Abstract
Bone cements are extensively employed in orthopaedics for joint arthroplasty, however implant failure in the form of aseptic loosening is known to occur after long-term use. The exact mechanism causing this is not well understood, however it is thought to arise from a combination of fatigue and chemical degradation resulting from the hostile in vivo environment. In this study, two commercial bone cements were aged in an isotonic fluid at physiological temperatures and changes in moisture uptake, microstructure and mechanical and fatigue properties were studied. Initial penetration of water into the cement followed Fickian diffusion and was thought to be caused by vacancies created by leaching monomer. An increase in weight of approximately 2% was experienced after 30 days ageing and was accompanied by hydrolysis of poly(methyl methacrylate) (PMMA) in the outermost layers of the cement. This molecular change and the plasticising effect of water resulted in reduced mechanical and fatigue properties over time. Cement ageing is therefore thought to be a key contributor in the long-term failure of cemented joint replacements. The results from this study have highlighted the need to develop cements capable of withstanding long-term degradation and for more accurate test methods, which fully account for physiological ageing.
Keywords: Bone cement, Mechanical properties, Fatigue, Diffusion, Biodegradation
Graphical abstract
Highlights
-
•
Two commercial bone cements were aged in Ringer's solution at 37 °C for 60 days.
-
•
Moisture uptake, mechanical, fatigue and microstructural properties were studied.
-
•
A maximum of 2% change in weight occurred due to Fickian diffusion after 30 days.
-
•
Hydrolysis of PMMA and reduced mechanical and fatigue properties were observed.
-
•
Cement degradation is thought to contribute to the failure of cemented implants.
1. Introduction
Cemented joint replacements are regarded as the “gold standard” for hip and knee arthroplasty and have been extensively employed in orthopaedic surgery since the late 1950s when they were first introduced by Sir John Charnley (Breusch and Malchau, 2005; Lewis, 1997). This procedure employs polymethyl methacrylate (PMMA) bone cement to secure the prosthesis to the bone and distribute stresses more evenly around the implant and is currently associated with a failure rate of approximately 10% after 15 years (Breusch and Malchau, 2005; Lewis, 1997). The exact mechanism behind implant failure is not well understood; however, aseptic loosening is known to be the main mode of failure and is thought to arise due to a combination of mechanical fatigue and chemical degradation (Haas et al., 1975; Coury et al., 2004; Pantucek, 1969; Herman et al., 1989; Pelletier et al., 1999). With an ageing population and the number of joint replacements on the increase (UK NJR, 2011), a study investigating the degradation of bone cement over time is of clinical relevance and may provide insights into the role cement ageing has on implant failure.
The mechanical performance of PMMA bone cements is dependent upon several factors, including the composition of the cement, the method of sterilisation, the mixing technique, bone-implant conditions and the in vivo environment (Lewis, 1997; Goldman et al., 1998; Shen et al., 1983; Graham et al., 2000; Jasty et al., 1990). The mixing technique can influence porosity, which in turn affects the mechanical performance of the cement. Porosity may also influence the chemical degradation of the cement as pores create pathways for fluid to penetrate and diffuse into the cement (Jasty et al., 1990; Ries et al., 2006).
Several authors have conducted studies on the in vivo behaviour of PMMA bone cement. Hughes et al. (2003) demonstrated that long-term implantation of PMMA bone cement is associated with a decrease in the molecular weight of the cement in hip retrievals, but not in knee retrievals. A reduction in the molecular weight of the cement is usually associated with a reduction in mechanical and fatigue performance (Graham et al., 2000). Bettencourt et al. (2004) demonstrated that the surface wettability of bone cement increased after in vivo use and it was hypothesised that this may contribute to loosening by inducing a host response towards the implant. A degradation mechanism was proposed by both Hughes and Bettencourt, whereby chain scission and hydrolysis of ester groups alters the PMMA molecule over time. A study by Oonishi et al. (2011) demonstrated that the bending strength of CMW bone cement decreased with increased time in vivo, however there was no correlation between changes in molecular weight and implantation time. Furthermore, chain scission and hydrolysis of PMMA in that study was found not to have occurred in retrieved CMW cement samples.
Polymers have been known to undergo changes in mechanical and rheological properties when submersed in fluid over time. The extent of these changes can be attributed to the degree of fluid uptake, the nature of the immersion fluid, the temperature, the composition of the polymer and the duration of immersion. Typical changes include an increase in mass and a lowering of the glass transition temperature (Tg), when a polymer changes from a glassy to a rubbery state (McCrum et al., 1997). Diffusion in polymers is a complex process and difficult to predict. It is known that the diffusion of a liquid into a polymer is affected by the physical properties of the polymer network and the interactions between the polymer and the liquid itself. A classification method was proposed by Alfrey et al. (1966), whereby diffusion is classified according to the polymer relaxation rate and liquid diffusion rate. The classifications fall into two categories, Fickian (Case I) and non-Fickian (Case II and anomalous) and can be related by Mt=ktn, where Mt is the amount of solvent absorbed per unit area at time t, k is a constant and n is a parameter related to the diffusion mechanism which lies between 1/2 and 1 (Masaro and Zhu, 1999).
The majority of polymers are known to follow a Fickian uptake curve (Fick's second law; Ashby and Jones, 2007), particularly when the temperature is above the glass transition point (Tg) and the polymer chains have high mobility, allowing for easy penetration of the liquid (Grinsted et al., 1992). This behaviour is characterised by a high polymer relaxation rate, Rrelax, and a low liquid diffusion rate, Rdiff (Rrelax⪢Rdiff). The diffusion distance of this mechanism is proportional to the square root of time and therefore the n parameter is 1/2. Fickian diffusion has been observed in polymers with plasticisers below the glass transition temperature and the literature shows a maximum value for total percentage weight change of roughly 2% (Grinsted et al., 1992; Nottrott, 2010). Interestingly, the diffusion into PMMA of methanol with water was found to follow Fickian diffusion below the glass transition temperature as water acted as a plasticiser, which increased chain mobility allowing for further water penetration (Grinsted et al., 1992).
Non-Fickian diffusion is usually observed in glassy polymers below the glass transition temperature, where the polymer chains are not mobile enough to permit diffusion of liquids. Two non-Fickian diffusion processes can occur, Case II and anomalous. These processes differ by the rates with which the liquid diffuses into the polymer. For Case II diffusion, the rate is much higher than the polymer relaxation rate (Rdiff⪢Rrelax), whilst for anomalous diffusion, the rates are roughly the same (Rdiff≈Rrelax). For Case II diffusion, the diffusion distance is directly proportional to time so n is equal to 1 and for anomalous diffusion, the rate is between that of Fickian diffusion and Case II diffusion so n is between 1/2 and 1 (Masaro and Zhu, 1999).
Water uptake in glassy polymers can arise through any of these diffusion processes. PMMA is considered a glassy polymer as it is hard, non-crystalline/amorphous and the polymer chains have little mobility below the Tg. It is thought that below the Tg, the diffusion of water into glassy polymers is limited. Upon heating a glassy polymer however, it will soften and become rubbery, giving the polymer chains increased mobility.
The actual mechanism of water uptake in polymers varies but can arise due to fluid occupying vacancies in the material, such as pores or voids, or by water diffusing between intermolecular spaces (Neogi, 1996). With PMMA bone cements, vacancies may be created by entrapped air during mixing and by leaching monomer. Water molecules are also small enough to diffuse into spaces within radiopacifier agglomerations and between the polymer molecules. The latter is more pronounced as a result of plasticisation and higher temperatures, which causes the polymer chains to relax and increase the intermolecular spaces. Fluid can therefore diffuse into bone cements either without any relationship to the polar molecules in the material (free volume theory) or it can be governed by specific molecular interactions (Bellenger and Verdu, 1989; Yiu et al., 2004).
Studies investigating antibiotic release from bone cements have postulated that water penetrates into the cement through surface cracks and voids, created by the release of gentamicin sulphate (Baker and Greenham, 1988; Torrado et al., 2001; Diez-Pena et al., 2002). Few studies however, have investigated the diffusion mechanisms of water into bone cements that do not contain antibiotics.
Although there is an extensive literature on PMMA ageing and degradation, studies investigating the effects of ageing on mechanical and fatigue properties are limited. The extent of moisture uptake in commercial PMMA bone cements, which may contribute to structural changes, is also a topic that has not been extensively explored. The aim of this study was to understand how commercial PMMA bone cement, without antibiotic, absorbs moisture in vitro and the effect this has on mechanical and fatigue properties. Furthermore, the study aimed to investigate whether the chemical changes hypothesised by other authors were observed when commercial cement is aged in vitro.
