Abstract
The novel guanidine compounds trans-[Pt(NH2Me)2{NH=C(NHMe)NR}2](Cl)2 (R = NEt2 [7], NC5H10 [8]) (trans-7,8) were synthesized by the nucleophilic addition of methylamine to dialkylcyanamide ligands of the push–pull nitrile complexes trans-[PtCl2(RCN)2] (R = NEt2, NC5H10). In vitro cytotoxicity tests conducted for the entire series of the guanidine complexes, i.e. trans-7,8, the neutral cis- or trans-[PtCl2{NH=C(NH2)R}2] (cis-1–3 and trans-1–3) and the cationic cis- or trans-[Pt(NH3)2{NH=C(NH2)R}2](Cl)2 (cis-4–6 and trans-4–6) (R = NMe2 [1,4], NEt2 [2,5], NC5H10 [3,6]) in two human cancer cell lines, CH1 (ovarian carcinoma) and SW480 (colon cancer), confirmed that the cytotoxicity of several trans-configured (trans-3,6) complexes is higher than that of cis-congeners (cis-3,6). Cellular platinum levels were analyzed by inductively coupled plasma mass spectrometry upon treatment of SW480 cells, revealing a dependence of cellular accumulation on the geometrical isomerism and the steric hindrance of the variable substituent R on the guanidine ligand. DNA interactions of selected guanidine complexes were studied in order to find hints for the possible reasons for their different activities. Changes induced to the electrophoretic mobility of a dsDNA plasmid confirmed the potency of the guanidine complexes (e.g. trans-1,3,5,6 and cis-1,3,4) to significantly alter DNA secondary structure, indicating DNA as a possible critical target of these compounds.
Keywords: Anticancer drug, Guanidine complexes, Cellular accumulation, DNA interactions, Structure–activity relationship
Graphical abstract
Structure–activity relationships, cellular accumulation and DNA interactions of a group of novel guanidine–platinum(II) complexes were studied. Thereby, some of them were identified as new examples of active platinum complexes with trans geometry, extending the knowledge on potential non-classic platinum drugs.
Highlights
-
•
A series of guanidine–Pt(II) complexes was extended with two new compounds.
-
•
Structure–activity relationships were inferred from cell culture studies.
-
•
New examples of active platinum complexes with trans geometry were identified.
1. Introduction
The three globally approved platinum based complexes – cisplatin, oxaliplatin, and carboplatin – play a major role in cancer chemotherapy [1]. The fundamental complex of the class is cis-[PtCl2(NH3)]2 (cis-DDP, cisplatin), which is supposed to exert its antitumor activity by distorting DNA functionality [2], [3]. However, along with a high curative potential in certain malignancies, there are principal drawbacks of cisplatin and its analogs such as undesirable side effects, mutagenic effects, and the occurrence of drug resistance [4], [5], [6]. The success of cisplatin has imposed a certain direction on the investigation of novel substances, in the first instance excluding compounds that may possess different and maybe unique modes of action. Today many active non-classic compounds, which violate the long accepted structure–activity relationships [7] for platinum based drugs [8], [9], [10], [11], are known from the literature. Above all, the presence of two monodentate or one bidentate exchangeable ligand(s) (commonly called leaving groups) coordinated in cis-geometry has been declared a requirement for antitumor activity. The significantly lower cytotoxicity of transplatin compared to that of cisplatin in vitro as well as its inactivity in vivo has been ascribed to its disfavored chelation reactions with DNA molecules due to the trans-standing leaving groups [12].
Nevertheless, numerous exceptions from the classic rules have been found in the last several years. The classes of trans-complexes exhibiting at least equal or even higher cytotoxicity than the corresponding cis-isomers are platinum(IV) species bearing pyridine-like ligands [13], platinum(II) complexes with iminoethers [14], [15], and platinum(II) compounds featuring piperidine or piperazine ligands [16], [17]. For the above groups, antitumor activity was confirmed by in vivo experiments in mice models. Other non-classic complexes are platinum(II) oxime species [18], [19], compounds bearing planar [20] and aliphatic amines [21], complexes containing various phosphoric groups [22], and complexes featuring the acetimine ligand [23]. Most of the listed platinum species are equally or even more cytotoxic than cisplatin.
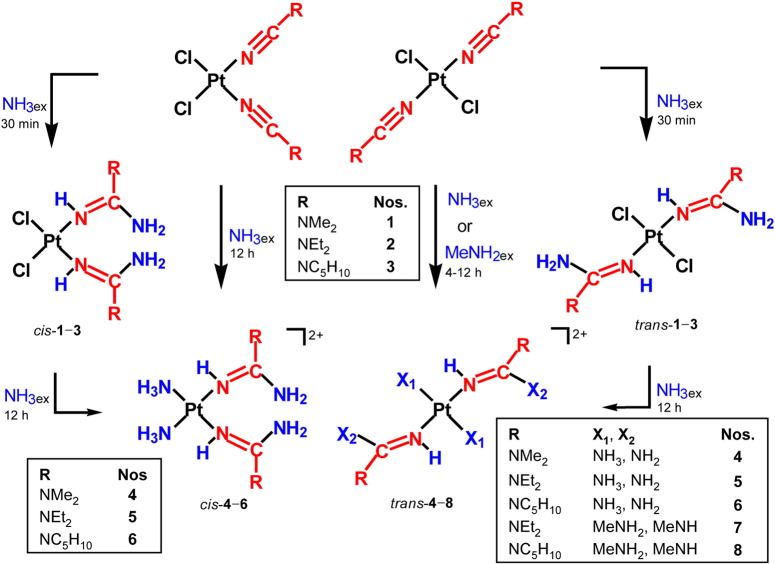
In this work, we studied (guanidine)2PtII complexes (Scheme 1), prepared by the nucleophilic addition of ammonia to the push–pull dialkylcyanamide ligands in cis- and trans-[PtCl2(RCN)2] (R = NMe2, NEt2, NC5H10). Neutral complexes cis-1–3 and trans-1–3 as well as cationic complexes cis-4–6 and trans-4–6, which contain NH3 instead of Cl− ligands, were synthesized and characterized previously [24]. Additionally, we have now synthesized the cationic guanidine compounds trans-[Pt(NH2Me)2{NH=C(NHMe)NR}2](Cl)2 (R = NEt2, NC5H10) (trans-7,8) by the nucleophilic addition of methylamine to dialkylcyanamides in the platinum(II) complexes trans-[PtCl2(RCN)2] (R = NEt2, NC5H10). Compounds trans-7,8 were purified and characterized by elemental analyses, electrospray ionization mass spectrometry (ESI-MS), IR, 1H and 13C{1H} NMR spectroscopy. The biological activity of the entire series of the guanidine species (cis-1–6 and trans-1–8) was studied, and the obtained results give novel insights into potential of non-classic platinum drugs.
