Abstract
Nephrolithiasis is a multifactorial disease caused by environmental, hormonal, and genetic factors. Genetic polymorphisms of ORAI1, which codes for the main subunit of the store-operated calcium (SOC) channel, were reported to be associated with the risk and recurrence of calcium nephrolithiasis. Inositol 1,4,5-trisphosphate (IP3) 3-kinase C (ITPKC) is a negative regulator of the SOC channel-mediated signaling pathway. We investigated the association between calcium containing nephrolithiasis and genetic variants of ITPKC gene in Taiwanese patients. 365 patients were recruited in this study. Eight tagging single nucleotide polymorphisms of ITPKC were selected for genotyping. ITPKC genotypes were determined by TaqMan assay. ITPKC plasmids were transfected into cells to evaluate the intracellular calcium mobilization. Our results indicated that rs2607420 CC genotype in the intron region of the ITPKC gene is associated with a lower eGFR by both Modification of Diet in Renal Diseases (P = 0.0405) and Cockcroft-Gault (P = 0.0215) equations in patients with calcium nephrolithiasis. Our results identify a novel polymorphism for renal function and highlight the importance of ITPKC as a key molecule to regulate calcium signaling.
1. Introduction
Urolithiasis is a global problem affecting almost all populations in the world. In developed countries, the prevalence rate of urolithiasis was reported to be 4%~20%. In Taiwan, the prevalence was reported to be 9.6% [1]. The lifetime risk of urolithiasis is about 10%~15% in the developed world, but the risk was as high as 20%~25% in the Middle East [2]. Furthermore, in 20%~75% of patients, the disease recurs within 10 years of the first episode [3]. Consequently, urolithiasis causes a burden on society and significantly influences patients' quality of life. Previous epidemiological studies described an association between obesity and nephrolithiasis [4].
Urolithiasis often involves the formation of stones containing calcium compounds, mainly calcium oxalate and calcium phosphate, which account for 70%~80% of reported cases of urolithiasis. Calcium urolithiasis is thought to have a physicochemical origin, involving processes such as nucleation, growth, aggregation, and retention of crystals in the urine. The crystals include inorganic (e.g., calcium, uric acid, phosphate, and citrate) and organic substances (the Tamm-Horsfall glycoprotein and osteopontin) [5]. Calcium nephrolithiasis is a type of calcium metabolism disorder. Several studies indicated that the crystals may injure renal epithelial cells through inflammatory reactions and apoptosis, resulting in stone formation [6–8]. It is thought to be a multifactorial disease influenced by environmental, hormonal, and genetic factors.
Our previous study indicated that genetic polymorphisms of ORAI1, which codes for the main subunit of the store-operated calcium (SOC) channel, were associated with the risk and recurrence of calcium nephrolithiasis in a Taiwanese population [9]. Inositol 1,4,5-trisphosphate (IP3) 3-kinase C (ITPKC) is a negative regulator of the SOC channel-mediated nuclear factor of activated T cell (NFAT) signaling pathway and is involved in immune modulation via phosphorylation of IP3 [10, 11]. A functional single-nucleotide polymorphism (SNP) of ITPKC (rs28493229) was found to be associated with susceptibility to Kawasaki disease and coronary artery lesion formation [11]. The purpose of this study was to determine whether SNPs (rs11673492, rs28493229, rs7257602, rs7251246, rs890934, rs10420685, rs2607420, and rs2290692) of ITPKC are associated with the recurrence, stone number, or kidney function of patients with nephrolithiasis.
2. Material and Methods
2.1. Patients and Methods
We enrolled 365 patients who fulfilled the diagnostic criteria for nephrolithiasis at Kaohsiung Medical University Hospital (KMUH). Radiographic and echographic documentation of urinary stones in these patients were collected. Stone samples were obtained either from spontaneous passage or by surgical manipulation. We also collected clinical information such as age, gender, family history of nephrolithiasis, and episodes of stone recurrence. The past history of the stone episode was traced back to the whole life as far as patients could remember. Patients with at least 2 symptomatic episodes (at least 6 months apart) or new stones after treatment were classified into the recurrent group, and those with only 1 episode were classified into the single group. All subjects provided informed consent. The study protocol conformed to the Declaration of Helsinki and the study was approved by the Institute Review Board of KMUH.