Two commercially available high viscosity cements were selected for this study: Cemex Isoplastic and Palacos R. These brands were selected due to their distinctly different properties and compositions, as shown in Table 1. These differences in physicochemical properties may impact the rate of water uptake, ageing and changes in mechanical properties over time. Similarly, the different compositions result in different setting parameters and viscosities during mixing, however this has not been investigated in this study. Nevertheless, both of these cements are currently employed in total hip and knee replacements as a grout between the bone and the implant and therefore this study has significant clinical relevance.
Table 1.
Composition of commercially available PMMA bone cements.
| Cemex Isoplastic | Palacos R | |
|---|---|---|
| Liquid | ||
| Total liquid (g) | 13.30 | 18.78 |
| Methyl methacrylate (%w/w) | 99.10 | 97.98 |
| N-N dimethyl-p-toluidine (%w/w) | 0.90 | 2.02 |
| Hydroquinone (ppm) | 75.00 | 60.00 |
| Powder | ||
| Total powder (g) | 40.00 | 40.00 |
| Polymethyl methacrylate (%w/w) | 84.30 | 84.50a |
| Barium sulphate (%w/w) | 13.00 | – |
| Zirconium dioxide (%w/w) | – | 15.00 |
| Benzoyl peroxide (%w/w) | 2.70 | 0.50 |
| Powder:liquid ratio | 3.01 | 2.13 |
Poly(methyl methacrylate/methyl acrylate) powder.
2. Materials and methods
2.1. Materials
Cemex Isoplastic was purchased from Exactech (Redditch, United Kingdom) and Palacos R was donated by Heraeus Medical GmbH (Newbury, United Kingdom). High-grade (>98%) calcium chloride (CaCl2), sodium chloride (NaCl), potassium chloride (KCl), potassium bromide (KBr) and sodium bicarbonate (NaHCO3) were obtained from Fisher Scientific UK Ltd. (Loughborough, UK). Krak-gages were purchased from Hartrun Corporation (Minnesota, USA).
2.2. Sample preparation
Cemex Isoplastic and Palacos R samples were prepared using the ISO5833 (Implants for surgery—Acrylic resin cements) standard as a guideline (Institution BS. ISO5833, 2002). Manual mixing was performed in a polypropylene mixing bowl with a polypropylene spatula according to the manufacturer's instructions. The cements were introduced into polytetrafluoroethylene (PTFE) moulds, which were clamped between stainless steel end plates to produce samples with the dimensions specified below for each test. After 30 min, the samples were removed from the moulds and dry sanded to the correct dimensions using 250 grit silicon carbide paper.
2.3. Storage conditions
Ringer's solution (8.6 mg/mL NaCl, 0.3 mg/mL KCl and 0.33 mg/mL CaCl2, buffered to a pH of 7.4 with NaHCO3) as specified by Davis (2003) was prepared using deionised water. Samples were aged in either air at 23 °C as specified by the ISO5833 standard or in 5 mL of isotonic fluid (Ringer's Solution, pH 7) at 37 °C, with the fluid replaced every 14 days.
2.4. Moisture uptake
Cylindrical compression samples (6 mm diameter and 12 mm length) were prepared and weighed to the nearest ±0.1 mg prior to ageing in air or Ringer's solution. Samples were aged in air at 23 °C or Ringer's solution at 37 °C and weighed at 6 h, 1, 2, 3, 7, 30 and 60 days. Surface moisture from samples stored in Ringer's solution was removed with a paper towel prior to weighing. Eq. (1) was used to calculate the total percentage change in weight for each sample. Five samples were used for each time point and ageing condition to obtain an average change in weight over time.
| (1) |
2.5. Mathematical modelling
Mathematical models were applied to the moisture uptake curves to better understand the diffusion mechanisms occurring in commercial bone cements. Eq. (2) was applied to the moisture uptake data at various time points (0–3 days and 3–60 days). Fickian (n=1/2) and Case II (n=1) diffusion equations were applied to the data and the goodness of fit was investigated using the curve fitting toolbox in Matlab (MathWorks, Cambridge, UK), which calculated coefficients by minimising the sum of squared residuals. An additional coefficient (b) was added to Eq. (2), which represents the leaching components from the cement, to better model the data obtained, as shown in Eq. (3).
| (2) |
| (3) |
2.6. Porosity
Cemex Isoplastic and Palacos R cement was prepared as previously outlined and two cement discs from each brand (10 mm diameter and 2 mm height) were formed using PTFE moulds. One sample was aged in air at 23 °C for 24 h and the other was aged in 5 mL of Ringer's solution at 37 °C for 60 days. The samples were then gold-coated using an E65x sputter coater (Emitech, Kent, UK) and the surface porosity was studied using an EBT1 scanning electron microscope (SEM Tech Ltd., Southampton, UK) at 15 KeV.
2.7. Mechanical properties
The compression and bending properties of cement samples were determined using a Zwick Roell ProLine table-top Z050/Z100 materials testing machine with TestXpert II software (Zwick Testing Machines Ltd., Herefordshire, UK) and the ISO5833 standard as a guideline after ageing in air at 23 °C for 1, 2, 3, 7, 30 and 60 days or Ringer's solution at 37 °C for the same time points. Cylindrical compression samples (6 mm diameter and 12 mm length) were tested at a constant cross-head speed of 20 mm/min. Rectangular bending samples (75 mm length, 10 mm width and 3.3 mm thickness) were tested at a constant cross-head speed of 5 mm/min in four-point bending. Five compression and five bending samples were tested to determine average compression and bending properties for each time point, ageing condition and cement brand.
2.8. Fracture toughness
Linear elastic fracture mechanics (LEFM) was used to determine the fracture toughness of cement samples aged in air for 24 h at 23 °C and Ringer's solution for 60 days at 37 °C. This allowed a comparison between fully saturated samples and those tested according to the ISO5833 standard specifications. Single-edge notched three-point bending samples (35 mm length, 10 mm width and 3 mm thickness) were prepared and a sharp chevron notch (4.5–5.5 mm in length) was created through the centre of the sample using a razor blade mounted onto a modified microtome, as described by Evans (2007). The initial crack length and specimen dimensions were measured using a travelling microscope (Pye Scientific, Cambridge, UK). The specimens were loaded at a crosshead speed of 5 mm/min in three-point bending, with the span between the rollers set to 40 mm, until failure occurred. The load and displacement were recorded and the critical stress intensity factor (Kic) of the cement was calculated as described in the ISO13586:2000 standard (Institution BS. ISO13586, 2000). Five samples were tested for each ageing condition and cement brand to produce mean fracture toughness values.
2.9. Fatigue properties
Fatigue crack growth tests were performed using disc compact tension (DCT) specimens, with dimensions conforming to the ASTM E399 standard (International ASfTaM, 2009) as described by Evans (2007). A modified microtome was used to cut a chevron notch, which ensured symmetrical crack growth. The initial crack length and specimen dimensions were measured using a travelling microscope (Pye Scientific, Cambridge, UK). In order to apply the load symmetrically across the sample, loose plates with oversize holes were employed, which allowed the loading pins to rotate freely without friction. The crack length was monitored using Krak-gages and a constant current supply and amplifier designed and built by Evans (2007) was used to obtain accurate crack growth measurements at low crack growth rates. Precision components were employed and the current regulator and preamplifier were kept at a constant temperature in an Instron environmental chamber (Instron SFL, Bristol, UK) to achieve very low noise and offset drift.
Four fatigue samples were prepared for each cement brand, two of which were tested after 24 h ageing in air at 23 °C and two of which were tested after 60 days ageing in Ringer's solution at 37 °C. All tests were carried out in an Instron environmental chamber at 37 °C using a 5 kN Dartec servohydraulic testing machine with an MTS FlexTest GT controller and MTS Multipurpose software (MTS, Eden Prairie, MN, USA).
