Highlights
-
•
PPG neurons express GLP-1 and project to autonomic control sites throughout the brain.
-
•
The distribution of PPG axon terminals mirrors the distribution of GLP-1 receptor cells throughout the CNS.
-
•
Brain-derived GLP-1 plays a role in suppression of hedonic and metabolic food intake.
Abstract
Glucagon-like peptide-1 (GLP-1) is both a peripherally expressed incretin and a centrally active neuropeptide. Brain derived GLP-1, produced in preproglucagon (PPG) neurons located in the nucleus of the solitary tract (NTS) and projecting to numerous brain regions, is ideally placed to activate central GLP-1 receptors in a range of autonomic control areas. In vivo analysis of central GLP-1 using GLP-1 receptor antagonists has demonstrated the control of a range of feeding responses mediated by GLP-1 receptor activation. Recent advances enabling identification and targeting of the neurons in the NTS has specifically implicated PPG neurons at the core of GLP-1 dependent central and peripheral control for short-term and long-term energy balance.
Current Opinion in Pharmacology 2013, 13:964–969
This review comes from a themed issue on Endocrine and metabolic diseases
Edited by Frank Reimann and Fiona M Gribble
For a complete overview see the Issue and the Editorial
Available online 24th September 2013
1471-4892/$ – see front matter, © 2013 Elsevier Ltd. All rights reserved.
Introduction
‘Gut hormones’ have increasingly been implicated in brain function [1,2]. One such example is glucagon-like peptide-1 (GLP-1), which in addition to being gut-derived, is also synthesised by preproglucagon (PPG) neurones in the brain. These are located primarily in a discrete region of the lower brainstem [3]. This review focuses on the recent advances in our understanding of the physiological significance of this cell population.
The vast majority of studies examining central GLP-1 effects have been performed on rodents and consequently we will focus on these. Although a small number of studies on the GLP-1 system have highlighted differences between mouse and rat [4,5] for most aspects they appear to be equivalent, and in this review we have treated studies on either as comparable. Furthermore, given that the amino acid sequence of GLP-1 is conserved throughout mammalian species [6] and that the distribution of PPG neurons in the non-human primate Macaca mulatta [7] is strikingly similar to that in rodents we make the assumption that most of these findings have clear relevance for GLP-1 action in man.
At present it is still controversial as to how the central GLP-1 system is linked to peripheral post-prandial GLP-1 release and whether gut-derived GLP-1 can enter the brain to a sufficient extent to activate central GLP-1 receptors (reviewed in [3,8,9]). Here, we will not address these issues, but consider the GLP-1 producing neurons as an independent cell population and examine the evidence as to the feasibility of the hypothesis that this cell population is the principal physiological source of endogenous GLP-1 interacting with GLP-1 receptors within the CNS. For this to be the case, there has to be anatomical evidence that the distribution of PPG cell axons and GLP-1 release sites matches the distribution of GLP-1 receptors in brain, functional evidence of endogenous release of GLP-1 within the CNS, and proof that inhibition or destruction of these PPG neurons prevents the central effects attributed to GLP-1. We examine these points in sequence.
Anatomical correlation between GLP-1 receptor expression and the distribution and projections of PPG neurons in the brain
It has been known for more than 20 years that GLP-1 is synthesised in mammalian brain [10–13]. Most published reports analysing the distribution of PPG neurons are from rat using either immunocytochemistry for GLP-1 or GLP-2 [3,10,14–16] or in situ hybridisation [17] to localise these neurons and their axon terminals. These studies demonstrated that PPG neurons are non-adrenergic neurons with their cell bodies located exclusively in the caudal nucleus of the solitary tract (NTS), the caudal medullary reticular formation and the olfactory bulb [14,17]. They also demonstrated a widespread projection pattern for these neurons with the highest density of terminals observed in the paraventricular nucleus (PVN) and the dorsomedial hypothalamus (DMH) [14,15,18]. Merchenthaler and colleagues [17] also concluded that all GLP-1 terminals outside the olfactory bulb must originate from the brainstem nuclei, because the olfactory bulb PPG neurons were located periglomerular, and were thus local interneurons. Two recent studies targeting the rostral forebrain confirmed by injection of Fluoro-Gold or RetroBeads into the nucleus accumbens (NAc) that GLP-1 immunoreactive neurons in the NTS project to this area [19••,20••].
