Abstract
Chick embryonic stem cells (cESCs) can be derived from cells obtained from stage X embryos (blastoderm stage); these have the ability to contribute to all somatic lineages in chimaeras, but not to the germ line. However, lines of stem cells that are able to contribute to the germ line can be established from chick primordial germ cells (cPGCs) and embryonic germ cells (cEGCs). This review provides information on avian stem cells, emphasizing different sources of cells and current methods for derivation and culture of pluripotent cells from chick embryos. We also review technologies for isolation and derivation of chicken germ cells and the production of transgenic birds.
Graphical abstract

Highlights
-
•
Chick embryonic stem cells (cESCs) can be derived from a variety of sources.
-
•
cESCs can contribute to all somatic cell types but not to the germ line.
-
•
germ cells can be isolated from early embryos, embryonic blood and gonads.
-
•
germ cells can establish self-renewing lines and contribute to the germline.
Introduction
Avian embryos are a powerful model to study developmental and stem cell biology (Stern, 1996, 2004a, 2005). They offer several advantages as a model for studying stem cell biology including their convenient size and ease of obtaining eggs (Berg et al., 1999), their year-round availability and ease of access to the embryo for manipulations, which among other applications led it to be used as a favourite model for toxicity testing since very early days (Halldin, 2005; Halldin et al., 2005). To date, avians are the only non-mammalian group from which stable embryonic stem cell and germ cell lines have been established. Both cES and chick embryonic germ (cEG) cells are considered to be pluripotent (Petitte et al., 2004), but cES cells have been shown to be able to contribute only to somatic tissues and not to the germ line (Pain et al., 1996), while chick embryonic germ cells can contribute to the germ line (van de Lavoir et al., 2006a). However, surprisingly little attention has been given to the biology of avian stem cells, especially regarding similarities and differences between chick embryonic stem (cES) cells, germ cells, and stem cells obtained from other embryonic and adult tissues. Here we provide information on avian stem cells, emphasizing sources, methods for derivation and culture of pluripotent cells from chick embryos.
The avian embryo spends its first 20 h or so in utero; the shell is deposited as the egg descends down the maternal oviduct (for review see Stern, 2004b). During this time, cell division occurs in a meroblastic pattern (open cleavage planes, from the centre out to the yolk) to generate a disc. By the time the egg is laid, the blastodisc comprises 20,000–50,000 cells arranged mainly as a single-cell-thick layer (epiblast) underlain by islands of more yolky cells (hypoblast — extraembryonic endoderm of the future yolk sac stalk) (Stern, 2004b). The entire embryo will arise from the centre of the epiblast, but it retains a remarkable ability to regenerate. Fragments of blastodisc can regenerate the entire embryo and re-establish polarity (Bertocchini et al., 2004; Bertocchini and Stern, 2012; Spratt and Haas, 1960), suggesting plasticity of the embryo and perhaps pluripotency of the component cells. It is from these early (pre-primitive streak) stages of development that cell lines analogous to mammalian embryonic stem cells (ESCs) can be established from cells dissociated from the central epiblast; these cells can be perpetuated in culture, perhaps indefinitely (Etches et al., 1996, 1997; Pain et al., 1996).
The biology of germ cells in bird embryos is particularly interesting and unique. Primordial germ cells (PGCs) appear to arise at pre-primitive streak stages (see above) by ingression from the epiblast, joining the hypoblast cells below (Ginsburg, 1997; Ginsburg and Eyal-Giladi, 1986, 1987; Karagenc et al., 1996; Petitte et al., 1997). The hypoblast forms a continuous layer of cells that then moves to the most anterior part of the embryo, under the pre-amnion, carrying the PGCs to this region, known as the Germinal Crescent. One remarkable feature is that primordial germ cells use the embryonic blood vasculature as a vehicle to migrate out of the germinal crescent, until they eventually settle in the embryonic gonads (Fujimoto et al., 1976; Kuwana and Rogulska, 1999; Nakamura et al., 2007; Nieuwkoop and Sutasurya, 1979). Another unique characteristic of the gonads in female birds is that the right ovary regresses, and only the left ovary remains functional in the adult (Romanoff, 1960; Smith and Sinclair, 2001, 2004). However even male embryos have a greater number of PGCs in the left gonad (Intarapat and Stern, 2013).
To date, it has been possible to establish long-term, self-renewing cultures of cells from pre-primitive streak stage embryos and from germ cells isolated from the blood vasculature or from the gonad. A few cell lines have also successfully been established from later embryos and adult tissues. This review surveys our current knowledge about stem cells from these various sources and their main biological properties.
Sources of chick stem cells
Endogenous stem cells in the early embryo
Endogenous stem cells were first discovered at early stages of chick embryo development by labelling a single cell in the organiser, Hensen's node, with fluorescent lineage tracers and following its descendants over time (Selleck and Stern, 1991, 1992; Stern et al., 1992). As the axis is laid down, the labelled cell deposits regularly spaced clusters of descendants along the axis (within the notochord and/or somite mesoderm), often with a single marked cell remaining at the site of labelling. Each cluster contains twice as many cells and displays about half the fluorescence intensity than the next cluster, consistent with the idea of resident cells that divide asymmetrically, one daughter remaining in place and the other contributing to emerging structures such as notochord and somites (Selleck and Stern, 1991, 1992; Stern et al., 1992) (Fig. 1A). The gradual ingression of cells from Hensen's node into the pre-somitic mesoderm, at a constant position in the cell cycle, has been related to somite formation, underlying the cell cycle model for somite formation (Collier et al., 2000; Palmeirim et al., 2008; Primmett et al., 1989; Stern et al., 2006). Since then, results consistent with this have been obtained in the mouse (Nicolas et al., 1996; Wilson et al., 2009).