Samples were cyclically loaded in load control with a sine wave at 5 Hz between 70 N and 7 N for Cemex Isoplastic and 100 N and 10 N for Palacos R (R-ratio of 0.1), allowing for measurements of crack growth rates through stress intensities of 0.3–0.9 MPa m1/2. Prior to loading, samples were subject to a precracking procedure to ensure an initial steady crack growth rate of 10−9 m/cycle. For precracking, a high cyclic load of 200 N at 5 Hz was applied and the load reduced by 10% after every 0.2 mm of crack growth. The crack length and number of cycles were measured during loading and the crack growth rate (da/dN in m/cycle) was calculated for every 0.2 mm of crack growth. Similarly, the corresponding stress intensity factor range (ΔK) for each sample and crack length (ranging from 6 to 12 mm, in steps of 0.2 mm) was calculated using Eq. (4), where Pmax is the maximum load, Pmin is the minimum load, b is the sample thickness, a is the crack length from centre point of the holes to the tip of the crack, W is the width of the specimen from centre point of the holes to the bottom of the specimen and α is a/W and is greater than 0.2.
| (4) |
For each sample, da/dN was plotted as a function of ΔK on a logarithmic scale. The Paris Law was applied to obtain coefficients ‘A’ and ‘m’ (as shown in Eq. (5)) for each sample group.
| (5) |
The fracture surfaces of the fatigue testing samples were gold coated using an E65x sputter coater (Emitech, Kent, UK) and imaged using an EBT1 scanning electron microscope (SEM Tech Ltd., Southampton, UK) at 15 KeV.
2.10. Structural changes
Structural changes were monitored by Fourier Transform Infrared Spectroscopy (FTIR) using a Perkin Elmer Spectrum One FT-IR Spectrometer with FT-IR Spectrum software (Perkin Elmer, Massachusetts, USA) between 4000 cm−1 and 450 cm−1 with a resolution of 4 cm−1. Samples were scanned after 24 h in air at 23 °C and 60 days in Ringer's solution at 37 °C. The surface of the bending samples was scanned using attenuated total reflectance (ATR) at 6 different sections on the sample, taking 30 scans at each section to obtain an average spectrum of the sample surface. To obtain a spectrum of the outer and inner layers of the sample, a sterile file was employed to remove a small cross section of the sample and 1 mg of the sample was combined and crushed with 100 mg of KBr to create discs. Two discs were prepared per sample and 30 scans per sample were performed.
2.11. Glass transition temperature
The glass transition temperature of samples was determined by dynamic mechanical analysis using a Rheometric Scientific V500 (TA Instruments Ltd., Elstree, UK). Small matchstick-like samples (30 mm length, 3 mm width and 2 mm height) were prepared and stored for 24 h in air at 23 °C and 60 days in Ringer's solution at 37 °C. Samples were loaded sinusoidally at a frequency of 1 Hz in a dual cantilever configuration and heated from 35 °C to 200 °C at a rate of 10 °C/min, whilst the applied stress and strain was measured. The phase lag (δ) between the stress (σ) and strain (ε) was measured and the storage modulus (E') and loss modulus (E″) were calculated using Eqs. (6) and (7) respectively. The complex viscosity (tan δ) was calculated as shown in Eq. (8) and plotted against temperature. The temperature at which tan δ was at its maximum was considered to be the glass transition temperature (Tg). Five samples were tested to obtain a mean value for Tg.
| (6) |
| (7) |
| (8) |
2.12. Vickers hardness tests
Two Cemex Isoplastic and two Palacos R disc shaped samples (10 mm diameter and 2 mm height) were prepared and the surfaces polished with a 4000 grit silicon carbide paper. One sample of each cement brand was stored in air for 24 h at 23 °C and one sample of each cement brand was stored in Ringer's solution for 60 days at 37 °C. The microhardness of the samples was tested according to the ISO6507-2 standard (Institution BS. ISO6507, 2005). Five indentations, 1 mm apart and 1 mm from the edges of the sample, were placed on both sides of each sample using a Zwick 3212 indenter fitted with a 136° square-based pyramid diamond indenter (Zwick Testing Machines Ltd., Herefordshire, UK) with a load of 200 g for 10 s. Indentations in the vicinity of large pores (>50 µm) were disregarded and repeated. For each indentation, two diagonal lengths were measured using a travelling microscope and averaged. Eq. (9) was employed to calculate the average hardness value (in MPa), where F is the load in kg (0.2) and d is the mean diagonal length of the indentation in mm.
| (9) |
2.13. Statistical analysis
An analysis of variance (ANOVA) was carried out to establish significant differences between groups of samples using the data analysis package in Excel (Microsoft, Reading, UK). Significance between groups was defined as those with a calculated p-value of less than 0.05.
3. Results
3.1. Moisture uptake
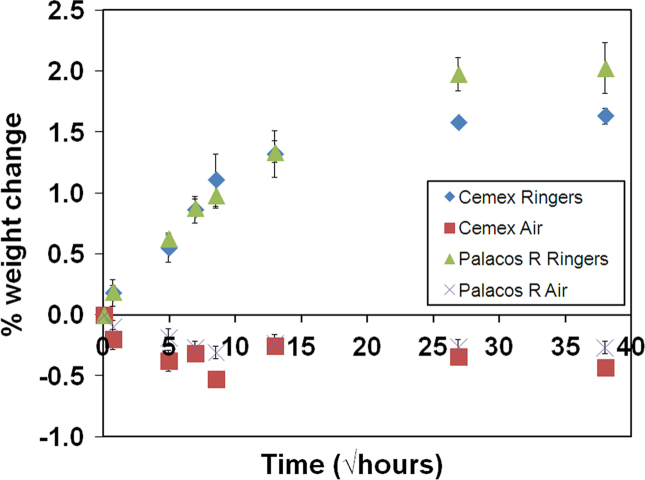
Fig. 1 shows the change in weight for Palacos R and Cemex Isoplastic stored in air at 23 °C and Ringer's solution at 37 °C, plotted against the square root of time. This type of plot was employed to demonstrate Fickian diffusion, indicated by a linear relationship between moisture uptake and the square root of time. Both commercial cements aged in Ringer's solution experienced a total weight increase of approximately 1.5–2%, whilst samples aged in air experienced a weight loss of approximately 0.3%. The linear portions for samples aged in Ringer's solution occurred over the first 3 days, after which water absorption continued at a slower rate. Equilibrium for both cements was reached after 30 days and overall Palacos R had the greatest moisture uptake at the end of the study (2% after 60 days).
Fig. 1.
Change in weight due to ageing in air at 23 °C and Ringer's solution at 37 °C over time. Both cements experienced a loss in weight when aged in air and an increase when aged in Ringer's solution. Palacos R experienced greater moisture uptake over 60 days when compared to Cemex cement.
3.2. Mathematical modelling of moisture uptake
Tables 2 and 3 demonstrate the results of modelling moisture uptake for Cemex Isoplastic and Palacos R respectively. For both cement brands the modified Eq. (3) (Mt=b+ktn) fitted the data most accurately. This additional constant (b) represents the leaching of monomer, which leaves vacancies into which the water diffuses. For the first 3 days, both cements fitted a Fickian diffusion model well (R-value≥0.96) and when using Eq. (3) (Mt=ktn) Cemex Isoplastic and Palacos R had n values of 0.526 and 0.354 respectively, signifying a close relationship to Fickian diffusion (n=0.5). After 3 days however, the diffusion slowed and was no longer Fickian. The additional coefficient did not improve the goodness of fit for both cements between 3 and 60 days in Ringer's solution. This suggests that the leaching monomer did not affect the moisture uptake after 3 days in Ringer's solution. The Case II diffusion equation produced the poorest fit for all the data points modelled and confirmed that water absorption was not linear with time.
Table 2.
Mathematical modelling of moisture uptake results for Cemex Isoplastic.
| Fickian | Case II | Eq. (3) | Modified Eq. (3) | |
|---|---|---|---|---|
| Mt=kt1/2 | Mt=kt | Mt=ktn | Mt=b+ktn | |
| Entire curve | ||||
| k | 0.056 | 0.001 | 0.380 | 1.581 |
| n | 0.500 | 1.000 | 0.211 | 0.091 |
| b | – | – | – | −1.346 |
| R-value | 0.2340 | −1.0631 | 0.9144 | 0.9445 |
| 0–3 Days | ||||
| k | 0.126 | 0.017 | 0.114 | 0.031 |
| n | 0.500 | 1.000 | 0.526 | 0.801 |
| b | – | – | – | 0.162 |
| R-value | 0.9688 | 0.8662 | 0.9693 | 0.9992 |
| 3–60 Days | ||||
| k | 0.052 | 0.001 | 0.798 | 0.463 |
| n | 0.500 | 1.000 | 0.100 | 0.137 |
| b | – | – | – | 0.400 |
| R-value | −9.2467 | −28.7763 | 0.9674 | 0.9636 |
Table 3.