2.2. DNA Extraction
DNA was extracted from blood samples collected from subjects. Blood cells were first treated with 0.5% sodium dodecyl sulfate lysis buffer and then with protease K (1 mg/mL) for 4 h at 60°C to digest the nuclear proteins. Total DNA was harvested using a Gentra (QIAGEN Inc., Valencia, CA) extraction kit and 70% alcohol precipitation, as in our previous study [12].
2.3. Genotyping
We selected seven tagging SNPs (rs11673492, rs7257602, rs7251246, rs890934, rs10420685, rs2607420, and rs2290692) with a minor allele frequency greater than 10% in the Han Chinese Beijing population from the HapMap database (http://hapmap.ncbi.nlm.nih.gov/). In addition, we included rs28493229 in this study which resulted from its association with the inflammation which has been demonstrated. The ITPKC gene structure was shown in Figure 1. Genotyping was carried out using the TaqMan allelic discrimination assay (Applied Biosystems, Foster City, CA). Briefly, we performed a polymerase chain reaction (PCR) using a 96-well microplate with an ABI 9700 thermal cycler (Applied Biosystems). The thermal cycle conditions were as follows: denaturing at 95°C for 10 min, followed by 40 cycles of denaturing at 92°C for 15 s, and annealing and extending at 60°C for 1 min. After the PCR, the fluorescence was measured and analyzed using System SDS software version 1.2.3 (Applied Biosystems, Foster City, CA).
Figure 1.
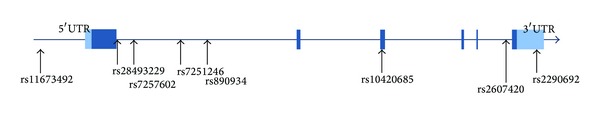
Graphical overview of the genotyped human ITPKC gene.
2.4. Calcium Imaging
Intracellular Ca2+ ([Ca2+]i) responses were induced by application of thapsigargin (TG) (Sigma-Aldrich, St. Louis, MO) in ITPKC-overexpressing HEK293 cells, according to the previously described methods [13]. Before the experiments, cells were stained with 1 μM Fluo-4-AM (Molecular Probes, Eugene, OR) at 37°C for 20 min and then washed with BSS buffer (5.4 mM KCl, 5.5 mM D-glucose, 1 mM MgSO4, 130 mM NaCl, 20 mM Hepes at pH 7.4, and 2 mM CaCl2). [Ca2+]i concentrations were estimated based on the ratio of fluorescence intensities emitted upon excitation with consecutive 3-s pulses of 488 nm light at a resolution of 1376 × 1038 pixels using an Olympus Cell ∧R IX81 fluorescence microscope (Olympus, Essex, UK) equipped with an MT 20 illumination system (Olympus) and UPLanApo 10x objective lens. The [Ca2+]i concentration was estimated based on calibration curves as follows. A Ca2+ calibration curve was created using a Ca2+ calibration buffer kit (Molecular Probes). This experiment was repeated five times. [Ca2+]i was calculated from Fluo-4 excited at 488 nm and imaged using an Olympus Cell ∧R IX81 fluorescence microscope and UPLanApo 10x objective lens at 20°C. Fluo-4 signals were calibrated by measuring the fluorescence intensity from microcuvettes containing 10 mM K2-EGTA (pH 7.20) buffered to various [Ca2+]i levels. The Ca2+ concentration was calculated using the following formula: [Ca2+]i = KD × ((F − F min)/(F max − F)). Plotting the fluorescence intensity versus [Ca2+]i yielded the calibration curve with the formula of [Ca2+]i = KD × ((F − F min)/(F max − F)), where KD is 345 nM, F is the Fluo-4 intensity, F max is 640, and F min is 21.7 for Fluo-4.
2.5. Statistical Analysis
SAS 9.1 for Windows (Cary, NC) was used for all statistical analyses. Statistical differences in genotypes and allelic frequencies between cases and controls were assessed by χ 2 test. A P value of <0.05 was considered to be statistically significant.
