Abstract
Background
Obsessive-compulsive disorder (OCD) is a psychiatric condition that typically manifests in compulsive urges to perform irrational or excessive avoidance behaviors. A recent account has suggested that compulsivity in OCD might arise from excessive stimulus-response habit formation, rendering behavior insensitive to goal value. We tested if OCD patients have a bias toward habits using a novel shock avoidance task. To explore how habits, as a putative model of compulsivity, might relate to obsessions and anxiety, we recorded measures of contingency knowledge, explicit fear, and physiological arousal.
Methods
Twenty-five OCD patients and 25 control subjects completed a shock avoidance task designed to induce habits through overtraining, which were identified using goal-devaluation. The relationship between habitual behavior, erroneous cognitions, and physiological arousal was assessed using behavior, questionnaires, subjective report, and skin conductance responses.
Results
A devaluation sensitivity test revealed that both groups could inhibit unnecessary behavioral responses before overtraining. Following overtraining, OCD patients showed greater avoidance habits than control subjects. Groups did not differ in conditioned arousal (skin conductance responses) at any stage. Additionally, groups did not differ in contingency knowledge or explicit ratings of shock expectancy following the habit test. Habit responses were associated with a subjective urge to respond.
Conclusions
These data indicate that OCD patients have a tendency to develop excessive avoidance habits, providing support for a habit account of OCD. Future research is needed to fully characterize the causal role of physiological arousal and explicit fear in habit formation in OCD.
Key Words: Avoidance, cognitive neuroscience, goal-directed learning, habit, obsessive-compulsive disorder, psychophysiology
Obsessive-compulsive disorder (OCD) is a paradox. In severe cases, patients spend most of their waking hours performing repetitive compulsive behaviors and struggling with disturbing obsessive thoughts and/or anxiety (1). But patients with OCD are not deluded; most recognize that their concerns are unrealistic and that their behavior is absurd or at least excessive (2). Researchers have thus far struggled to explain this ego-dystonic phenomenon: how a patient’s life can be taken over, for example, by an overwhelming compulsion to repeatedly flick a light switch, despite knowing that this action serves no real purpose.
In healthy humans, prolonged repetition of behavior instills habits, causing us to respond automatically under certain environmental situations, regardless of whether or not these actions produce useful outcomes (3). Although there is now a considerable body of literature describing appetitive habit formation, there is, to date, no published evidence that habits (as defined by the criterion of goal devaluation) can be formed in avoidance in healthy humans or nonhuman animals. Previous research suggests that OCD patients have a bias toward appetitive habit formation at the expense of goal-directed behavior, an imbalance that might contribute to the repetitive and seemingly senseless compulsions that exemplify the disorder (4). However, compulsions in OCD are avoidant rather than appetitive; therefore, if aberrant habit formation contributes to this symptom, excessive avoidance habits should be experimentally demonstrable in OCD.
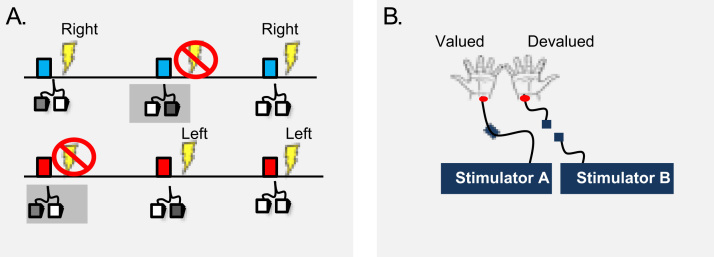
We used a shock avoidance paradigm wherein subjects could avoid receiving electric shocks by responding on the correct foot pedal in response to warning stimuli (Figure 1A). Following overtraining, we tested for habit formation using an instructed outcome devaluation procedure whereby we disconnected one of the subjects’ wrists from its stimulator (devalued), while leaving the other connected (valued) (Figure 1B), and measured the number of unnecessary avoidance responses to the now safe, devalued stimulus. By using an avoidance paradigm, it was possible to explore a number of functional predictions regarding the putative role of habits in the obsessive-compulsive cycle.
Figure. 1.
Task design. (A) Warning stimuli. The blue stimulus predicts a right shock, the red stimulus a left shock. If the correct avoidance response (e.g., left pedal to avoid left shock) is produced on time, subjects avoid shock. (B) Devaluation procedure. The electrodes on one side are disconnected from their connector (devalued), and the electrodes on the other side are unchanged (valued).
One of the longest standing accounts of OCD symptomatology is that compulsions are not habits but rather are rational avoidance responses triggered by irrational beliefs 5, 6, 7. Irrational beliefs are considered the product of cognitive bias in OCD, including, for example, the overestimation of threat (8), increased personal responsibility (5), and thought-action fusion (7). These beliefs are thought by some to form the basis of obsession and, in turn, anxiety in OCD, to which compulsions are a goal-directed avoidance response (9). In this experiment, we tested one possibility inspired by this account: that excessive behavioral repetition in OCD (4) might not be evidence for habit formation but rather is driven by a failure to learn about safety. To this end, we conducted a devaluation test before overtraining to assess if OCD patients exhibit a general failure to learn that what was once dangerous is now safe. Moreover, we recorded levels of self-report shock expectancy following the habit test to determine if responding could be defined as habitual, i.e., was evident in spite of low expectancy of shock. In addition to these two tests of safety learning, we used questionnaires to test explicit knowledge of task contingencies. This allowed us to test if a cognitive, specifically an instrumental learning, deficit in the OCD group might better explain habit-like responding in spite of outcome devaluation.
