Abstract
The emerging field of droplet microfluidics requires effective on-chip handling and sorting of droplets. In this work, we demonstrate a microfluidic device that is capable of sorting picoliter water-in-oil droplets into multiple outputs using standing surface acoustic waves (SSAW). This device integrates a single-layer microfluidic channel with interdigital transducers (IDTs) to achieve on-chip droplet generation and sorting. Within the SSAW field, water-in-oil droplets experience an acoustic radiation force and are pushed towards the acoustic pressure node. As a result, by tuning the frequency of the SSAW excitation, the position of the pressure nodes can be changed and droplets can be sorted to different outlets at rates up to 222 droplets s−1. With its advantages in simplicity, controllability, versatility, non-invasiveness, and capability to be integrated with other on-chip components such as droplet manipulation and optical detection units, the technique presented here could be valuable for the development of droplet-based micro total analysis systems (μTAS).
Keywords: Microfluidics, standing surface acoustic waves (SSAW), droplet sorting
Introduction
In recent years, droplet-based microfluidics1,2 has demonstrated tremendous potential in many chemical and biological assays due to its advantages of low sample consumption, high throughput, short mixing time, elimination of cross contamination, and capacity to be integrated with other lab-on-a-chip devices.3-8 In particular, the high throughput inherent in droplet microfluidics makes it extremely promising for various chemical or biological screenings.9-15 Droplet-based screening largely relies on precise isolation of individual droplets of interests from other droplets; thus it is essential to develop effective, on-chip droplet sorting techniques. Passive sorting methods such as hydrodynamic self-sorting16 and deterministic lateral displacement-based sorting17 make use of the size difference between droplets, but they suffer from an incapability to sort droplets with similar sizes. Meanwhile, many active droplet sorting techniques have been developed in recent years.18-33 Among the existing micro-object manipulation techniques, acoustic-based approaches offer significant advantages in compactness, versatility, and non-invasiveness. Recently, low-power acoustic waves have been extensively utilized in micro-object (e.g., bubble, particle, cell, organism, and droplet) manipulation due to their harmless nature to biological objects.32-59 In our previous studies, we have used standing surface acoustic waves (SSAW) to achieve focusing, separation, sorting, and patterning of microparticles and cells in microfluidics60-66.
In this work, we report a SSAW-based, on-chip, multichannel droplet sorting technique that takes advantages of the tunability and control65,66 offered by chirped interdigital transducers (IDTs). Our SSAW-based approach is fundamentally different from the recently demonstrated traveling surface acoustic wave (TSAW)-based droplet-sorting technique33: our SSAW-based droplet sorting method manipulates the objects (i.e., droplets) directly by the acoustic radiation force, while the TSAW-based method actuates the fluid (i.e., oil) that surrounds the objects through the acoustic streaming effect. Our method has excellent controllability and large range of translation, which renders it capable of precisely sorting cells into a great number (e.g., five) of outlet channels in a single step. This is a major advantage over most existing droplet-sorting methods, which typically only sort droplets into two outlet channels. Our approach is simple, versatile, and compact. We expect that it can be valuable in many chemical or biological analytical processes to facilitate the development of micro total analysis systems (μTAS)67-74.
Working mechanism
The integrated droplet-sorting device, shown in Fig. 1(a), consists of a flow-focusing, droplet-generation component2 and a SSAW-based, droplet-sorting component. The channel geometry was formed via standard soft lithography.75 In all of our devices, the height of the polydimethylsiloxane (PDMS) microchannel was 40 μm. The widths of the water-phase inlet, two oil-phase inlets and two sheath-flow inlets were 50 μm, 50 μm and 100 μm, respectively. The widths of the main channel and the five outlet channels were 200 μm and 45 μm, respectively. The two oil sheath flows were used to control the inter-droplet spacing. As shown in Fig. 1(a), the PDMS microchannel and a pair of narrow chirped IDTs (with period of 360–400 μm and aperture of 300 μm or 500 μm) were aligned in parallel. Chirped IDTs are a variation of regular interdigital transducers (IDTs) which contain a linear gradient in their finger period.65,66 As a result, the wavelength of the excited surface acoustic waves (SAWs) is dependent upon the input frequency of the RF signal, while the amplitude of the SAWs is controlled by input voltage. The use of chirped IDTs rather than regular IDTs in our experiment is due to their capability to change the pressure node distribution of the generated SSAW field by tuning the input frequency. This is the basis for sorting droplets into multiple outputs.
Figure 1.