3. Results
3.1. Demographic and Clinical Characteristics of Subjects
We included 365 nephrolithiasis patients in this study. Table 1 shows the demographic characteristics of subjects. The mean age of patients ± standard deviation was 54.3 ± 12.0 years, and 68.5% of patients were male (Table 1). In total, 8 tagging SNPs of ITPKC were selected in this study from the HapMap database (http://hapmap.ncbi.nlm.nih.gov/). These SNPs had at least 10% minimum allele frequencies (MAFs) in a Han Chinese Beijing population. A graphical overview of the genotyped polymorphisms is shown in Figure 1.
Table 1.
Basal characteristics of patients with nephrolithiasis and of normal controls.
| Characteristic | Patients with nephrolithiasis |
|---|---|
| Number of subjects | 365 |
| Gender: male, number (%) | 250 (68.5%) |
| Age (years)a | 54.3 ± 12.0 |
| Range | 24~86 |
aMean ± SD.
3.2. Association Study of ITPKC Genetic Polymorphisms with the Risk of Stone Numbers and Recurrence in Patients with Calcium Nephrolithiasis
Among cases, 113 patients had had recurrent episodes, and 116 patients had had a single episode. We tested whether the rs11673492, rs28493229, rs7257602, rs7251246, rs890934, rs10420685, rs2607420, and rs2290692 genotypes were associated with the stone number or recurrence of calcium nephrolithiasis. As shown in Table 2, we found no significant association of genotypes or allelic frequencies with stone numbers. No significant association was noted between genotypes and the recurrence of calcium nephrolithiasis (Table 2).
Table 2.
Association analysis of ITPKC single-nucleotide polymorphisms (SNPs) and clinical medical records data of patients with kidney stones.
| SNP | Genotype | Stone numbers (%) | P value | Stone frequency (%) | P value | ||
|---|---|---|---|---|---|---|---|
| Multiple | Single | Recurrence | Nonrecurrence | ||||
| rs11673492 | TT | 12 (9.7) | 10 (8.1) | 0.9050 | 11 (9.7) | 10 (8.7) | 0.4025 |
| CT | 58 (46.8) | 59 (47.6) | 46 (40.7) | 57 (49.6) | |||
| CC | 54 (43.5) | 55 (44.3) | 56 (49.6) | 48 (41.7) | |||
|
| |||||||
| rs28493229 | CG | 18 (14.8) | 10 (8.1) | 0.1032 | 16 (14.4) | 9 (7.9) | 0.1198 |
| GG | 104 (85.2) | 113 (91.9) | 95 (85.6) | 105 (92.1) | |||
|
| |||||||
| rs7257602 | GG | 32 (25.8) | 24 (19.2) | 0.3705 | 27 (23.9) | 23 (19.8) | 0.7665 |
| AG | 55 (44.4) | 65 (52.0) | 54 (47.8) | 58 (50.0) | |||
| AA | 37 (29.8) | 36 (28.8) | 32 (28.3) | 35 (30.2) | |||
|
| |||||||
| rs7251246 | GG | 38 (30.7) | 30 (24.0) | 0.2634 | 29 (25.7) | 33 (28.5) | 0.7521 |
| GA | 50 (40.3) | 63 (50.4) | 52 (46.0) | 55 (47.4) | |||
| AA | 36 (29.0) | 32 (25.6) | 32 (28.3) | 28 (24.1) | |||
|
| |||||||
| rs890934 | TT | 23 (18.5) | 29 (23.2) | 0.3919 | 23 (20.3) | 24 (20.7) | 0.9975 |
| GT | 58 (46.8) | 62 (49.6) | 55 (48.7) | 56 (48.3) | |||
| GG | 43 (34.7) | 34 (27.2) | 35 (31.0) | 36 (31.0) | |||
|
| |||||||
| rs10420685 | GG | 7 (5.6) | 5 (4.0) | 0.7842 | 6 (5.3) | 6 (5.2) | 0.8333 |
| AG | 43 (34.7) | 41 (33.1) | 37 (32.7) | 42 (36.5) | |||
| AA | 74 (59.7) | 78 (62.9) | 70 (62.0) | 67 (58.3) | |||