Scheme 1.
Synthesis of guanidine platinum(II) complexes.
2. Experimental section
2.1. Synthesis of starting complexes and cis/trans-1–6
Solvents were obtained from commercial sources and used without further purification and drying. The starting dialkylcyanimide platinum(II) complexes, [PtCl2(Et2NCN)2] and [PtCl2(C5H10NCN)2], were prepared according to the published methods [25] as mixtures of cis- and trans-isomers that were separated by column chromatography on SiO2 Merck 60 F254 (eluent: Me2CO:CHCl3 = 1:10, v/v). The synthesis of complexes cis/trans-1–6 was reported by Tyan et al. [24].
2.2. Synthesis of complexes trans-7,8
Complexes were synthesized via the amination of undried CH2Cl2 solutions (2 mL) of trans-[PtCl2(Et2NCN)2] (0.205 mmol; 120 mg) and trans-[PtCl2(C5H10NCN)2] (0.213 mmol; 130 mg) by gaseous methylamine (10-fold excess) for 4–5 h at room temperature (RT), which resulted in the formation of the cationic trans-[Pt(NH2Me)2{NH = C(NHMe)NR}2](Cl)2 (R = NEt2, NC5H10) (trans-7,8) species (Scheme 1). Discoloration of the initially yellow solutions and the precipitation of the colorless products were observed. The precipitates were separated by filtration and dried in air at RT; yields 87% (125.2 mg) and 83% (135.2 mg) for trans-7,8, respectively. Both complexes are well soluble in water and cell culture medium (viz. MEM).
trans-[Pt(NH2Me)2{NH = C(NHMe)N(Et)2}2]Cl2 (7) Anal. Calcd for C14H40N8Cl2Pt: C, 28.67; H, 6.87; N, 19.11. Found: C, 28.42; H, 6.90; N, 19.37. ESI+-MS, m/z (M refers to the molecular weight of C14H40N8Cl2Pt): 1137.49 [2M–Cl]+ (calc.: 1137.50), 551.26 [M–Cl]+ (calc.: 551.27), 520.22 [M–Cl–NH2Me]+ (calc.: 520.22), 452.20 [M–2NH2Me–2Cl–H]+ (calc.: 452.20), 257.65 [M–2Cl]2 + (calc.: 257.65). IR (KBr, selected bands, cm− 1): 3269 (m), 3194 (m), 3092 (m), ν(N—H); 2970 (m-w), 2936 (m-w), 2888 (m-w), ν(C—H); 1591 (s), ν(C N); 1282 (m-w), ν(C—N). 1H NMR [CDCl3, δ]: 7.43 (s, br, H, NH—), 5.58 (s, br, H, —NH—), 4.88 (s, br, 2H, —NH2—), 3.31 (q, 3JHH 6.9 Hz, 4H, —CH2— [in NEt2]), 3.01 (d, 3JHH 4.5 Hz, 3H, —CH3 [in NHMe]), 2.30 (t, 3JHH 6.2 Hz, 3H, —CH3 [in NH2Me]), 1.14 (t, 3JHH 6.9 Hz, 6H, —CH3 [in NEt2]). 13C{1H} NMR [CDCl3, δ]: 164.72 (C N), 43.61 (—CH2— [carbons in NEt2]), 33.18, 31.62 (—CH3 [carbons in NHMe/NH2Me]), 13.53 (—CH3 [carbons in NEt2]).
trans-[Pt(NH2Me)2{NH = C(NHMe)N(C5H10)}2]Cl2 (8) Anal. Calcd for C16H40N8Cl2Pt: C, 31.47; H, 6.60; N, 18.35. Found: C, 31.14; H, 6.71; N, 18.46. ESI+-MS, m/z (M refers to the molecular weight of C16H40N8Cl2Pt): 1185.49 [2M–Cl]+ (calc.: 1185.50), 575.26 [M–Cl]+ (calc.: 575.27), 544.22 [M–Cl–NH2Me]+ (calc.: 544.22), 538.27 [M–2Cl–H]+ (calc.: 538.27), 269.64 [M–2Cl]2 + (calc.: 269.65). IR (KBr, selected bands, cm− 1): 3418 (m), 3277 (m), 3089 (m), ν(N—H); 2936 (m-w), 2854 (m-w), ν(C—H); 1584 (s), ν(C N); 1256 (m-w), ν(C—N). 1H NMR [CDCl3, δ]: 7.62 (s, br, H, NH—), 5.42 (s, br, H, —NH—), 4.81 (s, br, 2H, —NH2—), 3.22 (s, 4H, α-CH2— [in NC5H10]), 2.95 (d, 3H, —CH3 [in NHMe]), 2.28 (t, 3H, 3JHH 6.3 Hz, —CH3 [in NH2Me]), 1.59 (s, br, 6H, β, γ-CH2— [in NC5H10]). 13C{1H} NMR [CDCl3, δ]: 166.07 (C N), 49.55 (α-CH2— [carbons in NC5H10]), 32.96, 31.36 (—CH3 [carbons in NHMe/NH2Me]), 26.10, 24.55 (β, γ-CH2— [carbons in NC5H10]).