(a) A schematic of the SSAW-based multichannel droplet sorter. The shaded area indicates the SSAW field. (b) An optical image of our SSAW-based multichannel droplet sorter. (c) A cross-section view showing the SSAW-based, multichannel droplet-sorting mechanism.
The acoustic radiation force in the SSAW field was utilized to sort droplets. The primary acoustic radiation force acting on any microsphere in a SSAW field can be expressed as76
| (1) |
| (2) |
where p0, λ, Vc, pc, pw, βc, and βw are the acoustic pressure, acoustic wavelength, volume of the sphere, density of the sphere, density of the fluid, compressibility of the sphere, and compressibility of the fluid, respectively. In the equations, φ(β,ρ) describes the acoustic contrast factor of the sphere and determines whether the spheres move to pressure nodes or antinodes. In our work, the sizes of the water droplets range from 40 to 50 Cm in diameter, which are less than the wavelength of the SAWs (360–400 μm), so the droplet can be approximated as a microsphere.
The mechanism of multichannel droplet sorting is illustrated in Fig. 1(c). When the chirped IDTs are excited at two different frequencies (f1 and f4), the SSAW with wavelengths of λ1/2 and λ4/2 (λ = c/f, where c is the SAW propagation velocity on the LiNbO3 substrate) are formed, respectively. Since the microchannel is intentionally positioned closer to one chirped IDTs than the other, the pressure node located inside the microchannel is the nth node from the centerline of the two IDTs. And the absolute distance between the node and the centerline at these two excitation frequencies are n(λ1/2) and n(λ4/2), respectively. Therefore, the translation of the droplet (Δx) at the pressure node can be described as Δx = n (λ1 – λ4)/2 = n (c/f1 – c/f4/2 for a frequency change from f1 to f4. As a result, droplets exit through different outlets as shown in Fig. 1(a). Therefore, our SSAW-based droplet sorter is capable of precisely and controllably sorting droplets to different outlets through the control of excitation frequencies.
Experiments
Device fabrication
A photographic image of our device is shown as Fig. 1(b). The fabrication procedure for the SSAW-based droplet-sorting device includes: (1) the fabrication of a SAW substrate; (2) the fabrication of a PDMS microchannel; (3) the bonding of the PDMS microchannel to the SAW substrate. To fabricate the SAW substrate, a double layer of chrome and gold (Cr/Au, 50 Å/500 Å) was deposited on a photoresist-patterned 128° Y-cut lithium niobate (LiNbO3) wafer (500 μm thick, double-side polished), followed by a lift-off technique to form the pair of chirped IDTs. The chirped IDTs in our experiment have 27 pairs of electrodes with the width of electrode and spacing gap increasing linearly from 90 to 100 μm by an increment of 1.25 μm, each width repeating three pairs. The PDMS microchannel was fabricated by standard soft-lithography using SU-8 photoresist mode. After holes were drilled on the PDMS microchannel for inlets and outlets with Harris Uni-Core 1.0 mm punch, the PDMS microchannel was treated with oxygen plasma in a plasma cleaner (Harrick Plasma) for 3 min with the SAW substrate. The PDMS microchannel was then aligned and bonded on the SAW substrate in between the chirped IDTs after which the whole device was cured at 65 °C for at least three days before experiments to ensure that the surfaces of the microchannel were hydrophobic.
System setup
All the experiments were conducted on the stage of an inverted microscope (Nikon Eclipse Ti-U). In order to eliminate the virtual image introduced by using a double-side polished LiNbO3 substrate, we placed a polarizer in the light path and adjusted it to a certain angle. For droplet generation, Fluorinert® FC-40 (Sigma) with 3 wt% DuPont Krytox 157 FS (ChemPoint) was used as continuous oil phase while deionized water was used as dispersed water phase. Fluid prepared in 1 ml plastic syringes (Becton, Dickinson and Company) was injected through polyethylene tubing (Becton, Dickinson and Company) into the microchannel using neMESYS syringe pumps (cetoni GmbH, Germany). An RF signal function generator (Agilent E4422B) was used to generate RF signals at desired frequencies, which were applied to the chirped IDTs after amplification by a power amplifier (Amplifier Research 100A250A) to generate the SSAW field.