Mathematical modelling of moisture uptake results for Palacos R.
| Fickian | Case II | Eq. (3) | Modified Eq. (3) | |
|---|---|---|---|---|
| Mt=kt1/2 | Mt=kt | Mt=ktn | Mt=b+ktn | |
| Entire curve | ||||
| k | 0.067 | 0.001 | 0.319 | 0.568 |
| n | 0.500 | 1.000 | 0.264 | 0.204 |
| b | – | – | – | −0.345 |
| R-value | 0.6462 | −0.3002 | 0.9719 | 0.9784 |
| 0–3 Days | ||||
| k | 0.123 | 0.016 | 0.217 | 0.132 |
| n | 0.500 | 1.000 | 0.354 | 0.451 |
| b | – | – | – | 0.095 |
| R-value | 0.9572 | 0.6390 | 0.9929 | 0.9961 |
| 3–60 Days | ||||
| k | 0.063 | 0.002 | 0.526 | 0.443 |
| n | 0.500 | 1.000 | 0.191 | 0.208 |
| b | – | – | – | 0.103 |
| R-value | −0.6748 | −5.3414 | 0.9078 | 0.9052 |
3.3. Porosity
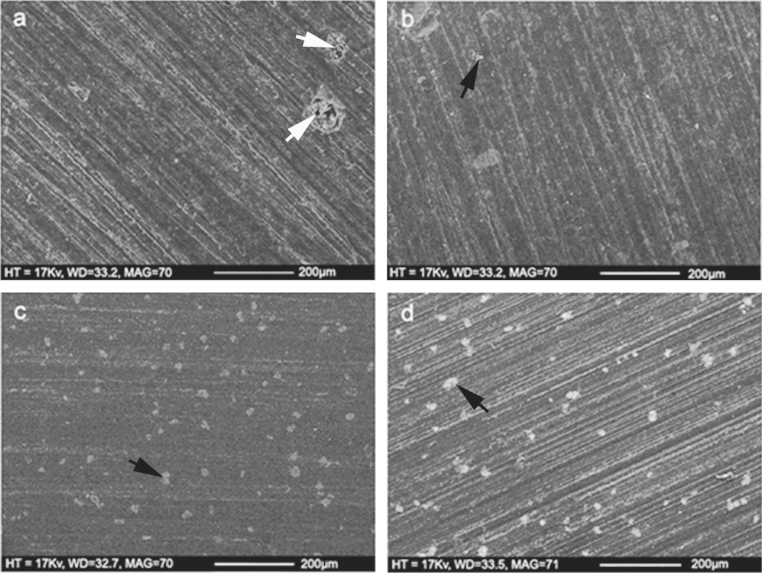
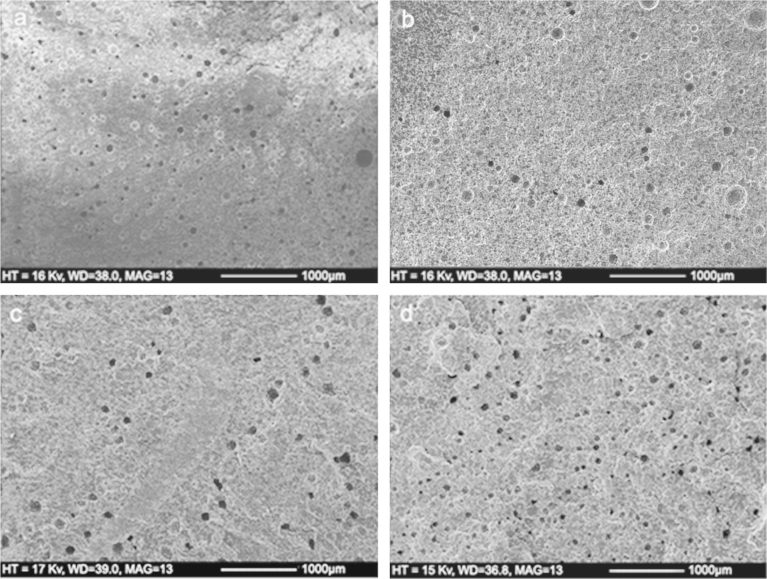
SEM images of the cement surfaces before and after storage in Ringer's solution for 60 days are shown in Fig. 2. For Cemex Isoplastic and Palacos R, surface imperfections were detected (white arrows), potentially as a result of the sample preparation. Radiopacifier particles were observed (black arrows), however few surface pores were detected on the surface of the samples both before and after ageing, demonstrating that diffusion was not predominantly driven by surface porosity.
Fig. 2.
Surface porosity for Cemex Isoplastic (a) before and (b) after ageing in Ringer's solution at 37 °C for 60 days, and for Palacos R (c) before and (d) after ageing in Ringer's solution at 37 °C for 60 days. There was no increase in surface porosity as a result of ageing in Ringer's solution for both cements. White arrows demonstrate surface imperfections before ageing and black arrows demonstrate radiopacifier particles.
3.4. Mechanical properties
Table 4 shows the compression and bending properties of Cemex Isoplastic and Palacos R after ageing in air at 23 °C and Ringer's solution at 37 °C. For both cement brands, samples aged in air resulted in significantly (ANOVA, p<0.05) higher compressive strengths than samples aged in Ringer's solution for the same amount of time. After ageing for 7, 30 and 60 days in Ringer's solution, Cemex Isoplastic bone cement samples had a significantly higher compressive strength than samples tested according to the ISO5833 standard (24 h in air). Palacos R samples stored in Ringer's solution were found to have significantly lower compressive strength values than the samples tested according to the standard. Ageing in Ringer's solution initially resulted in a higher bending strength and modulus for both cements when compared to samples aged in air. Over time however, both the bending strength and modulus of samples stored in Ringer's solution were found to decrease. The bending strength and modulus of Cemex Isoplastic after 60 days ageing in Ringer's solution were significantly lower than the values obtained according to the ISO5833 standard. For Palacos R cement samples, only ageing for 60 days in Ringer's solution significantly reduced the modulus.
Table 4.
Cemex Isoplastic and Palacos R compression and bending properties after ageing in air at 23 °C or Ringer's solution at 37 °C over time.
| 1 Day | 2 Days | 3 Days | 7 Days | 30 Days | 60 Days | |
|---|---|---|---|---|---|---|
| Cemex Isoplastic | ||||||
| Compressive strength | ||||||
| Air | 85.15±4.69 | 96.58±4.45b | 111.72±7.15b | 112.80±5.65b | 118.12±4.69b | 118.04±3.38b |
| Ringer's solution | 90.16±3.48 | 89.22±5.55a | 90.16±3.48a | 98.92±3.22a,b | 96.25±3.06a,b | 92.71±2.52a,b |
| Bending strength | ||||||
| Air | 45.95±7.21 | 48.54±7.68 | 44.11±1.56 | 55.05±3.67b | 50.41±3.93 | 44.37±4.24 |
| Ringer's solution | 59.08±6.85a,b | 69.30±1.59a,b | 61.93±5.82a,b | 47.05±3.76a | 47.09±1.31 | 36.36±1.50a,b |
| Bending modulus | ||||||
| Air | 3082.75±228.75 | 3296.18±119.09 | 3625.92±79.49b | 3767.31±22.56b | 3161.07±195.07 | 3518.78±130.88b |
| Ringer's solution | 4237.59±38.12a,b | 3136.27±67.60 | 4058.68±108.21a,b | 2942.61±165.51a | 2652.67±234.74a,b | 2442.90±230.39a,b |
| Palacos R | ||||||
| Compressive strength | ||||||
| Air | 104.56±2.92 | 99.05±4.05b | 90.28±6.25b | 93.35±9.92 | 99.32±8.50 | 100.81±9.16 |
| Ringer's solution | 71.87±2.25a,b | 81.97±3.57a,b | 78.61±4.71a,b | 75.23±6.06a,b | 73.32±5.98a,b | 75.49±5.73a,b |
| Bending strength | ||||||
| Air | 69.74±2.07 | 76.55±3.57b | 72.21±3.29 | 77.56±2.57b | 75.45±4.24b | 74.15±3.30b |
| Ringer's solution | 80.71±5.08a,b | 84.82±1.06a,b | 70.24±7.24 | 67.43±2.80a | 75.15±8.71 | 69.39±2.59a |
| Bending modulus | ||||||
| Air | 3414.03±123.38 | 3291.66±113.08 | 3633.25±103.57b | 3773.11±99.58b | 3312.37±191.85 | 3357.73±118.48 |
| Ringer's solution | 3443.85±110.02 | 3742.13±67.90a,b | 3680.07±72.87b | 3025.29±152.62a,b | 3058.37±138.97a,b | 3177.12±7.75a,b |
Significantly different from sample aged in air for same amount of time (ANOVA p<0.05).