Figure 1.

Endogenous stem cells in and around the primitive streak at early stages of chick development. A. From the full primitive streak stage (HH stage 4), the epiblast of Hensen's node contains a population of cells with properties suggesting that they are self-renewing, asymmetrically dividing stem cells. A single cell labelled in the median/anterior quadrant of the node generates clusters of labelled descendants in the notochord, about 2–4 somite-lengths apart. A single cell labelled a little more laterally generates similar clusters in the somites, 6–8 somites apart, suggesting that the cell cycle length is about twice the former (about 10 h) (Primmett et al., 1989). Occasionally, a cell labelled in the intermediate region can generate both types of clusters (Selleck and Stern, 1991, 1992). B. Next to the node, on each side of the epiblast, is a self-renewing region denominated “stem zone” which contains precursors for the caudal neural plate (which will give rise to the CNS, from hindbrain to tail) (Delfino-Machin et al., 2005). Eventually the node and stem zone seem to merge into a single domain containing mesendodermal precursors in the tail bud.
A separate region containing resident stem-cell-like cells has also been shown to exist in the epiblast just lateral to Hensen's node by Storey and colleagues (Akai et al., 2005; Delfino-Machin et al., 2005; Diez del Corral et al., 2003; Wilson et al., 2009) (Fig. 1B). This small region contributes cells to the caudally-elongating neural tube that will form the central nervous system from the hindbrain to the tail. Proliferation of the progenitor cells in the stem zone is maintained by FGF and Notch signalling and opposed by retinoids, causing cells to stop dividing, acquire Pax6 expression and differentiate into neurons (Akai et al., 2005; Delfino-Machin et al., 2005; Diez del Corral et al., 2003). Presumably both regions containing stem cells persist into the tail bud of later stage embryos, within a region where ectoderm and mesoderm merge into a solid mass and from which cells can contribute both to mesoderm (notochord and somite) and to the ventral neural tube. A striking demonstration of the self-renewing character of this region was provided by serial transplantation between GFP-transgenic and wild-type chick embryos: the grafted transgenic tail bud contributed extensively to the axis over at least two generations of host embryos (McGrew et al., 2008). Although in this latter case the phenomenon of self-renewal has not yet been studied at the single cell level (as in the node, see above), the results are consistent with this interpretation.
Chick embryonic stem cells (cESCs)
Most current work in the stem cell field is done using mammalian cells in vitro, particularly mouse and human; to date, the only non-mammalian system from which pluripotent embryonic stem cell lines can be established is the avian system, especially the chick. In chick, pluripotent cells have been isolated from several sources (summarised in Tables 1 and 2) and various sources of chick pluripotent stem cells from different stages of development and morphology of cESC, cPGCs and cEGCs are shown in Figs. 2–3.
Table 1.
Pluripotent stem cell types from avian embryos.
| Cell type | Source | Confirmation methods | References |
|---|---|---|---|
| ESCs | Stage X (EG&K) | EB formation, in vitro differentiation, somatic chimaeras | 1, 2, 3 |
| PGCs | Stages 14–17 (H&H) | Germline chimaeras | 4 |
| EGCs | Stage 28 (H&H) | EB formation, in vitro differentiation, somatic chimaeras | 5 |
| GSCs/SSCs | Juvenile 6 wk old and adult (24-wk old) male roosters | EB formation, in vitro differentiation | 6, 7 |
| iPSCs | Quail embryonic fibroblasts (embryonic day 11) | EB formation, in vitro differentiation, germline chimaera | 8 |
Abbreviations: ESCs = embryonic stem cells, PGCs = primordial germ cells, EGCs = embryonic germ cells, GSCs = germline stem cells, SSCs = spermatogonial stem cells, iPSCs = induced pluripotent stem cells; EG&K = stage (Eyal-Giladi and Kochav, 1976), H&H = stage (Hamburger and Hamilton, 1951). EB = embryoid body.
References: 1. Pain et al. (1996). 2. Boast and Stern (2012); van de Lavoir et al. (2006b). 3. Petitte et al. (2004). 4. van de Lavoir et al. (2006a). 5. Park and Han (2000). 6. Lee et al. (2006). 7. Jung et al. (2007). 8. Lu et al. (2012).
Table 2.