Characterization and data analysis
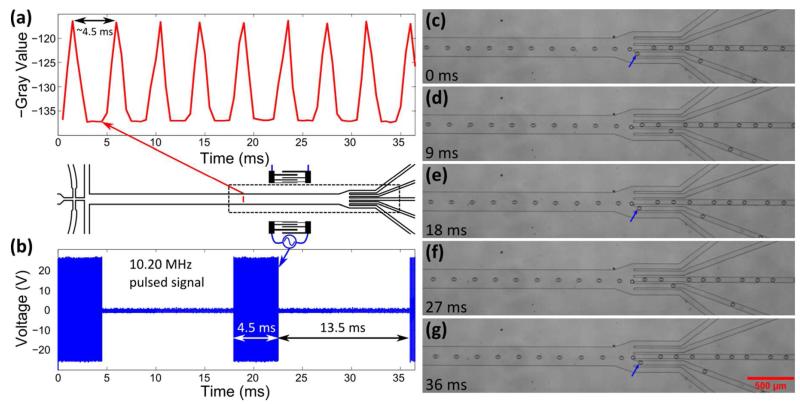
A fast camera (Photron SA4, Japan) was connected to the microscope for data acquisition at a speed of 2000 frames per second (fps) during the experiment. Recorded data was analyzed with Image J (NIH, Bethesda, MD, USA). In Fig. 3, the droplet position was traced by analyzing the gray value of the stacked image of 500 frames and plotted in MATLAB. In Fig. 5(a), the droplet generation was analyzed from the captured video with the “Plot Z-axis Profile” function and plotted in MATLAB. In Fig. 5(b), the input pulsed signal after amplification was recorded with a digital phosphor oscilloscope (load set at 1 MΩ) (Tektronix DPO4104) and plotted in MATLAB.
Figure 3.
The relationship between droplet lateral displacement in the y-direction and droplet position along the channel in the x-direction at different voltages under two SSAW frequencies, (a) 10.02 MHz and (b) 10.20 MHz. Black dashed line indicates the centerline of the microchannel. The origin of the coordinates was set at the lower channel wall 2400 μm away from the outlet region with the x-axis along the lower channel wall and the y-axis normal to the channel wall.
Figure 5.
Sorting of single droplets at rate of 222 droplets s−1. (a) shows the droplet generation. (b) shows the 10.20 MHz input pulsed signal. (c—g) show the sorting of three single droplets out of the stream (indicated by blue arrows).
Results and Discussion
SSAW-based droplet switching
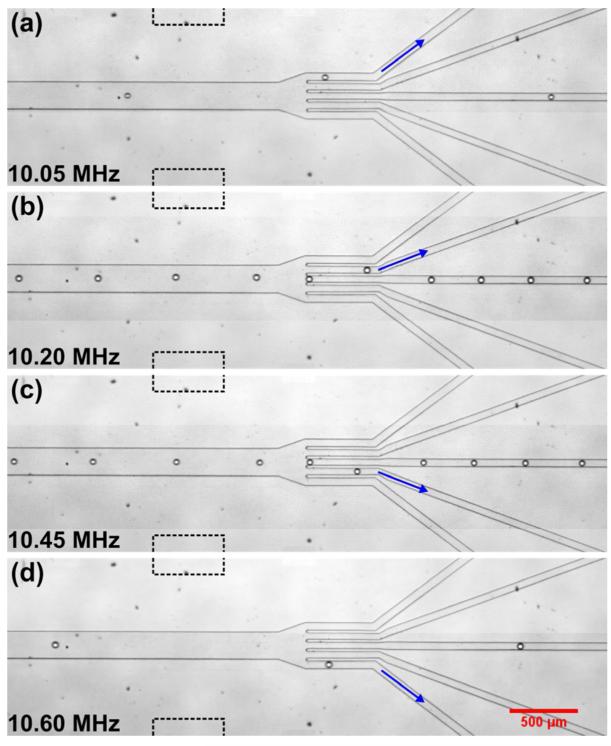
We first tested the device’s ability to switch droplet streams to each of the five outlet channels. Micrographs indicating directed droplet flows into five outlet channels under five different SSAW frequencies are shown in Fig. 2. In this experiment, a narrow chirped IDTs device with aperture of 500 μm was used. The flow rates of the water-phase inlet and two oil-phase inlets were 0.2 and 8.0 μl/min, respectively; no oil sheath flow was used. With a 40 × 50 μm cross section of the flow-focusing nozzle, droplets with diameter of ~50 μm were generated under these flow rates. At the beginning of the experiment we first tuned the input SSAW frequency to identify five working frequencies (f1 = 10.02 MHz, f2 = 10.20 MHz, f3 = 10.35 MHz, f4 = 10.46 MHz, and f5 = 10.60 MHz) under which droplets could be directed into five outlet channels when the SSAW was excited. Then, by applying an RF signal periodically sweeping among these five working frequencies with a mean peak-to-peak voltage of around 48.0 V to the chirped IDTs, we were able to switch droplet flows among the five outlets, as shown in Fig. 2(a—e). A video clip showing these results is available online in Supplemental Video 1. In our future work, a flow-focusing nozzle with smaller cross section will be used to generate droplets with smaller diameters (e.g., 30 μm). With this improvement, it is possible to further increase the number of outlets (e.g., 7 or more) in which droplets can be sorted in one step.
