Abstract
Retrieval from semantic memory is usually considered within a time window around 300–600 ms. Here we suggest that lexical access already occurs at around 100 ms. This interpretation is based on the finding that semantically rich and frequent words exhibit a significantly shorter topographical latency difference between the site with the shortest P1 latency (leading site) and that with the longest P1 latency (trailing site). This latency difference can be described in terms of an evoked traveling alpha wave as was already shown in earlier studies.
Abbreviations: + NOF, high number of features; − NOF, low number of features; WFREQU, word frequency; LATDIFF, latency difference between trailing and leading electrode
Keywords: P1, Alpha, Traveling-wave, Lexical access, Semantic memory
Highlights
-
•
P1 latency differences showed a travel movement from lateral to medial sites.
-
•
Concept words with high number of semantic features speed up P1 traveling.
-
•
P1 traveling indicates early lexical access at around 100 ms.
-
•
P1 latency differences reflect evoked traveling alpha wave.
Introduction
The aim of the present study is to investigate whether topographical latency differences of the P1 component of the visual event related potential (ERP) are associated with a specific type of cognitive process. Our hypothesis is that they might reflect early access to semantic memory. The motivation for this hypothesis is based on three different lines of evidence. The first refers to the observation that the P1 has a frequency characteristic in the alpha range and behaves like an evoked alpha traveling wave (Fellinger et al., 2012; Klimesch et al., 2007a) during access to semantic memory (Fellinger et al., 2012; Klimesch, 2011). The second is related to findings about the functionality of alpha (Klimesch, 1997, 1999, 2012) suggesting that alpha oscillations are associated with controlled knowledge access. The third line of evidence concerns predictions of a semantic network model (the connectivity model, cf. Klimesch, 1994) that describes the differential influence of the number of semantic features (representing the complexity of a semantic code) on memory access.
Semantic memory may be considered the core of long-term memory which represents the meanings of all kinds of information such as the meanings of words, geographic relationships, or mathematical knowledge (Anderson, 1983; Collins and Loftus, 1975; Klimesch, 1994). Activation of the semantic memory network is described in terms of spreading activation. One critical aspect (here) is how complexity influences the speed of spreading activation. Traditional models such as ACT or ACT* (Anderson, 1983) assume that activation hitting a node with many links (leading off from that node) is weakened (and processing speed is slowed down) proportionally to the number of links. This processing disadvantage became well-known as the fan effect. According to these models, complex codes are processed at a slower speed than less complex codes. The connectivity model (Klimesch, 1994), however, makes differential predictions that depend on the properties of spreading activation. In divergent processing stages complexity is associated with a slowing, but in convergent stages with an acceleration of spreading activation (see further below).
Short latency EEG responses around about 100 ms (such as the P1 and evoked alpha) may reflect early stimulus categorization that emerges as interaction or synthesis between bottom-up and top-down processes. The processing of visual information may be characterized by four consecutive time windows that are associated with different ERP components, sensory encoding (reflected by the C1at about 80 ms), early categorization (reflected by the P1at about 100 ms), stimulus identification (reflected by the N1 at about 150 ms) and conscious stimulus evaluation (reflected by the P3 at about 300 ms). In this context, early categorization is a process that precedes and enables identification and later processes (for a review cf. Klimesch, 2011).
In the present study we do not primarily focus on spreading activation within semantic memory but on the access to semantic memory, which is closely linked to lexical access (i.e., access to the graphemic/phonetic code of words). One essential question here is what kind of processes enables access to memory in general and to the lexicon in particular. Our hypothesis is that early categorization of stimulus information (regarding lexical and semantic information in our case) is the ‘key’ for memory access. We assume that the P1 may be considered as the EEG correlate reflecting this early categorization process that enables access to lexical and semantic memory.
Research on evoked EEG activity has demonstrated that sublexical and lexical features show differential effects already in the time window of the P1component of the visual ERP. For example several studies have shown differences in evoked ERP amplitudes at around 100 ms in response to orthographic neighborhood size, orthographic typicality, word length, letter n-gram frequency, word frequency, as well as semantic factors (see Dien, 2009 for a review; Hauk et al., 2006a,b; for a review Hauk and Pulvermüller, 2004; Hauk et al., 2009; Segalowitz and Zheng, 2009). Early differences in evoked activity in response to word length and frequency were also observed in MEG studies in the time window of the P1 (cf. e.g., Assadollahi and Pulvermüller, 2003). These findings suggest that lexical access occurs early, at around 100 ms. Other research groups, however, emphasize that early encoding processes of visually presented words are associated with a bottom-up analysis of visual features that enable access to visual word form (or low level feature) representations but not necessarily access to the lexicon (e.g. Dehaene et al., 2005; Pylkkänen and Marantz, 2003). These groups refer to evidence for a late time window of lexical access, at around 350 ms (cf. e.g., Pylkkänen et al., 2002; Solomyak and Marantz, 2009).
Evidence for early or late lexical access may depend largely on task type (e.g., requiring lexical or semantic decision), and thereby on the extent to which top-down strategies can be used to speed up encoding and/or decision/categorization processes. If sensory and (sub-) lexical features are closely interwoven, top-down processes can be effective even at low levels of stimulus processing. Evidence for this view is supported by an interesting corpus analysis of nouns and words showing phonological typicality effects (Farmer et al., 2006), thereby demonstrating that different lexical categories are already associated with ‘low level’ phonological properties.
Preliminary evidence for our hypothesis, that an evoked traveling alpha wave reflects access to semantic memory, comes from a recent study by Fellinger et al. (2012). The results showed that the speed of the traveling alpha wave (which coincides with the appearance of the P1) is related to semantic categorization speed in a way that a slow traveling movement of the P1 is significantly associated with a shorter reaction time (RT). Subjects had to categorize black and white pictures (whether showing a landscape or building). The physical properties of the pictures were kept constant by adjusting luminance, contrast and magnitude spectra. This procedure reduced or eliminated differences in surface features between the two categories but at the same time made the pictures rather difficult to recognize. The general conclusion was that the observed traveling alpha waves reflect access to semantic memory and that the speed of traveling is related to the complexity of this process — a complex process slows down traveling; a less complex process may speed it up. Considering the fact that the pictures were rather difficult to categorize, the interpretation was that a slow traveling process reflects a situation where many different semantic features are accessed because the meaning of the picture is complex and rather difficult to assess. Why such a process is associated with shorter RTs is rather difficult to answer. It could for instance be that a more complex process enables a more accurate categorization process which then operates to speed up RT. A more specific interpretation may be derived from the connectivity model (see the respective discussion in the last paragraphs of this section) which assumes that complexity slows down early access processes to semantic memory, but speeds up processes during later stages within semantic memory. In the study by Fellinger et al. (2012), however, no stimulus properties such as picture norms or related word norms were at hand to test this interpretation regarding the influence of stimulus complexity.
Here we proceed from the well-established finding that a variety of variables, such as word frequency, word length, as well as the number of semantic features (NOF) influence semantic categorization speed (as measured by RT). Several studies have shown that high word frequency and short word length result in faster lexical decision times (for a review cf. Brysbaert et al., 2011; Yap and Balota, 2009). Regarding semantic neighborhood density, similar findings were obtained. Pexman et al. (2003) found that high NOF words are easier to categorize in a sense that they speed up RT (also see Buchanan et al., 2001; Yates et al., 2003) compared to low NOF words. We predict that if the P1 reflects early categorization allowing memory access and if the traveling movement of the P1 is related to the ease of the access process, a higher traveling speed for words that are easy to categorize should be found.