Avian somatic and adult stem cells.
| Cell type | Culture conditions | Differentiation | Culture duration | References |
|---|---|---|---|---|
| Neural stem cells | Neurobasal A + EGF + FGF2 heparin | Neurosphere | 7–14 days | 1 |
| Mesenchymal stem cells | ||||
| Liver-MSCs | DMEM/F12 + FBS + bFGF | Neuronal/osteoblast cells | 7–8 days | 2 |
| Lung-MSCs | DMEM + FBS + HEPES | Adipogenic/osteogenic cells | 21 days | 3 |
| Bone marrow-MSCs | DMEM + FBS | Adipogenic/osteogenic/chondrogenic cells | 14–21 days | 4 |
| L-DMEM + FBS | Adipogenic/osteogenic/endothelial cells | 6–20 days | ||
| Umbilical cord-MSCs | L-DMEM + FBS | Adipogenic/osteogenic/cardiomyogenic cells | 7–21 days | 5 |
| Muscle stem cells | DMEM/F12 + FBS + bFGF | Myogenic/osteogenic/adipogenic | 6 days | 6 |
| Amniotic stem cells | DMEM/F12 + FBS | Neuronal/osteogenic/adipogenic/pancreatic like cells | 7–14 days | 7 |
| Germline stem cells | Modified-DMEM + FBS + CS + HEPES + LIF + FGF2 + IGF1 | EB formation/three germ layers formation/germline chimaera | 21 days | 8 |
| Induced pluripotent stem cells (quail) | H-DMEM + FBS | EB formation/three germ layers formation/chimaera | 7–39 days | 9 |
References: 1. Whalley et al. (2009); Reynolds and Rietze (2005). 2. Mu et al. (2013). 3. Khatri et al. (2010). 4. Khatri et al. (2009); Bai et al. (2012). 5. Bai et al.(2013a). 6. Bai et al.(2013b). 7. Gao et al. (2012). 8. Jung et al. (2007); Lee et al. (2006). 9. Lu et al. (2012).
Figure 2.
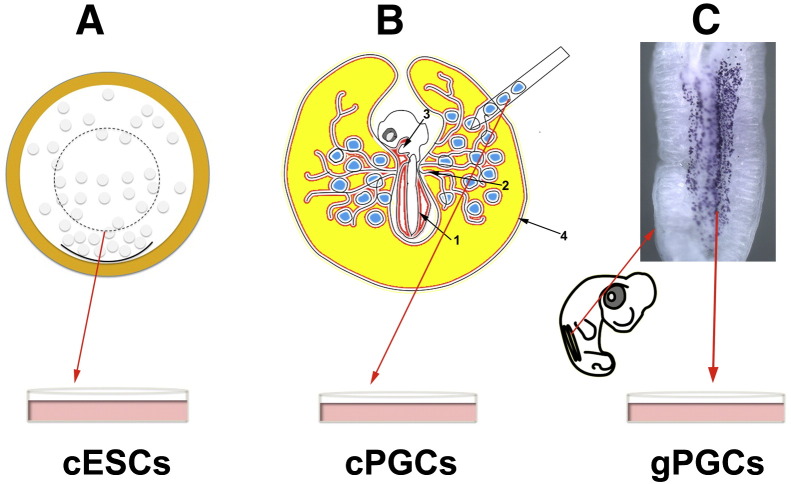
Sources of pluripotent stem cells from chick embryos at different stages of development. A. Chick embryonic stem cells (cESCs) can be derived from blastodermal cells at stage X (EG&K) (A). B. Circulating or blood derived-primordial germ cells (cPGCs) and chick embryonic germ cells (cEGCs) can be isolated from the dorsal aorta (1), vitelline artery (2), embryonic heart (3) or sinus terminalis (4) of stage 14 (H&H) embryos. At this stage, cPGCs use blood vessels as a route to migrate from the circulatory system to the future gonads. C. Gonadal primordial germ cells (gonocytes, gPGCs) and chick embryonic germ cells can be obtained from stage 25–28 (H&H) embryos. At this stage future gonads can be seen as bilateral ridges called gonadal or genital ridges where gPGCs have reached and settled inside to develop into functional gametes. GR = gonadal ridges; blue dots represent gPGCs.
Figure 3.
Morphology of chick embryonic stem cells (cESCs), circulating-primordial germ cells (cPGCs) and gonadal-primordial germ cells (gPGCs). A. The established cESC line 9N2 (Pain et al., 1996) has typical characteristics of undifferentiated embryonic stem cells with prominent large nucleus and relatively little cytoplasm. B. The established cPGC line NuGFP-02, isolated from embryonic blood (van de Lavoir et al., 2006a), can easily be distinguished from BRL feeder cells by having large cells with large nuclei and refractive granules in the cytoplasm. C. The established gPGC line 527, isolated from embryonic gonads (van de Lavoir et al., 2006a). Scale bar = 100 μm.
Isolation, culture and characterization of cESCs in vitro
As in mouse, cell lines can be established from cells obtained from very early chick embryos, prior to gastrulation. However, unlike murine ESCs, these chick ESCs have been shown to be able to contribute only to somatic lineages but not the germline (Lavial and Pain, 2010). Freshly isolated chick blastodermal cells retrieved from the area pellucida of stage X (Eyal-Giladi and Kochav, 1976; EG&K) embryos can contribute to all somatic tissues as well as the germline after injection into the subgerminal cavity of stage X (EG&K) recipient embryos (Carsience et al., 1993; Kagami et al., 1995; Kino et al., 1997; Petitte et al., 1990), but germline potency seems to be lost rapidly in culture. Thus, cESCs are more similar to murine epiblast stem cells (EpiSCs) (Lavial and Pain, 2010) and to human embryonic stem cells (hESCs) (Thomson et al., 1998) than to mESCs. This could be due to the fact that stage X is developmentally more advanced than the mouse ICM, from which mESCs are derived; it is possible that primordial germ cells have already left the epiblast by stage X, or that their lineage has already separated from somatic fates. That the ability of this cell population to contribute to the germline is lost upon culture suggests that early PGCs do not survive the culture conditions used in these studies, while the remaining epiblast-derived cells are already restricted to somatic lineages.