Figure 2.
(a—e) show the directed droplet flows into five outlet channels under five different SSAW frequencies. The blue arrow indices the target outlet and the dashed lines indicate the aperture position of the chirped IDTs relative to the microchannel.
The effect of input voltage on the lateral displacement of droplets across the microchannel was then investigated for two different SSAW frequencies (10.02 MHz and 10.20 MHz) using the same device under the same flow conditions. The RF signal was applied to the chirped IDTs with four different mean peak-to-peak voltages (30.0 V, 38.4 V, 48.0 V and 60.0 V), and the position of droplets within the last 2400 μm of the main channel at each voltage was recorded and plotted, as shown in Fig. 3. When the chirped IDTs were excited, higher input voltage generated stronger SSAW field with larger acoustic radiation force, which pushed droplets faster in the y-direction. This indicated that the velocity of droplet in the y-direction increased with the input voltage. Under the excitation of the 10.02 MHz RF signal, since the pressure node (the node nearest to the droplet stream) was located far from the centerline of the microchannel, the droplets never reached the pressure node and were still migrating in the y-direction as they left the SSAW field. Because the flow rates were constant, each droplet traveled through the SSAW field in the x-direction for the same amount of time regardless of the voltage. Therefore, the lateral displacement of droplets increased with the voltage [Fig. 3(a)]. As a result, the sorting outcome was also influenced: droplets exited through the uppermost outlet at 60.0 V and 48.0 V while through the second uppermost outlet at 38.0 V and 30.4 V (data not shown). In contrast, under the excitation of the 10.20 MHz RF signal, the pressure node was shifted nearer to the centerline of the microchannel. Hence, droplets would have reached the pressure node before leaving the SSAW field above certain voltage. As shown by the red curve in Fig. 3(b) (60.0 V), the lateral displacement of droplets did not increase after X = 1870 μm because droplets had already reached the pressure node. In this circumstance, droplets could never flow into the uppermost outlet regardless of the input voltage.
SSAW-based single droplet sorting
In order to develop a fluorescence-activated droplet sorter (FADS), the ability to sort a single droplet out of a stream is desirable. Therefore, we tested the feasibility of single droplet sorting by applying a pulsed RF signal to the narrow chirped IDTs. Micrographs demonstrating the diversion of single droplets from the main stream into four different target outlets are shown in Fig. 4. In this experiment, the same chirped IDTs device with aperture of 500 μm was used. When the SSAW was off, all the droplets flowed into the center outlet channel by default. When a pulsed signal of desired frequency with certain pulse width was applied, one could deflect a single droplet into the desired target outlet channel. In Figs. 4(b) and 4(c), the flow rates of the water-phase inlet, two oil-phase inlets and two sheath-flow inlets were 0.2, 8.0 and 1.7 μl/min, respectively. By applying a 15 ms wide pulsed signal of 10.20 MHz or 10.45 MHz with mean peak-to-peak voltage of 48.0 V, we successfully diverted a single droplet either upward or downward to the neighboring target outlet. In comparison, the sorting of a single droplet into the uppermost or lowermost target outlet is more difficult, since a larger lateral displacement of the droplet is required. To realize this, a longer pulse width and higher input voltage could help based on our discussion above. In Figs. 4(a) and 4(d), the flow rates of the water-phase inlet, two oil-phase inlets and two sheath-flow inlets were 0.1, 8.0 and 3.0 μl/min, respectively. Under this condition, we applied a 35 ms wide pulsed signal of 10.05 MHz or 10.60 MHz with mean peak-to-peak voltage of 54.0 V to sort a single droplet into the uppermost or lowermost target outlet. Four video clips showing these results are available online in Supplemental Videos 2—5.
Figure 4.
(a—d) show the sorting of single droplets into four different target outlets under pulsed RF signal. The dashed lines indicate the aperture position of the chirped IDTs relative to the microchannel.