2.3. Characterization of trans-7,8
Elemental analyses were carried out at the Department of Organic Chemistry, Saint Petersburg State University on a 185 V Hewlett Packard Carbon Hydrogen Nitrogen Analyzer. Infrared spectra (4000–400 cm− 1) were recorded on a Shimadzu FTIR-8400S spectrophotometer in KBr pellets.
1H and 13C{1H} NMR spectra were measured on a Bruker DPX 400 spectrometer at ambient temperature. Chemical shifts were measured relatively to the signals of the solvent CDCl3 (7.27 in the 1H NMR spectra and 77.4 in 13C{1H} NMR spectra).
Electrospray mass spectra were recorded on a Bruker micrOTOF instrument, equipped with an electrospray ion source type (ESI− or ESI+). For the mass spectrometric studies samples were dissolved in MeCN with an upper boundary of the concentration of 5–15 mg/mL. The resulting solution was supplied to the electrospray capillary by using a KDScientific syringe pump. The voltage generated at an electrode and the voltages on the capillary were − 500 V and − 4500 V (ESI+-MS) or 3500 V (ESI−-MS), respectively. The sample solution flow through the electrospray capillary was 3 mL/min. The output voltage of the capillary was ± 70 or ± 150 V. ESI+ and ESI− mass spectra were recorded in the range of 50 to 3000 m/z. Drying gas pressure in the spray was 0.4 bar, flow rate and temperature of the drying gas were 4.0 L/min and 180 °C, respectively.
2.4. Cell lines and growth conditions
Two human cancer cell lines were used for the studies, viz. CH1 (ovarian carcinoma) and SW480 (colon carcinoma), kindly provided by Lloyd R. Kelland (CRC Centre for Cancer Therapeutics, Institute of Cancer Research, Sutton, UK) and Brigitte Marian (Institute of Cancer Research, Department of Medicine I, Medical University of Vienna, Austria), respectively. Cells were grown in 75 cm2 culture flasks (Iwaki/Asahi Technoglass, Gyouda, Japan) as adherent monolayer cultures in complete medium [i.e., minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum, 1 mM sodium pyruvate, 4 mM l-glutamine, and 1% nonessential amino acids from 100× ready-to-use stock (all purchased from Sigma-Aldrich, Austria)]. Cell cultures were incubated at 37 °C in a moist atmosphere containing 5% CO2.
2.5. Cytotoxicity in cancer cell lines
The cytotoxic activity of test compounds was determined by using the colorimetric microculture MTT assay (MTT = 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide). CH1 and SW480 cells were collected from culture flasks by trypsinization and seeded in 100 μL aliquots in MEM into 96-well microculture plates (Iwaki/Asahi Technoglass, Gyouda, Japan) in cell densities of 3 × 103, 1 × 103 and 2.5 × 103 cells per well, respectively. After 24 h preincubation of the cells, the test compounds were dissolved and serially diluted in MEM, and 100 μL of solution were added per well. After continuous exposure for 96 h, drug solutions were replaced with 100 μL RPMI 1640 culture medium (supplemented with 10% heat-inactivated fetal bovine serum and 2 mM l-glutamine) plus 20 μL MTT solution in phosphate-buffered saline (5 mg/mL). After incubation for 4 h, the RPMI/MTT mixtures were removed, and the formazan crystals formed in viable cells were dissolved in 150 μL of DMSO per well. Optical densities were measured at a wavelength of 550 nm with a microplate reader (Tecan Spectra Classic), using a reference wavelength of 690 nm to correct for unspecific absorption. The quantity of viable cells was expressed by comparison of treated microcultures to untreated control microcultures, and 50% inhibitory concentrations (IC50) were calculated from concentration-effect curves by interpolation. Evaluation is based on means from at least three independent experiments, each comprising six microcultures per concentration level.
2.6. Cellular accumulation experiments
To determine the intracellular drug concentration, the method thoroughly described in Egger et al. [26] was used. Briefly, cells were seeded in 6-well plates (Iwaki/Asahi Technoglass, Gyouda, Japan) in a volume of 2.5 mL complete medium (MEM) in densities of about 3 × 105 cells per well. Cell microcultures were incubated in a moist atmosphere at 37 °C for 24 h prior to 2 h exposure to the test compounds. The number of viable cells was determined by using trypan blue (Sigma-Aldrich, Austria) exclusion in parallel microcultures during the exposure period. After exposure, the medium was removed, cells were washed with PBS and then lyzed with 0.5 mL sub-boiled HNO3 (conc.) per well for 1 h.
Cellular accumulation experiments in the non-adherent state were performed alternatively with those compounds that have a much stronger affinity to the polystyrene of the culture plates than to the cells (since non-adherent cells can easily be collected without detaching manipulations and transferred into other vessels prior to digestion, there is no need for measuring adsorption blanks). For this purpose, cells were harvested from the culture flasks, counted and transferred into 15 mL tubes in amounts of approximately 3 × 105 cells per tube (2.5 mL of cell suspension), which corresponds with the cell density in adherent-state experiments. Cells were exposed to the drugs for 2 h immediately after transfer, and the samples were shaken carefully every 15 min to keep the cells in suspension. After exposure, the cells were centrifuged, the medium was removed, cells were washed with PBS and then transferred into 1.5 mL tubes where they were lyzed with 0.5 mL sub-boiled HNO3 (conc.) per tube for 1 h. In all experiments, Pt concentrations were quantified by inductively coupled plasma mass spectrometry (ICP-MS) in the presence of 0.5 μM In standard. Results are based on three independent experiments, each consisting of three replicates.
