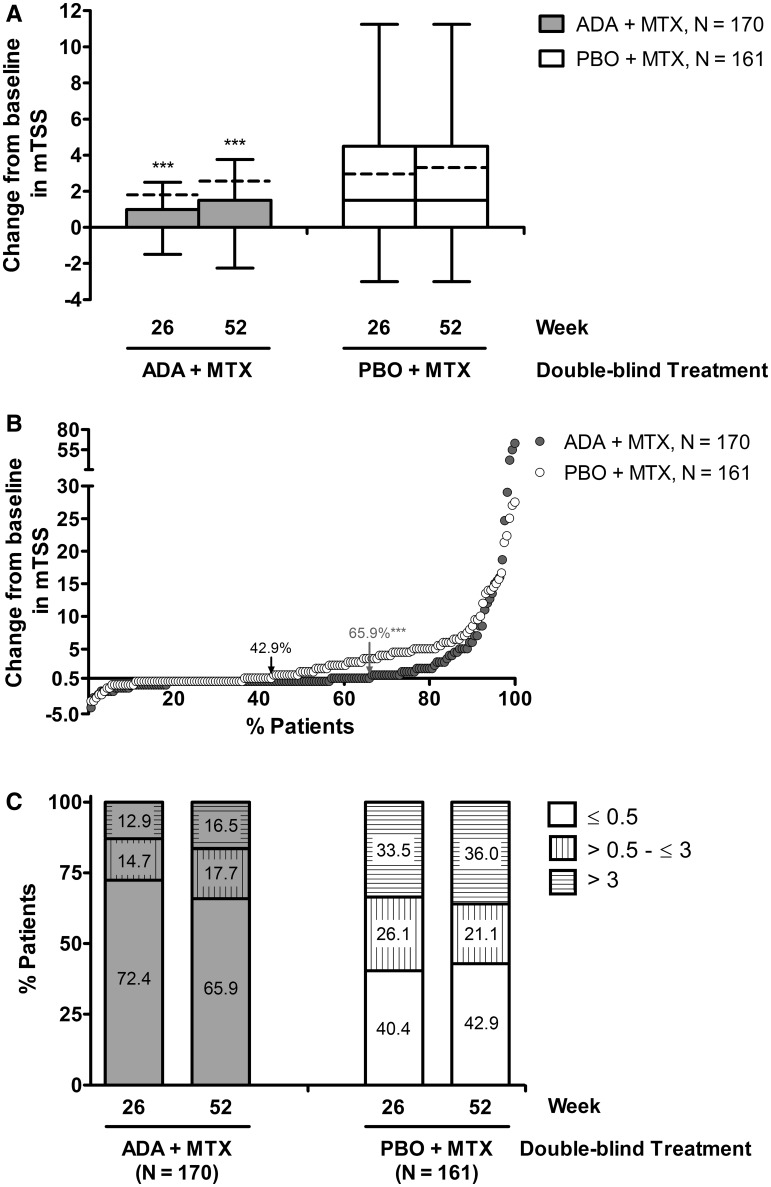
Following 26 weeks of blinded therapy, the mean change from baseline in mTSS was 1.79 and 2.93 for the adalimumab + MTX and placebo + MTX groups, respectively. The addition of OL adalimumab + MTX slowed further radiographic progression in both groups through week 52, resulting in mean changes from baseline in mTSS of 2.56 and 3.30, respectively. Still, the significant differences that were apparent in mean ΔmTSS between the initial treatment groups at week 26 persisted through week 52 (P < 0.001; Fig. 3A). Moreover, significantly more adalimumab + MTX–initiated patients were without radiographic progression through 52 weeks of treatment than patients who initially received placebo + MTX (65.9% vs 42.9%, P < 0.001; Fig. 3B), and significantly fewer exhibited clinically relevant radiographic progression through week 52 (16.5% vs 36.0%, P < 0.001; Fig. 3C).
Fig. 3.
Radiographic progression following up to 52 weeks of treatment with adalimumab (ADA) + MTX.