Beyond irrational belief, conditioned fear and anxiety are also thought to be important for OCD (10) and indeed can bias healthy individuals to behave habitually 11, 12. To test if OCD patients showed stronger conditioned arousal to warning stimuli during the devaluation test and whether this might cause overactive habit formation, we recorded skin conductance responses (SCRs) throughout the experiment. We predicted that OCD patients would be no more fearful of the conditioned stimuli than control subjects and that their behavioral habits would not be mediated by any such difference.
After the experiment, we recorded subjective accounts of why participants felt compelled to respond to the devalued stimulus during the critical habit test. Finally, to assess the ecological validity of overactive habit formation as a model of compulsivity, we asked subjects to rate the experiential urge to respond to the devalued stimulus in our critical habit test. If habits are more than just action slips, subjects should not only perform them following overtraining but also feel compelled to do so.
The primary hypothesis of this study was that OCD patients would show more behavioral habits than healthy control subjects following overtraining and these habits would be associated with a subjective urge to perform them. The secondary hypotheses were 1) both groups would show similar general sensitivity to devaluation (before overtraining), contingency knowledge, and shock expectancy following devaluation between groups; and 2) skin conductance responses, a putative proxy for physiological fear, would not differ between groups.
Methods and Materials
Twenty-Five OCD patients (11 male patients) and 25 healthy control subjects (11 male subjects) matched for age, IQ, handedness (left handed: four OCD patients, five control subjects), and years in education participated in this study (Table 1). Control subjects were recruited from the community, were unmedicated, and had never suffered from a psychiatric disorder. Obsessive-compulsive disorder patients were screened by a psychiatrist using an extended clinical interview to ensure they met the DSM-IV-Text Revision criteria for OCD, exceeding 12 on the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) (13), and had no comorbid psychiatric disorders, past or present. The only exceptions to this were two patients who had been previously diagnosed with depression and one patient who had prior alcohol dependence. We did not screen subjects for Axis II personality disorders, save for obsessive-compulsive personality disorder (OCPD), which was assessed using the Compulsive Personality Assessment Scale (14). We excluded patients for whom hoarding was the primary complaint. General exclusion criteria for both OCD patients and control subjects were current substance dependence, head injury, and current depression, indexed by scores exceeding 16 on the Montgomery-Åsberg Depression Rating Scale (15). Obsessive-compulsive disorder patients reported higher levels of depressive symptoms (though below clinical threshold), OCPD symptoms, and anxiety (Table 1). Eighteen patients were medicated (7 unmedicated; 11 selective serotonin reuptake inhibitor [SSRI]/serotonin reuptake inhibitor; 4 SSRI + antipsychotic; 1 SSRI + lithium bicarbonate; 1 SSRI + propranolol; 1 agomelatine). This study was approved by Cambridgeshire 2 Research Ethics Committee (10/H0308/27).
Table 1.
Demographic Information
| Measures | Control Subjects | OCD Patients | t | df | p |
|---|---|---|---|---|---|
| Age | 41.04 (13.22) | 40.6 (13.45) | .127 | 1,48 | .899 |
| Years in Education | 16.4 (2.19) | 15 (3.04) | 1.865 | 1,48 | .068 |
| NART | 36 (7.31) | 34.88 (7.14) | .548 | 1,48 | .587 |
| Y-BOCS | 0 | 22.76 (5.27) | |||
| MADRS | .96 (3) | 6.6 (3.7) | 5.875 | 1,48 | <.001 |
| STAI-State | 30.16 (5.83) | 44 (9.03) | 6.437 | 1,48 | <.001 |
| STAI-Trait | 32.44 (7.33) | 60 (8.67) | 12.140 | 1,48 | <.001 |
| OCI-R Total | 8.68 (8.4) | 33.16 (11.22) | 8.733 | 1,48 | <.001 |
| CPAS | 3.08 (3.81) | 10.28 (6.13) | 4.991 | 1,48 | <.001 |
Standard deviations are in parentheses.