|
| |||||||
| rs2607420 | CC | 10 (8.1) | 9 (7.2) | 0.9618 | 10 (8.9) | 9 (7.8) | 0.9191 |
| CT | 51 (41.5) | 52 (41.6) | 43 (38.4) | 47 (40.5) | |||
| TT | 62 (50.4) | 64 (51.2) | 59 (52.7) | 60 (51.7) | |||
|
| |||||||
| rs2290692 | GG | 38 (30.7) | 28 (22.4) | 0.2161 | 28 (24.8) | 32 (27.6) | 0.8602 |
| CG | 50 (40.3) | 63 (50.4) | 53 (46.9) | 54 (46.5) | |||
| CC | 36 (29.0) | 34 (27.2) | 32 (28.3) | 30 (25.9) | |||
3.3. Associations of ITPKC Genetic Polymorphisms with the Estimated Glomerular Filtration Rate (eGFR)
Previous studies indicated that renal stones may cause renal impairment and decrease renal function [14]. Therefore, we further calculated Modification of Diet in Renal Diseases- (MDRD-) based and Cockcroft-Gault- (C-G-) based eGFRs which are widely used tools to predict the renal function of nephrolithiasis patients [15, 16]. We tested the relationship between ITPKC genetic polymorphisms and renal function. Among the SNPs of ITPKC we tested, rs7251246 had a significant association (P = 0.0456) with a lower eGFR as calculated by C-G, while the rs2607420 CC genotype had a significant correlation with a lower eGFR as calculated by both MDRD (P = 0.0405) and C-G (P = 0.0215) (Table 3).
Table 3.
Association analysis of ITPKC single-nucleotide polymorphisms (SNPs) and clinical biochemical data of patients with kidney stones.
| SNP | Genotype | Sample number (%) | MDRD | P value | C-G | P value |
|---|---|---|---|---|---|---|
| (mL/min/1.73 m2) | (mL/min) | |||||
| rs11673492 | TT | 32 (8.8) | 77.6 ± 16.0 | 0.1471 | 71.4 ± 19.2 | 0.2342 |
| CT | 164 (45.1) | 84.9 ± 29.9 | 85.1 ± 33.0 | |||
| CC | 168 (46.1) | 84.0 ± 25.9 | 83.0 ± 28.2 | |||
|
| ||||||
| rs28493229 | CC | 0 (0.0) | — | 0.5622 | — | 0.8885 |
| CG | 42 (12.5) | 82.9 ± 26.4 | 83.2 ± 29.3 | |||
| GG | 295 (87.5) | 86.9 ± 34.3 | 82.1 ± 36.7 | |||
|
| ||||||
| rs7257602 | GG | 92 (25.3) | 80.5 ± 28.5 | 0.2855 | 77.9 ± 29.2 | 0.4558 |
| AG | 164 (45.0) | 86.8 ± 28.3 | 85.6 ± 29.3 | |||
| AA | 108 (29.7) | 80.1 ± 24.7 | 82.3 ± 31.2 | |||
|
| ||||||
| rs7251246 | GG | 96 (26.4) | 82.5 ± 22.4 | 0.0990 | 84.3 ± 28.9 | 0.0456* |
| GA | 164 (45.0) | 87.6 ± 29.9 | 87.4 ± 30.7 | |||
| AA | 104 (28.6) | 76.7 ± 27.3 | 73.2 ± 28.3 | |||
|
| ||||||
| rs890934 | TT | 77 (21.1) | 77.9 ± 29.0 | 0.4380 | 74.4 ± 23.3 | 0.1932 |
| GT | 180 (49.3) | 84.5 ± 28.9 | 84.0 ± 32.7 | |||
| GG | 108 (29.6) | 84.4 ± 23.6 | 86.0 ± 29.1 | |||
|
| ||||||
| rs10420685 | GG | 16 (4.4) | 90.9 ± 12.5 | 0.2153 | 89.5 ± 21.1 | 0.1545 |
| AG | 126 (34.6) | 86.5 ± 26.4 | 87.7 ± 31.7 | |||
| AA | 222 (61.0) | 80.2 ± 28.5 | 78.9 ± 29.3 | |||
|
| ||||||
| rs2607420 | CC | 27 (7.4) | 69.0 ± 16.4 | 0.0405* | 68.6 ± 18.2 | 0.0215* |
| CT | 143 (39.3) | 87.2 ± 26.6 | 89.2 ± 31.6 | |||
| TT | 194 (53.3) | 81.6 ± 27.9 | 79.2 ± 28.3 | |||
|
| ||||||
| rs2290692 | GG | 93 (19.4) | 83.1 ± 22.6 | 0.4016 | 84.8 ± 29.3 | 0.0784 |
| CG | 164 (34.2) | 85.8 ± 27.9 | 86.7 ± 30.8 | |||
| CC | 107 (46.4) | 78.9 ± 30.9 | 74.1 ± 28.5 | |||
*Significant (P < 0.05) values are in bold.