(A) Box and whisker Tukey plot of change from baseline to week 26 or 52 in mTSS. Boxes represent interquartile range (25–75%); whiskers represent 1.5 times the interquartile range; line represents the median; dashed line represents the mean. (B) Cumulative distribution of change from baseline to week 52 in mTSS. (C) The percentages of patients in remission experiencing radiographic non-progression (ΔmTSS ≤0.5), radiographic progression (ΔmTSS >0.5 to ≤3.0) or clinically relevant radiographic progression (ΔmTSS >3.0) at the indicated time points. ***Statistical significance at the P < 0.001 level.
Safety
A total of 325 patients received at least one dose of adalimumab + MTX, representing 232.5 PY of exposure (153.6 PY in the initial adalimumab + MTX group and 78.9 PY in the initial placebo + MTX group). The majority of patients experienced at least one AE during exposure to adalimumab + MTX, although relatively few (∼22%) were considered to be at least possibly related to the study drug; additionally, AEs described as serious or severe were rare (∼2%; Table 2). Throughout the study there were 305 infectious AEs reported, the most common being nasopharyngitis occurring in 29.8% of patients. Almost half of patients who experienced infectious AEs were assessed as probably or possibly related to adalimumab. A total of eight patients experienced nine serious infections during the course of the study. Serious infections included five cases of pneumonia reported in four patients, two cases of gastroenteritis, and one case each of bronchopneumonia and enteritis infectious. Five serious infections (3.3 E/100 PY) occurred in patients who received adalimumab + MTX throughout the study and four serious infections (5.1 E/100 PY) occurred in patients who received OL adalimumab + MTX only during the OL period. Hepatic and haematological events were relatively mild in severity and rare in frequency. Some elevations in liver function test levels were observed >2.5 times the upper limit of normal. Increased alanine aminotransferase was observed in 8.6% of patients, abnormal hepatic function in 7.4% and increased aspartate aminotransferase in 6.7%. A 55-year-old female who received initial adalimumab + MTX developed lupus-like syndrome and discontinued therapy at day 182 (week 26). There were no malignancies, tuberculosis, demyelinating disease or deaths during exposure to adalimumab + MTX.
Table 2.
Summary of adverse events
| Adalimumab + MTX (PY = 153.6), events (E/100 PY) | Placebo + MTX → adalimumab + MTX (PY = 78.9)a, events (E/100 PY) | |
|---|---|---|
| Any AE | 740 (481.8) | 439 (556.4) |
| AE at least possibly related to study drug | 147 (95.7) | 104 (131.8) |
| AE leading to discontinuation of study drug | 12 (7.8) | 5 (6.3) |
| Severe AE | 4 (2.6) | 1 (1.3) |
| Serious AE | 17 (11.1) | 10 (12.7) |
| Serious AE at least possibly related to study drug | 8 (5.2) | 4 (5.1) |
| Fatal AE | 0 | 0 |
| Infectious AE | 189 (123.0) | 116 (147.0) |
| Serious infectious AE | 5 (3.3) | 4 (5.1) |
| Opportunistic infection (excluding TB) | 1 (0.7) | 0 |
| TB | 0 | 0 |
| Malignancy | 0 | 0 |
| Injection site reaction | 29 (18.9) | 22 (27.9) |
| Lupus-like syndrome | 1 (0.7) | 0 |
| Allergic reaction | 3 (2.0) | 7 (8.9) |
| Haematological events | 12 (7.8) | 10 (12.7) |
| Elevated LFT level | 65 (42.3) | 30 (38.0) |
| Interstitial lung disease | 1 (0.7) | 2 (2.5) |
aPatient-years (PY) are only reported for the period treated with adalimumab. AE: adverse event; E: events; TB: tuberculosis; LFT: liver function test.
Discussion
Patients with aggressive forms of RA, as indicated by the presence of high disease activity and poor prognostic factors (e.g. autoantibody positivity, early erosive damage, etc.) are at risk for the rapid accumulation of irreversible damage and subsequent physical disability. Hence early intervention with effective therapy capable of suppressing inflammation and preventing disease progression is the cornerstone of disease management [16]. MTX is considered to be an anchor drug for the treatment of RA and international organizations recommend an initial trial of MTX for a duration of 3–6 months prior to treatment escalation to a biologic DMARD (e.g. TNF inhibitor) [5, 6]. For patients at greatest risk for disease progression, delaying the addition of a TNF inhibitor by 2 years can impact long-term outcomes [17]. Whether an MTX trial of more limited duration (e.g. 3–6 months) is associated with suboptimal outcomes remains unclear.