CPAS, Compulsive Personality Assessment Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; NART, National Adult Reading Test; OCD, obsessive-compulsive disorder; OCI-R, Obsessive-Compulsive Inventory-Revised (40); STAI, State-Trait Anxiety Inventory (39); Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
Procedure
After obtaining informed consent, participants were fitted with SCR and electrical stimulation electrodes. After the 5-minute SCR settling-in period, subjects completed a standard electric shock work-up procedure to reach a shock level that was unpleasant or annoying but not painful for each wrist. Conditioned stimuli (CS) were colored rectangles presented for 750 milliseconds. Two were warning stimuli that predicted shock to the left and right wrists respectively (CS1+, CS2+), and a third CS was safe, never predicting shock (CS3−). To avoid shock, subjects were required to respond on foot pedals while a CS+ was on the screen. The left pedal could avoid the shock to the left wrist, and likewise the right pedal could avoid the shock to the right wrist (Figure 1A). Correct avoidance responses cancelled an imminent shock but did not terminate the CS+. If the participant pressed the incorrect pedal to a CS+ or failed to respond within 750 milliseconds, they received a shock. If participants responded to the safe CS (CS3−), nothing happened; this was always safe. Intertrial intervals were 8 seconds, and the interval between stimulus termination and shock delivery varied randomly between 350 and 600 milliseconds. To eliminate SCR interference, behavioral responses (by foot) were recorded distally from the site of SCR recording (finger tips).
Conditioned stimulus presentation order was randomized and color counterbalanced across participants. Subjects were informed that their task in the entire experiment was to avoid receiving shocks. They were first presented with a Pavlovian demonstration of the stimulus-outcome contingencies (Table 2). Subjects were then instructed that they could avoid subsequent shocks by performing the correct avoidance response while the stimulus was on the screen. There were six avoidance practice trials before the experiment began (two per CS) (Table 2). The main task design consisted of four stages: a brief training session (3 trials per CS), a devaluation test in extinction (4 trials per CS) (devaluation sensitivity test), an extended training session (30 trials per CS), and a final devaluation test in extinction (4 trials per CS) (habit test) (Table 2). Shock outcomes were devalued by disconnecting the stimulator from the electrodes attached to one of the subjects’ wrists in full view of the participants. The electrodes attached to the subjects’ other wrists remained connected to the other stimulator and was thus valued (Figure 1B). Subjects were informed that they could no longer be shocked to the wrist that was disconnected and that their only task was to avoid receiving the remaining shock. Devaluation was conducted in extinction to control for additional reinforcement learning. Subjects behaving in a goal-directed manner should not respond to the CS that predicted the devalued outcome but maintain responding to avoid the valued outcome. In the habit test, the alternate wrist to that devalued during the devaluation sensitivity test was then devalued. The devaluation procedure was counterbalanced across subjects for left and right wrists. Half of the subjects had their left wrist devalued first, and the other half had their right wrist devalued first.
Table 2.
Trial Sequence
| Task Stage | CS1+ | CS2+ | CS3− |
|---|---|---|---|
| Pavlovian Exposure | 1 | 1 | 1 |
| Practice | 2 | 2 | 2 |
| Brief Training | 3 | 3 | 3 |
| Sensitivity to Devaluation Test | 4 | 4 | 4 |
| Extended Training | 30 | 30 | 30 |
| Habit Test | 4 | 4 | 4 |
The number of trials in which the two warning stimuli (CS1+ and CS2+) and the safe stimulus (CS3−) were presented over the task stages.
CS1+, CS2+, warning stimuli; CS3−, safe stimulus.
Following the behavioral experiment, subjects were tested on their explicit knowledge of stimulus-action-outcome associations experienced during training. Subjects also retrospectively rated visual analogue scales from 0 to 100 probing 1) their level of expectancy that a shock would follow the devalued CS; 2) the extent to which they experienced an urge to continue responding in spite of the devaluation; and 3) the extent to which they actively attempted to suppress this urge during the extinction test. Finally, we asked subjects to report why they continued to respond in the habit test or felt compelled to continue do so, even when they did not actually respond.
Data Analysis
Data were statistically analyzed using analysis of variance, Mann-Whitney U, chi-square, and Spearman’s rho. When correlations were conducted between clinical scales and task performance, only the 25 OCD patients were included. Skin conductance responses were measured using a Biopac system operating AcqKnowledge 4.1 (MP36R, Biopac Systems, Santa Barbara, California), sampling at 1000 Hz. Skin conductance responses were identified as the peak responses .5 to 4.5 seconds from stimulus onset, using 2 seconds before stimulus onset as baseline. Skin conductance responses were subject to a .02 umho threshold, and SCRs less than 5% of the max were excluded. Individual SCRs were z-transformed using the average and standard deviation of each subject's SCRs over the entire experiment. No SCRs were detected in five subjects who were excluded from SCR analysis as nonresponders. Additionally, SCR data from 1 OCD patient was lost due to a recording error, leaving 21 OCD patients and 23 control subjects for skin conductance analysis. The behavioral data and questionnaire analysis included all 50 participants. Analysis of behavior and skin conductance responses to the warning stimuli used the average responses to the two CS+ over both training periods combined (brief and extended training: 6 + 60 = 66 trials; Table 2). Analysis of false alarms to the CS−likewise used the average responses across both training stages (i.e., 33 trials).