Recently, Llewellyn-Smith and co-workers [21•,22•] have revisited the expression pattern of PPG neurons with the use of a transgenic mouse (PPG-YFP mouse) that expresses YFP under the control of the glucagon promoter [23•]. These mice show strong YFP fluorescence throughout the entire cytoplasm of the GLP-1 neurons and thus allowed the researchers to map the PPG neurons in mouse with unprecedented precision, showing not only cell bodies and terminals, but also the entire dendritic tree and axons [21•,22•]. These studies demonstrated mouse PPG cell bodies in the caudal NTS, the intermediate reticular formation mediodorsal of the nucleus ambiguus and along the midline ventral of the hypoglossal nucleus. Additionally, these mice have PPG neurons in the lumbar sacral spinal cord [24] and the granule cell layer of the olfactory bulb [25]. As has been suggested for the olfactory bulb PPG neurons in rat, these appear to be granule cells and thus local interneurons. Consequently, it is expected also in mouse, that all PPG cell projections, which are primarily to autonomic control areas, originate from brainstem PPG neurons. Tracing studies in rat thus far suggest no functional segregation between NTS and reticular PPG neurons [15,18].
In the absence of reliable antibodies for the GLP-1 receptor, the targets for GLP-1 in the brain have been mapped in rat either by identifying GLP-1 binding sites [26] or by in situ hybridisation for GLP-1 receptor mRNA [17]. GLP-1 receptors are found throughout the entire rostrocaudal extent of the CNS, from the olfactory bulb down to lamina 5–10 in the sacral spinal cord [17]. However, notable exceptions are cerebral cortex and cerebellum which are devoid of GLP-1 receptors. Interestingly, GLP-1 neurons do not project to these brain structures in rat or mouse [10,14,22•]. This good correlation between the expression of GLP-1 receptors and the presence of fibres from GLP-1-expressing neurons is also seen at a regional level; for example, within hypothalamus the arcuate nucleus receives many GLP-1-positive fibres and expresses high levels of GLP-1 receptor, whereas the neighbouring ventromedial nucleus has low levels of both [17,22•]. Similarly, Manton et al. [24] have recently reported that GLP-1 axons are found throughout the entire rostrocaudal extent of the ventral spinal cord in the PPG-YFP mouse with the highest density of terminals in lamina X and the intermediolateral nucleus (IML). These data correlate very well to the GLP-1 receptor expression pattern described by Merchenthaler et al. [17] in rat.
However, whilst Merchenthaler et al. report a moderate level of GLP-1 receptors within the caudal hippocampus of rat, no innervation of this area is seen in mouse [22•]. Given that effects of exogenous GLP-1 injection into the hippocampus have been observed [27,28] this raises the question of whether these receptors are activated under physiological conditions, and if so where would the GLP-1 originate from? One possible origin could be microglia involved in the response to inflammation of the brain. It has been reported that activated microglia express GLP-1, at least in culture [29]. The physiological relevance of this GLP-1 expression, though, remains to be established.
In conclusion, these anatomical findings suggest that projections from PPG neurons are appropriately placed to elicit effects on the vast majority of GLP-1 receptors expressed in the CNS.
Central application of GLP-1 receptor antagonists to explore the role of endogenous GLP-1 in brain
Most studies to date addressing the physiological role of central GLP-1 receptors have employed intracerebroventricular (i.c.v.) injections of high concentrations of GLP-1 or a GLP-1 receptor agonist. These studies have revealed a plethora of responses, such as suppression of food intake, improved blood glucose levels, nausea, increased taste aversion, alterations in blood pressure and heart rate, hypothermia, neuroprotection and effects on learning and memory [15,28,30–35].