The HOPEFUL-1 trial was designed to evaluate the 52-week clinical, functional and radiographic effectiveness of initial treatment with adalimumab + MTX vs adalimumab addition following up to 26 weeks of treatment with placebo + MTX in Japanese patients with early, aggressive RA not previously treated with MTX. Significant differences between treatment groups were noted for a panel of clinical, functional and radiographic endpoints following 26 weeks of blinded therapy [13]. Differences between treatment groups in clinical and functional parameters disappeared rapidly following the addition of OL adalimumab + MTX at week 26, with comparable levels of disease activity observed within 8 weeks of OL adalimumab addition. Despite slowed radiographic progression upon OL adalimumab + MTX treatment, significant structural damage accumulated in many patients exposed to 26 weeks of placebo + MTX [13], resulting in more severe progression in the initial placebo + MTX group that could not be completely reversed upon switching to adalimumab + MTX.
During the blinded period (the first 26 weeks) of this study, inflammation persisted to a greater extent in those who received placebo + MTX vs adalimumab + MTX [13]. The addition of adalimumab + MTX led to a rapid suppression of inflammation, irrespective of whether treatment was initiated with combination therapy or whether a 26-week trial of placebo + MTX was administered. Over the short term, the persistence of elevated disease activity experienced by those in the placebo + MTX group did not appear to translate into a functional difference, as the proportions of patients achieving a state of normal function at week 52 were not different between the two treatment strategies. However, other measures of mental/physical ability and productivity were not assessed in the current analysis, and the possibility remains that long-term advantages to the early adoption of adalimumab + MTX exist in this capacity. In contrast, quantitative differences in the accumulation of structural damage persisted through 52 weeks, despite all patients receiving OL adalimumab + MTX after week 26. This observation underscores the irreversible nature of erosive bone and cartilage loss present in RA patients. Unique to this trial was the prevalence of significant damage accumulation over a relatively short timeframe [13], a phenomenon not seen in recent clinical trials of MTX-naive populations [12, 18], and rather more consistent with observations from the PREMIER trial [8]. Typically, mean ΔmTSS values are driven by relatively few patients who accumulate significant damage over time, with the remainder of the population experiencing little, if any, damage. Although this was true for HOPEFUL-1, more than one third of patients in the initial placebo + MTX group experienced clinically relevant progression through week 52. Identifying those patients most at risk for damage accumulation continues to be challenging, as the reliability of prediction models that accurately classify patients based on pre-treatment characteristics is relatively poor [19, 20]. In this study population, an abnormal CRP baseline value was identified as an important predictor of damage accumulation in MTX-treated patients [13]. Expanded analyses combining baseline characteristics with genetic/biologic markers are needed to increase the accuracy that outcomes can be predicted.
Adalimumab + MTX treatment was generally well tolerated, and the safety profile was consistent with previously reported studies of Japanese RA patients and consistent with global trials of TNF inhibitors in combination with MTX.
As with all studies, important limitations exist. At the time the study was conducted, the maximum approved dose of MTX was 8 mg/week and MTX was not allowed as the first-line DMARD in Japanese patients with RA. In 2011 the maximum approved MTX dose was increased to 16 mg/week and was also allowed to be prescribed as a first-line DMARD. Therefore the possibility exists that more aggressive MTX doses, typically used in western trials, may have more adequately limited progression of structural damage than the lower doses used in this study, although clinical trial data supporting the optimal dose of MTX in combination with TNF inhibitors is lacking. In addition to a lower allowable dose of MTX, the average weight of the studied Japanese population differentiates this study from other global adalimumab studies. Despite a lower mg/kg MTX dosing, clinical and radiographic outcomes of adalimumab + MTX were robust, indicating that lower doses of MTX in combination with adalimumab are capable of significantly reducing the signs and symptoms of RA.