In the present study we used a living/non-living semantic decision task. Subjects were asked to give a ‘yes’ response to words representing a living object and a ‘no’ response to words denoting a non-living object. We assume that a semantic decision is based on a network search that aims to detect common features between the semantic code of the to be judged subordinate concept and that of the superordinate concept. According to the connectivity model proposed by Klimesch (1994), NOF – representing the complexity of a semantic code – speeds up semantic categorization, which is described as spreading activation between two (or more) codes. This processing advantage of NOF is, however, restricted to certain activation stages. In a simple case, when a single code is accessed, three activation stages – one with divergent and two with convergent activation – can be distinguished. In a first stage – the access stage – divergent activation flows from the concept node (also termed access node) to all directly connected feature nodes. During this access stage, NOF may lead to a slowing due to the dissipating influence of a fan effect (cf. detailed discussion in Klimesch, 1994 in chapter 8.4). In a second stage, activity flows from each feature node to all other feature nodes. At the end of this second activation stage convergent activation accumulates at each feature node. In a third stage, activity flows back and converges at the access node. The processing advantage occurs at the end of the second and third stage as activity converges and accumulates at the respective nodes (i.e. at the feature nodes in the second stage and the concept node in the third stage). The convergent activity (or echo) that spreads back to the access node is termed as indirect activation. Its amount is proportional to the number of features and its arrival at the access node signals the end of the search process. The time that indirect activation needs to arrive at the access node is reciprocal to its strength. Because activation strength depends on NOF, a search is faster for codes with many features as compared to codes with only a few features. This example characterizes the ‘standard case’, where a single code is accessed.
In a semantic categorization task, spreading activation between two codes is assumed. For a ‘yes’ response in a living/non-living judgment task (used in the present study) the critical prediction is that preactivation (operating in the second and third activation stage) speeds up spreading activation. Because the superordinate concept remains the same through the entire task, a top-down controlled processing mode can be established that activates semantic features which are typical and very common for living objects. Thus, the search process, emanating from the subordinate concept node will meet already preactivated feature nodes in the second activation stage that are shared between the two codes. The amount of preactivation increases with NOF because the activity of the preactivated node(s) is increased by activity flowing to this node from all remaining nodes. In the third stage, indirect activity (strengthened by preactivation) flows back to the access node. Due to the influence of preactivation, a positive search result for a ‘yes’ response is obtained faster than for a single code. It is important to note that an NOF-related processing advantage is predicted for the second and third activation stage but not for the first stage which reflects access to semantic features.
For a ‘no’ response, the first stage of activation is identical with that for a ‘yes’ response. Here too, the features of the (subordinate) concept code have to be accessed. But then the situation is quite different, because the two codes (that of the sub- and superordinate concept) will not share common features. According to the connectivity model, the lack of arrival of preactivated indirect activation is the criterion for giving a no response. The amount of indirect activation spreading back to the access node will equal the ‘standard case’ for a single code, indicating that the code is isolated (with respect to the superordinate concept network). A preactivated search process has a larger extent of activation and would arrive faster. Thus, evidence for a negative decision is available as soon as indirect activation, with a strength that signals the standard case, arrives at the access node.
Because there is evidence that the P1 reflects early categorization during access to semantic memory (for a review see Klimesch, 2011), we suggest that access to semantic features (reflecting the first activation stage described by the connectivity model) is associated with the P1 at leading sites (i.e. at electrodes where the P1 exhibits the shortest latency), whereas the P1 at trailing sites (the P1 with the longest latency) may be associated with the activation of the feature network (reflecting the second activation stage). The third activation stage with indirect activation accumulating at the access node provides evidence for the semantic decision and determines reaction time (RT).
With respect to the leading P1 the prediction is that NOF will increase latency due to an NOF-related fan effect. This prediction holds true for ‘yes’ and ‘no’ response trials. In contrast, NOF will have a differential effect on the latency of the trailing P1in ‘yes’ as compared to ‘no’ response trials. As preactivation can only operate to speed spreading activation in trials with a ‘yes’ response, we predict that spreading activation (reflected by the latency difference between the leading and trailing P1) will show strong influence of NOF in ‘yes’ response trials only. For the present study, we selected words with different visual forms and surface features from McRae et al's. (2005) “semantic feature production norms”. An example is shown in Table 1.
Table 1.
Examples of semantic features for a concept word with high and low number of visual-form and surface features (+ NOF/− NOF).
| Feature | Category | Feature | Category |
|---|---|---|---|
| Eagle [+ NOF] | |||
| A bird | Taxonomic | Has feathers | Visual-form and surface |
| A carnivore | Taxonomic | Has wings | Visual-form and surface |
| A predator | Taxonomic | Is bald | Visual-form and surface |
| Builds nests | Encyclopedic | Is endangered | Encyclopedic |
| Eats | Visual-motion | Is large | Visual-form and surface |
| Flies | Visual-motion | Lives in mountains | Encyclopedic |
| Lays eggs | Encyclopedic | Symbol of freedom | Encyclopedic |
| Has a beak | Visual-form and surface | Symbol of u.s. | Encyclopedic |
| Has claws | Visual-form and surface | ||
| Hamster [− NOF] | |||
| A mammal | Taxonomic | Runs on wheels | Visual-motion |
| A pet | Taxonomic | Has a tail | Visual-form and surface |
| A rodent | Taxonomic | Has fur | Visual-form and surface |
| An animal | Taxonomic | Is brown | Visual-color |
| Drinks | Visual-motion | Is small | Visual-form and surface |
| Drinks water | Visual-motion | Is soft | Tactile |
| Eats | Visual-motion | Lives in cages | Encyclopedic |
Early access models would make similar predictions regarding differential effects in early evoked EEG activity. The main difference to the connectivity model is the distinction of different processing stages and the differential effects of NOF within different stages during access to semantic memory which is made by the latter model.
Materials and methods
Subjects
An original sample of 40 subjects participated in the present study after having given informed consent. Three subjects had to be excluded, one due to excessive movement artifacts, and two due to a reported earlier brain injury. The remaining 37 subjects (19 females, 18 males; mean age 24.3 years, SD = 3.3 years) who had normal or corrected-to-normal vision, did not report neurological diseases and were not on psychotropic medication. All subjects were students of the University of Salzburg and were compensated by course credits. The experiment was conducted according to the code of ethics (WorldMedicalAssociation, 1996).
Stimulus material and task
Subjects performed a semantic (living vs. non-living) judgment task. For each word they had to decide whether it represented a living or non-living object. The ‘living category’ consisted of animals and fruits, the ‘non-living category’ of tools and everyday objects. Stimuli were words, taken from McRae's et al's. (2005) “semantic feature production norms” provided for 541 concept words. These norms were obtained by asking subjects to list features for each concept word. The features could reflect physical (sensory) and functional properties (usage) or encyclopedic knowledge. A feature had to be listed by at least 5 out of 30 subjects to be included in the collection of norms. According to Cree and McRae's (2003) taxonomy, the collected data were categorized into the following feature types: visual-color, visual form and surface, visual-motion, other sensory properties (smell, sound, tactile, and taste), functional, and encyclopedic information. When subjects reported an adjective–noun description, then the respective feature information was counted separately. As an example, the description ‘has four legs’ was considered to comprise two features (‘has legs’ and ‘has 4 legs’). Examples of features for two living concept words are given in Table 1. For our purpose, stimuli could unambiguously be translated into German.