Whilst these studies demarcate the potential scope for effects of endogenous GLP-1 released from PPG neurons, the question remains, whether some of the effects observed are due to supraphysiological concentrations of GLP-1 producing a pattern of GLP-1 receptor activation that would not occur under physiological conditions. This question is best addressed by the injection of a GLP-1 receptor antagonist into brain in the absence of any exogenous GLP-1 or GLP-1 analogue.
In their landmark paper, more than 15 years ago, Turton and colleagues [36••] demonstrated that not only does i.c.v. injection of GLP-1 produce a reduction in food intake in rat but also that i.c.v. injection of the truncated exendin fragment (9–39; Ex9), a GLP-1 receptor antagonist, strongly increased food intake and body weight in satiated animals whilst having no effect on starved rats [36••,37] (see also [38]). This demonstrated not only a physiological role for endogenous GLP-1, but also showed that endogenous release varies with the animal's feeding state.
Several GLP-1 receptor antagonists [39] have been used to explore the role of endogenous GLP-1 in brain. Most of these studies have focused on food intake, showing that central administration of GLP-1 receptor antagonists can cause increased feeding in stressed animals, attenuate c-fos expression in the brainstem and decrease LiCl-induced anorexia and stress hormone levels [40–43]. Others have shown reduced glucose tolerance [44•] or impaired the insulin-dependent suppression of hepatic glucose production [45] when central GLP-1 receptors were blocked. However, whilst these studies indicate a physiological role for endogenous GLP-1 in brain, i.c.v. injection of these antagonists has precluded dissection of the specific neuronal populations involved. To overcome this limitation, several recent papers used site-specific injections into the brain parenchyma to allow the examination of specific brain nuclei [19••,20••,38,46,47••].
Delineating ascending pathways: injection of antagonists into forebrain sites
Most of these studies have focused on the dissection of GLP-1 effects on food intake. Schick et al. demonstrated that lateral hypothalamic microinjections of Ex9 significantly augmented food intake in satiated rats only [38]. More recent studies were designed to dissect suppression of metabolically driven food intake from conditioned taste aversion and from reward system driven appetite. These papers explored the influence of the nucleus accumbens (NAc) and the ventral tegmental area (VTA) known to be involved with reward and motivation [19••,20••,46,48] (Figure 1). Specific targeting of the NAc core rather than the shell region with Ex9 was shown to increase food intake up to 2 hours after animals entered the dark phase of the circadian cycle [19••]. Similarly, a unilateral injection of Ex9 into the VTA increased high fat diet intake in rats at 3 and 6 hours post-injection [20••]. Further analysis of the effects of GLP-1R blockade in the NAc core on the intake of palatable food suggested that GLP-1 receptor activation in the NAc affects meal size rather than meal frequency [46] whereas the opposite was observed for GLP-1 receptor activation in the hindbrain [47••].
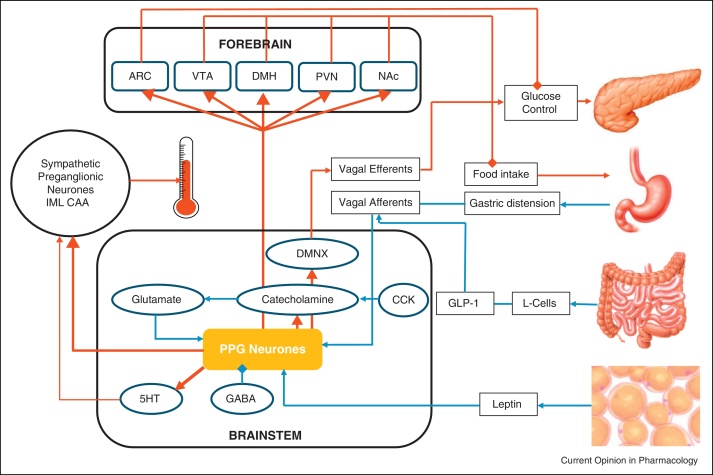
Figure 1.