Results
Behavior
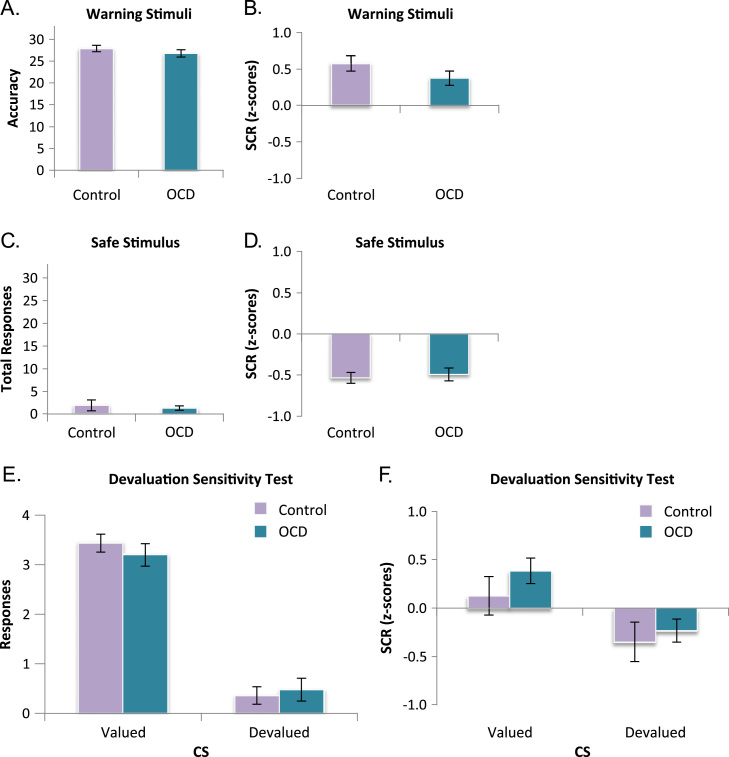
There was no difference between groups in proficiency of task performance, as reflected by equivalent response accuracy to the warning stimuli, F1,48 = 1.004, p = .321 (Figure 2A), false alarms to safe stimulus, and overall reaction times, Fs<1 (Figure 2C). In the devaluation sensitivity test, before overtraining, both groups responded more to the valued than the devalued stimulus, F1,48 = 200.08, p<.001 (Figure 2E). There was no difference between groups or group by stimulus interaction, F<1, indicating that OCD patients were unimpaired in their ability to learn about the safety of the devalued stimulus and withhold unnecessary responses accordingly.
Figure 2.
Training accuracy and general devaluation sensitivity. Error bars denote SEM. (A, B) Discriminative avoidance learning from training sessions. There were no group differences in total avoidance performance or skin conductance responses (SCRs) to warning stimuli. (C, D) Rate of false alarm responses and SCRs to safe stimulus did not differ between groups. (E, F) Devaluation sensitivity test. There were no differences in behavioral or physiological (SCR) sensitivity to devaluation. CS, conditioned stimulus; OCD, obsessive-compulsive disorder.
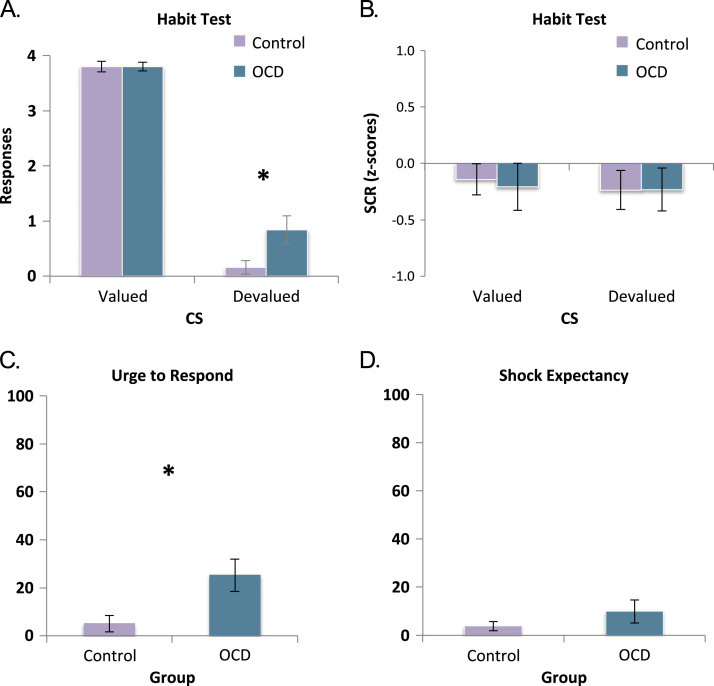
In the habit test, following overtraining, although both groups showed a strong devaluation effect, OCD patients showed greater stimulus-response habit learning than control subjects (Figure 3A), evidenced by greater avoidance of a stimulus that was no longer predictive of shock (devalued). There was a significant main effect of group and a group-by-stimulus interaction, both F1,48 = 4.725, p = .035. These effects were driven by the persistent responding to the devalued stimulus by OCD patients (mean = .84, SE = .25) compared with control subjects (mean = .16, SE = .12), F1,48 = 5.695, p = .021. Responses to the valued stimulus did not differ between groups (OCD: mean = 3.8, SE = .08; control subjects: mean = 3.8, SE = .1). Overall, there was greater responding to the valued compared with the devalued stimulus, indicating that there was a significant devaluation effect in both groups, F1,48 = 445.095, p<.001. The majority of subjects did not continue to respond in the habit test, with just nine OCD patients and two control subjects making any responses. Given that responses were not normally distributed, we conducted a Mann-Whitney U test to confirm the results of our analysis of variance using a nonparametric test. This confirmed that relative to control subjects, OCD patients responded more to the devalued stimulus (U50 = 223.5, z =−2.384, p = .017) and there was no difference in responses to the valued stimulus (U50 = 302.5, z =−.291, p = .771). A chi-square test confirmed that more individuals in the OCD group responded during the habit test (χ21 = 5.71, p = .017).