2.7. Alterations in DNA secondary structure
To assess drug–DNA interactions, the pPUC19 DNA plasmid (Fermentas, UK) was incubated with test compounds for different periods of time (i.e., from 15 min to 3 h). Electrophoresis was performed in 1% agarose gel for 2 h at 80 V and 55 mA in 1× TBE buffer (0.45 M Tris borate, 0.01 M EDTA, pH 8.3, purchased from Eppendorf AG, Germany). PEQLAB electrophoresis chambers were used. Staining was performed in ethidium bromide (EtBr) solution in 1× TBE (0.2 μg/mL) for 20 min. Images were taken under UVE light in the GelDoc-It Imaging System (UVP).
3. Results
3.1. Cytotoxicity in cancer cells
A cisplatin sensitive ovarian carcinoma cell line (CH1) and intrinsically cisplatin resistant (but oxaliplatin sensitive) colon carcinoma cell line (SW480) were chosen for the experiments. The inhibitory potency of complexes cis-1–6 and trans-1–8 on proliferation and viability of these human cancer cells was characterized by using the spectrophotometric MTT assay (Table 1), and the following structure-activity relationships were inferred from the data.
Table 1.
IC50 values (μM; mean ± standard deviation) of cis-1–6 and trans-1–8 in two cancer cell lines (CH1 and SW480).
|
1 |
2 |
3 |
4 |
|||||
|---|---|---|---|---|---|---|---|---|
| cis | trans | cis | trans | cis | trans | cis | trans | |
| CH1 | 2.7 ± 0.4 | 5.2 ± 1.1 | 3.0 ± 0.4 | 23 ± 4 | 4.4 ± 0.8 | 7.4 ± 1.3 | 21 ± 6 | 210 ± 70 |
| SW480 | 36 ± 2 | 36 ± 10 | 32 ± 7 | 62 ± 5 | 32 ± 5 | 19 ± 3 | 370 ± 40 | > 640 |
| 5 | 6 | 7 | 8 | cisplatin | transplatin | |||
| cis | trans | cis | trans | trans | trans | |||
| CH1 | 39 ± 12 | 25 ± 4 | 40 ± 2 | 8.2 ± 2 | 140 ± 40 | 330 ± 60 | 0.14 ± 0.03 | 15 ± 2 |
| SW480 | 42 ± 6 | 110 ± 20 | 46 ± 2 | 55 ± 1 | 241 ± 3 | > 640 | 3.3 ± 0.4 | 19 ± 3 |
Cytotoxicities of the trans-configured complexes are mostly not higher than those of the corresponding cis-congeners with a few notable exceptions. The most active compound in the cell line SW480 is trans-3 with the most sterically encumbered substituent R (R = NC5H10, IC50 = 19 μM). Cytotoxic potency of this compound exceeds that of cis-3, which is one of the most active complexes with cis-configuration (cis-3, IC50 = 32 μM).
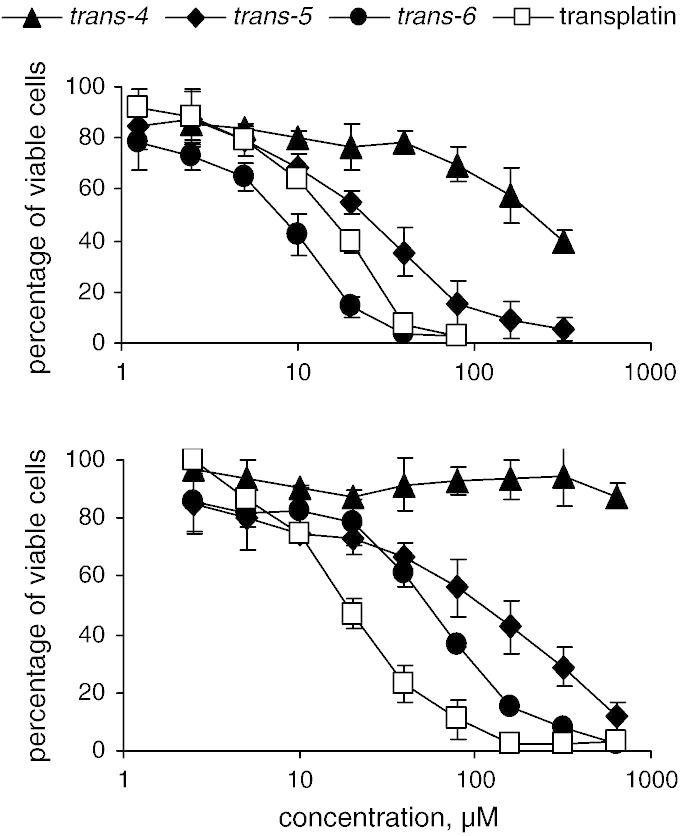
Among the cationic complexes, trans-5 and especially trans-6 exhibit lower IC50 values in CH1 cells than the corresponding cis-isomers. Furthermore, cytotoxic potencies of trans-4–6 correlate with the steric hindrance of substituents R, with bulkier groups (R = NC5H10 > NEt2 > NMe2) yielding higher cytotoxicities in both cell lines, approaching or even exceeding that of transplatin (Fig. 1). However, the cis-configured complexes are by one to two orders of magnitude less potent than cisplatin.
Fig. 1.
Concentration-effect curves of trans-4,5,6 and transplatin in CH1 (top) and SW480 (bottom) cells. Cytotoxicity correlates with the steric hindrance of the substituents R (R = NMe2 in trans-4, NEt2 in trans-5, NC5H10 in trans-6 and cis-6).
Curves for transplatin are taken from Ref. [10].
In general, cytotoxicity of neutral cis-/trans-1–3 is higher than that of cationic guanidine complexes cis-/trans-4–6, which confirms the higher potency of molecules containing labile ligands. However, the cytotoxic potency of trans-6 is reaching that of the corresponding neutral couple cis-/trans-3, despite the lack of distinctly labile ligands.