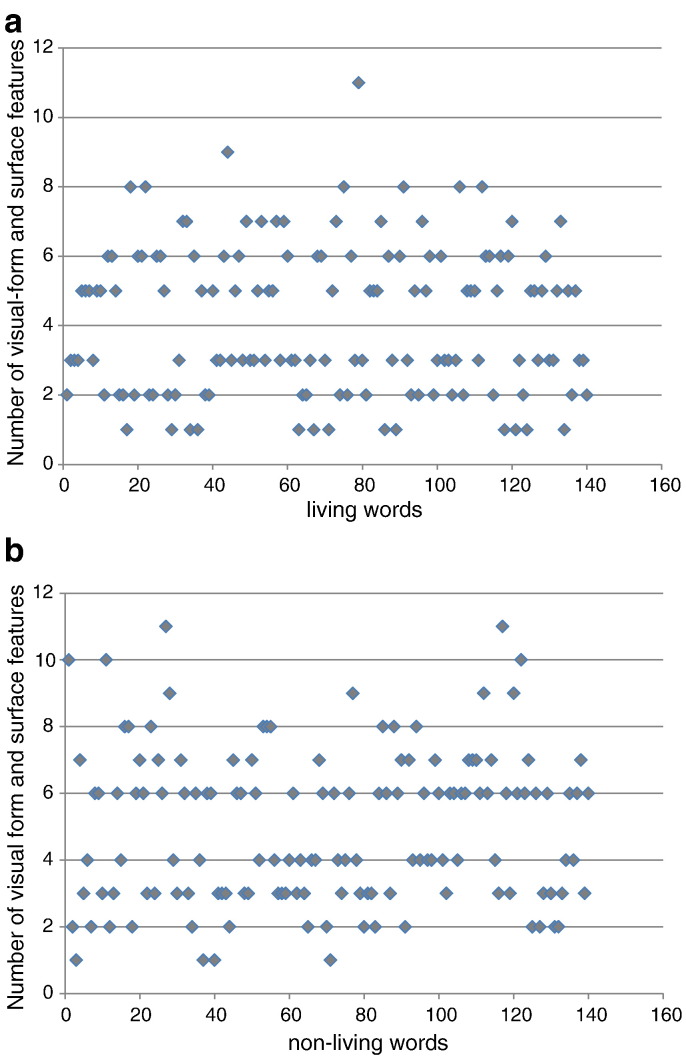
We selected words with a reasonably large variation in NOF. Inspection revealed that some types of features were not adequate for distinguishing words with a large vs. small number of features (termed NOF +/− in the following), because for some types the median was close to 1 (as was the case for, e.g., the number of visual color features or sound features). An appropriately large variation was found for the number of visual form and surface features. For living words the median was 4 (mean = 4.2, SD = 1.9, min = 1, max = 11). For non-living words the median was 5 (mean = 4.9, SD = 2.2, min = 1, max = 11). The distribution of NOF is illustrated in Fig. 1. The whole set of stimuli is listed in Appendix A. We split the set into four groups of 70 items each, living words with + NOF (more than 4) and − NOF (less than 4), and non-living words with + NOF (more than 5) and − NOF (less than 5).
Fig. 1.
Distribution of NOF for visual form and surface feature category is illustrated for living words (a) and non-living words (b).
Statistical analyses of the selected words (based on a 2-way ANOVA with the factors CATEGORY: living vs. non-living and NOF: ±) showed significant main effects for CATEGORY (F(1,69) = 43.95, p < .001) and for NOF (F(1,69) = 838.42, p < .001). The interaction was not significant. The respective means indicate that non-living words have more features than living words and + NOF words have more features than − NOF words (non-living + NOF: M = 6.97, SD = 1.3; non-living − NOF: M = 2.99, SD = 0.9; living + NOF: M = 6.03, SD = 1.2; living − NOF: M = 2.27, SD = 0.8).
We estimated word frequency (WFREQU) by the “WortschatzUniversität Leipzig” dataset (vocabulary of the University of Leipzig, available at http://wortschatz.uni-leipzig.de/). The algorithm calculates the frequency class, which is computed in relation to the most frequent word in the whole set of references (corpus). The higher the score, the rarer the words are. For living words, NOF was correlated with frequency class (r = − .31, p < .001). No significant correlation was found between NOF and word length (number of letters) (r = − .15, p > .05). For the non-living words, no significant correlation was found between NOF and frequency (r = − .14, p > .05) and word length (number of letters) (r = .05, p > .05).
Procedure
Subjects were seated in a comfortable chair in front of a computer monitor (75 Hz refresh rate) at a distance of about 130 cm. To familiarize the participants with the task, a training session was run before the experimental session. A gray fixation cross appeared at the center of a black screen. The interval between the onset of the fixation cross and the onset of the word varied between 400 and 600 ms in 50 ms intervals in order to reduce onset expectations. The fixation cross was replaced by a word written in upper-case-letters either belonging to the living or non-living category. The word was presented for 1000 ms in dark gray (horizontal angle: 2.8°–4.3°; vertical angle = 0.66°) within a bright gray box (9.7° × 2.6°) to ensure comfortable reading and to hold visual surface features constant between trials. Subjects indicated by button press on the keyboard with their right index finger whether the word denoted a living (“yes-response”) or non-living object (“no-response”). A total of 140 living and 140 non-living words (with 70 + NOF and 70 − NOF words each) were presented in randomized order.
EEG recordings
EEG was recorded using 64-channel BrainAmp amplifier (BrainProducts, Inc., Gilching, Germany) was used for EEG recording. EEG-signals were online referenced against the nose and subsequently (off-line) re-referenced to digitally averaged ([A1 + A2]/2) ear lobes. Band-pass filters were set at 0.5 to 100 Hz and a notch filter at 50 Hz. Signals were digitized at a sampling rate of 500 Hz. 60 Ag–AgCl-electrodes were mounted using an EasyCap at the following positions: Fp1, Fp2, AF7, AF3, AFz, AF4, AF8, F7, F5, F3, F1, Fz, F2, F4, F6, F8, FT7, FC5, FC3, FC1, FCz, FC2, FC4, FC6, FT8, T7, C5, C3, C1, Cz, C2, C4, C6, T8, TP7, CP5, CP3, CP1, CPz, CP2, CP4, CP6, TP8, P7, P5, P3, P1, Pz, P2, P4, P6, P8, PO7, PO3, POz, PO4, PO8, O1, Oz, O2. Impedances were kept below 8 kΩ. To control for vertical and horizontal eye movements two bipolar EOG-channels were mounted. After re-referencing, epochs containing eye artifacts were corrected by the Gratton & Coles method (Gratton et al., 1983) and muscle artifacts were rejected. BrainVisionAnalyzer (BrainProducts, Inc.) was used for data analyses. Epochs consisted of EEG segments ranging from − 600 to 1000 ms relative to the stimulus. EEG analyses are based on a 37 × 280 data matrix for each electrode. The 37 rows represent the subjects while the 280 columns represent the set of words (140 living/non-living, consisting of 70 + NOF and 70 − NOF words each). ERPs were calculated either by averaging over the respective subsets of + NOF and − NOF words (analysis 1: word averaged ERPs), or by averaging each word separately across all subjects (analysis 2: subject averaged ERPs). The latter analysis has the advantage that correlation analyses can be carried out between latency values and word norms. Analysis were done for the living and non-living words separately.
EEG analysis: Detection of the P1-component
Analysis 1 (word averaged ERPs): Detection of the P1-component
For this analysis, ERPs were averaged over the 70 + NOF and the 70 − NOF words separately for the living and the non-living items. We used two data sets, one filtered between 4 and 20 Hz and another between 7.5 and 12.5 Hz (denoted the ‘alpha filtered data’ in the following). The segmented data were averaged and peaks were detected semi-automatically. The typical latency of the visual P1 is between 80 and 120 ms post-stimulus. Because we focus on a ‘topographical traveling movement’ of the P1, we used an extended window of about 70 and 165 ms for P1-peak detection on 17 posterior electrodes (P7, P5, P3, P1, Pz, P2, P4, P6, P8, PO7, PO3, POz, PO4, PO8, O1, Oz, O2). Visual inspection of ERPs revealed that in some cases the supposed P1 appeared after 165 ms on certain electrodes (in 7 subjects for the living data, and in 6 for the non-living data). Because the delayed latency could be the result of a traveling movement, we decided to include these values. For the 4 to 20 Hz data, peak detection was difficult in 4 subjects due to double-peaks as a consequence of a superimposed beta rhythm. In such cases, the mean of the two peaks was chosen. Finally, if no clear P1-component existed (< 0.5 μV), missing values were assigned as listed in Table 2.