Physiologically relevant inputs to and projections from brainstem PPG neurons. Brainstem PPG neurons receive inputs (blue) related to short-term and long-term energy status. Inputs include electrical satiety signals via the vagal nerve from the stomach and gut, hormonal signals like CCK from the gut, or leptin from adipose tissue. Outputs (red) from these neurons are directed towards various forebrain sites with emphasis on food intake and glucose control. Local and descending outputs from these neurons travel to dorsal vagal efferent neurons, serotonergic (5-HT) neurons and catecholaminergic neurons in the NTS and ventrolateral medulla. These outputs might be involved in the regulation of blood glucose in the case of vagal neurons and thermoregulation for 5-HT neurons. Additionally, there are strong direct projections from PPG neurons to sympathetic preganglionic neurons in the central autonomic area (CAA) and the intermediolateral cell column (IML) in the spinal cord. ARC, arcuate nucleus; VTA, ventral tegmental area; DMH, dorsomedial hypothalamus; PVN, paraventricular nucleus; NAc, nucleus accumbens; DMNX, dorsal motor nucleus of the vagus; CCK, cholecystokinin.
Descending pathways: injection of antagonists into brainstem
Early evidence that hindbrain GLP-1 receptors are involved in different aspects of food intake than those in the hypothalamus was provided by Grill et al. [49] who showed that lipopolysaccharide (LPS) anorexia is alleviated by blocking GLP-1 receptors accessible from the 4th, but not the 3rd, ventricle. Subsequently, it was shown that both 4th ventricular and local caudal NTS delivery of Ex9 increased food intake in satiated rats, indicating a role for hindbrain GLP-1 receptors in ‘metabolic’ food intake [47••]. The same study also demonstrated that the reduction in food intake caused by gastric distension is reversed by 4th ventricular Ex9, but not when caused by duodenal nutrient infusion [47••]. These findings suggest that it is unlikely to be duodenally released GLP-1 entering the brainstem that activates the GLP-1 receptors, but rather electrical signals (presumably vagal) from the stomach that activate PPG neurons in the NTS. These in turn release GLP-1 locally, leading to the observed effects (Figure 1).
At present, little is known about which cell types in the lower brainstem express GLP-1 receptors, and thus what downstream pathways are likely to be involved. A recent study has identified some of the cell types receiving close appositions from PPG axons within the brainstem [21•]. These include about 30% of cholinergic dorsal vagal neurons, a similar proportion of catecholaminergic A1/C1 and A2/C2 neurons, and the majority of serotonergic neurons in the raphe pallidus and the parapyramidal tract. These cell populations would provide both descending and ascending projections that could potentially account for effects on food intake, thermoregulation, blood pressure, heart rate, insulin release, among others.
What inputs do GLP-1 neurons receive?
Until the development of the PPG-YFP mouse by Reimann and colleagues [23•], PPG neurons could only be identified post hoc by immunocytochemistry. This limited functional analysis of this cell population to the use of immunoreactivity to c-fos or equivalent markers of neuronal activation [15,50,51]. Such studies demonstrated that PPG neurons were activated by gastric distension [51], leptin [52], LiCl and oxytocin [53], placing the PPG neurons at the core of central GLP-1 effects observed in relation to these stimuli.