At last, we evaluated the throughput of our SSAW-based droplet sorter by using the chirped IDTs with smaller aperture (300 μm), which gave us a better chance to sort a single droplet out from its neighboring droplets. These results are shown in Fig. 5. In this experiment, droplets (diameter ~ 40 μm) were generated roughly every 4.5 ms, corresponding to a droplet generation rate of 222 droplets s−1 [Fig. 5(a)]. In order to sort single droplets out of the stream, we applied a 10.20 MHz periodically oscillating pulsed signal with a pulse width of 4.5 ms and pulse period of 18 ms to the chirped IDTs (duty cycle is 25%) [Fig. 5(b)]. As a consequence, a single droplet was sorted into the target outlet about every 18 ms, as shown in Fig. 5(c—g). A video clip showing these results is available online in Supplemental Video 6. The sorting performance of our SSAW-based approach is promising since whenever the SSAW was on during the pulse of 4.5 ms, one droplet was sorted out. Therefore, we have every reason to believe that with the integration of an optical-detection unit to detect the target droplet and a trigger-control unit to control the SSAW generation, we can better match the SSAW generation with the target droplet to achieve single droplet sorting with high accuracy. To further increase the throughput in our future work, there are several possible approaches. The first approach is to fabricate chirped IDTs with an even smaller aperture (e.g., 100 μm) and more pairs of electrodes. This modified design can help to generate a narrower SSAW field while reducing the effect of acoustic dispersion at the edge. Thus, it can help to sort single droplets with closer inter-droplet distances at higher throughput. The second approach is to apply a pulsed signal with a higher voltage. Under higher voltages, the acoustic radiation force is larger, which pushes the droplet at a higher velocity in the y-direction. Therefore, a shorter time or pulse width (and thus less translational distance of the droplet in the x-direction) will be needed to sort the single droplet into a certain target outlet. This can also lead to higher-throughput single droplet sorting. The third approach to increase the sorting throughput is to decrease the distance between the two IDTs and bond the microchannel closer to the IDTs. This can also reduce the effect of acoustic dispersion to increase the sorting throughput.
Conclusions
In summary, we have successfully developed and tested a SSAW-based multichannel droplet sorter. Our device integrates on-chip, water-in-oil droplet formation with SSAW-based, multichannel droplet sorting. Our device takes advantages of the excellent controllability and tunability offered by chirped IDTs and can sort picoliter droplets into five (or more) outputs at rates up to 222 droplets s−1 without external labeling. With further optimization, this device can be integrated with an optical-detection module and other on-chip functions to enable a SSAW-based, micro fluorescence-activated droplet sorter (μFADS).
Supplementary Material
Acknowledgement
The authors would like to thank Joey Rufo for the help in preparing the manuscript and ChemPoint for providing DuPont Krytox 157 FS surfactant. This research was supported by National Institutes of Health (NIH) Director’s New Innovator Award (1DP2OD007209-01), the National Science Foundation, and the Penn State Center for Nanoscale Science (MRSEC) under grant DMR-0820404. Components of this work were conducted at the Penn State node of the NSF-funded National Nanotechnology Infrastructure Network (NNIN).
Footnotes
Associated Contents
Video clips available in supplementary information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Thorsen T, Roberts RW, Arnold FH, Quake SR. Physical Review Letters. 2001;86:4163. doi: 10.1103/PhysRevLett.86.4163. [DOI] [PubMed] [Google Scholar]
