Abstract
Beta oscillations are involved in movement and have previously been linked to levels of the inhibitory neurotransmitter GABA. We examined changes in beta oscillations during rest and movement in primary motor cortex (M1). Amplitude and frequency of beta power at rest and movement-related beta desynchronization (MRBD) were measured during a simple unimanual grip task and their relationship with age was explored in a group of healthy participants. We were able to show that at rest, increasing age was associated with greater baseline beta power in M1 contralateral to the active hand, with a similar (non-significant) trend in ipsilateral M1. During movement, increasing age was associated with increased MRBD amplitude in ipsilateral M1 and reduced frequency (in contralateral and ipsilateral M1). These findings would be consistent with greater GABAergic inhibitory activity within motor cortices of older subjects. These oscillatory parameters have the potential to reveal changes in the excitatory–inhibitory balance in M1 which in turn may be a useful marker of plasticity in the brain, both in healthy ageing and disease.
Keywords: Ageing, Motor, Beta, Oscillations, MEG, Plasticity
Highlights
-
•
Changes in motor cortex beta oscillations are linked with changes in GABA.
-
•
Changes in GABA-related cortical inhibition are linked with plasticity.
-
•
Older subjects had higher resting beta power and greater beta decrease during grip.
-
•
Beta oscillations are useful markers of cortical inhibition and plasticity.
Introduction
The capacity for neuroplastic change is altered in the ageing brain (Chollet, 2013; Kolb and Teskey, 2012). A key determinant of the capacity for plasticity in the adult brain is the balance between GABAergic inhibition and glutamatergic excitation (Benali et al., 2008) and a shift of balance away from inhibition tends to increase plasticity (Hensch, 2005).
Age-related changes in intracortical excitation and inhibition of primary motor cortex (M1) at rest have been explored in a number of transcranial magnetic stimulation (TMS) studies. Short-interval intracortical inhibition (SICI) and long-interval intracortical inhibition (LICI) are TMS protocols designed to probe inhibitory mechanisms mediated by GABA-A and GABA-B receptors respectively. Results from these studies are contradictory. Some demonstrate decreased inhibition with age (Heise et al., 2013; Hortobágyi et al., 2006; Marneweck et al., 2011), whereas others suggest an increase in inhibition (McGinley et al., 2010; Smith et al., 2009). Furthermore, SICI and LICI are most commonly measured at rest and so may not reflect the dynamic changes during movement that are important for task-dependent plasticity.
More recently, there has been interest in investigating cortical inhibitory mechanisms using magnetoencephalography (MEG) and electroencephalography (EEG). MEG can detect the oscillatory signals generated by changes in the post-synaptic fields of glutamatergic pyramidal cells. Pyramidal cells are reciprocally connected to GABAergic interneurons and so changes in oscillatory signals are dependent on the balance between inhibition and excitation within these microcircuits (Yamawaki et al., 2008). As such, oscillatory changes could reflect the potential for both local and network plasticity (Traub et al., 2004). Oscillations in the beta frequency band (15–30 Hz) are known to be important in movement. In M1, they are present at rest and are suppressed during movement (movement-related beta desynchronization — MRBD) (Pfurtscheller and Lopes da Silva, 1999).
Evidence that the properties of beta oscillations are related to GABAergic activity comes from pharmacological manipulations of GABA prior to and during movement in both animals and humans (Hall et al., 2010, 2011; Roopun et al., 2006; Yamawaki et al., 2008). Diazepam, a GABA-A agonist, increased the amplitude of baseline beta power (Hall et al., 2010) and accentuated MRBD (Hall et al., 2011). It also increased SICI in healthy controls (Florian et al., 2008). Tiagabine, a GABA-reuptake inhibitor (Muthukumaraswamy et al., 2012), increased amplitude of baseline beta power and enhanced MRBD. Furthermore, the frequency of beta oscillations have been found to decrease with administration of benzodiazepine concurrent with an increase in baseline beta power amplitude (Jensen et al., 2005). These results suggest that higher baseline beta power, decreased beta frequency and greater amplitude of MRBD reflect increased levels of intracortical GABAergic inhibition. We can therefore use these findings to make inferences about changes in GABAergic inhibition from measurements of beta oscillatory power.