Table 2.
Frequency distribution of missing values for each electrode.
| P7/P8 | PO7/PO8 | P5/P6 | P3/P4 | PO3/PO4 | P1/P2 | O1/O2 | Pz/POz/Oz | ||
|---|---|---|---|---|---|---|---|---|---|
| 7.5–12.5 Hz | |||||||||
| HIGH | Living | 0/2 | 0/0 | 0/1 | 0/0 | 0/0 | 0/0 | 1/2 | 0/0/2 |
| Nonliving | 0/4 | 0/1 | 1/2 | 2/1 | 0/0 | 3/3 | 1/1 | 4/1/3 | |
| LOW | Living | 0/2 | 0/1 | 3/4 | 3/1 | 1/1 | 2/2 | 1/3 | 2/3/3 |
| Nonliving | 2/2 | 0/1 | 4/3 | 5/3 | 1/1 | 5/4 | 0/2 | 4/1/3 | |
| 4–20 Hz | |||||||||
| HIGH | Living | 0/1 | 0/0 | 0/0 | 1/1 | 0/1 | 1/2 | 1/2 | 2/1/2 |
| Nonliving | 2/4 | 0/1 | 2/4 | 2/4 | 1/1 | 3/5 | 1/2 | 5/2/2 | |
| LOW | Living | 1/1 | 0/0 | 4/3 | 7/4 | 1/3 | 8/8 | 1/2 | 7/5/3 |
| Nonliving | 4/3 | 0/0 | 4/4 | 6/7 | 2/4 | 6/6 | 1/2 | 6/3/2 | |
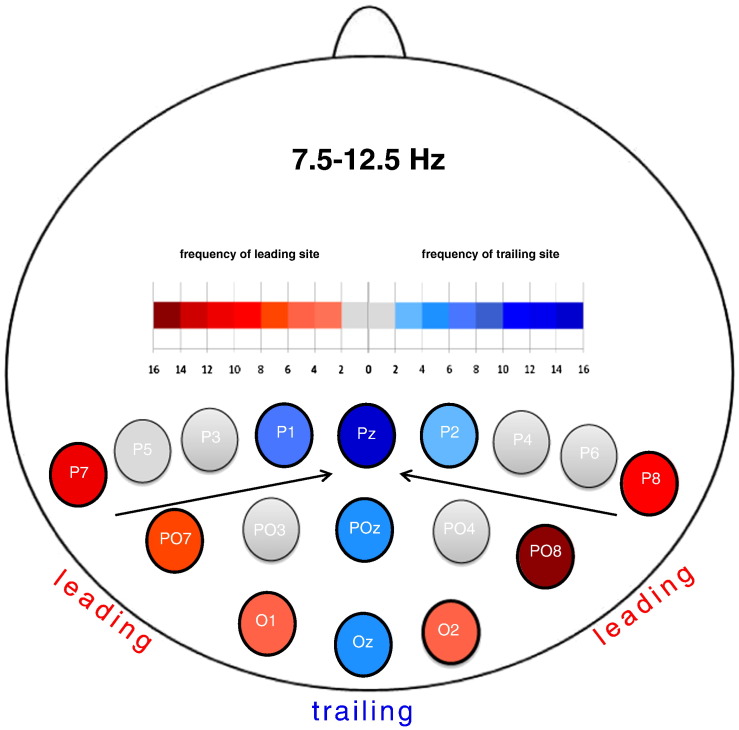
Following peak detection, for each subject the electrode with the shortest and the electrode with the longest latency were identified as leading and trailing sites, respectively. The latency difference (LATDIFF) between these sites was calculated as a coarse measure of traveling. Fig. 2 shows which electrodes were obtained as leading and trailing sites.
Fig. 2.
Frequency distribution of leading and trailing sites. For each subject the leading and trailing electrodes were determined for yes-responses and the high and low number of feature condition (NOF). The observed frequencies are color coded (reddish for leading and bluish for trailing). Note the clear lateral to medial traveling movement.
To control for the influence of electrode distance on latency differences, we also calculated for each (leading–trailing) electrode pair traveling speed. For each pair, electrode distance in mm was divided by LATDIFF in ms. The resulting variable is termed traveling SPEED.
Analysis 2 (subject averaged ERPs): Detection of the P1-component
The procedure for this analysis is identical to that for analysis 1 with the exception that ERPs were obtained by averaging across subjects. This yielded 138 ERPs for the living words for each electrode. Two words had to be excluded due to an extremely small trial number of 13 and 11, respectively. The mean number of trials was 31.9 (SD = 2.9). For the non-living words we obtained 139 ERPs (one word was excluded due to a small trial number of 16) with a mean number of trials of 31.8 (SD 2.9). The P1 analysis for the subject averaged ERPs was based on the alpha filtered (7.5 to 12.5 Hz) data only.
Statistical analysis
For the behavioral data, correlations were calculated between reaction time (RT) and word norms. For analyses 1 and 2, statistical tests were performed separately for the living and non-livings words. To test for NOF related differences in LATDIFF and traveling speed, we performed t-tests and ANOVAs between high and low NOF words for the data of analysis 1. The ANOVAs comprised two factors, NOF (±) and SITE (leading/trailing). The dependent measure was latency. ANOVAs and t-tests were calculated separately for the 4 to 20 Hz and the alpha filtered data. For the data of analysis 2 we used primarily correlations between LATDIFF and word norms (NOF and WFREQU).
We also tested whether the topographical distribution of P1 peak latencies shows a continuous and directional distribution which has to be expected if the P1 exhibits a traveling movement. For this purpose, we have ranked the P1 latencies for each subject and the 17 electrodes from the shortest (leading) to the longest (trailing) latency site. These P1 latencies were correlated with the distance (in mm) of each site to the trailing position. In the case of a perfect directional traveling movement, the two variables must be negatively correlated because the leading position shows the shortest latency but the longest distance to the trailing site. For each of the following rank positions latency increases but distance decreases. Thus, a significant negative correlation would indicate a continuous, directional traveling pattern.
Results
Behavioral data
The overall mean percentage of correct responses was 95%. The percentages of correct responses (and SD) for the 4 conditions, living + NOF, living NOF-, non-living + NOF and non-living − NOF are in this order, 95.3% (SD = 4.2), 94.6 (SD = 3.6), 96.3 (SD = 4.2), 95.9 (SD = 3.5).
The mean RT for the 4 conditions, living + NOF, living − NOF, non-living + NOF and non-living − NOF are in this order 661.4 (SD = 77.6), 681.4 (SD = 79.9), 700.7 (SD = 89.1), and 710.8 (SD = 92.8). For the living and non-living words significant correlations between NOF and RT were obtained (r = − .25, p < .01 for living and r = − .17, p < .05 for non-living). These correlations do not differ significantly, as determined by a t-test performed on the Fisher Z-transformed correlation coefficients.
Leading and trailing sites
Over the entire sample of subjects PO8 was identified as the most probable leading site, whereas Pz was most likely to be the trailing site. When inspecting the traveling pattern for each dataset, 7 subjects out of 37 showed a shift of the traveling site more to the left (e.g. P3/PO3), or more to the right site (e.g. P4/PO4), or even more posterior (POz, Oz). Another 4 subjects showed an opposite traveling direction from medial to lateral sites. Fig. 2 shows which electrodes were obtained on average as leading and trailing sites for the filtered living words. The mean latencies and standard deviations are listed in Table 3.