- (2).Anna SL, Bontoux N, Stone HA. Applied Physics Letters. 2003;82:364. [Google Scholar]
- (3).Guo F, Lapsley MI, Nawaz AA, Zhao Y, Lin S-CS, Chen Y, Yang S, Zhao X-Z, Huang TJ. Analytical Chemistry. 2012;84:10745. doi: 10.1021/ac302623z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Huebner A, Sharma S, Srisa-Art M, Hollfelder F, Edel JB, deMello AJ. Lab on a Chip. 2008;8:1244. doi: 10.1039/b806405a. [DOI] [PubMed] [Google Scholar]
- (5).Guo MT, Rotem A, Heyman JA, Weitz DA. Lab on a Chip. 2012;12:2146. doi: 10.1039/c2lc21147e. [DOI] [PubMed] [Google Scholar]
- (6).Theberge AB, Courtois F, Schaerli Y, Fischlechner M, Abell C, Hollfelder F, Huck WTS. Angewandte Chemie (International ed. in English) 2010;49:5846. doi: 10.1002/anie.200906653. [DOI] [PubMed] [Google Scholar]
- (7).Griffiths AD, Tawfik DS. Trends in Biotechnology. 2006;24:395. doi: 10.1016/j.tibtech.2006.06.009. [DOI] [PubMed] [Google Scholar]
- (8).Song H, Chen DL, Ismagilov RF. Angewandte Chemie (International ed. in English) 2006;45:7336. doi: 10.1002/anie.200601554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Clausell-Tormos J, Lieber D, Baret J-C, El-Harrak A, Miller OJ, Frenz L, Blouwolff J, Humphry KJ, Köster S, Duan H, Holtze C, Weitz DA, Griffiths AD, Merten CA. Chemistry & Biology. 2008;15:427. doi: 10.1016/j.chembiol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- (10).Fallah-Araghi A, Baret J-C, Ryckelynck M, Griffiths AD. Lab on a Chip. 2012;12:882. doi: 10.1039/c2lc21035e. [DOI] [PubMed] [Google Scholar]
- (11).Miller OJ, El-Harrak A, Mangeat T, Baret J-C, Frenz L, El Debs B, Mayot E, Samuels ML, Rooney EK, Dieu P, Galvan M, Link DR, Griffiths AD. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:378. doi: 10.1073/pnas.1113324109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Neuži P, Giselbrecht S, Länge K, Huang TJ, Manz A. Nature Reviews Drug Discovery. 2012;11:620. doi: 10.1038/nrd3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Schaerli Y, Wootton RC, Robinson T, Stein V, Dunsby C, Neil MAA, French PMW, deMello AJ, Abell C, Hollfelder F. Analytical Chemistry. 2009;81:302. doi: 10.1021/ac802038c. [DOI] [PubMed] [Google Scholar]
- (14).Srisa-Art M, deMello AJ, Edel JB. Analytical Chemistry. 2007;79:6682. doi: 10.1021/ac070987o. [DOI] [PubMed] [Google Scholar]
- (15).Mao X, Huang TJ. Lab on a Chip. 2012;12:1412. doi: 10.1039/c2lc90022j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chabert M, Viovy J-L. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3191. doi: 10.1073/pnas.0708321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Joensson HN, Uhlén M, Svahn HA. Lab on a Chip. 2011;11:1305. doi: 10.1039/c0lc00688b. [DOI] [PubMed] [Google Scholar]
- (18).Zhang K, Liang Q, Ai X, Hu P, Wang Y, Luo G. Lab on a Chip. 2011;11:1271. doi: 10.1039/c0lc00484g. [DOI] [PubMed] [Google Scholar]
- (19).Shemesh J, Bransky A, Khoury M, Levenberg S. Biomedical Microdevices. 2010;12:907. doi: 10.1007/s10544-010-9445-y. [DOI] [PubMed] [Google Scholar]
- (20).Robert de Saint Vincent M, Wunenburger R, Delville J-P. Applied Physics Letters. 2008;92:154105. [Google Scholar]
- (21).Niu X, Zhang M, Wu J, Wen W, Sheng P. Soft Matter. 2009;5:576. [Google Scholar]
- (22).Niu X, Zhang M, Peng S, Wen W, Sheng P. Biomicrofluidics. 2007;1:44101. doi: 10.1063/1.2795392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Link DR, Grasland-Mongrain E, Duri A, Sarrazin F, Cheng Z, Cristobal G, Marquez M, Weitz DA. Angewandte Chemie (International ed. in English) 2006;45:2556. doi: 10.1002/anie.200503540. [DOI] [PubMed] [Google Scholar]
- (24).Lee C-Y, Lin Y-H, Lee G-B. Microfluidics and Nanofluidics. 2008;6:599. [Google Scholar]