One key question is which brain regions we should examine for such movement related changes. FMRI studies have shown that healthy ageing is associated with more widespread activation of motor areas during movement, and specifically less ‘deactivation’ in the ipsilateral M1 in older subjects (Talelli et al., 2008; Ward and Frackowiak, 2006; Ward, 2006; Ward et al., 2008). We therefore examined task-related changes in beta oscillations in M1 both contralateral and ipsilateral to the moving hand. Many previous studies have suggested that there is a reduced capacity for plasticity (at rest) with increasing age (Fathi et al., 2010; Sawaki et al., 2003; Tecchio et al., 2008) most likely due to greater cortical inhibition. In this study, we were interested in investigating age-related changes in beta oscillations (as a marker of the balance between excitation and inhibition) at rest and during movement, because of their potential relevance for practice-dependent cortical plasticity.
We expected to find that older subjects would demonstrate increased baseline beta power and MRBD amplitude along with a decrease in beta frequency as a reflection of a reduced potential for plasticity in the motor cortices.
Materials and methods
Subjects
Thirty-two healthy participants (mean age 51 ± 21 years, range 22–82 years; 11 female, 2 left-handed) took part in this study. Full written consent was obtained from all participants in accordance with the Declaration of Helsinki. The study was approved by the Joint Ethics Committee of the Institute of Neurology, UCL and National Hospital for Neurology and Neurosurgery, UCL Hospitals NHS Foundation Trust, London.
Behavioural testing
All participants were scored on the Nine Hole Peg Test (NHPT), Box and Blocks test and Grip strength in order to cover a range of upper limb motor abilities, from dexterity to power.
Motor task
Participants performed visually cued isometric hand grips with their dominant hand using a manipulandum during MEG recording. Prior to scanning, maximum voluntary contraction (MVC) was obtained for each participant. Sixty trials were performed. The cue to perform a hand grip was the appearance of a ‘force thermometer’ on the screen which provided continuous visual feedback about the force exerted. The target force was set at 30% of their MVC and displayed visually. Each grip was sustained for 3 s with an interstimulus interval that jittered between 3 and 7 s.
MEG recording
MEG signals were measured continuously at 600 Hz during the task using a whole-head CTF Omega 275 MEG system (CTF, Vancouver, Canada). Head localization was monitored continuously during the recordings in order to check for excessive movement. The MEG data were pre-processed offline using SPM8 (Wellcome Trust Centre for Neuroimaging, www.fil.ion.ucl.ac.uk/spm) (Litvak et al., 2011). Data were down-sampled to 300 Hz and were filtered from 5 to 100 Hz. Data were epoched from − 3 s to + 3 s where time 0 indicated onset of the visual cue for analysis. Data with large eye blinks or other artefacts were excluded.
Structural MRI recording
A 3 T Siemens Trio scanner (Siemens, Erlangen, Germany) was used to acquire high resolution T1-weighted anatomical images (1.3 × 1.3 × 1.3 mm voxels); 176 partitions, FoV = 256 × 240, TE = 2.48 ms, TR = 7.92 ms, FA = 16°). Structural MRIs could not be obtained in four of the participants due to MRI contraindications.
Data processing and analysis
Lead fields were computed using a single-shell head model (Nolte, 2003) based on an inner skull mesh derived by inverse-normalizing a canonical mesh to the subject's individual MRI image (Mattout et al., 2007). For subjects without an individual MRI the canonical mesh was affine-transformed to fit their MEG fiducials. Coregistration between the MRI and MEG coordinate systems used three fiducial points: nasion, left and right pre-auricular. Whilst acquiring the structural MRI, fiducial points were marked with vitamin-E capsules in order to coregister with the MEG fiducials.