Many germline-associated genes in chick have been reported including Deadend (Aramaki et al., 2007), Dazl (Rengaraj et al., 2010), Piwi (Kim et al., 2012) and the chick vasa homologue (Cvh), which was the first of these genes cloned in chick (Tsunekawa et al., 2000). Cvh expression has been detected from stage X (EG&K) until adult stages (Tsunekawa et al., 2000); in the latter, expression is restricted to functional male and female gametes (Tsunekawa et al., 2000).
The limited ability of cESCs to contribute to the germline lineage could be explained by early germline determination and the reduction of germline potency in vitro (Lavial et al., 2009). Cvh might be a key gene for germ cell specification and determination in chick that enables cPGCs to enter the germline. Indeed, cESCs transfected with Cvh plasmid and cultured in differentiation medium in vitro adopt a germ cell fate (Lavial et al., 2009). This suggested that Cvh may be sufficient to confer cESCs with the ability to contribute to the germline in vivo by colonising the embryonic gonads, expressing specific germline and meiotic markers (Lavial et al., 2009).
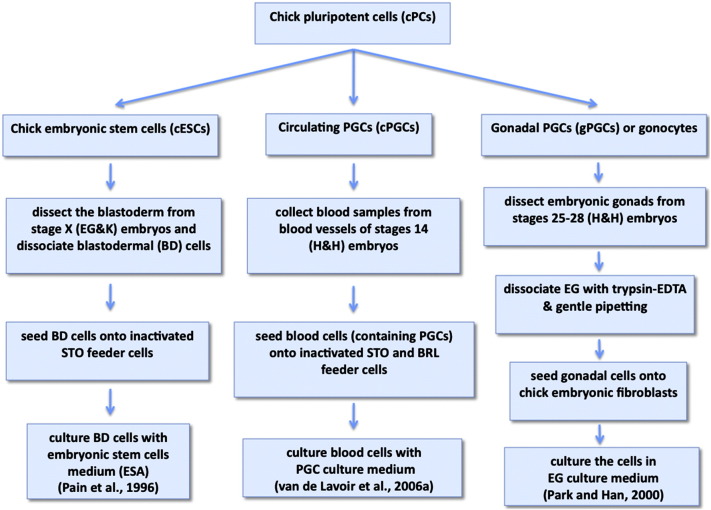
Chick ESCs were first isolated from stage X blastodermal cells by culturing them on inactivated STO feeder cells in embryonic stem cell medium (ESA) containing growth factors and cytokines including bFGF, IGF-1, mSCF, IL-6, IL-11, CNTF, OSM and LIF (Aubel and Pain, 2013; Pain et al., 1996). Like mESCs, cESCs can be maintained in an undifferentiated state in the presence of LIF (Horiuchi et al., 2004, 2006). Current methods for isolating cESCs are summarised in Fig. 4.
Figure 4.
Methods for isolation and derivation of chick pluripotent cells. Table summarising the main features of current methods for deriving cell lines from different sources.
Several characteristics have been shown to be shared between cESCs and their mESC counterparts. First, alkaline phosphatase (AP) activity is exhibited by cESCs (Pain et al., 1996; van de Lavoir and Mather-Love, 2006; van de Lavoir et al., 2006b). Several immunological markers are also expressed, including stage-specific embryonic antigens (SSEA) SSEA1, SSEA3 and SSEA4 (Knowles et al., 1978; Pain et al., 1996; Shevinsky et al., 1982; Solter and Knowles, 1978). Expression of chick homologues of Oct3/4 (cPouV) and cNanog has also been reported in cESCs (Lavial et al., 2007).
Being able to differentiate is one of the characteristics of ESCs; cESCs can generate nerve cells, haematopoietic cells and muscle cells and can also form embryoid bodies when plated onto low adherence plates in medium without LIF (Pain et al., 1996). Removal of LIF from the culture medium causes loss of SSEA1 (Pain et al., 1996), cPouV and cNanog expression (Lavial et al., 2007).
Chick somatic and adult stem cells
Neural stem cells
Neural stem cells (NSCs) are multipotent cells with the ability to self-renew and to differentiate into various cell types of the nervous system such as neurons and glia (Alvarez-Buylla et al., 2001; Temple, 2001). In adult birds, it was reported long ago that precursor cells located in the ventricular zone of the forebrain give rise to new neurons (Goldman and Nottebohm, 1983; Nottebohm, 1985; Paton and Nottebohm, 1984). Radial glia in contact with the ventricle appear to act as stem cells, which give rise to new neuroblasts (Alvarez-Buylla et al., 1990, 1998).