Another important limitation is that the therapeutic regimen of the initial placebo + MTX group was switched to OL adalimumab + MTX at week 26 for all patients regardless of whether a good response was observed during the blinded period. Switching only those patients with an inadequate response to initial placebo + MTX may have yielded a better approximation of the harm of delayed adalimumab + MTX therapy, as was performed in the OPTIMA trial [12]. Furthermore, this analysis pooled patients who received rescue adalimumab + MTX therapy with those who were able to tolerate 26 weeks of placebo + MTX. Lastly, the relatively short follow-up time precluded long-term analysis of the socio-economic impacts and perhaps unique long-term benefit/risk profiles associated with the different treatment strategies for this population.
While the addition of OL adalimumab + MTX following the blinded period was able to improve clinical and functional outcomes comparable to patients initiated on combination therapy, undeniable benefits pertaining to the prevention of radiographic progression and associated joint damage exist. Early intervention with adalimumab + MTX is important to minimize irreversible structural damage in many Japanese patients with early, aggressive RA not previously treated with MTX.
Rheumatology key messages.
Japanese patients with aggressive RA benefit from early adalimumab combination therapy.
The addition of adalimumab after 26 weeks of MTX monotherapy improves clinical and functional outcomes in patients with RA.
Delayed treatment cannot recover radiographic damage occurring in the first 26 weeks in patients with RA.
Acknowledgements
AbbVie contributed to the design of the study and participated in the collection, analysis and interpretation of the data, and in the writing, reviewing and approval of the final version. The authors would like to thank all the patients, investigators and support staff who participated in the study, and Douglas E. Dylla, PhD, and Benjamin Wolfe, PhD, both of AbbVie, for medical writing support.
Funding: This work was supported by AbbVie GK (Tokyo, Japan) and Eisai (Tokyo, Japan).
Disclosure statement: N.M. has received research grants from AbbVie GK, Astellas Pharmaceutical, Banyu Pharmaceutical, Chugai Pharmaceutical, Daiichi Sankyo Pharmaceutical, Eisai, Janssen Pharmaceuticals, Mitsubishi Tanabe Pharma, Takeda Pharmaceutical and Teijin. H.Y. has received research grants from AbbVie GK, Bristol-Myers Squibb, Chugai Pharmaceutical, Eisai, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma, Otsuka Pharmaceutical, Pfizer Japan, Takeda Industrial Pharmaceutical and UCB Japan, and speakers honoraria/consulting fees from AbbVie GK, Bristol-Myers Squibb, Chugai Pharmaceutical, Eisai, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma, Otsuka Pharmaceutical, Pfizer Japan, Takeda Pharmaceutical and UCB Japan. V.A. is a full-time employee of AbbVie and may hold AbbVie stock or stock options. H.A. is an employee of Eisai, Tokyo, Japan. Y.T. has received consulting fees, speaking fees and/or honoraria from Mitsubishi-Tanabe Pharma, AbbVie GK, Eisai, Chugai Pharmaceutical, Janssen Pharmaceutical K.K., Santen Pharmaceutical, Pfizer Japan, Astellas Pharma, Daiichi-Sankyo, GlaxoSmithKline K.K., Astra-Zeneca, Otsuka Pharmaceutical, Actelion Pharmaceuticals Japan, Eli Lilly Japan K.K., Nippon Kayaku, UCB Japan, Quintiles Transnational Japan, Ono Pharmaceutical and Novartis Pharma K.K. Y.T. has received research grants from Bristol-Myers Squibb, MSD K.K., Chugai Pharmaceutical, Mitsubishi-Tanabe Pharma, Astellas Pharma, AbbVie GK, Eisai and Janssen Pharmaceutical K.K. N.I. has received research grants from Astellas Pharmaceutical, Chugai Pharmaceutical, Eisai, Mitsubishi Tanabe Pharmaceutical, Takeda Pharmaceutical, AbbVie and Janssen Pharmaceutical. M.M. has received research grants from AbbVie GK, Eli Lilly Japan K.K., GlaxoSmithKline K.K., Pfizer Japan, Bristol-Myers Squibb and Otsuka Pharmaceutical. S.U. has received research grants from AbbVie GK. T.M. has received research grants from Chugai Pharmaceutical, Bristol-Myers