Oscillatory changes in the beta band (15–30 Hz) between rest and grip were localised using the Linearly Constrained Maximal Variance (LCMV) beamformer (Hillebrand and Barnes, 2005; Vrba and Robinson, 2001) as part of the SPM8 software. The beamforming method is based on the linear projection of sensor data using a spatial filter computed from the lead field of the source of interest and the data covariance (Van Veen et al., 1997). We computed the data covariance matrix using two time windows (passive and active). The passive time window was taken from − 2.5 s to 0 s with 0 as the onset of the visual cue to move. The active time window was from 0.5 s to 3 s following the visual cue onset. We then made volumetric t-statistic images per subject using a grid spacing of 10 mm. At each location, the source orientation was taken to be in the direction yielding maximal signal variance (Sekihara et al., 2004). From these t-statistic images, we extracted the source signal from the location of peak change in beta power (15–30 Hz) within the primary motor cortices both contralateral and ipsilateral to the moving hand. Morlet-wavelet time–frequency analysis was used to explore the changes in beta across a trial from these locations, data were epoched again in order to visualise changes before and after the movement using the time window − 1 s to + 5 s. The spectrograms were rescaled in order to show percentage change from baseline (− 1 to 0 s) and averaged across trials. The mean percentage decrease in beta power (MRBD) was then extracted from the 3 s movement period for each participant. The absolute baseline beta power (− 1 s to 0 s) was also obtained. These beta parameters were then correlated with age.
Results
All subjects were able to perform the grip task adequately. The average and range for the behavioural tests were as follows: NHPT average = 0.74 pegs per second, range = 0.53–0.96, Box and Blocks test average = 61 blocks per minute, range = 44–91, Grip strength average = 75 kg, range = 46–117 kg.
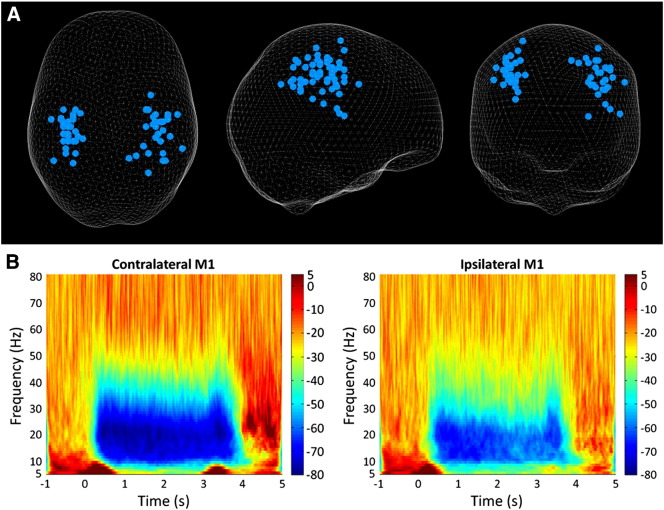
A change in beta power was seen between rest and grip in all participants in M1 both contralateral and ipsilateral to the moving hand. The location of these peaks can be seen in Fig. 1A. Fig. 1B shows a group average spectrogram spanning from 5 to 80 Hz across a trial with 1 s baseline, 3 s of grip and 2 s post-grip. MRBD during grip can be seen clearly and appears stronger in contralateral M1 than ipsilateral M1.
Fig. 1.
A) Glass brain showing peak change in beta power between rest and grip in both the contralateral and ipsilateral M1 (grip was performed with the right hand — left hand grips were flipped in the sagittal plane so that all data could be included on the same plot) with each dot representing an individual. Results are displayed on a ‘glass brain’ and shown from above (left), from the right side (middle) and from behind (right). B) Group average time–frequency spectrogram showing the changes in power from 5 to 80 Hz across 1 trial comprising 1 s baseline, 3 s grip and 2 s post-grip. The colour indicates the percentage change in power from baseline at that frequency with red indicating high power and blue indicating low power. The decrease in beta power during the grip can be seen as well as the rebound following movement cessation.