The PPG-YFP mouse allowed identification of PPG neurons in living tissue and in the first study that directly recorded the electrical activity of GLP-1-expressing cells, Hisadome and colleagues discovered that leptin directly depolarises these neurones in the nucleus of the solitary tract (NTS) and that, whilst these neurons do not express GLP-1 receptors, at least a proportion of PPG neurons receive monosynaptic input from the solitary tract [54••] (i.e. vagal afferent fibres). These results further supported the notion that gut-derived GLP-1 would act in the periphery, rather than directly on PPG neurons in order to elicit central GLP-1 release (Figure 1). A recent study on human subjects that had undergone truncal vagotomy further supports this hypothesis [55]. Subsequently, Hisadome et al. reported that CCK and noradrenaline increased the activity of GLP-1-expressing neurons by enhancing glutamatergic drive [56] (Figure 1). These results demonstrated that GLP-1 neuronal activity is modulated by both long-term and short-term satiety signals. It now remains to be established whether there is a separation of these neurons into discrete subpopulations that responds to either short-term or long-term signals, and similarly whether the projection targets for the individual PPG neurons correlate with the specific inputs they receive.
Can we interfere with the function of GLP-1 neurons in vivo, and what are the consequences?
Finally, in order to unequivocally determine the importance of the central GLP-1 system, it needs to be completely separated from the peripheral system. There are two key questions to be answered. Firstly, are central GLP-1 receptors only accessible for CNS derived GLP-1, and secondly, do the hindbrain GLP-1 neurons fulfil this role? The pharmacological studies employing the central injection of GLP-1 receptor antagonists address these questions only partially, because whilst they demonstrate the action of endogenous GLP-1, they cannot rule out the possibility that gut-derived GLP-1 is responsible for the observed effects on central GLP-1 receptors. Similarly, the global knockout of either the glucagon gene, or the GLP-1 receptor gene, affects both central and peripheral systems. Additionally, such a genetic approach is prone to developmental compensation. To circumvent such problems, Barrera et al. [57••] employed RNA interference, delivered by stereotaxic injection of lentivirus into the NTS, to knock down GLP-1 expression. With this approach they achieved a reduction of preproglucagon mRNA levels by 50% in NTS and by 30% in the PPG cell terminals in PVN. They observed hyperphagia and weight gain compared to control animals that received injections of scrambled shRNA. However, these controls only regained preoperative weight 28 days after surgery. Nevertheless, these data indicate that endogenous GLP-1 derived from PPG cells has a physiological role in the regulation of energy balance and it is experiments like these that will hopefully give us a more complete and detailed picture of the physiological importance of the PPG neurons over the coming years.
Conclusions
We suggest that the effects of neuropeptide GLP-1 (released by PPG neurons) are distinct from the effects of incretin GLP-1 (released by enteroendocrine cells) and that the PPG neurons constitute a central signalling network that integrates peripheral and central signals for both long and short term nutritional and digestional status. GLP-1 neurons might produce an output signal to feeding and autonomic circuits which optimises digestion and assimilation of nutrients and regulates calorific intake.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Work in our laboratory is supported by the Medical Research Council (MRC) UK, Diabetes UK and the European Foundation for the Study of Diabetes (EFSD). We would also like to thank Dr Simon Cork for valuable discussions and creative input.