Significantly different from sample aged according to ISO5833 standard (24 h in air).
3.5. Fracture toughness, glass transition temperature and Vickers hardness
Table 5 shows the fracture toughness, glass transition temperature and hardness for Cemex Isoplastic and Palacos R after ageing in air for 24 h at 23 °C and Ringer's solution for 60 days at 37 °C. All three properties were significantly reduced (ANOVA, p<0.05) after ageing in Ringer's solution for 60 days at 37 °C when compared to samples tested after 24 h in air at 23 °C.
Table 5.
Fracture toughness, glass transition temperature and hardness of Cemex Isoplastic and Palacos R after ageing in air for 24 h at 23 °C or Ringer's solution for 60 days at 37 °C.
| Critical stress intensity factor,Kic(MPa m1/2) | Glass transition temperature,Tg(°C) | Hardness HV30/10 (MPa) | |
|---|---|---|---|
| Cemex Isoplastic | |||
| Air 24 h | 1.68±0.14 | 127.29±1.63 | 24.78±2.01 |
| Ringer's solution 60 days | 1.38±0.08a | 121.58±1.42a | 19.10±1.98a |
| Palacos R | |||
| Air 24 h | 2.46±0.19 | 126.17±3.25 | 19.76±1.84 |
| Ringer's solution 60 days | 1.50±0.31a | 117.90±1.23a | 17.47±2.58a |
Significantly different from sample aged in air for 24 h (ANOVA p<0.05).
3.6. Fatigue properties
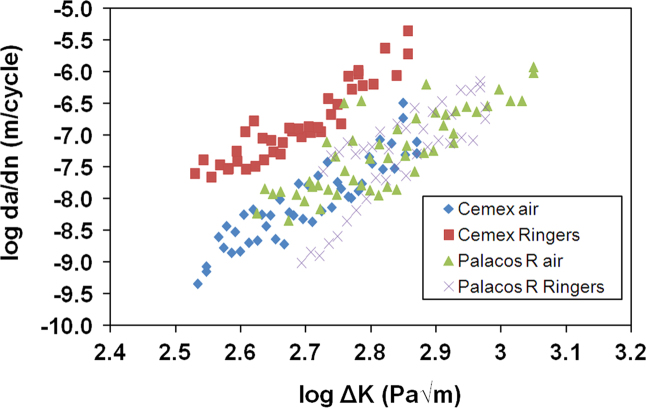
The crack growth rates for the fatigue tests are shown in Fig. 3. The crack growth rates increased for Cemex Isoplastic after storage in Ringer's solution for 60 days at 37 °C. It is unclear, however, whether ageing in Ringer's solution at 37 °C affected the fatigue properties of Palacos R. Table 6 demonstrates the coefficients obtained when the Paris Law was applied to the fatigue data. The ‘A’ coefficient represents the crack growth at ΔK=1 MPa m1/2 and the ‘m’ coefficient is the slope of the plot, which represents the change in crack growth rate. Both cements experienced an increase in the ‘A’ and ‘m’ coefficients for both cements, demonstrating a reduction in crack growth resistance. All fatigue samples were found to have correlation coefficients above 0.60 when the Paris Law was applied, demonstrating a moderate to strong positive correlation between the data and the Paris Law. Palacos R had lower correlation coefficients when compared to Cemex Isoplastic, due to higher variation in the crack growth rates of the samples tested. The fracture surfaces of both Cemex Isoplastic and Palacos R fatigue samples were rougher after ageing in Ringer's solution and both cements were found to have pores both before and after ageing on the fracture surface due to entrapped air during mixing (Fig. 4).
Fig. 3.
Crack growth rates over different stress intensities for Cemex Isoplastic and Palacos R before and after ageing in Ringer's solution for 60 days at 37 °C. Ageing Cemex cement in Ringer's solution increased the crack growth rates, however further analysis was required to establish changes in the Palacos cement.
Table 6.
Fatigue test constants of Cemex Isoplastic and Palacos R after ageing in air at 23 °C for 24 h or Ringer's solution at 37 °C for 60 days.
|
A |
m |
Correlation coefficient |
||||
|---|---|---|---|---|---|---|
| 24 h Air | Ringer's solution 60 days | 24 h Air | Ringer's solution 60 days | 24 h Air | Ringer's solution 60 days | |
| Cemex Isoplastic | 5.39E−07 | 3.54E−05 | 5.92 | 7.57 | 0.82 | 0.79 |
| Palacos R | 3.73E−07 | 7.34E−07 | 4.65 | 7.33 | 0.67 | 0.66 |
Fig. 4.
SEM images of fracture surfaces for Cemex Isoplastic after (a) 24 h in air at 23 °C and (b) 60 days in Ringer's solution at 37 °C; and Palacos R after (c) 24 h in air at 23 °C and (d) 60 days in Ringer's solution at 37 °C. Porosity was observed before and after ageing and both cements demonstrated increased fracture surface roughness.
3.7. Structural changes
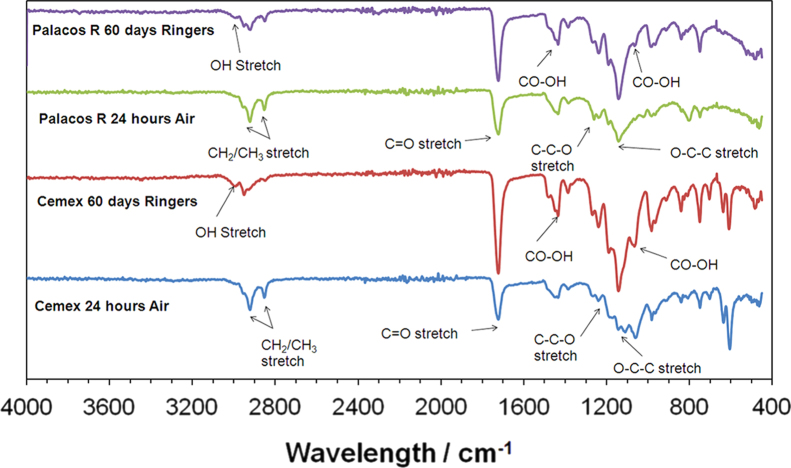
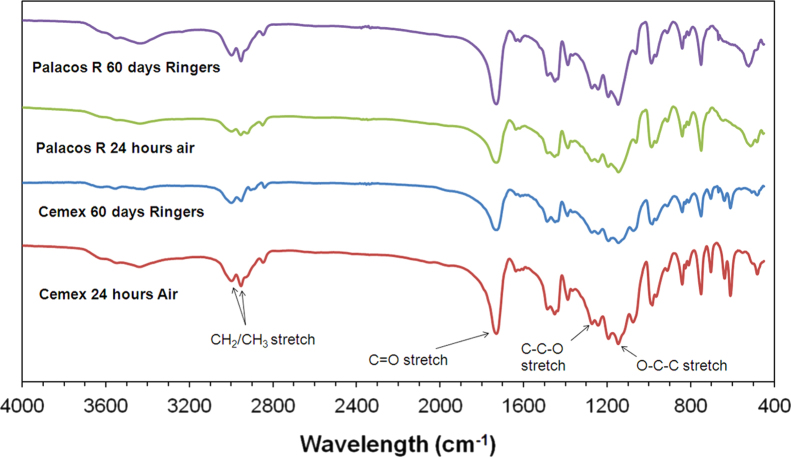
FTIR peaks representative of PMMA were observed in Cemex Isoplastic and Palacos R samples using both ATR and KBr techniques for all time points (Figs. 5 and 6). The peaks were between 2850 cm−1 and 2950 cm−1 for methylene (CH2) and methyl (CH3) stretches; at 1400 cm−1 and 1450 cm−1 for methyl groups (CH3); between 1300 cm−1 and 1500 cm−1 for the CH bend; at 1730 cm−1 for the C=O stretch; at 1240 cm−1 for the C–C–O stretch; at 1140 cm−1 for the O–C–C stretch; and between 1300 cm−1 and 900 cm−1 for the C–O stretch. Differences were observed between the spectra of Cemex Isoplastic and Palacos R and are attributed to the different radiopacifiers employed and the presence of methyl acrylate (MA) in Palacos R. Increases in peak intensity were observed, demonstrating an increase in the number of bonds formed. A shift in the position of certain peaks was also observed, demonstrating a change in one chemical bond in favour of another. The changes in peak intensity and position however, were only observed in ATR surface measurements. After 60 days ageing, a decrease in the intensity of peaks located between 2850 cm−1 and 2950 cm−1 was observed and is characteristic of a transition from unsaturated alkenes (C=C) to saturated alkanes (C–C), as occurs in the polymerisation of MMA to PMMA, and also indicates the absence of unreacted monomer, which leaches out of the cement over time. An increase in the intensity of peaks at 1730 cm−1 and between 900 cm−1 and 1300 cm−1 were observed, demonstrating the formation of C=O and C–O bonds. Similarly an increase in the intensity of the O–C–C stretch at 1140 cm−1 was recorded. Peaks at 2800 cm−1 decreased in intensity and a peak at 3000 cm−1 formed, demonstrating a change from CH bonds to OH bonds. Similarly, peaks at 1430 cm−1 and 1060 cm−1 were found to increase in intensity for ATR scans after ageing, demonstrating the potential formation of carboxyl groups. The changes however, were only present on surface ATR scans and not KBr scans, which examined the bulk cross-section of cement samples.