Table 3.
P1 latencies of the leading and trailing electrodes for the broadly and the alpha filtered data.
| 4–20 Hz | M | SD | MED | MIN | MAX | 7.5–12.5 Hz | M | SD | MED | MIN | MAX | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High NOF | ||||||||||||
| LIVING | Leading | 114.8 | 12.6 | 115 | 72 | 145 | Leading | 114.5 | 14.7 | 115 | 72 | 153 |
| Trailing | 142.4 | 18.3 | 144 | 100 | 181 | Trailing | 142.6 | 18.0 | 146 | 93 | 174 | |
| NONLIVING | Leading | 115.9 | 11.2 | 116 | 86 | 143 | Leading | 115.2 | 15.7 | 116 | 76 | 155 |
| Trailing | 144.1 | 18.7 | 146 | 97 | 180 | Trailing | 144.1 | 18.4 | 146 | 99 | 180 | |
| Low NOF | ||||||||||||
| LIVING | Leading | 113.2 | 14.1 | 116 | 76 | 135 | Leading | 113.0 | 15.7 | 113 | 84 | 153 |
| Trailing | 145.4 | 17.7 | 142 | 110 | 191 | Trailing | 146.1 | 17.7 | 145 | 110 | 178 | |
| NONLIVING | Leading | 112.5 | 13.6 | 117 | 70 | 134 | Leading | 114.2 | 13.4 | 114 | 75 | 140 |
| Trailing | 140.7 | 16.0 | 143 | 95 | 163 | Trailing | 142.4 | 17.8 | 144 | 99 | 175 | |
M = Mean; SD = Standard Deviation; MED = Median; MIN = shortest P1 latency; MAX = longest P1 latency.
Analysis 1: P1- LATDIFF for the 4–20 Hz and alpha filtered data
For the broadly filtered data, paired-sample t-test showed significant mean differences for + NOF and − NOF living words (t(36) = − 2.1, p < .05). The respective means indicate that + NOF words showed smaller LATDIFF between trailing and leading electrodes (M = 27.54, SE = 2.57) than words with − NOF (M = 32.24, SE = 3.39). For the non-living words, + NOF (M = 28.20, SE = 2.61) and − NOF (28.16, SE = 2.73) did not differ significantly (t(36) = 0.019).
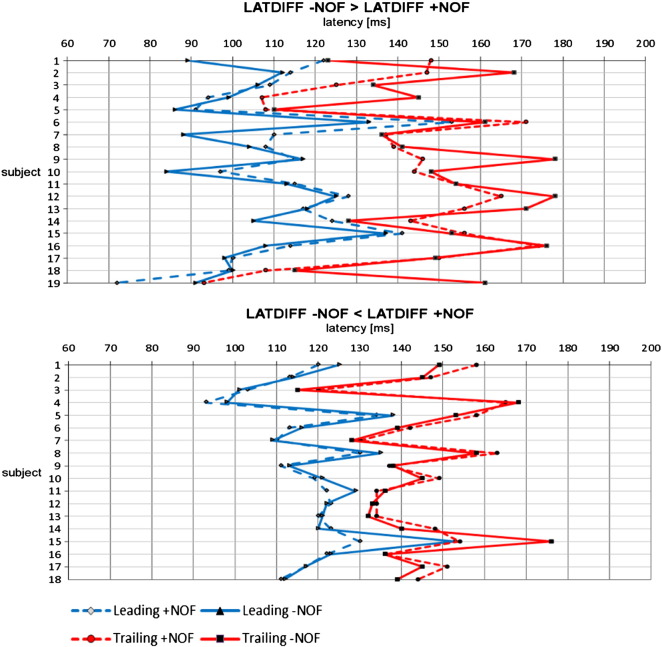
For the alpha filtered data, paired-sample t-test yielded significant mean differences for + NOF and − NOF living words (t(36) = − 2.3, p < .05). Living words with + NOF showed smaller LATDIFF between trailing and leading electrodes (M = 28.05, SE = 2.26) compared to living words with − NOF (M = 33.08, SE = 3.05). The mean latency difference between + NOF and − NOF of about 5 ms is very small. Inspection of the latency difference for each subject revealed a range of − 14 to + 49 ms (− NOF minus + NOF), indicating that some subjects show smaller LATDIFF for − NOF compared to + NOF. The magnitude and distribution of latency differences between leading and trailing sites supporting and not supporting our hypothesis is shown in Fig. 3. For the non-living words, + NOF (M = 28.95, SE = 2.35) and − NOF (28.14, SE = 2.13) did not differ significantly (t(36) = 0.49).
Fig. 3.
Distribution of latency differences over all subjects supporting and not supporting our hypothesis. LATDIFF− NOF > LATDIFF+ NOF indicates that words with a + NOF show a smaller latency difference between leading and trailing sites than − NOF. LATDIFF− NOF < LATDIFF+ NOF shows the opposite effect.
With respect to SPEED as dependent measure, neither the 4–20 Hz living data (t(36) = 0.29) and non-living data (t(36) = 0.71), nor the alpha filtered living data (t(36) = 1.42) and non-living data (t(36) = − 1.3) showed significant differences between + NOF and − NOF words.
P1 latencies at leading and trailing sites; 4–20 Hz filtered data
The analysis of the P1-latencies at leading and trailing sites for the living words exhibited a significant main effect for SITE (F(1,36) = 114.5, p < .001). Leading sites showed shorter latencies (M = 113.97, SE = 2.05) as compared to trailing sites (M = 143.88, SE = 2.76). A significant interaction was obtained for the interaction SITE × NOF (F(1,36) = 4.4, p < .05). For − NOF words the latency at the leading electrode was shorter and the latency at the trailing electrode was longer compared to the + NOF words (− NOF leading: M = 113.16, SE = 2.35; − NOF trailing: M = 145.41, SE = 2.95; + NOF leading: M = 114.81, SE = 2.11; + NOF trailing: M = 142.35, SE = 3.04).
For the non-living words a significant main effect for SITE (F(1,36) = 133.2, p < .001) was also found, with leading sites showing shorter latencies (M = 114.20, SE = 1.91) than trailing sites (M = 142.39, SE = 2.78). A significant main effect for NOF was found (F(1,36) = 13.8, p < .01). − NOF words exhibited generally shorter latencies at leading and trailing sites (leading: M = 112.51, SE = 2.23; trailing: M = 140.68, SE = 2.63) compared to + NOF words (leading: M = 115.89, SE = 1.85; trailing: M = 144.10, SE = 3.08). The interaction SITE × NOF was not significant (F(1,36) = 0.00, p = .99).
P1 latencies at leading and trailing sites; alpha filtered data
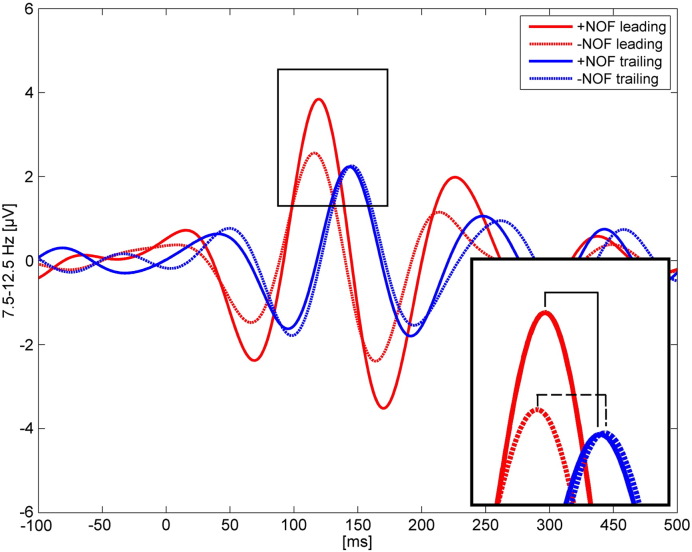
The respective ANOVA for the living words showed a significant main effect for SITE (F(1,36) = 155.1, p < .001). Leading sites showed shorter latencies (M = 113.74, SE = 2.40) than trailing sites (M = 144.31, SE = 2.67). Again, the interaction between SITE and NOF was significant (F(1,36) = 5.4, p < .05). Just as for the broadly filtered data, the latency at the leading electrode was shorter and the latency at the trailing electrode was longer compared to the + NOF words (− NOF leading: M = 112.97, SE = 2.6; − NOF trailing: M = 146.05, SE = 2.96; + NOF leading: M = 114.51, SE = 2.45; + NOF trailing: M = 142.57, SE = 3.00). The results are summarized in Fig. 4.