References
- 1.De Silva A. The gut hormones PYY 3–36 and GLP-1 7–36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14:700–706. doi: 10.1016/j.cmet.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy K.G., Bloom S.R. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 3.Vrang N., Larsen P.J. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: role of peripherally secreted and centrally produced peptides. Prog Neurobiol. 2010;92:442–462. doi: 10.1016/j.pneurobio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Lachey J.L. The role of central glucagon-like peptide-1 in mediating the effects of visceral illness: differential effects in rats and mice. Endocrinology. 2005;146:458–462. doi: 10.1210/en.2004-0419. [DOI] [PubMed] [Google Scholar]
- 5.Huo L. Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology. 2008;149:492–497. doi: 10.1210/en.2007-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kieffer T.J., Habener J.F. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 7.Vrang N., Grove K. The brainstem preproglucagon system in a non-human primate (Macaca mulatta) Brain Res. 2011;1397:28–37. doi: 10.1016/j.brainres.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Barrera J.G. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol. 2011;7:507–516. doi: 10.1038/nrendo.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trapp S., Hisadome K. Glucagon-like peptide 1 and the brain: central actions-central sources? Auton Neurosci. 2011;161:14–19. doi: 10.1016/j.autneu.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Jin S.L. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol. 1988;271:519–532. doi: 10.1002/cne.902710405. [DOI] [PubMed] [Google Scholar]
- 11.Conlon J.M. Glucagon-like polypeptides in canine brain. Diabetes. 1979;28:700–702. doi: 10.2337/diab.28.7.700. [DOI] [PubMed] [Google Scholar]
- 12.Loren I. Gut-type glucagon immunoreactivity in nerves of the rat brain. Histochemistry. 1979;61:335–341. doi: 10.1007/BF00508455. [DOI] [PubMed] [Google Scholar]
- 13.Drucker D.J., Asa S. Glucagon gene expression in vertebrate brain. J Biol Chem. 1988;263:13475–13478. [PubMed] [Google Scholar]
- 14.Larsen P.J. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 15.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999;277:R582–R590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- 16.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merchenthaler I., Lane M., Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Vrang N. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res. 2007;1149:118–126. doi: 10.1016/j.brainres.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Dossat A.M. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31:14453–14457. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; First study to show a direct link between NTS PPG neurons and modulation of food intake involving the nucleus accumbens.
- Alhadeff A.L., Rupprecht L.E., Hayes M.R. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153:647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study establishes the ventral tegmental area as another part of the mesolimbic system that is modulated by GLP-1 input from the NTS.
- Llewellyn-Smith I.J. Preproglucagon (PPG) neurons innervate neurochemically identified autonomic neurons in the mouse brainstem. Neuroscience. 2013;229:130–143. doi: 10.1016/j.neuroscience.2012.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pinpoints potential anatomical correlates for the action of brain derived GLP-1 within the brainstem.
- Llewellyn-Smith I.J. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111–121. doi: 10.1016/j.neuroscience.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]; Description of PPG neurons and their projections in mouse. Utilises a transgenic YFP-mouse and thus shows complete cytosolic staining of PPG neurons, including dendrites, axons and terminals.
- Reimann F. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Generated the PPG-YFP mouse.
- 24.Manton R. Preproglucagon neurons innervate spinal autonomic neurons. Proc Physiol Soc. 2012;27:PC134. [Google Scholar]
- 25.Thiebaud N. Expression and activity of glucagon-like peptide 1 in the mouse olfactory bulb. Association of Chemoreception Sciences (AChemS) Chemical Senses. 2013:64. abstract #P110 p. [Google Scholar]
- 26.Goke R. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 27.McClean P.L. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci. 2011;31:6587–6594. doi: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.During M.J. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 29.Iwai T. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res. 2006;55:352–360. doi: 10.1016/j.neures.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Knauf C. Role of central nervous system glucagon-like peptide-1 receptors in enteric glucose sensing. Diabetes. 2008;57:2603–2612. doi: 10.2337/db07-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knauf C. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest. 2005;115:3554–3563. doi: 10.1172/JCI25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabou C. Brain glucagon-like peptide-1 regulates arterial blood flow, heart rate, and insulin sensitivity. Diabetes. 2008;57:2577–2587. doi: 10.2337/db08-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto H. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. Am J Physiol. 1999;277(Pt 2):R1537–R1540. doi: 10.1152/ajpregu.1999.277.5.R1537. [DOI] [PubMed] [Google Scholar]
- 35.Hayes M.R., Skibicka K.P., Grill H.J. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149:4059–4068. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton M.D. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]; The first comprehensive study demonstrating that both exogenous and endogenous GLP-1 in the brain reduces food intake. Also demonstrated that endogenous release is only seen in satiated rats.