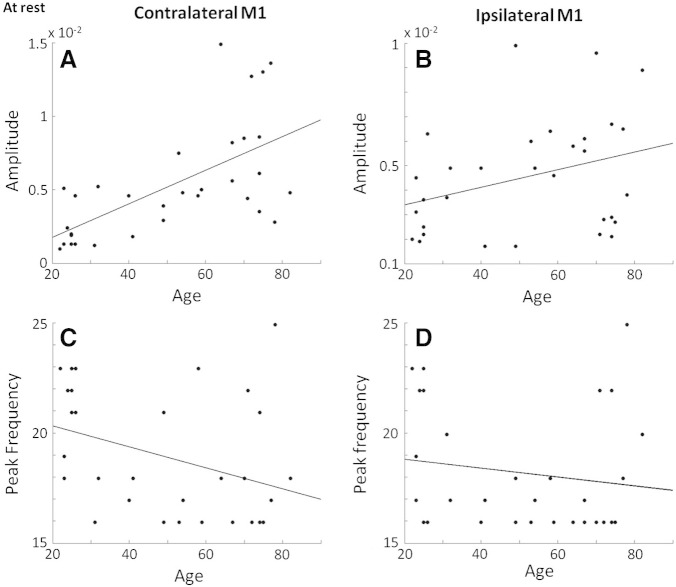
At rest, baseline beta amplitude in contralateral M1 correlated positively with age (R = 0.63, p = 0.0001, 95% CI = 0.49–0.77) (Fig. 2A). There was a similar yet non-significant trend in ipsilateral M1 (R = 0.33, p = 0.07, 95% CI = 0.05–0.56) (Fig. 2B). Peak beta frequency did not alter significantly with age in either contralateral or ipsilateral M1, although there was a non-significant trend (R = − 0.36, p = 0.06, 95% CI = − 0.66– − 0.05) towards a decrease in frequency with increasing age in contralateral M1 (Fig. 2C).
Fig. 2.
A) Significant positive correlation (R = 0.64, p = 0.00008) between contralateral M1 baseline beta power and age across the control group (n = 32). B) Ipsilateral M1 baseline beta power against age, this correlation was not significant but did show a trend (R = 0.35, p = 0.06). C) Contralateral M1 peak beta frequency at rest against age, this correlation did not reach significance (R = − 0.31, p = 0.09). D) Ipsilateral M1 peak beta frequency at rest against age, this correlation was not significant (R = 0.05, p = 0.79).
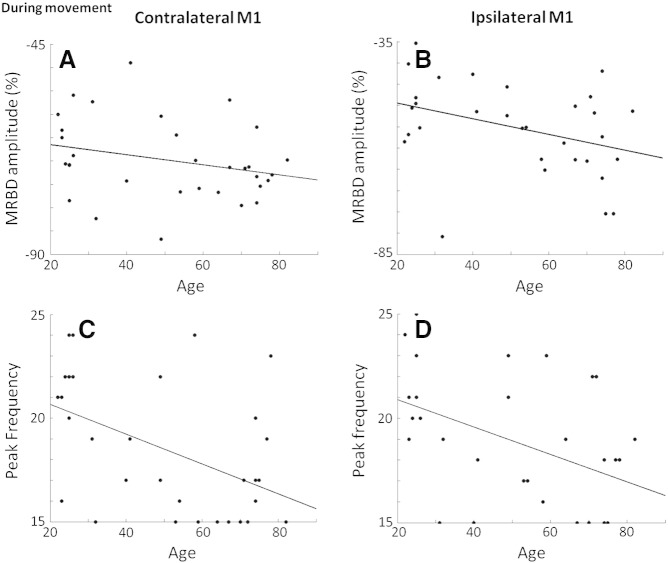
The amplitude of MRBD in ipsilateral M1, but not contralateral M1, correlated negatively with age (R = − 0.37, p = 0.04, 95% CI = − 0.63– − 0.06) so that in older subjects, a larger percentage decrease in beta power was seen (Fig. 3B). The peak frequency of MRBD correlated negatively with age in both contralateral M1 (R = − 0.48, p = 0.01, 95% CI = − 0.69– − 0.22) and ipsilateral M1 (R = − 0.45, p = 0.01, 95% CI = 0.5–0.02) (Figs. 3C & D). The behavioural test scores did not correlate with age, baseline beta power, peak frequency of baseline, percentage decrease in MRBD, or peak frequency of MRBD in either contralateral or ipsilateral M1.
Fig. 3.
A) Contralateral M1 MRBD amplitude against age, this correlation was not significant (R = − 0.27, p = 0.13). B) Significant correlation between ipsilateral M1 MRBD amplitude and age (R = − 0.37, p = 0.04). C) Significant negative correlation between peak frequency of MRBD in contralateral M1 and age (R = − 0.48, p = 0.01). D) Significant negative correlation between peak frequency of MRBD in ipsilateral M1 and age (R = − 0.45, p = 0.01).