Fig. 5.
FTIR spectra using ATR for Cemex Isoplastic and Palacos R after 24 h in air at 23 °C and Ringer's solution at 37 °C for 30 and 60 days. Several changes were observed for both cements indicating chemical changes as a result of ageing.
Fig. 6.
FTIR spectra using KBr for Cemex Isoplastic and Palacos R after 24 h in air at 23 °C and Ringer's solution at 37 °C for 30 and 60 days. No chemical changes were observed when scanning a cross-section of the cement samples.
4. Discussion
The mechanism of moisture uptake and ageing in PMMA bone cements is of clinical relevance as changes to the material properties over time may affect the performance of the cement and influence the likelihood of failure.
Three processes are thought to occur simultaneously during the ageing of bone cement: the cement polymerises over a prolonged period of time, increasing the strength and modulus of the cement; unreacted monomer and other components will diffuse out of the cement and water will penetrate in.
The increase in weight over time for samples aged in Ringer's solution was caused by the diffusion of water into the cement (N’Diaye et al., 2012). Similar levels of moisture uptake (1.8–2%) have been reported with other polymers (Nottrott, 2010; Schmitt et al., 2004). Although PMMA is a hydrophobic polymer, studies have shown it to absorb up to 2% of its weight in water (Kusy et al., 2001; McCabe and Rusby, 2004; Smith et al., 2011). A recent study by N’Diaye et al. (2012) demonstrated that PMMA swells when submerged in water. This increase in mass may be beneficial in reducing shrinkage stresses associated with bone cements. The commercial bone cements achieved a total moisture uptake of between 1.5% and 2.3%. Palacos R had a greater moisture uptake when compared to Cemex Isoplastic due to the presence of methyl acrylate, which made the cement more hydrophilic. The composition of the cement therefore plays a role in the extent of moisture uptake. Mathematical modelling demonstrated that the initial diffusion of water into the cement followed Fickian diffusion, but only up to 3 days in Ringer's solution. This result is similar to a study by Grinsted et al. (1992) where Fickian diffusion was observed in polymers below the glass transition point due to the plasticising effect of water. Variation in water absorption was large as shown by the standard deviations for the initial moisture uptake. The decrease in weight experienced by samples aged in air occurred as a result of residual monomer from the polymerisation reaction evaporating over time. Although a decrease in weight was demonstrated for samples aged in air, the amount of MMA evaporating fluctuated, with no linear relationship between change in weight and time. It can be assumed that samples aged in Ringer's solution also experience leaching of the residual monomer. This is further highlighted by the improved goodness of fit for the mathematical models using the additional coefficient for monomer loss (b). The goodness of fit however, was only improved for samples aged in Ringer's solution for up to 3 days, after which no improvement was observed when using an additional coefficient. Therefore it is thought that the leaching monomer does not influence moisture uptake beyond 3 days immersion. The initial leaching of monomer creates vacancies in the cement, which are replaced by water molecules and this results in a dramatic increase in moisture uptake over the first 3 days, which slows as the amount of leached monomer reduces. After the initial uptake of water, diffusion between the polymer molecules is thought to occur. This period of moisture uptake may be influenced by molecular changes in the cement and the relaxation of the polymer chains. Similarly, porosity within the cement may prolong the moisture uptake process by creating pathways for further diffusion.
Several changes occurred to the mechanical properties of the commercial cements as a result of ageing. Most notably, long-term ageing in Ringer's solution was found to reduce the strength and modulus of the cement when compared to samples tested in air. Water may act as a plasticiser, inducing structural changes and degradation of the polymer matrix as described by other authors (Oonishi et al., 2011; Nottrott et al., 2007). The initial moisture uptake may have enhanced the bending properties of bone cement, however after long-term ageing the uptake of water was found to be detrimental to the performance of the cement. Ageing in air resulted in more stable and enhanced mechanical properties as unreacted monomer either evaporated or polymerised slowly to form strengthening interpenetrating molecular networks. Although the cement will initially provide mechanical stability for the implant, after long-term use it is expected that the cement strength will decrease as demonstrated in this study and in extreme cases, loosening or fracture of the cement may occur.
The results obtained for the compression and bending tests highlight the limitations of the ISO5833 standard. Significantly different results were obtained when samples were aged according to the standard than when samples were aged in an isotonic fluid at physiological temperatures. The ISO5833 standard does not accurately predict the clinical performance of bone cement and better-defined experiments are required which fully address the effects of physiological ageing. These may incorporate, higher temperatures or more corrosive environments to accelerate the moisture uptake and ageing of the cement prior to testing. Similarly, a physiologically relevant fluid must be employed as ageing in air does not replicate the conditions in vivo accurately.
Fatigue samples stored in Ringer's solution demonstrated rougher fracture surfaces and had poorer fatigue properties. Schmitt et al. (2004) found that long-term soaking in Ringer's solution improves fatigue crack propagation resistance. It is thought that water absorption increases polymer chain mobility and can enhance crack tip blunting. This however, was not observed in this study as fracture toughness and fatigue properties were reduced after ageing in Ringer's solution. Degradation of the cement as a result of ageing may have caused the observed decrease in fracture toughness and fatigue properties. Microcracks developed either as a result of cement shrinkage during polymerisation or high impact loads may therefore decrease the strength of the cement and potentially lead to failure, as the fracture toughness of the cement decreases due to ageing.
The outermost surface of bone cement experiences the most water penetration and therefore is more prone to degradation and structural changes as a result of ageing in isotonic fluid. Although no surface porosity was observed, hardness tests on the surface of samples demonstrated that the material softened over time when aged in Ringer's solution. Furthermore, FTIR scans highlighted structural changes on the surface of samples (using ATR), but not through the bulk (cross-section) of the cement (using KBr). Degradation at the outer surface of the cement, as experienced in this study, would be detrimental to the cement–implant and cement–bone interfaces and could encourage loosening of the implant. The increase in intensity of peaks from ATR scans may be attributed to molecular bonds continuously forming on the outermost layers of the cement. This is consistent with previous studies that state when bone cement is mixed, polymerisation occurs most rapidly in the centre of the cement mass, where the most heat is generated, whilst the outermost layers polymerise more slowly (Stanczyk and van Rietbergen, 2004; Mazzullot et al., 1991).
Several properties of the commercial bone cements have been investigated in this study, however the dimensions of the various test samples differed due to the requirements of each test. This resulted in different surface to volume ratios, which may have influenced the rate of fluid uptake. Therefore, samples with different dimensions aged for the same amount of time may have experienced different levels of water uptake. To overcome this issue, fracture toughness, Vickers hardness and glass transition temperature samples were all aged for 60 days to ensure that the maximum amount of water uptake took place before testing. This allowed for a comparison between material properties of fully saturated samples.