Fig. 4.
Examples for the alpha filtered ERPs at selected leading and trailing sites that were observed for words with + NOF and − NOF. The ERPs are averaged over those subjects with PO8 as leading and Pz as trailing site. The inset exhibits the enlarged P1 peaks. Note the small leading to trailing latency differences between + NOF and − NOF, marked by the black bold and dashed horizontal lines in the inset.
For the non-living words, a significant main effect for SITE (F(1,36) = 187.8, p < .001) was found, with leading sites showing shorter latencies (M = 114.72, SE = 2.35) than trailing sites (M = 143.26, SE = 2.90). Factor NOF closely failed to reach significance (F(1,36) = 3.3, p = .077) with − NOF words showing shorter leading and trailing latencies (leading: M = 114.24, SE = 2.20; trailing: M = 142.38, SE = 2.92) as compared to + NOF words (leading: M = 115.19, SE = 2.58; trailing: M = 144.14, SE = 3.03). The interaction SITE × NOF was not significant (F(1,36) = 0.24, p = .63).
Result analysis 2
The correlation analysis of the living words yielded significant effects between LATDIFF and NOF and between LATDIFF and WFREQU (r = −. 16 and r = .14 respectively; p < .05, one-sided) showing that short latency differences are associated with many semantic features and high word frequency. The correlation with SPEED exhibits a significant correlation with NOF (r = .17; p < .025, one sided) only. The latter finding shows that high traveling speed is associated with a large number of semantic features. Due to the fact that NOF and WFREQU are strongly correlated (r = − .33; p < .001, one-sided) partial correlations failed to show a direct significant association between LATDIFF and SPEED.
Inspection of the respective scatter diagrams revealed that NOF is quite unevenly distributed between words. NOF varies between 1 and 11, but a medium number of NOF is much more frequent than very high values in particular. In order to obtain a more even distribution we categorized words in 7 categories by collapsing the few high feature number words into one category. Then, within each category the values for LATDIFF, SPEED, NOF and WFREQU were averaged. We found highly significant effects between LATDIFF and NOF and between LATDIFF and WFREQU (r = − .81 and r = .93 respectively; p < .025 and p < .01 respectively, one-sided). This indicates that NOF is a variable that is quite limited in differentiating between words. The correlations between SPEED and NOF and between SPEED and WFREQU were similar (r = .85 and r = − .90 respectively; p < .01 and p < .01 respectively, one-sided).
With regard to the non-living words, neither the correlation between LATDIFF and NOF, nor the correlation between LATDIFF and WFREQU was significant (r = .02 and r = − .08 respectively; p > .05, one-sided). For SPEED, neither the correlation with NOF, nor the correlation with WFREQU was significant (r = .01 and r = .05 respectively; p > .05, one-sided).
Results of the test of a directional spreading movement
As explained in the Materials and method section, we correlated two variables, the ranked latencies and the corresponding electrode distances of each ranked site to the trailing site. These correlations were calculated for each subject for 17 electrodes. The analyses yielded significant negative correlations for 34 of the 37 subjects of our sample for the + NOF living words. The mean correlation coefficient was r = − .76 (with a variation between − 0.48, p < .05 and − 0.94, p < .001) For the − NOF living words, significant negative correlations were obtained for 30 subjects. The mean correlation coefficient was r = − .77 (with a variation between − .56, p < .05 and − .93, p < .001).
For the non-living + NOF words significant, negative correlations were found for 33 subjects with a mean correlation coefficient of r = − .79 (ranking from − .53, p < .05 until − .94, p < .001). For the − NOF non-living words 32 significant, negative correlations were obtained with a mean of r = − .79, correlation coefficients varied between − .52, p < .05 and − .95, p < .001.
Summary of the results
The behavioral data confirmed and replicated that NOF was significantly negatively correlated with RT, showing that short RT's were associated with a large number of semantic features (+ NOF). The ERP data of analysis 1 demonstrated that topographical latency differences of the P1 (as expressed by variable LATDIFF) were indeed significantly associated with NOF in the predicted way: For ‘yes’ response trials, short latency differences were associated with a large number of features (+ NOF) whereas long differences were associated with a small number of features (− NOF). In ‘no’ response trials, NOF did not have a significant influence on LATDIFF. In the alpha band, these findings were observed in an analogous way.
The analysis of absolute P1 latencies revealed an interesting interaction between NOF and SITE for ‘yes’ response trials indicating a differential influence of NOF on leading and trailing sites. Here, + NOF exhibited a tendency to increase latency at leading sites but to decrease latency at trailing sites. For ‘no’ response trials, however, latencies at leading and trailing sites were generally longer for + NOF words. Again, the alpha filtered data showed very similar results.
Analysis 2 confirmed the significant association between NOF and LATDIFF in ‘yes’ response trials, but showed that WFREQ and NOF are highly correlated and that their influence on LATDIFF cannot be dissociated from each other.
It is important to note that NOF had an influence on the P1 latencies already at leading sites (for ‘yes’ and ‘no’ response trials) as analysis 1 revealed. This means that the findings for LATDIFF should not be interpreted in terms of traveling speed as the findings from analysis 2 with variable SPEED might suggest.
Discussion
The most interesting finding is a differential influence of NOF on P1 latencies in ‘yes’ and ‘no’ response trials. For living words, in ‘yes’ response trials, the difference between leading and trailing P1 latencies (as measured by LATDIFF) is shorter for + NOF as compared to NOF- words suggesting a facilitating effect of NOF. For non-living words in ‘no’ response trials LATDIFF does not differ between + NOF and − NOF words. Most importantly, however, P1 latencies (at leading and trailing sites) are generally longer for + NOF as compared to − NOF words, suggesting a de-facilitating effect of + NOF in ‘no’ response trials. The key to understanding these seemingly conflicting results is that the decrease in LATDIFF in ‘yes’ response trials for + NOF words is due to reduced P1 latencies at trailing sites but increased latencies at leading sites. In other words, + NOF is generally associated with increased P1 latencies (and − NOF with decreased latencies) at leading sites in ‘yes’ and ‘no’ response trials. In ‘yes’ response trials only, the trailing P1 latencies exhibit shorter latencies for + NOF as compared to − NOF words (cf. the significant interaction between SITE and NOF as reported in the P1 latencies at leading and trailing sites; 4–20Hz filtered data and P1 latencies at leading and trailing sites; alpha filtered data sections).
These findings can easily be interpreted within the framework of the connectivity model as outlined in the introduction section. The de-facilitating effect of + NOF on leading sites may reflect the dissipating effect of a fan that increases with the number of features during the access stage. The facilitating effect of + NOF at trailing sites is specific for ‘yes’ response trials and may reflect the enhancing influence of preactivation during the second activation stage.