If these p-values are Bonferroni corrected for multiple comparisons across the two brain areas being investigated (corrected threshold of p = 0.025) then the correlation between CM1 baseline beta and age survives, as do the correlations between peak frequency of MRBD and age in both CM1 and IM1.
Discussion
In this study, we used MEG to assess age-related changes in cortical oscillations at rest and during movement. Cortical oscillations are dependent on the balance between GABAergic inhibition and glutamatergic excitation within the cortex (Traub et al., 2004; Yamawaki et al., 2008), and as such might reflect the capacity for neuroplastic change. Evidence from previous work in humans suggests that increased levels of intracortical GABAergic inhibition lead to higher resting beta power and greater amplitude of MRBD, together with a decrease in beta frequency (Hall et al., 2011; Jensen et al., 2005; Muthukumaraswamy et al., 2012). We were able to show that at rest, the motor cortices of older subjects tended to exhibit greater beta power and (in contralateral M1 at least) reduced beta frequency. During movement, the motor cortices of older subjects were more likely to exhibit increased MRBD amplitude and reduced beta frequency. This is in keeping with previous work by Gaetz et al. (2010) which examined oscillatory changes during development and found an increase in MRBD with increasing age from young children to adolescents.
There are two caveats to these results. Firstly, though we were primarily interested in the beta frequency band, we note that the correlation between power and age in CM1 at rest was also found in the mu (7–14 Hz) frequency band (R = 0.45, p = 0.01). This may mean that the age-related changes we see in the beta band could be a harmonic of mu (Pfurtscheller and Lopes da Silva, 1999). However, none of the other mu oscillatory parameters correlated with age. Secondly, MRBD can be affected by the amplitude of baseline beta power (Lemm et al., 2009) and although we used the percentage change between rest and grip to take account of this, the change in MRBD may still be partly accounted for by alterations in baseline beta power.
Overall these results suggest an age-related increase in GABAergic inhibition in both contralateral and ipsilateral M1, most noticeably during movement, and to a lesser extent at rest.
Heise et al. (2013) examined SICI both at rest and during movement preparation in contralateral M1 in a large group of healthy participants and found a decrease in SICI (less inhibition) at rest in older participants. In addition, increasing age was associated with a reduced ability to decrease inhibition during movement preparation. This differs from our results, in which we saw higher baseline beta levels and a decrease in peak beta frequency with age which are both thought to reflect an increase in GABAergic inhibition. One explanation for this might be that SICI and MEG signals are measuring different aspects of the GABAergic system. Stimulation of synaptic GABAA receptors produces a phasic highly transient effect, but extrasynaptic GABAA receptors mediate a slower tonic inhibitory current (Carmichael, 2012), as do GABAB receptors which are metabotropic (Benarroch, 2012). Whilst we know that SICI specifically assesses synaptic GABAA function (Florian et al., 2008), it may be that the MEG signal is the result of a combination of these different GABAergic processes.
We have previously reported with fMRI that no age-related change was seen in the magnitude of motor task-related activation in contralateral M1, but there was less ‘deactivation’ in ipsilateral M1 in older subjects (Ward et al., 2008), consistent with the idea that there is greater involvement of ipsilateral hemisphere in motor control with ageing (Boudrias et al., 2012). We were therefore interested to look at changes in beta band oscillations in ipsilateral M1 and found enhanced MRBD amplitude and lower peak frequency during movement, which is likely to reflect the same age-related increased intracortical inhibition seen in contralateral M1.
Interestingly, motor performance as measured by NHPT, Box and Blocks and Grip strength was not found to correlate with age as has been seen in other studies (Potvin et al., 1980; Rossini et al., 2007). It may be that chronological age is not the best indicator of biological ageing in this case as we saw a range of motor performance scores both in younger and older participants. Nevertheless, these are relatively simple tests and age-effects might be seen with more complex motor tasks (Smith et al., 1999). No correlation was found between the beta parameters and the motor performance scores. This was not unexpected as decreased plasticity does not necessarily equate to poor performance, but is likely to impact more on motor learning which can be slower in older adults (Wu and Hallett, 2005).