- (25).Guo F, Ji X-H, Liu K, He R-X, Zhao L-B, Guo Z-X, Liu W, Guo S-S, Zhao X-Z. Applied Physics Letters. 2010;96:193701. [Google Scholar]
- (26).Baroud C, Delville J-P, Gallaire F, Wunenburger R. Physical Review E. 2007;75:1. doi: 10.1103/PhysRevE.75.046302. [DOI] [PubMed] [Google Scholar]
- (27).Baret J-C, Miller OJ, Taly V, Ryckelynck M, El-Harrak A, Frenz L, Rick C, Samuels ML, Hutchison JB, Agresti JJ, Link DR, Weitz DA, Griffiths AD. Lab on a Chip. 2009;9:1850. doi: 10.1039/b902504a. [DOI] [PubMed] [Google Scholar]
- (28).Ahn K, Kerbage C, Hunt TP, Westervelt RM, Link DR, Weitz DA. Applied Physics Letters. 2006;88:024104. [Google Scholar]
- (29).Ahn B, Lee K, Panchapakesan R, Oh KW. Biomicrofluidics. 2011;5:24113. doi: 10.1063/1.3604393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ahn B, Lee K, Louge R, Oh KW. Biomicrofluidics. 2009;3:44102. doi: 10.1063/1.3250303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Abate AR, Agresti JJ, Weitz DA. Applied Physics Letters. 2010;96:203509. [Google Scholar]
- (32).Lee C, Lee J, Kim HH, Teh S-Y, Lee A, Chung I-Y, Park J, Shung KK. Lab on a Chip. 2012;12:2736. doi: 10.1039/c2lc21123h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Franke T, Abate AR, Weitz DA, Wixforth A. Lab on a Chip. 2009;9:2625. doi: 10.1039/b906819h. [DOI] [PubMed] [Google Scholar]
- (34).Ahmed D, Mao X, Juluri BK, Huang TJ. Microfluidics and Nanofluidics. 2009;7:727. [Google Scholar]
- (35).Ahmed D, Mao X, Shi J, Juluri BK, Huang TJ. Lab on a Chip. 2009;9:2738. doi: 10.1039/b903687c. [DOI] [PubMed] [Google Scholar]
- (36).Alvarez M, Yeo LY, Friend JR, Jamriska M. Biomicrofluidics. 2009;3:14102. doi: 10.1063/1.3055282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Franke T, Braunmüller S, Schmid L, Wixforth A, Weitz DA. Lab on a Chip. 2010;10:789. doi: 10.1039/b915522h. [DOI] [PubMed] [Google Scholar]
- (38).Li H, Friend JR, Yeo LY. Biomedical Microdevices. 2007;9:647. doi: 10.1007/s10544-007-9058-2. [DOI] [PubMed] [Google Scholar]
- (39).Li H, Friend JR, Yeo LY. Physical Review Letters. 2008;101:1. doi: 10.1103/PhysRevLett.101.084502. [DOI] [PubMed] [Google Scholar]
- (40).Lin S-CS, Mao X, Huang TJ. Lab on a Chip. 2012;12:2766. doi: 10.1039/c2lc90076a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Liu Y, Lim K-M. Lab on a Chip. 2011;11:3167. doi: 10.1039/c1lc20481e. [DOI] [PubMed] [Google Scholar]
- (42).Meng L, Cai F, Chen J, Niu L, Li Y, Wu J, Zheng H. Applied Physics Letters. 2012;100:173701. [Google Scholar]
- (43).Meng L, Cai F, Jin Q, Niu L, Jiang C, Wang Z, Wu J, Zheng H. Sensors and Actuators B. 2011;160:1599. [Google Scholar]
- (44).Meng L, Cai F, Zhang Z, Niu L, Jin Q, Yan F, Wu J, Wang Z, Zheng H. Biomicrofluidics. 2011;5:044104. doi: 10.1063/1.3652872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Nam J, Lee Y, Shin S. Microfluidics and Nanofluidics. 2011;11:317. [Google Scholar]
- (46).Nam J, Lim H, Kim C, Kang JY, Shin S. Biomicrofluidics. 2012;6:024120. doi: 10.1063/1.4718719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Nam J, Lim H, Kim D, Shin S. Lab on a Chip. 2011;11:3361. doi: 10.1039/c1lc20346k. [DOI] [PubMed] [Google Scholar]
- (48).Orloff ND, Dennis JR, Cecchini M, Schonbrun E, Rocas E, Wang Y, Novotny D, Simmonds RW, Moreland J, Takeuchi I, Booth JC. Biomicrofluidics. 2011;5:44107. doi: 10.1063/1.3661129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Petersson F, Aberg L, Swärd Nilsson A-M, Laurell T. Analytical Chemistry. 2007;79:5117. doi: 10.1021/ac070444e. [DOI] [PubMed] [Google Scholar]
- (50).Petersson F, Nilsson A, Jönsson H, Laurell T. Analytical Chemistry. 2005;77:1216. doi: 10.1021/ac048394q. [DOI] [PubMed] [Google Scholar]
- (51).Qi A, Yeo LY, Friend JR, Ho J. Lab on a Chip. 2010;10:470. doi: 10.1039/b915833b. [DOI] [PubMed] [Google Scholar]