Cationic guanidine complexes trans-7,8 – derived from the nucleophilic addition of MeNH2 to the metal-bound dialkylcyanamides – have shown a rather moderate level of cytotoxicity in both cell lines, apparently indicating that the methyl substituted am(m)ine ligands are less favorable for the cytotoxic potency of guanidine platinum complexes.
3.2. Cellular accumulation
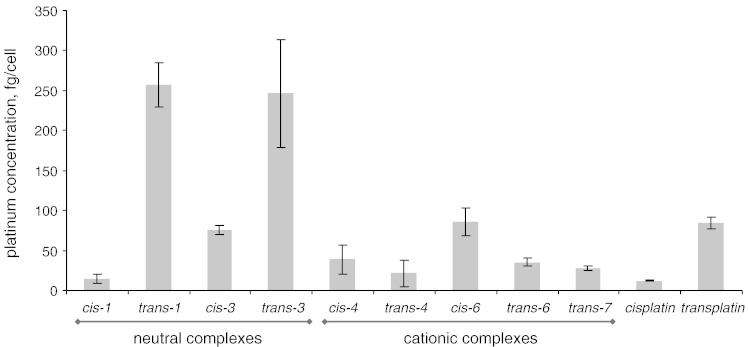
In order to figure out whether cytotoxicity correlates with cellular accumulation of the compounds, cellular platinum levels were analyzed by ICP-MS upon treatment of SW480 cells for 2 h with 50 μM of cis-1,3,4,6, trans-1,3,4,6,7, as well as the reference compounds, viz. cisplatin and transplatin (Fig. 2).
Fig. 2.
Cellular accumulation in adherent SW480 cells during 2 h exposure with 50 μM cis- and trans-configured Pt complexes (cis-1,3,4,6; trans-1,3,4,6,7; cisplatin, transplatin).
Values for cisplatin/transplatin are taken from Ref. [27].
The dependence of cellular accumulation on coordination geometry in the case of neutral guanidine complexes (cis-1,3, trans-1,3) correlates well with the reference couple (cisplatin, transplatin), with trans-configured compounds being accumulated more efficiently by the cells. However, the cytotoxicities of the trans-configured (trans-1,3) neutral guanidine complexes in SW480 cells are comparable or only slightly superior to those of their cis-isomers (cis-1,3). For the cationic guanidine complexes (cis-4,6, trans-4,6) the reverse relationship was observed.
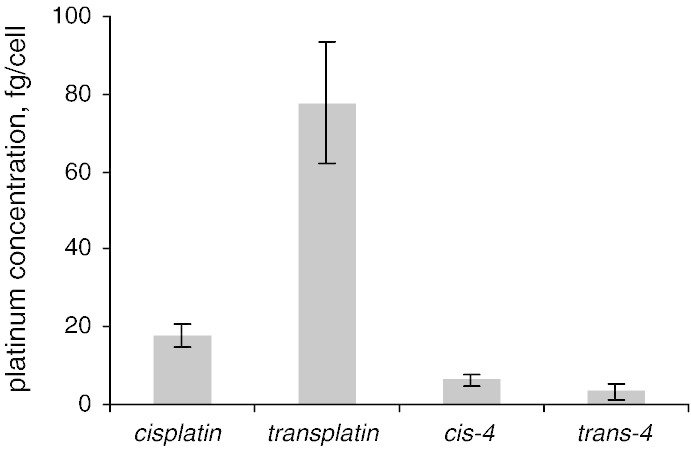
Experiments with adherent cell microcultures posed the problem that a few substances (cis-4, trans-4) displayed much higher affinity to the plastics of the dishes then to the cells. In these cases, the wells used as adsorption/desorption controls, which were exposed to the test substances, but were free from cells showed a high concentration of Pt. The latter indicates that substantial amounts of compounds were adsorbed to the surface of the wells and became randomly desorbed by the preparation, resulting in high deviations (Fig. 2). To overcome this problem, we performed experiments with non-adherent cells. This avoided desorption of adsorbed compounds by separating the exposure and lyzing steps from other by using using different vessels. The modified experiment gave an appropriate repeatability, and the values obtained for cisplatin and transplatin correspond well with those measured in the adherent state. For compounds cis-4 and trans-4, the same dependence on geometric isomerism, as in case of cis-/trans-6, was observed (Fig. 3).
Fig. 3.
Cellular accumulation of cis-4, trans-4 (50 μM, 2 h exposure) in non-adherent SW480 cells compared to the cisplatin/transplatin couple.
3.3. Alteration of DNA secondary structure
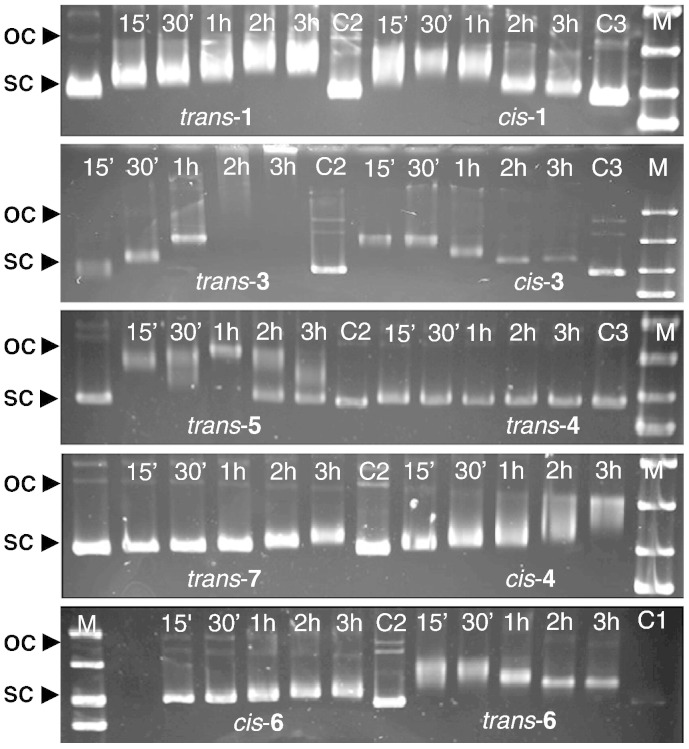
Since the difference in cytotoxic potency between cisplatin and transplatin has been ascribed to different capacities of forming particular DNA cross-links, DNA interactions of selected compounds were studied to find hints for possible reasons for their different activities. For this purpose, effects on electrophoretic mobility of a dsDNA plasmid, indicating the impact on DNA secondary structure in cell-free conditions, were investigated. The pUC19 plasmid was exposed to compounds for different time periods (15 min–3 h), and then the mobility of DNA was studied in an agarose gel. In particular, the retardation of the supercoiled form of the plasmid in the gel allows making conclusions about the capacity of forming adducts that lead to local unwinding of DNA. Monofunctional and bifunctional adducts can result in retardation of the plasmid DNA (due to higher mass, decrease of positive charge or untwisting of the supercoiled form) or in acceleration of the plasmid DNA due to bending and/or twisting. The composite action of all these factors leads to the observed pattern (Fig. 4).