In this study we make inferences about GABAergic inhibition indirectly through measurement of cortical beta oscillations. However, we did not measure GABA directly. Currently only MR spectroscopy provides a method for this, but MRS cannot measure dynamic changes in GABA before and during handgrip. TMS is another tool for indirectly assessing GABAergic inhibition, but again, it is unusual to use TMS paired pulse paradigms during movement. Assessing neurotransmitter activity in the human cortex is clearly challenging and there is no gold standard. Despite that, we believe our results can be interpreted in light of previous pharmaco-MEG studies which suggest a link between properties of cortical beta oscillations and GABAergic inhibitory activity. Future studies might usefully investigate how ageing influences these types of pharmacological challenge. Clearly further work is required to get a more complex picture of the relationship between cortical oscillations, cortical inhibition and excitation, and ageing or pathology.
Conclusions
The results from this study demonstrate age-related changes in the properties of motor cortex beta oscillations at rest and during movement. Previous work suggests that beta oscillations are sensitive to levels of GABA in the brain (Gaetz et al., 2011; Hall et al., 2011; Muthukumaraswamy et al., 2012) and support the view that our results reflect greater levels of GABAergic activity in motor cortices of older subjects and therefore potentially reduced task-dependent plasticity. The study of oscillatory signals in the brain may provide a method for studying the mechanisms of intracortical plasticity in both healthy ageing and disease. New approaches will allow us to examine the balance between excitation and inhibition by modelling changes in GABAergic and glutamatergic function using neural mass models of in-vivo spectral responses (Bastos et al., 2012; Moran et al., 2009, 2011).
Role of the funding source
This research was supported by the European Commission under the 7th Framework Program-HEALTH-Collaborative Project Plasticise (Contract no. 223524) www.plasticise.eu (Dr. Rossiter), and The Wellcome Trust (Dr. Nick Ward).
Conflict of interest statement
There is no conflict of interest.
References
- Bastos A.M., Usrey W.M., Adams R.A., Mangun G.R., Fries P., Friston K.J. Canonical microcircuits for predictive coding. Neuron. 2012;76:695–711. doi: 10.1016/j.neuron.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benali A., Weiler E., Benali Y., Dinse H.R., Eysel U.T. Excitation and inhibition jointly regulate cortical reorganization in adult rats. J. Neurosci. 2008;28:12284–12293. doi: 10.1523/JNEUROSCI.1952-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E.E. GABAB receptors: structure, functions, and clinical implications. Neurology. 2012;78:578–584. doi: 10.1212/WNL.0b013e318247cd03. [DOI] [PubMed] [Google Scholar]
- Boudrias M.-H., Gonçalves C.S., Penny W.D., Park C.-H., Rossiter H.E., Talelli P., Ward N.S. Age-related changes in causal interactions between cortical motor regions during hand grip. NeuroImage. 2012;59:3398–3405. doi: 10.1016/j.neuroimage.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael S.T. Brain excitability in stroke: the yin and yang of stroke progression. Arch. Neurol. 2012;69:161–167. doi: 10.1001/archneurol.2011.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet F. Pharmacologic approaches to cerebral aging and neuroplasticity: insights from the stroke model. Dialogues Clin. Neurosci. 2013;15:67–76. doi: 10.31887/DCNS.2013.15.1/fchollet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi D., Ueki Y., Mima T., Koganemaru S., Nagamine T., Tawfik A., Fukuyama H. Effects of aging on the human motor cortical plasticity studied by paired associative stimulation. Clin. Neurophysiol. 2010;121:90–93. doi: 10.1016/j.clinph.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Florian J., Müller-Dahlhaus M., Liu Y., Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J. Physiol. 2008;586:495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W., Macdonald M., Cheyne D., Snead O.C. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: age predicts post-movement beta rebound. NeuroImage. 2010;51:792–807. doi: 10.1016/j.neuroimage.2010.01.077. [DOI] [PubMed] [Google Scholar]