Squibb, Nippon Kayaku, Otsuka Pharmaceutical, Takeda Pharmaceutical, Eli Lilly Japan K.K., Eli Lilly and Company, Astellas Pharma Pfizer Japan, AstraZeneca K.K. and Santen Pharmaceutical. T.T. has received grants from Abbott Japan, Astellas Pharma, Bristol-Myers K.K., Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma, Pfizer Japan, Sanofi-Aventis K.K., Santen Pharmaceutical, Takeda Pharmaceutical, Teijin Pharma, AbbVie GK, Asahikasei Pharma and Taisho Toyama Pharmaceutical; speaking fees from Abbott Japan, Bristol-Myers K.K., Chugai Pharmaceutical, Eisai, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma, Pfizer Japan, Takeda Pharmaceutical, Astellas Pharma and Diaichi Sankyo; and consultant fees from Astra Zeneca K.K., Eli Lilly Japan K.K., Novartis Pharma K.K., Mitsubishi Tanabe Pharma, Asahi Kasei Medical K.K., AbbVie GK and Daiichi Sankyo. H.K. is a full-time employee of AbbVie and may hold AbbVie stock or stock options.
References
- 1.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381–90. doi: 10.1002/art.21405. [DOI] [PubMed] [Google Scholar]
- 2.Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263–9. doi: 10.1016/S0140-6736(04)16676-2. [DOI] [PubMed] [Google Scholar]
- 3.Soubrier M, Puechal X, Sibilia J, et al. Evaluation of two strategies (initial methotrexate monotherapy vs its combination with adalimumab) in management of early active rheumatoid arthritis: data from the GUEPARD trial. Rheumatology. 2009;48:1429–34. doi: 10.1093/rheumatology/kep261. [DOI] [PubMed] [Google Scholar]
- 4.Verstappen SM, Jacobs JW, van der Veen MJ, et al. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann Rheum Dis. 2007;66:1443–9. doi: 10.1136/ard.2007.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–75. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Vollenhoven RF, Ernestam S, Geborek P, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet. 2009;374:459–66. doi: 10.1016/S0140-6736(09)60944-2. [DOI] [PubMed] [Google Scholar]
- 8.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 9.Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272–83. doi: 10.1002/art.24638. [DOI] [PubMed] [Google Scholar]
- 10.Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–81. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 11.St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432–43. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- 12.Kavanaugh A, Fleischmann RM, Emery P, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis. 2013;72:64–71. doi: 10.1136/annrheumdis-2011-201247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi T, Yamanaka H, Ishiguro N, et al. Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202433. Advance Access published 11 January 2013,, doi: 10.1136/annrheumdis-2012-202433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–11. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- 16.Lard LR, Visser H, Speyer I, et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med. 2001;111:446–51. doi: 10.1016/s0002-9343(01)00872-5. [DOI] [PubMed] [Google Scholar]
- 17.van der Heijde D, Breedveld FC, Kavanaugh A, et al. Disease activity, physical function, and radiographic progression after longterm therapy with adalimumab plus methotrexate: 5-year results of PREMIER. J Rheumatol. 2010;37:2237–46. doi: 10.3899/jrheum.100208. [DOI] [PubMed] [Google Scholar]
- 18.Emery P, Fleischmann R, van der Heijde D, et al. The effects of golimumab on radiographic progression in rheumatoid arthritis: results of randomized controlled studies of golimumab before methotrexate therapy and golimumab after methotrexate therapy. Arthritis Rheum. 2011;63:1200–10. doi: 10.1002/art.30263. [DOI] [PubMed] [Google Scholar]
- 19.Smolen JS, Van Der Heijde DM, St Clair EW, et al. Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: results from the ASPIRE trial. Arthritis Rheum. 2006;54:702–10. doi: 10.1002/art.21678. [DOI] [PubMed] [Google Scholar]
- 20.Vastesaeger N, Xu S, Aletaha D, et al. A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rheumatology. 2009;48: 1114–21. doi: 10.1093/rheumatology/kep155. [DOI] [PubMed] [Google Scholar]