Fig. 4.
Mobility of the plasmid pUC19 after the exposure to 100 μM of different cis-/trans- configured compounds. C —control, M —marker. Bright bands correspond to the supercoiled form (SC) of the plasmid, whereas bands of the open circular (OC) form are mostly too weak to allow interpretation.
Among all tested compounds, only trans-4 does not alter the electrophoretic mobility of the plasmid significantly, suggesting that if this compound interacts with DNA at all it does so without altering DNA secondary structure, excluding all forms of cross-links. This is consistent with the lack of marked cytotoxicity in the MTT assay (Table 1). All the other drugs are capable of DNA binding to varying degrees. Compound cis-1 causes a more pronounced retardation of electrophoretic migration of the supercoiled plasmid after shorter incubation than trans-1, suggesting faster and/or stronger untwisting. Furthermore, a reversion of this retardation (probably caused by counter-twisting from the negatively supercoiled through the untwisted to the positive supercoiled state) is already observable after 30 min exposure to cis-1, but not before 2 h exposure to trans-1. A similar dependence on geometric isomerism applies to the pair cis-3/trans-3. However, upon 3 h exposure to trans-3 the plasmid becomes immobile and remains in the starting well during electrophoresis. This might be caused by interhelical cross-linking of plasmids to larger aggregates, which are not able to enter the separation gel. Remarkably, cationic species trans-5–7 (bearing N-donor ligands only) also exhibit a capacity of altering DNA secondary structure, even though the lack of readily exchangeable ligands would suggest that DNA interaction of these compounds should primarily be of an electrostatic kind.
Generally, there is no direct correlation between the cytotoxicity and the alteration of dsDNA for most of the drugs with regard to time required and the extent of the shift. The cytotoxicity of complexes trans-4,5,6 increases with steric hindrance of the substituent R and partially correlates with the ability of altering DNA secondary structure. The most cytotoxic of these complexes (trans-5,6) were shown to exert faster and stronger effects on DNA, while the least cytotoxic (trans-4) does not have any significant impact on the secondary structure of plasmid DNA. Weak impact on plasmid DNA may explain the lower cytotoxicity of trans-7 and trans-4 compared to the other cationic complexes. In the case of trans-7, it might be due to steric hindrance in interacting with DNA rather than the extent of cellular uptake, which is similar to both the weakly cytotoxic trans-4 and rather cytotoxic trans-6.
4. Discussion
Compounds cis-1–6 and trans-1–8 belong to the new class of potential anticancer drugs based on guanidine (or, in a broader sense, amidine) platinum(II) complexes. For many of these amidine species, it was reliably shown that the cytotoxic activity of trans-complexes is comparable or superior to that of the corresponding cis-isomers [28], [29], [30]. Moreover, a similar dependence of cytotoxicity on geometric isomerism was observed in the case of bis(oxime)platinum(II) species [Pt(X)2(R2C=NOH)2](NO3)2 (R = Me, Et, and n-Pr; X = NH3, Cl) [18]. The bis(oxime) platinum complexes, structurally related to the guanidine complexes, albeit bearing different N-donor organic ligands, exhibit a very similar cytotoxicity pattern. The trans-configured neutral bis(oxime) complexes are mostly superior in cytotoxicity to their cis-congeners, while the cytotoxic potency of trans-configured cationic bis(oxime) complexes increases with the bulkiness of the substituent R. Similarly, there is a correlation between the cytotoxic potency of the guanidine compounds and the steric hindrance of the variable substituent R on the guanidine ligand. Cytotoxicities of the cationic complexes are in some cases comparable to those of the corresponding neutral complexes, despite the lack of distinctly labile ligands. The cytotoxic potencies of the tested complexes are inferior to that of cisplatin, but many of them have IC50 values in the same order of magnitude as carboplatin, another clinically active, approved platinum-based drug (compare with Ref. [31]).
The behavior of cationic trans-6, containing only N-donor ligands, is the most surprising with its high cytotoxicity comparable to that of the neutral species cis-/trans-1–3, which contain two easily exchangeable chlorido ligands. Both cellular accumulation and impact on DNA of trans-6 are discrepant from those of neutral trans-congeners (trans-1,3), raising the question whether this compound acts by a different mechanism. The presence of the N–H moieties in guanidine complexes entails pronounced abilities for various types of complementary H-bonding interactions when approaching DNA, which may in connection with coordinative binding lead to the formation of new types of adducts. A high cytotoxic potency was already shown for several similar examples of double charged cationic amidine compounds by other authors [18], [30].