3.4. Effects of ITPKC Overexpression in Calcium Signaling in HEK293 Cells
The mechanism by which ITPKC influences cellular signaling is not clear. Thus, we transfected ITPKC plasmids into cells and evaluated the intracellular calcium mobilization. As shown in Figure 2, overexpression of ITPKC slightly increased the calcium release (first calcium peak). Importantly, the sustained calcium influx was reduced (second calcium peak).
Figure 2.
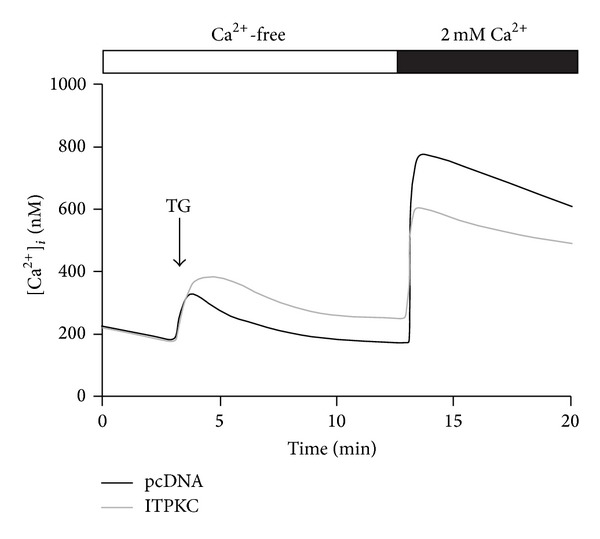
Effects of ITPKC on calcium influx in HEK293 cells. Thapsigargin (TG; 2 μM) was applied in a Ca2+-free BSS solution. The extracellular Ca2+ concentration abruptly increased from 0 to 2 mM which triggered the store-operated (SOC) channel influx.
4. Discussion
Nephrolithiasis is caused by a variety of conditions, including metabolic disorders and anatomical defects. It is considered a metabolic disorder commonly associated with type 2 diabetes, obesity, dyslipidemia, and hypertension. Most cases of nephrolithiasis are idiopathic; in such cases, there is undoubtedly a genetic predisposition, but both environmental and lifestyle factors may play important roles [17]. Almost 40% of patients who develop a stone for the first time will develop a second stone within 3 years of the first episode if prophylactic measures are not taken. The physicochemical theory of stone formation considers urine a supersaturated solution in which homogeneous or heterogeneous nucleation can lead to initiation of crystal formation, which can then aggregate and grow into a stone [17]. A well-known theory is Randall's plaques; this theory proposes that subepithelial interstitial calcium-based deposits act as nuclei for stone formation. These plaques, which are composed of apatite (calcium phosphate, not calcium oxalate), originate adjacent to the thin limbs of the loops of Henle. As they grow in size, they may injure the renal papillary duct epithelium and serve as sites for intratubular adhesion and growth of calcium oxalate crystals [17]. A positive family history of kidney stones is strongly associated with an increased risk of stone formation; the relative risk is 2~3 times higher in such individuals than in individuals without a family history of kidney stones [6].