Figure 3.
Habits in obsessive-compulsive disorder (OCD) patients. Error bars denote SEM. (A) Habit test. Behavioral responses made to the valued stimulus did not differ, F<1, but OCD patients responded significantly more to the stimulus that explicitly no longer predicted shock (devalued) than control subjects. (B) Skin conductance response (SCR) of OCD patients and control subjects during critical habit test did not differ. (C) Obsessive-compulsive disorder patients reported a significantly stronger urge to make avoidance responses in spite of devaluation, F1,48 = 7.016, p = .011. (D) No group difference in explicit shock expectancy in light of the devaluation procedure. *Significant at p < .05. CS, conditioned stimulus.
The rate of habitual responding in OCD patients correlated marginally with overall Y-BOCS scores, r = .357, p = .08. This was driven by the obsessions subscale on the Y-BOCS r = .397, p = .049, and not the compulsions subscale, r = .102, p = .627. Other nonsignificant trends in positive correlations existed between habit responses and the Obsessive-Compulsive Inventory-Revised obsessions (r = .357, p = .08) and hoarding (r = .387, p = .056) subscales and state anxiety, r = .352, p = .084. There was no significant correlation between habit responses and trait anxiety, r = .11, p = .602, or OCPD symptoms (Compulsive Personality Assessment Scale scores), r =−.061, p = .771. As only a subset of OCD patients continued to respond in the habit test, we compared OCD patients who responded and those who did not. We found no significant differences in any of the clinical variables recorded, including symptom subtypes. Finally, as many patients were medicated, we tested for differences between medicated and unmedicated patient responses to the devalued stimulus. Although we found no difference (Fs<1), given the small sample size (unmedicated 7, medicated 18), this does not eliminate the possibility of a medication effect.
Skin Conductance
The behavioral data discussed in this section pertain only to subjects for whom SCR data could be analyzed. Nevertheless, the main results remain significant in this smaller subset (e.g., stimulus-by-group interaction: F1,42 = 5.224, p = .027). To test if habitual responding in the OCD group was the result of heightened conditioned arousal to threatening stimuli or general arousal in response to stimulus presentation (SCRs to the safe stimulus), we compared SCRs between groups during training. There was no difference in SCRs between OCD (mean = .374, SE = .098) and control subjects (mean = .574, SE = .104) to the warning stimuli, F1,42 = 1.96, p = .169 (Figure 2B), or to the safe stimulus, F<1, (OCD: mean =−.535, SE = .068; control subjects: mean =−.493, SE = .081) (Figure 2D). We also compared SCRs during our two extinction tests to assess whether our OCD habit effect was caused by a failure to decrease (extinguish) conditioned arousal in light of devaluation. The data obtained during these tests were highly variable given the low number of trials, and therefore these results should be interpreted with caution. There was no difference in overall SCR between OCD patients (mean = .076, SE = .117) and control subjects (mean =−.112, SE = .117) in the devaluation sensitivity test, F1,42 = 1.29, p = .263, or in the habit test (OCD: mean =−.219, SE = .158; control subjects: mean =−.189, SE = .116), F<1, nor were there any interactions between group and CS type, Fs<1. In the devaluation sensitivity test, both groups exhibited greater SCRs to the valued relative to the devalued CS, F1,42 = 10.27, p = .003 (Figure 2F). However, in the habit test, following extended training, the main effect of stimulus (i.e., the difference between the valued and devalued CS) was not significant, F<1 (Figure 3B). In the habit test, there was no significant correlation between OCD patients’ SCRs to the devalued CS and the number of behavioral responses they made toward that CS, r21 =−.248, p = .279.
Explicit Knowledge
To test if the observed difference in sensitivity to devaluation following extended training was the result of impairments in contingency knowledge in the OCD group, we tested subjects’ awareness of explicit associative contingencies. We found no difference between OCD patients and control subjects in their knowledge of the response (OCD: 100% accuracy; control subjects: mean = 98.7%, SE = 1.3), F1,48 = 1, p = .322, or outcome (OCD: 96%, SE = 4; control subjects: 98.7%, SE = 1.3), F<1, contingencies from the task. Likewise, there were no group differences in post hoc ratings of shock expectancy following devaluation, F1,48 = 1.371, p = .247 (Figure 3D), indicating that our devaluation procedure had been equally effective in both groups (also see behavior in devaluation sensitivity test).