Polymers have been known to undergo hydrolysis when immersed in acidic conditions at high temperatures. The acid acts as a catalyst for the reaction and under weak acidic conditions, hydrolysis of the polymer may occur slowly over time. As OH groups were found to form when bone cement was immersed in Ringer's solution at 37 °C, it is possible that hydrolysis of the ester groups (C=O) of PMMA caused the change in polymeric structure. In vivo any localised acidic pH in the area of the implant may further accelerate this reaction. This process results in the formation of methanol (CH3OH) and carboxylic acid (–COOH) (as shown in Fig. 7a and b for MMA and PMMA respectively). A reduction in the glass transition temperature was also observed as a result of ageing, indicating chemical degradation. This theory has been postulated by other authors and demonstrated using a variety of techniques (Hughes et al., 2003; Bettencourt et al., 2004). Bettencourt et al. (2004) found changes in the wetting properties and the molecular structure of artificially aged PMMA bone cement using contact angle measurements and X-ray photoelectron spectroscopy (XPS). Hughes et al. (2003) used gel permeation chromatography (GPC) to demonstrate changes in molecular weight and FTIR to demonstrate chemical changes in artificially and physiologically-aged cement. The changes observed by Hughes and Bettencourt were similar to the results of this study. Oonishi et al. (2011) also used FTIR to study structural changes of retrieved CMW1 cement samples. CMW1 cement however, did not undergo structural changes, as experienced with Cemex Isoplastic and Palacos R in this study. Structural changes in Cemex Isoplastic and Palacos R were further highlighted by a reduction in the glass transition temperature. Since the glass transition temperature is dependent upon molecular weight it can be assumed that a reduction in the molecular weight would also have occurred as a result of ageing. These findings correlate well with the majority of ageing studies on PMMA (Hughes et al., 2003; Bettencourt et al., 2004; McCrum et al., 1997; Arnold and Venditti, 2001). A reduction in both these properties would result in polymer chains having greater mobility at body temperature. This greater mobility can be attributed to scission of polymer chains, via the hydrolysis mechanism outlined above, and would result in a less rigid cement, as observed in the results. Furthermore, increased surface wettability, caused by hydrolysis of PMMA has been hypothesized by Bettencourt et al. (2004), to induce a host response towards the implant, which may contribute to its loosening.
Fig. 7.
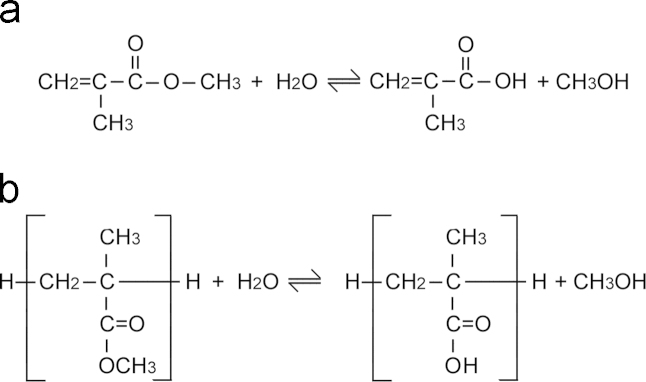
The proposed mechanisms by which hydrolysis of ester groups of (a) MMA and (b) PMMA occurs.
Although a variety of properties of aged bone cement have been investigated, there are several limitations to this study. The samples were unloaded when aged in a simple isotonic fluid. In vivo it is expected that the cement will be subjected to a continuous loading and unloading regime, potentially increasing its rate of degradation. It is also expected that the environment experienced in vivo is more hostile with the cement interacting with cells, enzymes, lipid material and wear particles. This may have a greater impact on the changes experienced in terms of molecular, mechanical and crack propagation properties. It is debated whether mixing techniques significantly influence cement porosity (Graham et al., 2000; Linden, 1989; Messick et al., 2007). As manual mixing was employed in this study, it is not known if samples prepared using vacuum mixing techniques would produce different results due to variations in cement porosity. Employing a variety of mixing techniques would have provided useful information on moisture uptake and ageing as a consequence of mixing technique and porosity. The mechanical and fatigue tests would also be influenced by factors such as porosity and the presence of stress raisers, such as voids and agglomerations and therefore mixing technique is an important factor to consider. Static tests do not replicate the loading conditions that occur in vivo. Nevertheless, the range of in vitro tests employed, which cover properties and loading conditions relevant to in vivo circumstances, can be used to establish baseline performance for comparative purposes.
To prevent ageing of the cement, it is recommended that a more hydrophobic cement be developed. Modifying the cement with a silane, as demonstrated by Gbureck et al. (2005), has been shown to improve the bond strength with the metallic implant and prevent hydrolysis of the interface. Employing hydrophobic radiopacifiers, such as the iodine-based radiopacifiers developed by Lewis et al. (2005), over conventional hydrophilic radiopacifiers, such as barium sulphate, may also slow the rate of water uptake and therefore long-term ageing.
5. Conclusions
Polymers have been known to undergo time-dependant changes in mechanical and rheological properties when submerged in aqueous fluid. The extent of these changes can be attributed to the amount of fluid uptake, the type of fluid, the surrounding temperature, the composition of the polymer and the immersion time (McCrum et al., 1997). The mechanisms of moisture uptake in two commercial bone cements have been investigated and a reduction in strength and hardness, an increase in fatigue crack growth rate and structural changes were observed as a result of in vitro ageing in Ringer's solution at 37 °C. Initial diffusion of water into PMMA bone cement was found to be Fickian and was thought to be governed by the leaching of unreacted monomer from the cement, which created vacancies thereby occupied by water molecules. A maximum change in weight of 2% after 30 days was observed due to moisture uptake, which was accompanied by structural changes in the outermost layer of the cement, where high levels of diffusion are thought to occur and where the cement polymerises most slowly. This adversely affected the mechanical and fatigue properties of the commercial cements, with significant differences observed between samples tested according to the standard and those tested after ageing in isotonic medium. The effects of ageing and moisture uptake are thought to contribute to the long-term failure of PMMA bone cement and therefore, methods to prevent cement degradation and more accurate testing techniques must be developed, which fully account for physiological ageing.
Acknowledgements
The authors would like to thank Dr. Panagiota Manti of the Cardiff School of History, Archaeology and Religion for her assistance with the FTIR measurements, Mrs. Wendy Rowe of the Cardiff School of Dentistry for undertaking the SEM imaging and Heraeus Medical GmbH for providing Palacos R cement for this study. This work was supported by the Arthritis Research UK Biomechanics and Bioengineering Centre at Cardiff, through funding from Arthritis Research UK and Cardiff University.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Alfrey T., Gurnee E.F., Lloyd W.G. Diffusion in glassy polymers. J. Polym. Sci., Part C: Polym. Symptoms. 1966;12(1):249–261. [Google Scholar]
- Arnold J.C., Venditti N.P. Effects of environment on the creep properties of a poly(ethylmethacrylate) based bone cement. J. Mater. Sci. Mater. Med. 2001;12(8):707–717. doi: 10.1023/a:1011272626846. [DOI] [PubMed] [Google Scholar]
- Ashby M.F., Jones D.R.H. Elsevier; Oxford: 2007. Engineering Materials 1: An Introduction to Properties, Applications and Design. [Google Scholar]
- Breusch S.J., Malchau H. Springer; Berlin: 2005. The Well-Cemented Total Hip Arthroplasty: Theory and Practice. [Google Scholar]
- Baker A.S., Greenham L.W. Release of gentamicin from acrylic bone cement. Elution and diffusion studies. J. Bone Jt. Surg. 1988;70(10):1551–1557. [PubMed] [Google Scholar]
- Bettencourt A., Calado A., Amaral J., Alfaia A., Vale F.M., Monteiro J. Surface studies on acrylic bone cement. Int. J. Pharm. 2004;278(1):181–186. doi: 10.1016/j.ijpharm.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Bellenger V., Verdu J. Structure–properties relationship for densely crosslinked epoxy-amine systems based on epoxide or amine mixtures. J. Mater. Sci. 1989;24:63–68. [Google Scholar]
- Coury A.J., Levy R.J., McMillin R., Pathak Y., Ratner B.D., Schoen F.J. Elsevier Academic Press; London: 2004. Degradation of Materials in the Biological Environment. Biomaterials Science: An Introduction to Materials in Medicine; pp. 411–429. [Google Scholar]
- Davis E.J.R. ASM International; Materials Park, Ohio: 2003. Handbook of Materials for Medical Devices. [Google Scholar]
- Diez-Pena E., Frutos G., Frutos P., Barrales-Rienda J.M. Gentamicin sulphate release from a modified commercial acrylic surgical radiopaque bone cement. I. Influence of the gentamicin concentration on the release process mechanism. Chem. Pharm. Bull. 2002;50(9):1201–1208. doi: 10.1248/cpb.50.1201. [DOI] [PubMed] [Google Scholar]
- Evans S. Fatigue crack propagation under variable amplitude loading in PMMA and bone cement. J. Mater. Sci. Mater. Med. 2007;18(9):1711–1717. doi: 10.1007/s10856-007-3021-x. [DOI] [PubMed] [Google Scholar]
- Goldman, M., Pelletier, B., Muller, S., Ries, M., Pruitt, L., (Eds.), 1998. Comparison of the effects of sterilization techniques on acrylic bone cement: implications for mechanical failure of total joint replacement. In: Proceedings of the Transactions of 44th Annual Meeting. Orthopaedic Research Society, New Orleans.