Calcium nephrolithiasis is a type of calcium metabolic disorder, and most renal stones contain calcium. Formation of stones may also result from injury and inflammation of the renal tubular epithelium. Recent study indicated that crystals deposition or formation inside the kidney not only directly causes renal epithelial cell damage but also may induce immune response [18]. Crystals act as a stimulant that trigger innate immunity and lead to generate several cytokines such as IL-1β and IL-18 that drive inflammatory response [18]. Genetic factors that regulate calcium metabolism and inflammation are possibly linked with nephrolithiasis. Genetic polymorphisms of CLDN14, CASR, OPN, ORAI1, and VDR were reported to be involved in calcium nephrolithiasis in humans [19–23]. Our previous study indicated that genetic polymorphisms of ORAI1 are associated with susceptibility to calcium urolithiasis [6]. ORAI1 is a pore subunit of the SOC channel involved in different physiological functions, including immune responses and inflammatory reactions [24]. Indeed, calcium influx through the SOC channel can regulate the secretion of proinflammatory molecules such as arachidonic acid and leukotriene C4 [25]. ITPKC is an upstream gene of the SOC channel that may influence its activation via IP3 phosphorylation, which in turn regulates immune responses [26]. Therefore, we supposed that genetic variant of ITPKC may contribute to influence the calcium influx and, furthermore, may induce the activation of T cells which provoke inflammatory reaction. By using fluorescence-based calcium detection, we provide the first evidence to support a functional role of ITPKC in calcium signaling (Figure 2).
In this study, we found no significant associations between genotypes of ITPKC (rs11673492, rs28493229, rs7257602, rs7251246, rs890934, rs10420685, rs2607420, and rs2290692) and calcium nephrolithiasis. However, we cannot rule out the possibility that other low-frequency genetic polymorphisms of ITPKC may contribute to calcium nephrolithiasis. Importantly, we found that rs2607420 was significantly associated with kidney function (MDRD and C-G). Although we had no evidence to prove that rs2607420, the intronic SNP, directly affects the splicing efficiency or influences the affinity of transcription factor binding site, however, another intronic SNP of ITPKC, rs28493229, had demonstrated that the variant of rs28493229 is associated with the mRNA expression level of ITPKC [11]. Thus, the variant of rs2607420 may result in enhancing the risk of renal injury through changing ITPKC expression level. Even so, the effects of ITPKC on calcium nephrolithiasis and kidney functions are still unclear. More research is needed to determine the molecular basis of ITPKC and the pathogenesis of calcium nephrolithiasis.
In conclusion, we found that rs2607420 in the intron region of the ITPKC gene is associate with estimated creatinine clearance in patients with calcium nephrolithiasis.
Acknowledgments
This work was supported by grants from Chi-Mei Medical Center, Kaohsiung Medical University Research Foundation (101CM-KMU-08), and Taipei Medical University (TMU101-AE1-B14).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Wei-Chih Kan and Yii-Her Chou contributed equally to the paper.
References
- 1.Lee Y-H, Huang W-C, Tsai J-Y, et al. Epidemiological studies on the prevalence of upper urinary calculi in Taiwan. Urologia Internationalis. 2002;68(3):172–177. doi: 10.1159/000048445. [DOI] [PubMed] [Google Scholar]
- 2.Pak CYC. Kidney stones. The Lancet. 1998;351(9118):1797–1801. doi: 10.1016/S0140-6736(98)01295-1. [DOI] [PubMed] [Google Scholar]
- 3.Basiri A, Shakhssalim N, Khoshdel AR, et al. Familial relations and recurrence pattern in nephrolithiasis: new words about old subjects. Urology Journal. 2010;7(2):81–86. [PubMed] [Google Scholar]
- 4.Semins MJ, Shore AD, Makary MA, Magnuson T, Johns R, Matlaga BR. The association of increasing body mass index and kidney stone disease. Journal of Urology. 2010;183(2):571–575. doi: 10.1016/j.juro.2009.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basavaraj DR, Biyani CS, Browning AJ, Cartledge JJ. The role of urinary kidney stone inhibitors and promoters in the pathogenesis of Calcium containing renal stones. EAU-EBU Update Series. 2007;5(3):126–136. [Google Scholar]