In chick, NSCs have not been found to be present in substantial numbers in the central nervous system until after embryonic day 5; SOX9 appears to be crucial for the formation and maintenance of NSCs (Scott et al., 2010). Cells with NSC properties can also be isolated from adult CNS; these cells are able to form clusters of cells known as neurospheres in long term in vitro culture. The majority of in vitro experiments use neurospheres as an assay for the presence of NSCs (Reynolds and Rietze, 2005). Neurospheres can be generated for example by culturing chick spinal cord with neurobasal A medium supplemented with EGF and FGF-2 (Whalley et al., 2009). However, little is known about the molecular mechanisms that regulate the maintenance, proliferation and differentiation of NSCs in the adult chick CNS.
Recently, the lateral ventricle of chick embryonic brain was used to assess the behaviour of human neural stem cell (hNSC) properties in vivo (Kharazi et al., 2013). hNSCs successfully engrafted into chick embryonic brain, introducing a new model for studying human stem cells in the nervous system (Kharazi et al., 2013).
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) (also known as mesenchymal progenitors, stromal stem cells or multipotent mesenchymal stromal cells) were first described from bone marrow cultures as cells having fibroblast-like shape, colony forming ability and adherent to plastic (Dominici et al., 2006; Friedenstein et al., 1982, 1987). They have been shown to be able to self-renew and to differentiate into a variety of cell types (Bruder and Caplan, 1990; Bruder et al., 1994; Jaiswal et al., 1997; Javazon et al., 2004; Lee et al., 2010; Owen and Friedenstein, 1988; Pittenger et al., 1999; Prockop, 2009; Reese et al., 1999; Saito et al., 1995; Wakitani et al., 1994, 1995; Yoo et al., 1998; Yoshimura et al., 2006; Young et al., 1998), which led to the proposal of the “mesengenic progress”, defined as the ability of bone marrow derived MSCs to give rise to mesenchymal lineages including bone, cartilage, muscle, marrow, tendon, ligament and connective tissue (Caplan, 1994). Several makers have been proposed to characterize MSCs including CD73/SH3 (Barry et al., 2001; Chartoff et al., 2011), CD90/Thy1 (Giuliani et al., 2011) and CD105/SH2/Endoglin (Barry et al., 1999; Chartoff et al., 2011; Kern et al., 2006; Ninagawa et al., 2011; Trivedi and Hematti, 2008). Other commonly used indicators include absence of expression of haematopoietic (CD34 and CD45) and endothelial (CD31) markers, co-expression of CD105, CD90/Thy-1, CD73, CD44/HCAM, CD166/ALCAM, CD29 (Pittenger et al., 1999) and CD146/MSCA-1 (Sorrentino et al., 2008). MSCs can also be isolated from various somatic organs such as lung (Chow et al., 2011; Gong et al., 2012), heart (Anzalone et al., 2013; Vasa et al., 2001), umbilical cord (Lee et al., 2004; Tsagias et al., 2011; Weiss and Troyer, 2006; Zhang et al., 2011) and bone marrow (Kastrinaki et al., 2008; Romanov et al., 2005; L. Wang et al., 2009). Because of their great multipotentiality, there has been great hope that MSCs can be used for regenerative medicine. This has been explored for diseases of the CNS (stroke, Multiple Sclerosis, Amyotrophic Lateral Sclerosis) (Estes et al., 2006; Mahmood et al., 2003; Riordan et al., 2009), the bone marrow (GvHD) (Bernardo et al., 2011; Le Blanc et al., 2008), the heart (chronic/AMI) (Pittenger and Martin, 2004; Toma et al., 2002), the lung (asthma, Cystic Fibrosis) (Bonfield and Caplan, 2010; Bonfield et al., 2010; Solchaga et al., 2005), muscle (Muscular Dystrophy) (Bosch et al., 2000; Lee et al., 2000), pancreas (diabetes) (Vija et al., 2009) and for alleviation of spinal cord injury (Chopp and Li, 2002; Neuhuber et al., 2005).
Like their mammalian counterparts, chick MSCs have been isolated from different organs including liver (Mu et al., 2013), lung (Khatri et al., 2010), bone marrow (Bai et al., 2013a; Khatri et al., 2009, 2010; Khatri and Sharma, 2009) and umbilical cord Wharton's jelly (Bai et al., 2013b). Chick MSCs from different organs can be differentiated into osteogenic and adipogenic lineages. However, whether MSCs from different tissue sources are actually the same cell type, and whether they have equivalent potency, is unknown. To date, it has been reported that umbilical cord-derived cMSCs can differentiate into cardiomyogenic cells (Bai et al., 2013b), and that bone marrow-derived cMSCs can differentiate into endothelial (Bai et al., 2013a) and chondrogenic cells (Khatri et al., 2009).
Muscle stem cells
The growth of adult skeletal muscle depends on the proliferation and differentiation of muscle progenitor cells derived from specialised muscle stem cells, the satellite cells (Brack and Rando, 2012; Buckingham, 2006; Montarras et al., 2005; Relaix et al., 2005). In both chick and mouse, satellite cells and muscle progenitors reside under the basal lamina (Cossu and Biressi, 2005; Halevy et al., 2006; Yablonka-Reuveni, 1995). Muscle progenitors have been shown to arise from the dermomyotome until late during embryogenesis and eventually become located under the basal lamina of muscles (Picard and Marcelle, 2013). It was suggested that the cellular strategies driving muscle growth in embryonic and fetal stages have been conserved during the evolution of amniotes (reptiles, birds and mammals) (Picard and Marcelle, 2013). Two distinct populations of muscle progenitor cells appear to coexist throughout amniote development; a common feature is that Pax7 expressing cells co-exist with a major fast-cycling population and Myf5 expressing cells (Picard and Marcelle, 2013), consistent with a model (Rudnicki et al., 2008) of muscle homeostasis during development.