- 37.Meeran K. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology. 1999;140:244–250. doi: 10.1210/endo.140.1.6421. [DOI] [PubMed] [Google Scholar]
- 38.Schick R.R. Peptides that regulate food intake: glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1427–R1435. doi: 10.1152/ajpregu.00479.2002. [DOI] [PubMed] [Google Scholar]
- 39.Montrose-Rafizadeh C. High potency antagonists of the pancreatic glucagon-like peptide-1 receptor. J Biol Chem. 1997;272:21201–21206. doi: 10.1074/jbc.272.34.21201. [DOI] [PubMed] [Google Scholar]
- 40.Thiele T.E. Central infusion of glucagon-like peptide-1-(7–36) amide (GLP-1) receptor antagonist attenuates lithium chloride-induced c-Fos induction in rat brainstem. Brain Res. 1998;801:164–170. doi: 10.1016/s0006-8993(98)00584-8. [DOI] [PubMed] [Google Scholar]
- 41.Seeley R.J. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci. 2000;20:1616–1621. doi: 10.1523/JNEUROSCI.20-04-01616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tauchi M. Role of central glucagon-like peptide-1 in hypothalamo–pituitary–adrenocortical facilitation following chronic stress. Exp Neurol. 2008;210:458–466. doi: 10.1016/j.expneurol.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinzig K.P. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23:6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval D.A. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046–2054. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrated a clear link between GLP-1 receptor activation in the hypothalamic arcuate nucleus and blood glucose homeostasis.
- 45.Burmeister M.A. Acute activation of central GLP-1 receptors enhances hepatic insulin action and insulin secretion in high-fat-fed, insulin resistant mice. Am J Physiol Endocrinol Metab. 2012;302:E334–E343. doi: 10.1152/ajpendo.00409.2011. [DOI] [PubMed] [Google Scholar]
- 46.Dossat A.M. Nucleus accumbens GLP-1 receptors influence meal size and palatability. Am J Physiol Endocrinol Metab. 2013;67:E1314–E1320. doi: 10.1152/ajpendo.00137.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M.R., Bradley L., Grill H.J. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150:2654–2659. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrated the physiological importance of GLP-1 action within the brainstem.
- 48.Dickson S.L. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32:4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grill H.J. Attenuation of lipopolysaccharide anorexia by antagonism of caudal brain stem but not forebrain GLP-1-R. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1190–R1193. doi: 10.1152/ajpregu.00163.2004. [DOI] [PubMed] [Google Scholar]
- 50.Maniscalco J.W., Rinaman L. Overnight food deprivation markedly attenuates hindbrain noradrenergic, glucagon-like peptide-1, and hypothalamic neural responses to exogenous cholecystokinin in male rats. Physiol Behav. 2013;121:35–42. doi: 10.1016/j.physbeh.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vrang N. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol. 2003;285:R470–R478. doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- 52.Elias C.F. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol. 2000;423:261–281. [PubMed] [Google Scholar]
- 53.Rinaman L., Rothe E.E. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R99–R106. doi: 10.1152/ajpregu.00008.2002. [DOI] [PubMed] [Google Scholar]
- Hisadome K. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like peptide 1 neurons. Diabetes. 2010;59:1890–1898. doi: 10.2337/db10-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first study to show the electrical functional properties of NTS PPG neurons. Also demonstrated that PPG neurons do not express GLP-1 receptors.
- 55.Plamboeck A. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1117–G1127. doi: 10.1152/ajpgi.00035.2013. [DOI] [PubMed] [Google Scholar]
- 56.Hisadome K. CCK stimulation of GLP-1 neurons involves {alpha}1-adrenoceptor-mediated increase in glutamatergic synaptic inputs. Diabetes. 2011;60:2701–2709. doi: 10.2337/db11-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera J.G. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci. 2011;31:3904–3913. doi: 10.1523/JNEUROSCI.2212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study constitutes the first successful attempt of selectively modulating endogenous GLP-1 release in brain in vivo.