- 6.Robertson WG, Peacock M, Nordin BEC. Calcium crystalluria in recurrent renal-stone formers. The Lancet. 1969;2(7610):21–24. doi: 10.1016/s0140-6736(69)92598-7. [DOI] [PubMed] [Google Scholar]
- 7.Bigelow MW, Wiessner JH, Kleinman JG, Mandel NS. Calcium oxalate-crystal membrane interactions: dependence on membrane lipid composition. Journal of Urology. 1996;155(3):1094–1098. [PubMed] [Google Scholar]
- 8.Khan SR, Kok DJ. Modulators of urinary stone formation. Frontiers in Bioscience. 2004;9:1450–1482. doi: 10.2741/1347. [DOI] [PubMed] [Google Scholar]
- 9.Chou Y-H, Juo S-HH, Chiu Y-C, et al. A polymorphism of the ORAI1 gene is associated with the risk and recurrence of calcium nephrolithiasis. Journal of Urology. 2011;185(5):1742–1746. doi: 10.1016/j.juro.2010.12.094. [DOI] [PubMed] [Google Scholar]
- 10.Sauer K, Cooke MP. Regulation of immune cell development through soluble inositol-1,3,4,5- tetrakisphosphate. Nature Reviews Immunology. 2010;10(4):257–271. doi: 10.1038/nri2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onouchi Y, Gunji T, Burns JC, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nature Genetics. 2008;40(1):35–42. doi: 10.1038/ng.2007.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang KD, Chang J-C, Chuang H, et al. Gene-gene and gene-environment interactions on IgE production in prenatal stage. Allergy. 2010;65(6):731–739. doi: 10.1111/j.1398-9995.2009.02260.x. [DOI] [PubMed] [Google Scholar]
- 13.Hsu WL, Tsai MH, Lin MW, et al. Differential effects of arsenic on calcium signaling in primary keratinocytes and malignant (HSC-1) cells. Cell Calcium. 2012;52(2):161–169. doi: 10.1016/j.ceca.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Gambaro G, Favaro S, D’Angelo A. Risk for renal failure in nephrolithiasis. American Journal of Kidney Diseases. 2001;37(2):233–243. doi: 10.1053/ajkd.2001.21285. [DOI] [PubMed] [Google Scholar]
- 15.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Annals of Internal Medicine. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Johri N, Cooper B, Robertson W, Choong S, Rickards D, Unwin R. An update and practical guide to renal stone management. Nephron. 2010;116(3):c159–c171. doi: 10.1159/000317196. [DOI] [PubMed] [Google Scholar]
- 18.Mulay SR, Evan A, Anders HJ. Nephrology, Dialysis, Transplantation. European Renal Association; 2013. Molecular mechanisms of crystal-related kidney inflammation and injury. Implications for cholesterol embolism, crystalline nephropathies and kidney stone disease. [DOI] [PubMed] [Google Scholar]
- 19.Sayer JA. Renal stone disease. Nephron. 2011;118(1):p35–p44. doi: 10.1159/000320902. [DOI] [PubMed] [Google Scholar]
- 20.Thorleifsson G, Holm H, Edvardsson V, et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nature Genetics. 2009;41(8):926–930. doi: 10.1038/ng.404. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira LG, Pereira AC, Heilberg IP. Vitamin D receptor and calcium-sensing receptor gene polymorphisms in hypercalciuric stone-forming patients. Nephron. 2010;114(2):c135–c144. doi: 10.1159/000254386. [DOI] [PubMed] [Google Scholar]
- 22.Zerwekh JE, Hughes MR, Reed BY, et al. Evidence for normal vitamin D receptor messenger ribonucleic acid and genotype in absorptive hypercalciuria. Journal of Clinical Endocrinology and Metabolism. 1995;80(10):2960–2965. doi: 10.1210/jcem.80.10.7559881. [DOI] [PubMed] [Google Scholar]
- 23.Nishio S, Hatanaka M, Takeda H, Iseda T, Iwata H, Yokoyama M. Analysis of urinary concentrations of calcium phosphate crystal- associated proteins: α2-HS-glycoprotein, prothrombin F1, and osteopontin. Journal of the American Society of Nephrology. 1999;10(supplement 14):S394–S396. [PubMed] [Google Scholar]
- 24.Braun A, Varga-Szabo D, Kleinschnitz C, et al. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood. 2009;113(9):2056–2063. doi: 10.1182/blood-2008-07-171611. [DOI] [PubMed] [Google Scholar]
- 25.Chang W-C, Parekh AB. Close functional coupling between Ca2+ release-activated Ca2+ channels, arachidonic acid release, and leukotriene C4 secretion. The Journal of Biological Chemistry. 2004;279(29):29994–29999. doi: 10.1074/jbc.M403969200. [DOI] [PubMed] [Google Scholar]
- 26.Kuo H-C, Chang W-C. Genetic polymorphisms in Kawasaki disease. Acta Pharmacologica Sinica. 2011;32(10):1193–1198. doi: 10.1038/aps.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]