The correlational analysis has shown that WFREQ and NOF are highly correlated. The results of partial correlations revealed that the influence of these variables on LATDIFF and SPEED cannot be dissociated. Thus, at first glance, it appears an open question whether the observed findings (regarding P1 latencies) are due to the influence of word frequency and/or the number of semantic features. But when considering the observed de-facilitating effects, an interpretation that favors the influence of word frequency is unlikely. It would be difficult to explain why the access to semantic memory should be slower for high frequency words and faster for low frequency words. In contrast, a de-facilitating influence of NOF can easily be explained in terms of a fan effect operating during the access stage to semantic memory. The connectivity model predicts a facilitating effect of NOF only for spreading activation in semantic memory where activation can converge and accumulate but not for the access stage where complexity is associated with a fan effect that slows spreading activation.
When interpreting latency differences in terms of an evoked traveling alpha wave, the data imply that access to semantic memory is associated with an alpha wave. In the present study, we have only used the alpha filtered ERPs to demonstrate that the observed pattern of P1 latency differences can be described as a traveling alpha wave. In support of this notion we could show that these latency differences exhibit a continuous distribution that has to be expected if the P1 represents the positive peak of a spreading alpha wave. In two studies performed earlier (Fellinger et al., 2012; Klimesch et al., 2007a), we have shown, however, that (i) the topographic distribution of alpha phase – measured on a single trial basis – behaves like a traveling wave, (ii) this traveling movement is confined to the time window of the P1–N1 complex, and (ii) no other frequency (with the exception of slow beta) did exhibit a traveling movement in that time window.
If the suggested interpretation of the influence of NOF on semantic memory and P1 latencies is valid, the conclusion is that lexical access occurs in the time window of the latency of the leading P1. Our findings are well in line with the ERP studies of word recognition indicating early lexical-(semantic) access around 100 to 160 ms poststimulus (Hauk et al., 2006a,b; Pulvermüller et al., 2001; Sereno and Rayner, 2003; Sereno et al., 1998). Segalowitz and Zheng even found P1 differences between words and pseudo words at about 100 ms (Segalowitz and Zheng, 2009). These findings support the assumption that the P1 component reflects early stimulus categorization providing access to memory (Klimesch, 2011). They favor ‘early access’ models (e.g., Pulvermüller et al., 2001) as opposed to ‘late access’ models (e.g., Dehaene et al., 2005).
Spreading activation within semantic memory can be (at least in part) associated with P1 traveling movement. According to the connectivity model lexical access is associated with the activation of concept nodes. These are considered units that serve as an interface between a semantic network on the one hand and a sublexical (phonemic/graphemic) and sensory network on the other hand. Concept nodes are the core units of lexical knowledge in the sense that they represent semantic and word form information. They establish a network that can be accessed from semantic memory or from sensory information via sublexical features. The important point here is the idea that different networks are ‘mapped’ onto each other. The sublexical network is mapped on the lexical network (i.e. a concept node or word form) network which in turn is mapped onto a semantic network. The implication is that concept nodes serve as an interface between networks representing different types of knowledge. This view is very close to the idea that the ‘lexicon’ should be understood in terms of a ‘lexical interface’ (Hickok and Poeppel, 2007). It also bears a close resemblance to the traditional lexeme/lemma distinction in linguistics (for an excellent review of this and related questions cf. Gow, 2012). The emphasis on a network representation of lexical information is well compatible with other models, particularly with the assumption proposed by Pulvermüller that words are represented by interconnected cell assemblies (for an overview cf. Pulvermüller, 1999).
The observed topography of the traveling alpha wave represents a dorsal activation pattern. For visually presented words, however, we would have to expect a ventral spreading activation pattern, because it is well-established that visual word perception follows a more ventral path with a ‘hub’ in the ventral occipito-temporal cortex (for reviews cf. Gow, 2012; Price and Devlin, 2011). There are at least two possible reasons why a dorsal activation pattern was observed in the present study. Both explanations are based on evidence that alpha is an inhibitory oscillation (cf. Jensen et al., 2012; Klimesch et al., 2007b). The first explanation refers to the functional meaning of alpha amplitude (or power). The assumption is that alpha power will be larger over regions that are not involved in the processing of the respective task. In agreement with this interpretation alpha power is larger over parietal regions when a task engages the ventral stream (Jokisch and Jensen, 2007). The second interpretation refers to the functional meaning of alpha phase with respect to the timing of excitatory events. According to the inhibition timing hypothesis (Klimesch et al., 2007b), alpha reflects a rhythmic cycling between minimal inhibition (maximal excitation) and maximal inhibition (minimal excitation). The terms ‘maximal’ and ‘minimal’ do not refer to amplitude but to phase, i.e., to those phase values marking the positive and negative turning points of the alpha wave. A spreading alpha wave may, thus, be considered a moving time window of increased inhibition followed by decreased inhibition. During the latter phase excitation may spread from one brain region to another. The two interpretations are by no means mutually exclusive, they can easily be combined. As an example, it may well be the case that there are two spreading directions, one in the ventral, and one in the dorsal stream. The ventral activation is generally difficult to detect with the EEG (and particularly with the montage we used). In addition, as the ventral path is more strongly involved than the dorsal path, alpha amplitude will be smaller in the ventral as compared to the dorsal path. This, in addition, is a factor that makes it difficult to detect ventral activity.
When calculating cortical traveling speed in m per second (analogous to Klimesch et al., 2007a), we divide the distance (approximately 8 cm) between leading (e.g., PO8) and trailing (e.g. Pz) sites by the respective latency difference. Then, by applying a correction factor (f = 2) for cortical folding (as e.g. suggested by Nunez et al., 2001), we obtain a value of about ~ 6 m/s for + NOF and ~ 5 m/s for − NOF words. These values are well in line with earlier reports (e.g., Burkitt et al., 2000; Klimesch et al., 2007a; Nunez et al., 2001).
We have to emphasize that in a statistical sense the impact of NOF on topographical latency differences is small. The mean latency difference between + NOF and − NOF is in the magnitude of about 5 ms with a standard error of about 3 ms (SD of about 17). This weak statistical effect does not necessarily indicate a weak influence of NOF and/or word frequency on traveling speed. The measurement of traveling speed by scalp electrodes is extremely difficult because of the extensive gyrification of the cortex, volume conduction and individual variations in cortical morphology and electrode placement. Depending on the direction of the traveling movement and the location of electrodes, some electrode pairs will be better suited than others to record the resulting latency differences. Those pairs that are well-suited will indeed reflect the full magnitude of the traveling movement (in terms of latency difference). Those that are not or less suited will completely mask or weaken the respective latency differences. In agreement with these considerations, the individual latency differences as plotted in Fig. 3 show that those reflecting a pronounced traveling movement are those that are in line with the predicted NOF effect. This may indicate that only those electrode pairs that are actually capable of recording large latency differences are those that adequately reflect the traveling movement.
The most important conclusion is that topographical latency differences of the P1 may be described in terms of a traveling alpha wave with leading and trailing sites. Leading sites may reflect lexical access, whereas trailing sites may reflect activation of semantic features. More complex spreading activation processes within semantic memory (such as semantic comparison or decision processes) may occur in later time windows.
Acknowledgments
This research was supported by the Austrian Science Foundation (FWF Project P21503-B18). The first author, Andrea Zauner, of this article was financially supported by the Doctoral College “Imaging the Mind” of the Austrian Science Fund (FWF W1233-G17). Julia Lechinger is an associated member of the Doctoral College “Imaging the Mind”.
Conflict of interest
None of the authors have conflicts of interest.
Appendix A. Supplementary data
Supplementary material.