- Gaetz W., Edgar J.C., Wang D.J., Roberts T.P.L. Relating MEG measured motor cortical oscillations to resting γ-aminobutyric acid (GABA) concentration. NeuroImage. 2011;55:616–621. doi: 10.1016/j.neuroimage.2010.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S.D., Barnes G.R., Furlong P.L., Seri S., Hillebrand A. Neuronal network pharmacodynamics of GABAergic modulation in the human cortex determined using pharmaco-magnetoencephalography. Hum. Brain Mapp. 2010;31:581–594. doi: 10.1002/hbm.20889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S.D., Stanford I.M., Yamawaki N., McAllister C.J., Rönnqvist K.C., Woodhall G.L., Furlong P.L. The role of GABAergic modulation in motor function related neuronal network activity. NeuroImage. 2011;56:1506–1510. doi: 10.1016/j.neuroimage.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Heise K.-F., Zimerman M., Hoppe J., Gerloff C., Wegscheider K., Hummel F.C. The aging motor system as a model for plastic changes of GABA-mediated intracortical inhibition and their behavioral relevance. J. Neurosci. 2013;33:9039–9049. doi: 10.1523/JNEUROSCI.4094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hillebrand A., Barnes G.R. Beamformer analysis of MEG data. Int. Rev. Neurobiol. 2005;68:149–171. doi: 10.1016/S0074-7742(05)68006-3. [DOI] [PubMed] [Google Scholar]
- Hortobágyi T., del Olmo M.F., Rothwell J.C. Age reduces cortical reciprocal inhibition in humans. Exp. Brain Res. 2006;171:322–329. doi: 10.1007/s00221-005-0274-9. [DOI] [PubMed] [Google Scholar]
- Jensen O., Goel P., Kopell N., Pohja M., Hari R., Ermentrout B. On the human sensorimotor–cortex beta rhythm: sources and modeling. NeuroImage. 2005;26:347–355. doi: 10.1016/j.neuroimage.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Kolb B., Teskey G.C. Age, experience, injury, and the changing brain. Dev. Psychobiol. 2012;54:311–325. doi: 10.1002/dev.20515. [DOI] [PubMed] [Google Scholar]
- Lemm S., Müller K.-R., Curio G. A generalized framework for quantifying the dynamics of EEG event-related desynchronization. PLoS Comput. Biol. 2009;5:e1000453. doi: 10.1371/journal.pcbi.1000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V., Mattout J., Kiebel S., Phillips C., Henson R., Kilner J., Barnes G., Oostenveld R., Daunizeau J., Flandin G., Penny W., Friston K. EEG and MEG data analysis in SPM8. Comput. Intell. Neurosci. 2011;2011:852961. doi: 10.1155/2011/852961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marneweck M., Loftus A., Hammond G. Short-interval intracortical inhibition and manual dexterity in healthy aging. Neurosci. Res. 2011;70:408–414. doi: 10.1016/j.neures.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Mattout J., Henson R.N., Friston K.J. Canonical source reconstruction for MEG. Comput. Intell. Neurosci. 2007:67613. doi: 10.1155/2007/67613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley M., Hoffman R.L., Russ D.W., Thomas J.S., Clark B.C. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp. Gerontol. 2010;45:671–678. doi: 10.1016/j.exger.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R.J., Stephan K.E., Seidenbecher T., Pape H.-C., Dolan R.J., Friston K.J. Dynamic causal models of steady-state responses. NeuroImage. 2009;44:796–811. doi: 10.1016/j.neuroimage.2008.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R.J., Symmonds M., Stephan K.E., Friston K.J., Dolan R.J. An in vivo assay of synaptic function mediating human cognition. Curr. Biol. CB. 2011;21:1320–1325. doi: 10.1016/j.cub.2011.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Myers J.F.M., Wilson S.J., Nutt D.J., Lingford-Hughes A., Singh K.D., Hamandi K. The effects of elevated endogenous GABA levels on movement-related network oscillations. NeuroImage. 2012;66C:36–41. doi: 10.1016/j.neuroimage.2012.10.054. [DOI] [PubMed] [Google Scholar]