To interpret the cellular accumulation results, several important aspects have to be taken into account. (i) On the one hand, the rotational symmetry of the trans-configured complexes implies the lack of polarity, making them more lipophilic. Thereby, the neutral trans-compounds probably have a higher ability to penetrate lipid membranes, which is consistent with their higher cellular accumulation observed experimentally. On the other hand, the cis-complexes possess a pronounced polarity, lower lipophilicity and apparently lower cellular permeability as a consequence. (ii) Another important difference between the cis- and trans-complexes is their three-dimensional geometry. While trans-complexes maintain the planar structure in solution, steric hindrance of the adjacent ligands in the cis-complexes can force their rotation out of the coordination plane. The planar structure is sterically more favorable for passage through membranes. (iii) The cationic am(m)ine complexes are kinetically inert, and the ligands can be hardly exchanged with H2O. Taking all these points into account, the higher cellular accumulation of the cis-configured cationic guanidine complexes in comparison with their trans-isomers is unexpected and requires further investigation. Concerning the difference in accumulation of complexes featuring the same geometry, we suggest that the permeability for complexes with more sterically encumbered substituents R is higher due to their pronounced lipophilicity.
Overall, nine of ten studied complexes were shown to be capable of altering the DNA secondary structure strongly (most likely by cross-linking), indicating DNA to be a possible target for the guanidine platinum(II) species. In both pairs of neutral complexes (cis/trans-1,3), the velocity and/or extent of structural changes imposed on plasmid DNA is dependent on the geometry of the complex. In accordance with the previously reported behavior of dichlorobis(acetoxime)platinum(II) complexes but in contrast to that of cis-/transplatin [10], the cis isomers are more effective in this respect. Steric constraints on platinum–DNA interactions imposed by the larger guanidine ligands (or acetoxime ligands, both as compared to ammine) might be a reason for slower kinetics of DNA binding in the case of trans-isomers. For the surprisingly strong impact of the cationic complexes trans-5 and trans-6 (containing only N-donor ligands supposed to be stably coordinated) on DNA secondary structure, there is no obvious explanation, however.
5. Conclusions
A series of guanidine platinum(II) complexes cis-/trans-[PtX2(RCN)2](Cl2) (R = NMe2, NEt2, NC5H10; X = NH3, Cl; cis-/trans-1–6), was extended with two novel compounds, viz. trans-[Pt(NH2Me)2{NH=C(NHMe)NR}2](Cl)2 (R = NEt2, NC5H10) (trans-7,8). Structure–activity relationships were inferred from bioanalytical experiments. In vitro cytotoxicity tests in two human cancer cell lines, CH1 (cisplatin-sensitive) and SW480 (intrinsically cisplatin-resistant), confirmed that the cytotoxicity of the trans-configured complexes is, in some cases, comparable or higher than that of the cis-congeners in contrast to the behavior of cisplatin/transplatin. Substantial cytotoxicity was revealed for cationic compound trans-6, despite the lack of distinctly labile ligands. Cellular accumulation was shown to be dependent on geometrical isomerism of the compounds, favoring the higher accumulation of the trans-configured neutral trans-[PtCl2{NH=C(NH2)R}2] and the cis-configured cationic cis-[Pt(NH3)2{NH=C(NH2)R}2](Cl)2 (R = NMe2, NC5H10) complexes over their counterparts. Guanidine species trans-1,3,5,6,7 and cis-1,3,4,6 were shown to alter DNA secondary structure to a different extent and, considering cellular accumulation and cytotoxicity data, DNA can be suggested as a main target. Further investigations of DNA interactions (and other potential intracellular targets) may shed some light on the mode of action of these compounds. A better understanding of the mode of action of amidine platinum(II) antitumor agents, may, in turn, help to rationally modify their chemical and biological properties in order to optimize their anticancer activity.
Abbreviations
- ESI-MS
electrospray ionization mass spectrometry
- EtBr
ethidium bromide
- ICP-MS
inductively coupled plasma mass spectrometry
- IR
infrared spectroscopy
- MEM
(Eagles's) minimum essential medium
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
- PBS
(Dulbecco's) phosphate-buffered saline
Acknowledgments
The authors are indebted to the Austrian Science Fund (FWF; project no. L567) and the Russian Fund for Basic Research (grant 12-03-33071) for the financial support. The authors also acknowledge Saint Petersburg State University for a research grant (2012–2013, 12.39.1050.2012).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Michael A. Jakupec, Email: michael.jakupec@univie.ac.at.
Bernhard K. Keppler, Email: bernhard.keppler@univie.ac.at.