- Grinsted R.A., Clark L., Koenig J.L. Study of cyclic sorption–desorption into poly(methyl methacrylate) rods using NMR imaging. Macromolecules. 1992;25(4):1235–1241. [Google Scholar]
- Gbureck U., Grübel S., Thull R., Barralet J.E. Modified PMMA cements for a hydrolysis resistant metal–polymer interface in orthopaedic applications. Acta Biomater. 2005;1(6):671–676. doi: 10.1016/j.actbio.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Graham J., Pruitt L., Ries M., Gundiah N. Fracture and fatigue properties of acrylic bone cement: the effects of mixing method, sterilization treatment, and molecular weight. J. Arthroplast. 2000;15(8):1028–1035. doi: 10.1054/arth.2000.8188. [DOI] [PubMed] [Google Scholar]
- Herman J.H., Wendy W.G., Anderson D., Appel A.M., Hopson N. Polymethylmethacrylate-induced release of bone-resorbing factors. J. Bone Jt. Surg. 1989;71(10):1530–1541. [PubMed] [Google Scholar]
- Hughes K.F., Ries M.D., Pruitt L.A. Structural degradation of acrylic bone cements due to in vivo and simulated ageing. J. Biomed. Mater. Res., Part A. 2003;65A(2):126–135. doi: 10.1002/jbm.a.10373. [DOI] [PubMed] [Google Scholar]
- Haas S.S., Brauer G.M., Dickson G. A characterization of polymethylmethacrylate bone cement. J. Bone Jt. Surg. (Am) 1975;57(3):380–391. [PubMed] [Google Scholar]
- Institution BS. ISO13586:2000, 2000. Plastics: determination of fracture toughness (Gic and Kic). Linear elastic fracture mechanics (LEFM) approach.
- Institution BS. ISO6507:2005, 2005. Metallic materials—Vickers hardness test—Part 1: test method.
- Institution BS. ISO5833:2002, 2002. Implants for surgery: acrylic resin cements.
- International ASfTaM, 2009. ASTM E399—09e2 Standard Test Method for Linear-Elastic Plane-Strain Fracture Toughness of Metallic Materials. ASTM International.
- Jasty M., Davies J.P., O’Connor D.O., Burke D.W., Harrigan T.P., Harris W.H. Porosity of various preparations of acrylic bone cements. Clin. Orthop. Relat. Res. 1990;259:122–129. [PubMed] [Google Scholar]
- Kusy R.P., Whitley J.Q., Kalachandra S. Mechanical properties and interrelationships of poly(methyl methacrylate) following hydration over saturated salts. Polymer. 2001;42(6):2585–2595. [Google Scholar]
- Lewis G., van Hooy-Corstjens C.S.J., Bhattaram A., Koole L.H. Influence of the radiopacifier in an acrylic bone cement on its mechanical, thermal, and physical properties: barium sulfate-containing cement versus iodine-containing cement. J. Biomed. Mater. Res., Part B. 2005;73B(1):77–87. doi: 10.1002/jbm.b.30176. [DOI] [PubMed] [Google Scholar]
- Lewis G. Properties of acrylic bone cement: state of the art review. J. Biomed. Mater. Res. 1997;38(2):155–182. doi: 10.1002/(sici)1097-4636(199722)38:2<155::aid-jbm10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Linden U. Fatigue properties of bone cement: comparison of mixing techniques. Acta Orthop. 1989;60(4):431–433. doi: 10.3109/17453678909149312. [DOI] [PubMed] [Google Scholar]
- Messick K.J., Miller M.A., Damron L.A., Race A., Clarke M.T., Mann K.A. Vacuum-mixing cement does not decrease overall porosity in cemented femoral stems: an in vitro laboratory investigation. J. Bone Jt. Surg. (Br) 2007;89(8):1115–1121. doi: 10.1302/0301-620X.89B8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzullot S., Paolini M., Verdi C. Numerical simulation of thermal bone necrosis during cementation of femoral prostheses. J. Math. Biol. 1991;29(5):475–494. doi: 10.1007/BF00160473. [DOI] [PubMed] [Google Scholar]
- McCrum N.G., Buckley C.P., Bucknall C.B. 2nd ed. Oxford University Press; Oxford: 1997. Principles of Polymer Engineering. [Google Scholar]
- McCabe J.F., Rusby S. Water absorption, dimensional change and radial pressure in resin matrix dental restorative materials. Biomaterials. 2004;25(18):4001–4007. doi: 10.1016/j.biomaterials.2003.10.088. [DOI] [PubMed] [Google Scholar]
- Masaro L., Zhu X.X. Physical models of diffusion for polymer solutions, gels and solids. Prog. Polym. Sci. 1999;24(5):731–775. [Google Scholar]
- Nottrott M., Molster A.O., Gjerdet N.R. Time dependent mechanical properties of bone cement. An in vitro study over one year. J. Biomed. Mater. Res., Part B. 2007;83B(2):416–421. doi: 10.1002/jbm.b.30811. [DOI] [PubMed] [Google Scholar]
- Nottrott M. Acrylic bone cements. Acta Orthop. 2010;81(S341):1–27. doi: 10.3109/17453674.2010.487929. [DOI] [PubMed] [Google Scholar]
- N’Diaye M., Pascaretti-Grizon F., Massin P., Basle M.F., Chappard D. Water absorption of poly(methyl methacrylate) measured by vertical interference microscopy. Langmuir. 2012;28(31):11609–11614. doi: 10.1021/la302260a. [DOI] [PubMed] [Google Scholar]
- Neogi P. In: Diffusion in Polymers. Neogi P., editor. CRC Press; New York: 1996. [Google Scholar]
- Oonishi H., Akiyama H., Takemoto M., Kawai T., Yamamoto K., Yamamuro T. The long-term in vivo behavior of polymethyl methacrylate bone cement in total hip arthroplasty. Acta Orthop. 2011;82(5):553–558. doi: 10.3109/17453674.2011.625538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier, B., Hughes, K., Gundiah, N., Muller, S., Pruitt, L., Ries, M., (Eds.), 1999. A study of the in vivo molecular degradation of acrylic bone cements used in cemented total joint arthroplasties. In: Proceedings of the Transactions of the 45th Annual Meeting. Orthopaedic Research Society, Anaheim.
- Pantucek M. On the metabolic pathway of methylmethacrylate. Federation Eur. Biochem. Soc. 1969;2(2):206–208. doi: 10.1016/0014-5793(69)80020-7. [DOI] [PubMed] [Google Scholar]
- Ries M.D., Young E., Al-Marashi L., Goldstein P., Hetherington A., Petrie T. In vivo behavior of acrylic bone cement in total hip arthroplasty. Biomaterials. 2006;27(2):256–261. doi: 10.1016/j.biomaterials.2005.05.103. [DOI] [PubMed] [Google Scholar]
- Shen, J., Chen, C.C., Saver, J.A., (Eds.), 1983. Fatigue of PMMA effects of molecularweight, watercontent and frequency. In: Fatigue in Polymers Conference Proceedings. London.
- Schmitt S., Krzypow D.J., Rimnac C.M. The effect of moisture absorption on the fatigue crack propagation resistance of acrylic bone cement. Biomed. Tech. (Berl) 2004;49(3):61–65. doi: 10.1515/BMT.2004.012. [DOI] [PubMed] [Google Scholar]
- Smith K.E., Trusty P., Wan B., Gall K. Long-term toughness of photopolymerizable (meth)acrylate networks in aqueous environments. Acta Biomater. 2011;7(2):558–567. doi: 10.1016/j.actbio.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Stanczyk M., van Rietbergen B. Thermal analysis of bone cement polymerisation at the cement–bone interface. J. Biomech. 2004;37(12):1803–1810. doi: 10.1016/j.jbiomech.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Torrado S., Frutos P., Frutos G. Gentamicin bone cements: characterisation and release (in vitro and in vivo assays) Int. J. Pharm. 2001;217(1–2):57–69. doi: 10.1016/s0378-5173(01)00587-7. [DOI] [PubMed] [Google Scholar]
- UK NJR . National Joint Registry for England and Wales. 8th Annual Report 2011. National Joint Registry UK; Hertfordshire: 2011. [Google Scholar]
- Yiu C.K., King N.M., Pashley D.H., Suh B.I., Carvalho R.M., Carrilho M.R. Effect of resin hydrophilicity and water storage on resin strength. Biomaterials. 2004;25(26):5789–5796. doi: 10.1016/j.biomaterials.2004.01.026. [DOI] [PubMed] [Google Scholar]