References
- Anderson J.R. A spreading activation theory of memory. J. Verbal Learn. Verbal Behav. 1983:261–295. [Google Scholar]
- Assadollahi R., Pulvermüller F. Early influences of word length and frequency: a group study using MEG. Neuroreport. 2003;14:1183–1187. doi: 10.1097/00001756-200306110-00016. [DOI] [PubMed] [Google Scholar]
- Brysbaert M., Buchmeier M., Conrad M., Jacobs A.M., Bölte J., Böhl A. The word frequency effect: a review of recent developments and implications for the choice of frequency estimates in German. Exp. Psychol. 2011;58:412–424. doi: 10.1027/1618-3169/a000123. [DOI] [PubMed] [Google Scholar]
- Buchanan L., Westbury C., Burgess C. Characterizing semantic space: neighborhood effects in word recognition. Psychon. Bull. Rev. 2001;8:531–544. doi: 10.3758/bf03196189. [DOI] [PubMed] [Google Scholar]
- Burkitt G.R., Silberstein R.B., Cadusch P.J., Wood A.W. Steady-state visual evoked potentials and travelling waves. Clin. Neurophysiol. 2000;111:246–258. doi: 10.1016/s1388-2457(99)00194-7. [DOI] [PubMed] [Google Scholar]
- Collins A.M., Loftus E.F. A spreading-activation theory of semantic processing. Psychol. Rev. 1975;82:407–428. [Google Scholar]
- Cree G., McRae K. Analyzing the factors underlying the structure and computation of the meaning of chipmunk, cherry, chisel, cheese, and cello (and many other such concrete nouns) J. Exp. Psychol. Gen. 2003;132:163–201. doi: 10.1037/0096-3445.132.2.163. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Cohen L., Sigman M., Vinckier F. The neural code for written words: a proposal. Trends Cogn. Sci. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Dien J. The neurocognitive basis of reading single words as seen through early latency ERPs: a model of converging pathways. Biol. Psychol. 2009;80:10–22. doi: 10.1016/j.biopsycho.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Farmer T.A., Christiansen M.H., Monaghan P. Phonological typicality influences on-line sentence comprehension. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12203–12208. doi: 10.1073/pnas.0602173103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellinger R., Gruber W., Zauner A., Freunberger R., Klimesch W. Evoked traveling alpha waves predict visual-semantic categorization-speed. Neuroimage. 2012;59:3379–3388. doi: 10.1016/j.neuroimage.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow D.W., Jr. The cortical organization of lexical knowledge: a dual lexicon model of spoken language processing. Brain Lang. 2012;121:273–288. doi: 10.1016/j.bandl.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G., Coles M.G., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hauk O., Pulvermüller F. Effects of word length and frequency on the human event-related potential. Clin. Neurophysiol. 2004;115:1090–1103. doi: 10.1016/j.clinph.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Hauk O., Davis M.H., Ford M., Pulvermüller F., Marslen-Wilson W.D. The time course of visual word recognition as revealed by linear regression analysis of ERP data. Neuroimage. 2006;30:1383–1400. doi: 10.1016/j.neuroimage.2005.11.048. [DOI] [PubMed] [Google Scholar]
- Hauk O., Patterson K., Woollams A., Watling L., Pulvermüller F., Rogers T.T. [Q:] When would you prefer a SOSSAGE to a SAUSAGE? [A:] At about 100 msec. ERP correlates of orthographic typicality and lexicality in written word recognition. J. Cogn. Neurosci. 2006;18:818–832. doi: 10.1162/jocn.2006.18.5.818. [DOI] [PubMed] [Google Scholar]
- Hauk O., Pulvermüller F., Ford M., Marslen-Wilson W.D., Davis M.H. Can I have a quick word? Early electrophysiological manifestations of psycholinguistic processes revealed by event-related regression analysis of the EEG. Biol. Psychol. 2009;80:64–74. doi: 10.1016/j.biopsycho.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Jensen O., Bonnefond M., VanRullen R. An oscillatory mechanism for prioritizing salient unattended stimuli. Trends Cogn. Sci. 2012;16:200–206. doi: 10.1016/j.tics.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Jokisch D., Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J. Neurosci. 2007;27:3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. Erlbaum Associates; Hillsdale, N.J.: 1994. The structure of Long-term Memory: A Connectivity Model of Semantic Processing L. [Google Scholar]
- Klimesch W. EEG-alpha rhythms and memory processes. Int. J. Psychophysiol. 1997;26:319–340. doi: 10.1016/s0167-8760(97)00773-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Evoked alpha and early access to the knowledge system: the P1 inhibition timing hypothesis. Brain Res. 2011;1408:52–71. doi: 10.1016/j.brainres.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012;16:606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W., Hanslmayr S., Sauseng P., Gruber W.R., Doppelmayr M. P1 and traveling alpha waves: evidence for evoked oscillations. J. Neurophysiol. 2007;97:1311–1318. doi: 10.1152/jn.00876.2006. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- McRae K., Cree G.S., Seidenberg M.S., McNorgan C. Semantic feature production norms for a large set of living and nonliving things. Behav. Res. Methods. 2005;37:547–559. doi: 10.3758/bf03192726. [DOI] [PubMed] [Google Scholar]
- Nunez P.L., Wingeier B.M., Silberstein R.B. Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum. Brain Mapp. 2001;13:125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pexman P., Holyk G., Monfils M.-H. Number-of-features effects and semantic processing. Mem. Cogn. 2003;31:842–855. doi: 10.3758/bf03196439. [DOI] [PubMed] [Google Scholar]
- Price C.J., Devlin J.T. The Interactive Account of ventral occipitotemporal contributions to reading. Trends Cogn. Sci. 2011;15:246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F. Words in the brain's language. Behav. Brain Sci. 1999;22:253–279. [PubMed] [Google Scholar]
- Pulvermüller F., Assadollahi R., Elbert T. Neuromagnetic evidence for early semantic access in word recognition. Eur. J. Neurosci. 2001;13:201–205. doi: 10.1046/j.0953-816x.2000.01380.x. [DOI] [PubMed] [Google Scholar]
- Pylkkänen L., Marantz A. Tracking the time course of word recognition with MEG. Trends Cogn. Sci. 2003;7:187–189. doi: 10.1016/s1364-6613(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Pylkkänen L., Stringfellow A., Marantz A. Neuromagnetic evidence for the timing of lexical activation: an MEG component sensitive to phonotactic probability but not to neighborhood density. Brain Lang. 2002;81:666–678. doi: 10.1006/brln.2001.2555. [DOI] [PubMed] [Google Scholar]
- Segalowitz S.J., Zheng X. An ERP study of category priming: evidence of early lexical semantic access. Biol. Psychol. 2009;80:122–129. doi: 10.1016/j.biopsycho.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Sereno S.C., Rayner K. Measuring word recognition in reading: eye movements and event-related potentials. Trends Cogn. Sci. 2003;7:489–493. doi: 10.1016/j.tics.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Sereno S.C., Rayner K., Posner M.I. Establishing a time-line of word recognition: evidence from eye movements and event-related potentials. Neuroreport. 1998;9:2195–2200. doi: 10.1097/00001756-199807130-00009. [DOI] [PubMed] [Google Scholar]
- Solomyak O., Marantz A. Lexical access in early stages of visual word processing: a single-trial correlational MEG study of heteronym recognition. Brain Lang. 2009;108:191–196. doi: 10.1016/j.bandl.2008.09.004. [DOI] [PubMed] [Google Scholar]
- WorldMedicalAssociation Declaration of Helsinki. Br. Med. J. 1996;313:1445–1449. [Google Scholar]
- Yap M.J., Balota D.A. Visual word recognition of multisyllabic words. J. Mem. Lang. 2009;60:502–529. [Google Scholar]
- Yates M., Locker L., Simpson G. Semantic and phonological influences on the processing of words and pseudohomophones. Mem. Cogn. 2003;31:856–866. doi: 10.3758/bf03196440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.