Additional Assessments
Obsessive-compulsive disorder patients reported experiencing a more intense premonitory urge to perform unnecessary habitual foot presses than control subjects, F1,48 = 7.016, p = .011 (Figure 3C). This intensity of urge correlated with the number of responses made to the devalued stimulus in the habit test by the OCD group, r25 = .528, p = .007. Patients who responded to the devalued stimulus in the habit test reported a greater urge than those who did not, F1,23 = 8.706, p = .007. Some subjects, two control subjects and eight OCD patients, who experienced an urge to respond, reported that they attempted to suppress it during the test. Of these two control subjects, both successfully suppressed their responses. Of the eight OCD patients who attempted to suppress responses, six were successful and two were not. Post hoc explanations for responding to the devalued stimulus manifested primarily as irrational threat beliefs in OCD patients, e.g., “I thought I might still be shocked” (Table 3). Like obsessions in OCD, these beliefs were in many cases directly ego-dystonic or contradictory to the patients’ explicit knowledge of task contingency and their ratings of shock expectancy taken moments earlier.
Table 3.
Subjective Accounts for Urge to Perform Habits in OCD Patients and Control Subjects
|
n of Cases |
||||
|---|---|---|---|---|
| Subjective Accounts | OCD | Control | χ2 | p |
| Threat Beliefs (“I thought it could still shock me”) | 9 | 2 | 5.71 | .017 |
| Accidental Slips (“I lost concentration”) | 6 | 3 | 1.22 | .269 |
| Other | 1 | 2 | .355 | .552 |
| NA | 9 | 17 | 5.128 | .024 |
Comments are from all subjects who responded on either pedal (correct or incorrect) or felt an urge to respond when presented with the devalued stimulus in the habit test.
NA, not applicable; OCD, obsessive-compulsive disorder.
Discussion
This study is the first experimental demonstration of habits in avoidance using the goal devaluation procedure. We observed that OCD patients have a bias toward developing avoidance habits and that these habits are related to a subjective urge to respond. These results are consistent with prior reports of impaired goal-directed learning in OCD during appetitive instrumental learning (4) and economic choice (16). Avoidance habits were not the result of any measurable differences in contingency knowledge, explicit threat appraisal, or physiological arousal, suggesting that habits might be an independent contributor to the disorder. The observation that habits were associated with a subjective urge to respond is novel and suggests that habits can, at times, manifest as more than just accidental slips. This finding lends further support to the habit hypothesis of OCD 17, 18.
We investigated the notion that excessive responding in OCD patients is due to a failure to adjust (i.e., devalue) goal-directed behavior, rather than indicative of stimulus-response habit. To test this, we examined sensitivity to a shock devaluation manipulation before overtraining. We found no difference in behavior between the groups in this test, suggesting that before overtraining, OCD patients and control subjects alike believed that the devalued stimulus was safe and were capable of updating their behavioral responses accordingly. This finding was corroborated by the ratings of shock expectancy taken after the habit test, wherein both groups reported very low expectancy. Together, these data indicate that responses to the devalued stimulus in the OCD group are indicative of habit and cannot be construed as goal-directed, purposeful avoidance.
Milad and Rauch (10) recently proposed that OCD may be associated with failures in conditioned fear extinction, noting that although this is not a sufficient account of the phenomenology of OCD, such an impairment could likely be involved in maintaining compulsions. Consistent with this view, anxiety is thought to induce habits by biasing cognitive control systems toward salient stimuli and away from goal-directed actions (12). Obsessive-compulsive disorder patients showed no difference in physiological conditioning or extinction following outcome devaluation, and SCRs were not predictive of habit responses. Therefore, a simple stress-habit account does not appear to explain the excessive behavioral habits observed in the OCD group in this study. However, this finding does not rule out the possibility that stress or anxiety mediated the habit responses observed. The SCR data acquired during our habit test was particularly variable and caution should be taken when interpreting a null result. Future research should address this question using a paradigm that can fully (e.g., temporally) disambiguate Pavlovian fear responses from instrumental responding.
Previous studies have shown that OCD is also associated with deficits in general response inhibition 19, 20, abnormal orbitofrontal cortex activation during reversal learning (21), and switching between rules (extradimensional set-shifting) (22), findings that are consistent with the results of the present study. The overreliance on avoidance habits observed in this study takes these findings concerning behavioral flexibility a step forward, revealing that apparent inhibitory failures emerge over time and with repetition. Furthermore, these data offer a means of explaining the ego-dystonic nature of OCD symptoms, i.e., stimuli, rather than goals, control behavior. Apparently discongruent with our prediction, however, habit responding did not correlate with the compulsions subscale of the Y-BOCS but did correlate marginally with obsessions. Future research should investigate this and would perhaps have greater success in characterizing the relationship between habit, compulsions, and obsessions by adopting a continuous test (23), rather than using outcome devaluation.