- Nolte G. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys. Med. Biol. 2003;48:3637–3652. doi: 10.1088/0031-9155/48/22/002. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Lopes da Silva F.H. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Potvin A.R., Syndulko K., Tourtellotte W.W., Lemmon J.A., Potvin J.H. Human neurologic function and the aging process. J. Am. Geriatr. Soc. 1980;28:1–9. doi: 10.1111/j.1532-5415.1980.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Roopun A.K., Middleton S.J., Cunningham M.O., LeBeau F.E.N., Bibbig A., Whittington M.A., Traub R.D. A beta2-frequency (20–30 Hz) oscillation in nonsynaptic networks of somatosensory cortex. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15646–15650. doi: 10.1073/pnas.0607443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini P.M., Rossi S., Babiloni C., Polich J. Clinical neurophysiology of aging brain: from normal aging to neurodegeneration. Prog. Neurobiol. 2007;83:375–400. doi: 10.1016/j.pneurobio.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Sawaki L., Yaseen Z., Kopylev L., Cohen L.G. Age-dependent changes in the ability to encode a novel elementary motor memory. Ann. Neurol. 2003;53:521–524. doi: 10.1002/ana.10529. [DOI] [PubMed] [Google Scholar]
- Sekihara K., Nagarajan S.S., Poeppel D., Marantz A. Asymptotic SNR of scalar and vector minimum-variance beamformers for neuromagnetic source reconstruction. IEEE Trans. Biomed. Eng. 2004;51:1726–1734. doi: 10.1109/TBME.2004.827926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.D., Umberger G.H., Manning E.L., Slevin J.T., Wekstein D.R., Schmitt F.A., Markesbery W.R., Zhang Z., Gerhardt G.A., Kryscio R.J., Gash D.M. Critical decline in fine motor hand movements in human aging. Neurology. 1999;53:1458–1461. doi: 10.1212/wnl.53.7.1458. [DOI] [PubMed] [Google Scholar]
- Smith A.E., Ridding M.C., Higgins R.D., Wittert G.A., Pitcher J.B. Age-related changes in short-latency motor cortex inhibition. Exp. Brain Res. 2009;198:489–500. doi: 10.1007/s00221-009-1945-8. [DOI] [PubMed] [Google Scholar]
- Talelli P., Ewas A., Waddingham W., Rothwell J.C., Ward N.S. Neural correlates of age-related changes in cortical neurophysiology. NeuroImage. 2008;40:1772–1781. doi: 10.1016/j.neuroimage.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecchio F., Zappasodi F., Pasqualetti P., De Gennaro L., Pellicciari M.C., Ercolani M., Squitti R., Rossini P.M. Age dependence of primary motor cortex plasticity induced by paired associative stimulation. Clin. Neurophysiol. 2008;119:675–682. doi: 10.1016/j.clinph.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Traub R.D., Bibbig A., LeBeau F.E.N., Buhl E.H., Whittington M.A. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu. Rev. Neurosci. 2004;27:247–278. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- Van Veen B.D., van Drongelen W., Yuchtman M., Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 1997;44:867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Vrba J., Robinson S.E. Signal processing in magnetoencephalography. Methods. 2001;25:249–271. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- Ward N.S. The neural substrates of motor recovery after focal damage to the central nervous system. Arch. Phys. Med. Rehabil. 2006;87:S30–S35. doi: 10.1016/j.apmr.2006.08.334. [DOI] [PubMed] [Google Scholar]
- Ward N.S., Frackowiak R.S.J. The functional anatomy of cerebral reorganisation after focal brain injury. J. Physiol. 2006;99:425–436. doi: 10.1016/j.jphysparis.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Ward N.S., Swayne O.B.C., Newton J.M. Age-dependent changes in the neural correlates of force modulation: an fMRI study. Neurobiol. Aging. 2008;29:1434–1446. doi: 10.1016/j.neurobiolaging.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Hallett M. The influence of normal human ageing on automatic movements. J. Physiol. 2005;562:605–615. doi: 10.1113/jphysiol.2004.076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki N., Stanford I.M., Hall S.D., Woodhall G.L. Pharmacologically induced and stimulus evoked rhythmic neuronal oscillatory activity in the primary motor cortex in vitro. Neuroscience. 2008;151:386–395. doi: 10.1016/j.neuroscience.2007.10.021. [DOI] [PubMed] [Google Scholar]