References
- 1.Kelland L. Nat. Rev. Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 2.Wang D., Lippard S.J. Nat. Rev. Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 3.Jung Y.W., Lippard S.J. Chem. Rev. 2007;107:1387–1407. doi: 10.1021/cr068207j. [DOI] [PubMed] [Google Scholar]
- 4.Pourahmad J., Hosseini M.J., Eskandari M.R., Shekarabi S.M., Daraei B. Xenobiotica. 2010;40:763–771. doi: 10.3109/00498254.2010.512093. [DOI] [PubMed] [Google Scholar]
- 5.Quasthoff S., Hartung H.P. J. Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 6.Wu F., Lin X., Okuda T., Howell S.B. Cancer Res. 2004;64:8029–8035. doi: 10.1158/0008-5472.CAN-03-3942. [DOI] [PubMed] [Google Scholar]
- 7.Cleare M.J., Hoeschele J.D. Platinum Met. Rev. 1973;17:2–13. [Google Scholar]
- 8.Brabec V., Vrana O., Novakova O., Kleinwachter V., Intini F.P., Coluccia M., Natile G. Nucleic Acids Res. 1996;24:336–341. doi: 10.1093/nar/24.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sbovata S.M., Bettio F., Mozzon M., Bertani R., Venzo A., Benetollo F., Michelin R.A., Gandin V., Marzano C. J. Med. Chem. 2007;50:4775–4784. doi: 10.1021/jm070426p. [DOI] [PubMed] [Google Scholar]
- 10.Zorbas-Seifried S., Jakupec M.A., Kukushkin N.V., Groessl M., Hartinger C.G., Semenova O., Zorbas H., Kukushkin V.Y., Keppler B.K. Mol. Pharmacol. 2007;71:357–365. doi: 10.1124/mol.106.030726. [DOI] [PubMed] [Google Scholar]
- 11.Amtmann E., Zoller M., Wesch H., Schilling G. Cancer Chemother. Pharmacol. 2001;47:461–466. doi: 10.1007/s002800000261. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y., Vinje J., Pacifico C., Natile G., Sletten E. J. Am. Chem. Soc. 2002;124:12854–12862. doi: 10.1021/ja027251n. [DOI] [PubMed] [Google Scholar]
- 13.Martinez A., Lorenzo J., Prieto M.J., de Llorens R., Font-Bardia M., Solans X., Aviles F.X., Moreno V. ChemBioChem. 2005;6:2068–2077. doi: 10.1002/cbic.200500108. [DOI] [PubMed] [Google Scholar]
- 14.Coluccia M., Nassi A., Boccarelli A., Giordano D., Cardellicchio N., Locker D., Leng M., Sivo M., Intini F.P., Natile G. J. Inorg. Biochem. 1999;77:31–35. doi: 10.1016/s0162-0134(99)00139-7. [DOI] [PubMed] [Google Scholar]
- 15.Casini A., Gabbiani C., Mastrobuoni G., Pellicani R.Z., Intini F.P., Arnesano F., Natile G., Moneti G., Francese S., Messori L. Biochemistry. 2007;46:12220–12230. doi: 10.1021/bi701516q. [DOI] [PubMed] [Google Scholar]
- 16.Nguewa P.A., Fuertes M.A., Iborra S., Najajreh Y., Gibson D., Martinez E., Alonso C., Perez J.M. J. Inorg. Biochem. 2005;99:727–736. doi: 10.1016/j.jinorgbio.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Najajreh Y., Ardeli-Tzaraf Y., Kasparkova J., Heringova P., Prilutski D., Balter L., Jawbry S., Khazanov E., Perez J.M., Barenholz Y., Brabec V., Gibson D. J. Med. Chem. 2006;49:4674–4683. doi: 10.1021/jm060238j. [DOI] [PubMed] [Google Scholar]
- 18.Scaffidi-Domianello Y.Y., Meelich K., Jakupec M.A., Arion V.B., Kukushkin V.Y., Galanski M., Keppler B.K. Inorg. Chem. 2010;49:5669–5678. doi: 10.1021/ic100584b. [DOI] [PubMed] [Google Scholar]
- 19.Scaffidi-Domianello Y.Y., Legin A.A., Jakupec M.A., Arion V.B., Kukushkin V.Y., Galanski M., Keppler B.K. Inorg. Chem. 2011;50:10673–10681. doi: 10.1021/ic2010612. [DOI] [PubMed] [Google Scholar]
- 20.Ma E.S., Bates W.D., Edmunds A., Kelland L.R., Fojo T., Farrell N. J. Med. Chem. 2005;48:5651–5654. doi: 10.1021/jm050539d. [DOI] [PubMed] [Google Scholar]
- 21.Quiroga A.G., Cubo L., de Blas E., Aller P., Navarro-Ranninger C. J. Inorg. Biochem. 2007;101:104–110. doi: 10.1016/j.jinorgbio.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Kalinowska U., Matlawska K., Checinska L., Domagala M., Kontek R., Osiecka R., Ochocki J. J. Inorg. Biochem. 2005;99:2024–2031. doi: 10.1016/j.jinorgbio.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Boccarelli A., Intini F.P., Sasanelli R., Sivo M.F., Coluccia M., Natile G. J. Med. Chem. 2006;49:829–837. doi: 10.1021/jm050986t. [DOI] [PubMed] [Google Scholar]
- 24.Tyan M.R., Bokach N.A., Wang M.J., Haukka M., Kuznetsov M.L., Kukushkin V.Y. Dalton Trans. 2008:5178–5188. doi: 10.1039/b806862c. [DOI] [PubMed] [Google Scholar]
- 25.Bokach N.A., Pakhomova T.B., Kukushkin V.Y., Haukka M., Pombeiro A.J. Inorg. Chem. 2003;42:7560–7568. doi: 10.1021/ic034800x. [DOI] [PubMed] [Google Scholar]
- 26.Egger A.E., Rappel C., Jakupec M.A., Hartinger C.G., Heffeter P., Keppler B.K. J. Anal. At. Spectrom. 2009;24:51–61. doi: 10.1039/B810481F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartel C., Bytzek A.K., Scaffidi-Domianello Y.Y., Grabmann G., Jakupec M.A., Hartinger C.G., Galanski M., Keppler B.K. J. Biol. Inorg. Chem. 2012;17:465–474. doi: 10.1007/s00775-011-0869-5. [DOI] [PubMed] [Google Scholar]
- 28.Marzano C., Sbovata S.M., Bettio F., Michelin R.A., Seraglia R., Kiss T., Venzo A., Bertani R. J. Biol. Inorg. Chem. 2007;12:477–493. doi: 10.1007/s00775-006-0202-x. [DOI] [PubMed] [Google Scholar]
- 29.Michelin R.A., Sgarbossa P., Sbovata S.M., Gandin V., Marzano C., Bertani R. ChemMedChem. 2011;6:1172–1183. doi: 10.1002/cmdc.201100150. [DOI] [PubMed] [Google Scholar]
- 30.Marzano C., Mazzega Sbovata S., Gandin V., Colavito D., Del Giudice E., Michelin R.A., Venzo A., Seraglia R., Benetollo F., Schiavon M., Bertani R. J. Med. Chem. 2010;53:6210–6227. doi: 10.1021/jm1006534. [DOI] [PubMed] [Google Scholar]
- 31.Varbanov H.P., Jakupec M.A., Roller A., Jensen F., Galanski M., Keppler B.K. J. Med. Chem. 2013;56:330–344. doi: 10.1021/jm3016427. [DOI] [PMC free article] [PubMed] [Google Scholar]