Finally, we recorded subjective accounts of why subjects either continued to respond or felt compelled to do so (but did not actually make the response) when presented with the devalued stimulus. This exploratory measure revealed OCD patients and control subjects reported post hoc irrational threat beliefs pertaining to the devaluation. It was particularly striking that these threat beliefs emerged in spite of the earlier experience of safety following devaluation in the devaluation sensitivity test and were in stark contrast to explicit ratings of shock expectancy taken moments before this subjective account. We speculate that experiencing an urge to perform, or performing, irrational avoidance habits could potentially reinforce, or even engender, irrational fear. In situations of cognitive dissonance, where behavior contradicts belief, humans are known to alter beliefs to match behavior (24). Within this framework, irrational obsessive thoughts in OCD might function to resolve the internal conflict arising from experiencing an otherwise nonsensical urge to avoid. However, it may be ultimately impossible to disentangle irrational beliefs from habit behavior, as these features might concurrently strengthen over time (e.g., as cognitive habits). As our task was not optimized to assess the development of irrational beliefs and only a subset of subjects reported these threat-related beliefs, future studies will be necessary to directly test these proposals.
The neural circuits underlying the balance between habitual and goal-directed behavior have been well defined in the neuroscience literature, overlapping with the frontostriatal circuits known to be involved in OCD 25, 26, 27. Although this study provides evidence for overactive habit formation in OCD, it does not resolve the issue of whether deficient inhibitory process in frontal regions mediate this effect (from the top down) 28, 29 or whether overactive habit formation is, in fact, mediated in the striatum (bottom up), where goal-directed actions and habits are subserved by the caudate nucleus and the putamen, respectively 30, 31, 32, 33. Future neuroimaging studies should address this question.
That most patients were medicated with SSRIs poses a limitation for this study. However, analysis comparing medicated and unmedicated patients revealed no effect of medication status on habit formation. Nonetheless, such a comparison is limited, given the low number of unmedicated patients, and therefore future studies should test their role in habit formation directly. To date, no research in humans has directly assessed the role of serotonin in habit formation, but work in animals has shown decreased sensitivity to outcome devaluation as a result of serotonin receptor antagonism or serotonin depletion 34, 35. It therefore would appear unlikely that chronic SSRIs themselves would enhance habit formation.
This notion that excessive habit formation may be a contributor to OCD is consistent with recent data suggesting that compulsivity might play a more prominent role in OCD than previously assumed (36). These data are compatible with a possible shift in the classification of OCD as an anxiety disorder in the forthcoming issue of the DSM (37) and have implications for the development of new behavioral and pharmacologic interventions focusing on suppressing habits. Furthermore, understanding how the urge to perform habits might contribute to compulsive urge in OCD may offer a new insight of immediate clinical value not only to the cognitive-behavioral treatment of the OCD but also potentially to other disorders of maladaptive habit formation, such as drug addiction (38).
This research was funded by a Wellcome Trust Grant ( 089589/Z/09/Z ) awarded to T.W. Robbins, B.J. Everitt, A.C. Roberts, J.W. Dalley, and B.J. Sahakian, and it was conducted at the Behavioural and Clinical Neuroscience Institute, which is supported by a joint award from the Medical Research Council and Wellcome Trust ( G00001354 ). CMG is supported by a studentship from the Medical Research Council. AMA-S and SM-Z are supported by the Wellcome Trust Grant above. VV is a Wellcome Trust Fellow.
We are grateful to the obsessive-compulsive disorder patients and healthy volunteers who participated in this study. We thank Anthony Dickinson for his advice.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at 10.1016/j.biopsych.2013.02.002.
Supporting information
Supplementary Material
References
- 1.American Psychiatric Association . American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. [Google Scholar]
- 2.Foa E.B., Kozak M.J., Goodman W.K., Hollander E., Jenike M.A., Rasmussen S.A. DSM-IV field trial: Obsessive-compulsive disorder. Am J Psychiatry. 1995;152:90–96. doi: 10.1176/ajp.152.1.90. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson A. Actions and habits: The development of behavioural autonomy. Philos Trans R Soc Lond B Biol Sci. 1985;308:67–78. [Google Scholar]
- 4.Gillan C.M., Papmeyer M., Morein-Zamir S., Sahakian B.J., Fineberg N.A., Robbins T.W., de Wit S. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry. 2011;168:718–726. doi: 10.1176/appi.ajp.2011.10071062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salkovskis P.M. Obsessional-compulsive problems: A cognitive-behavioural analysis. Behav Res Ther. 1985;23:571–583. doi: 10.1016/0005-7967(85)90105-6. [DOI] [PubMed] [Google Scholar]
- 6.Purdon C., Clark D.A. Metacognition and obsessions. Clin Psychol Psychother. 1999;6:102–110. [Google Scholar]
- 7.Rachman S. A cognitive theory of obsessions. Behav Res Ther. 1997;35:793–802. doi: 10.1016/s0005-7967(97)00040-5. [DOI] [PubMed] [Google Scholar]