In the chick, satellite cells have been isolated from Beijing fatty chicken (Bai et al., 2012). These cells were characterized molecularly using MyoD, Pax7 and desmin as myogenic markers and were found to differentiate into osteocytes and adipocytes after exposure to bone morphogenetic (BMPs) and adipogenic factors, respectively (Bai et al., 2012).
Amniotic stem cells
Like mammals, avian embryos contain extra-embryonic membranes including allantois, yolk sac, chorion and amnion. The cavity enclosed by the amnion contains fluid and a population of stem cells that expresses Oct4 (Prusa et al., 2003). Cells isolated from the mammalian amniotic cavity can generate clonal cell lines and can differentiate into primary germ cell lineages (De Coppi et al., 2007). These cells are known as amniotic fluid stem cells (AFSCs) and express c-kit surface antigen (CD117), the receptor of stem cell factor (Zsebo et al., 1990). Because of the ease of isolation using relatively non-invasive methods in humans, AFSCs are considered a promising source of stem cells for regenerative medical applications (Bajada et al., 2008).
Although the vast majority of studies on AFSCs have been done in mammals (Carraro et al., 2008; De Coppi et al., 2007; Elwan and Sakuragawa, 1997; Fuchs et al., 2004; Perin et al., 2007; Steigman et al., 2009), stem cells have also recently been isolated from chick amnion (Gao et al., 2012). Chick amniotic epithelial cells (cAECs) isolated from 6 day old chick embryos not only express CK19 (an epithelial cell marker) but also the pluripotency-associated genes Oct4, Nanog and Sox2 (Gao et al., 2012). Moreover, they have been successfully induced to differentiate into pancreatic islet-like cells, osteoblasts, adipocytes and neural-like cells (Gao et al., 2012), suggesting that cAECs are multipotent.
Germline stem cells
In chick, germline stem cells have been studied mainly in connection with the production of transgenic animals. Several techniques for transgenesis using male germline stem cells, also called spermatogonial stem cells (SSCs), have been described. These include testis-mediated gene transfer (Li et al., 2008), transplantation of transfected SSCs (Li et al., 2008), electrotransfection (Yu et al., 2010), allogeneic transplantation (Yu et al., 2010), lentiviral infection (Liu et al., 2010) and busulfan treatment (Tagirov and Golovan, 2012).
Since the first report of isolation of chick germline stem cells from testes (Jung et al., 2007), a testis-mediated system has been devised for transgenic technology (Han, 2009; Lee et al., 2006). This facilitates the isolation and derivation of pluripotent cell lines from adult stages. Both PGCs and SSCs can be differentiated into adipocytes, neuron-like cells and osteoblasts in vitro (B. Li et al., 2010; B.C. Li et al., 2010) and both express similar gene markers (Jung et al., 2005, 2007).
Although there are many publications about male germline stem cells in chick, there is as yet no evidence for an equivalent female germline stem cell. However, chick ovarian cells have been cultured for toxicology (Liu et al., 2006; Xie and Zhang, 2004), endocrinology (Liu et al., 2005; Sirotkin and Grossmann, 2007; Velazquez et al., 2006) and cancer-related (Giles et al., 2006) studies.
Avian induced pluripotent stem cells (iPSCs)
Direct reprogramming has been established recently for converting differentiated somatic cells into pluripotent ES-like cells. This method is useful for medical applications and avoids ethical issues associated with the use of human eggs or embryonic tissues. It has been hypothesised that fusion of somatic cells with ES cells can generate ES-like cells by inducing the pluripotent state and de-differentiation (Yamanaka, 2008). Key factors able to induce mouse embryonic and human fibroblasts into iPSCs have been discovered; these factors can maintain pluripotency both in vitro and in vivo (Takahashi et al., 2007a, 2007b; Takahashi and Yamanaka, 2006). The original method used for testing pluripotency-inducing factors involved retrovirus-mediated transfection; when four transcription factors (Oct-4, Sox2, c-Myc and Klf4) were transfected into mouse fibroblasts, a pluripotent stem cell state was induced. Cells generated this way are known as induced pluripotent stem cells (iPSCs) (Takahashi and Yamanaka, 2006), and these transcription factors are now often referred to as “Yamanaka factors”. iPSCs generated by this method are similar to mESCs including having an ES cell-like morphology, proliferation and the ability to form teratomas (Takahashi and Yamanaka, 2006).
iPSCs have been successfully generated from several mammalian species including human (Imamura et al., 2012; Okita et al., 2013; Okita and Yamanaka, 2010; Qu et al., 2012; Sommer et al., 2012; Takahashi et al., 2007b; Yu et al., 2009, 2010; Zhou et al., 2012), mouse (Imamura et al., 2012; Okita et al., 2008; Takahashi et al., 2007a; Takahashi and Yamanaka, 2006), common marmoset (Imamura et al., 2012; Wiedemann et al., 2012), monkey (Wunderlich et al., 2012; Zhong et al., 2011) and pig (Liu et al., 2012; Rodriguez et al., 2012; West et al., 2010). The combination of factors used differs a little among different groups and for different species; for example, Thompson's group uses Oct-4, Sox2, Nanog and Lin28 transfected into human foreskin fibroblasts with lentiviral vectors (Yu et al., 2007). However, iPSCs can be generated successfully in various mammalian species using human reprogramming factors, suggesting that the reprogramming mechanisms are conserved in mammals.