- (52).Tan M, Friend JR, Yeo LY. Physical Review Letters. 2009;103:1. [Google Scholar]
- (53).Wood CD, Cunningham JE, O’Rorke R, Wa□ti C, Linfield EH, Davies AG, Evans SD. Applied Physics Letters. 2009;94:054101. [Google Scholar]
- (54).Wood CD, Evans SD, Cunningham JE, O’Rorke R, Wa□ti C, Davies AG. Applied Physics Letters. 2008;92:044104. [Google Scholar]
- (55).Xie Y, Ahmed D, Lapsley MI, Lin SS, Nawaz AA, Wang L, Huang TJ. Analytical Chemistry. 2012;84:7495. doi: 10.1021/ac301590y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Xie Y, Zhao C, Zhao Y, Li S, Rufo J, Yang S, Guo F, Huang TJ. Lab on a Chip. 2013;13:1772. doi: 10.1039/c3lc00043e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Mao X, Huang TJ. Lab on a Chip. 2012;12:4006. doi: 10.1039/c2lc90100e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Ahmed D, Chan CY, Lin S-CS, Muddana HS, Nama N, Benkovic SJ, Huang TJ. Lab on a Chip. 2013;13:328. doi: 10.1039/c2lc40923b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Lin S-CS, Mao X, Huang TJ. Lab on a Chip. 2012;12:2766. doi: 10.1039/c2lc90076a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Shi J, Yazdi S, Lin S-CS, Ding X, Chiang I-K, Sharp K, Huang TJ. Lab on a Chip. 2011;11:2319. doi: 10.1039/c1lc20042a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Shi J, Mao X, Ahmed D, Colletti A, Huang TJ. Lab on a Chip. 2008;8:221. doi: 10.1039/b716321e. [DOI] [PubMed] [Google Scholar]
- (62).Shi J, Huang H, Stratton Z, Huang Y, Huang TJ. Lab on a Chip. 2009;9:3354. doi: 10.1039/b915113c. [DOI] [PubMed] [Google Scholar]
- (63).Shi J, Ahmed D, Mao X, Lin S-CS, Lawit A, Huang TJ. Lab on a Chip. 2009;9:2890. doi: 10.1039/b910595f. [DOI] [PubMed] [Google Scholar]
- (64).Ding X, Shi J, Lin S-CS, Yazdi S, Kiraly B, Huang TJ. Lab on a Chip. 2012;12:2491. doi: 10.1039/c2lc21021e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Ding X, Lin S-CS, Lapsley MI, Li S, Guo X, Chan CY, Chiang I-K, Wang L, McCoy JP, Huang TJ. Lab on a Chip. 2012;12:4228. doi: 10.1039/c2lc40751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Ding X, Lin S-CS, Kiraly B, Yue H, Li S, Chiang I, Shi J, Benkovic SJ, Huang TJ. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11105. doi: 10.1073/pnas.1209288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Arora A, Simone G, Salieb-Beugelaar GB, Kim JT, Manz A. Analytical Chemistry. 2010;82:4830. doi: 10.1021/ac100969k. [DOI] [PubMed] [Google Scholar]
- (68).Wheeler AR, Throndset WR, Whelan RJ, Leach AM, Zare RN, Liao YH, Farrell K, Manger ID, Daridon A. Analytical Chemistry. 2003;75:3581. doi: 10.1021/ac0340758. [DOI] [PubMed] [Google Scholar]
- (69).Ng AHC, Choi K, Luoma RP, Robinson JM, Wheeler AR. Analytical Chemistry. 2012;84:8805. doi: 10.1021/ac3020627. [DOI] [PubMed] [Google Scholar]
- (70).Pamme N, Manz A. Analytical Chemistry. 2004;76:7250. doi: 10.1021/ac049183o. [DOI] [PubMed] [Google Scholar]
- (71).Riahi R, Mach KE, Mohan R, Liao JC, Wong PK. Analytical Chemistry. 2011;83:6349. doi: 10.1021/ac2012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Sin MLY, Liu T, Pyne JD, Gau V, Liao JC, Wong PK. Analytical Chemistry. 2012;84:2702. doi: 10.1021/ac203245j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Hughes AJ, Lin RKC, Peehl DM, Herr AE. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5972. doi: 10.1073/pnas.1108617109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Karns K, Herr AE. Analytical Chemistry. 2011;83:8115. doi: 10.1021/ac202061v. [DOI] [PubMed] [Google Scholar]
- (75).Xia Y, Whitesides GM. Annual Review of Materials Science. 1998;28:153. [Google Scholar]
- (76).Yosioka K, Kawasima Y. Acustica. 1955;5:167. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.