- 8.Foa E., Kozak M. In: Anxiety and the Anxiety Disorders. Tuma A., Maser J., editors. Lawrence Erlbaum Associates; Hillsdale NJ: 1985. Treatment of anxiety disorders: Implications for psychopathology; pp. 421–452. [Google Scholar]
- 9.Mowrer O.H. Two-factor learning theory: Summary and comment. Psychol Rev. 1951;58:350–354. doi: 10.1037/h0058956. [DOI] [PubMed] [Google Scholar]
- 10.Milad M.R., Rauch S.L. Obsessive-compulsive disorder: Beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwabe L., Wolf O.T. Stress prompts habit behavior in humans. J Neurosci. 2009;29:7191–7198. doi: 10.1523/JNEUROSCI.0979-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eysenck M.W., Derakshan N., Santos R., Calvo M.G. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 13.Goodman W.K., Price L.H., Rasmussen S.A., Mazure C., Fleischmann R.L., Hill C.L. The Yale-Brown Obsessive-Compulsive Scale (Y-BOCS): Part 1 Development, use and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 14.Fineberg N.A., Sharma P., Sivakumaran T., Sahakian B., Chamberlain S. Does obsessive-compulsive personality disorder belong within the obsessive-compulsive spectrum? CNS Spectr. 2007;12:467–482. doi: 10.1017/s1092852900015340. [DOI] [PubMed] [Google Scholar]
- 15.Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 16.Gillan CM, Sharon M-Z, Kaser M, Fineberg NA, Sule A, Sahakian BJ, et al. (in press): Counterfactual processing of economic action-outcome alternatives in obsessive-compulsive disorder: Further evidence of impaired goal-directed behavior. Biol Psychiatry. [DOI] [PMC free article] [PubMed]
- 17.Graybiel A.M., Rauch S.L. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 18.Robbins T.W., Gillan C.M., Smith D.G., de Wit S., Ersche K.D. Neurocognitive endophenotypes of impulsivity and compulsivity: Towards dimensional psychiatry. Trends Cogn Sci. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Menzies L., Achard S., Chamberlain S.R., Fineberg N., Chen C.H., del Campo N. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–3236. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- 20.Morein-Zamir S., Papmeyer M., Gillan C.M., Crockett M.J., Fineberg N.A., Sahakian B.J., Robbins T.W. Punishment promotes response control deficits in obsessive-compulsive disorder: Evidence from a motivational go/no-go task. Psychol Med. 2013;43:391–400. doi: 10.1017/S0033291712001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chamberlain S.R., Menzies L., Hampshire A., Suckling J., Fineberg N.A., del Campo N. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–422. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain S.R., Fineberg N.A., Menzies L.A., Blackwell A.D., Bullmore E.T., Robbins T.W., Sahakian B.J. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164:335–338. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daw N.D., Niv Y., Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci. 2005;8:1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- 24.Festinger L. Row Peterson; Evanston, IL: 1957. A Theory of Cognitive Dissonance. [Google Scholar]
- 25.Balleine B.W., O'Doherty J.P. Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saxena S., Rauch S.L. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000;23:563–586. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- 27.de Wit S., Watson P., Harsay H.A., Cohen M.X., van de Vijver I., Ridderinkhof K.R. Corticostriatal connectivity underlies individual differences in the balance between habitual and goal-directed action control. J Neurosci. 2012;32:12066–12075. doi: 10.1523/JNEUROSCI.1088-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valentin V.V., Dickinson A., O'Doherty J.P. Determining the neural substrates of goal-directed learning in the human brain. J Neurosci. 2007;27:4019–4026. doi: 10.1523/JNEUROSCI.0564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Wit S., Corlett P.R., Aitken M.R., Dickinson A., Fletcher P.C. Differential engagement of the ventromedial prefrontal cortex by goal-directed and habitual behavior toward food pictures in humans. J Neurosci. 2009;29:11330–11338. doi: 10.1523/JNEUROSCI.1639-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tricomi E., Balleine B.W., O'Doherty J.P. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci. 2009;29:2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin H.H., Knowlton B.J., Balleine B.W. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- 32.Yin H.H., Ostlund S.B., Knowlton B.J., Balleine B.W. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- 33.Jentsch J.D., Taylor J.R. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 34.Clarke H.F., Walker S.C., Dalley J.W., Robbins T.W., Roberts A.C. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- 35.Altman H., Normile H. Enhancement of the memory of a previously learned aversive habit following pretest administration of a variety of serotonergic antagonists in mice. Psychopharmacology (Berl) 1986;90:24–27. doi: 10.1007/BF00172866. [DOI] [PubMed] [Google Scholar]
- 36.Williams M.T., Farris S.G., Turkheimer E., Pinto A., Ozanick K., Franklin M.E. Myth of the pure obsessional type in obsessive-compulsive disorder. Depress Anxiety. 2011;28:495–500. doi: 10.1002/da.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Psychiatric Association (2012): DSM-V development. Available at: http://www.dsm5.org/Pages/Default.aspx. Accessed December 15th, 2013
- 38.Everitt B.J., Dickinson A., Robbins T.W. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 39.Spielberger C.D. Mind Garden; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory STAI. [Google Scholar]
- 40.Foa E.B., Huppert J.D., Leiberg S., Langner R., Kichic R., Hajcak G., Salkovskis P.M. The Obsessive-Compulsive Inventory: Development and validation of a short version. Psychol Assess. 2002;14:485–496. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material