The first non-mammalian iPSCs were recently generated from quail using embryonic fibroblasts (QEFs) transfected with lentiviral vectors containing human POU5F1, NANOG, SOX2, LIN28, KLF4, and C-MYC (Lu et al., 2012). This is a larger number of reprogramming factors than routinely used for mammalian cells (selected by combining the Yamanaka and Thompson sets of factors). It seems likely that a smaller subset should also be successful, but this has not yet been explored systematically. Nevertheless, this pioneering study reveals that reprogramming somatic cells by a few transcription factors is not a unique property of mammalian cells, and, since human proteins work on quail cells, that the molecular mechanisms of reprogramming may be largely conserved beyond mammals. Like their mammalian counterparts, quail iPSCs exhibit pluripotent stem cell characteristics including differentiation into derivatives of the primary germ layers, neural differentiation, embryoid body formation and importantly the ability to produce germ line chimaeras (Lu et al., 2012). It should therefore be possible to generate chick iPSCs.
Applications and technologies related to chick stem cells
Isolation and derivation of chick embryonic stem cells
Methods for isolation and derivation of cESC lines were first reported by Pain et al. (1996). They used cells derived from the chick blastoderm before gastrulation (stages IX–XI), dissociated mechanically in ESA medium, to establish lines. In culture, the cells expressed ECMA-7 and SSEA-1 and were able to differentiate into somatic tissues in vitro and to contribute to the germline in vivo (Etches et al., 1997). For longer-term culture, the cells were grown on a feeder layer of inactivated STO cells (a mouse embryonic fibroblast cell line), supplemented with a cocktail of growth factors (see above). Under these conditions, cESCs exhibited similar characteristics to mESCs (see Table 1). Since then, another method was designed to improve the ease of isolation and establishment of the cell lines and the efficiency of production of somatic chimaeras from cultured cells (van de Lavoir and Mather-Love, 2006; van de Lavoir et al., 2006b). This method also uses cells dissociated from the area pellucida rather than from the whole embryo (which contains a large extraembryonic area opaca region) used for the earlier studies. To maintain undifferentiated cESCs in culture for long periods, Leukaemia Inhibitory Factor (LIF) appears to be important (Nichols et al., 1994; Smith and Hooper, 1987). Both natural LIF (secreted by BRL cells) (van de Lavoir and Mather-Love, 2006; van de Lavoir et al., 2006b) and synthetic LIF (Pain et al., 1996) have been used successfully for maintaining cESCs in an undifferentiated state. van de Lavoir's and Pain's methods both relied on murine LIF (mLIF), but other groups have sought to use conspecific, recombinant chicken LIF (cLIF) which was reported to promote alkaline phosphatase and EMA-1 expression (Horiuchi et al., 2004, 2006). Other recent methods use enzymatic dissociation to obtain the cells (Zhang et al., 2013), combined with the use of chick embryonic fibroblasts (CEFs) as feeder cells instead of STO cells, along with culture in medium without added growth factors. cESCs grown this way express alkaline phosphatase and SSEA-1 and can contribute to somatic tissues in chimaeras.
Methods for culture of other avian somatic and adult stem cells are summarised in Table 2. A wide variety of isolation conditions, media and feeder layers have been used by different groups. However no rigorous, systematic comparison of these conditions has been undertaken for cells from any of these different tissue sources and it is therefore impossible to determine which of these conditions are important for specific properties of the stem cell lines, or whether these vary according to the originating tissue type.
Isolation and derivation of chick germ cell lines
Several attempts have been made to isolate chick germ cells and to establish cell lines from them. It was first demonstrated that chick PGCs can be cultured from pre-primitive-streak stage embryos (Karagenc et al., 1996); factors secreted by STO cells were found to enhance their maintenance (Karagenc and Petitte, 2000). PGCs can also be obtained from the germinal crescent of primitive streak stage embryos, which can be successfully transfected by retroviruses; recipient embryos injected with such transfected PGCs grew to sexual maturity and produced offspring containing the foreign DNA (Vick et al., 1993).
Chick PGCs have some unique characteristics that distinguish them from their mammalian counterparts, such as their use of the blood circulation as a migratory route to the embryonic gonad. This allows PGCs to be isolated from embryonic blood. This was achieved relatively recently (van de Lavoir et al., 2006a). Blood-derived PG cells remained undifferentiated after prolonged culture in the presence of LIF, SCF and bFGF (van de Lavoir et al., 2006a); cells established by this method exhibit good germline transmission after injection into stage 13–15 embryos, but do not contribute to somatic tissues, suggesting that they are committed to a germline fate. Genetically modified PGCs have been created from these cells (van de Lavoir et al., 2006a), offering a method for production of transgenic lines of birds.
Several attempts have also been made to isolate gonadal-derived germ cells from later embryonic stages (Chang et al., 1995, 1997; Ha et al., 2002; Park and Han, 2000; Park et al., 2003; Shiue et al., 2009; Suraeva et al., 2008; J. Wang et al., 2009; Wu et al., 2010) (see Table 3). However, very few germline chimaeras were obtained after injecting cultured gonad-derived germ cells into recipient embryos (Chang et al., 1995, 1997; Ha et al., 2002; Park et al., 2003). IGF and IL-11 were reported to be essential for gonadal PGCs to maintain germline potency and colony formation (Chang et al., 1995; Park and Han, 2000). Although no systematic comparison has yet been undertaken, these findings suggest that blood-derived and gonadal-derived germ cells may differ in their ability to be cultured for long periods and in their capacity to establish germline chimaeras. A method for isolating and deriving chick embryonic germ cells is summarised in Fig. 4.
Table 3.
Comparison of methods for isolation and derivation of blood-derived and gonadal-derived PGCs.
| Cells | Feeder layer | Sera/growth factors/cytokines | References |
|---|---|---|---|
| cPGCs | Irradiated BRL | FBS, CS/bFGF, SCF/secreted LIF from BRL | van de Lavoir et al. (2006a) |
| FBS, CS/–/– | Yamamoto et al. (2007) | ||
| FBS, CS/bFGF, SCF, hLIF | Choi et al. (2010) | ||
| Irradiated STO | FBS, CS/FGF, SCF/secreted LIF from BRL | Macdonald et al. (2010) | |
| gPGCs | CEF | FBS, CS/bFGF, SCF, IGF-I/mLIF, IL-11 | Park and Han (2000) |
| GSC | FBS, CS/bFGF, SCF/mLIF | Suraeva et al. (2008) | |
| CEF | FBS, CS/bFGF, SCF, IGF-I/mLIF, IL-11 | Shiue et al. (2009) | |
| Inactivated MEF | FBS, CS/bFGF, SCF, IGF-I/mLIF | J. Wang et al. (2009) | |
| CEF | FBS/bFGF/mLIF | Wu et al. (2010) |
Abbreviations: BRL = buffalo rat liver cells, STO = Sandoz inbred mouse-derived thioguanine-resistant and ouabain-resistant fibroblast, CEF = chicken embryonic fibroblasts, GSC = gonadal stromal cells, MEF = mouse embryonic fibroblasts, FBS = Fetal bovine serum, CS = chicken serum, bFGF = basic fibroblast growth factor, SCF = stem cell factor, IGF-I = insulin growth factor type I, mLIF = murine leukaemia inhibitory factor, IL-11 = interleukin-11.
Production of transgenic birds
Although retroviral vectors have been used to transduce exogenous genes into early chick blastoderms and somatic stem cells, and constructs including LacZ can be transfected into cells of the blastodisc to produce chick chimaeras (Inada et al., 1997; Naito et al., 1991, 1994), the transgene has not yet been shown to be transmitted through the germline (Bosselman et al., 1989). In contrast, lentiviral vectors have been used with considerable success to generate several transgenic lines of birds (both chick and quail) (Bosselman et al., 1989; Fraser et al., 1993; Hunter et al., 2005; Lillico et al., 2005; McGrew et al., 2004; Poynter et al., 2009a, 2009b, 2009c; Poynter and Lansford, 2008; Sang, 2004; Sato and Lansford, 2013; Sato et al., 2010; Seidl et al., 2013). The method is now almost routine and several laboratories are now establishing transgenic lines of birds for example expressing GFP (cytoplasmic or membrane-localized) ubiquitously or driven by tissue-specific promoters, and other cell lines such as reporters for various signalling pathways for research applications. A particularly promising technique for transgenesis was recently described, using the Tol2 and piggyBac transposons, allowing the introduction of large inserts (Macdonald et al., 2012).
Despite this success for transgenic bird construction, lentiviral transduction does not easily allow targeted mutagenesis at a selected gene locus because the transgene inserts randomly at multiple sites. Although carefully designed breeding can separate lines and purify those with a single integration, it is still not possible to target specific gene loci using this method. For this, a cell-based method using homologous recombination in ESC or PGC would be desirable. This has not yet been achieved, despite the fact that homologous recombination is possible, and even relatively straightforward, in avian cells (Acloque et al., 2001, 2004; Ishiai et al., 2012; Takata et al., 2006, 2009). A major hurdle remains the availability of cell lines, susceptible to homologous recombination (and which can be maintained in culture so that selection can be used to isolate lines that have undergone successful recombination, as in mouse), and able to contribute to the germ line.
Blastoderm-derived cells were first used for creating chimaeric chickens after transplanting these cells to the central zone (Marzullo, 1970) or the subgerminal cavity of early embryos (Pain et al., 1996; van de Lavoir and Mather-Love, 2006; van de Lavoir et al., 2006b; Zhang et al., 2013). Some success has been obtained with early blastodermal cells containing PGCs from stage X embryos, leading to the production of both somatic and germline chimaeras (Carsience et al., 1993; Etches et al., 1996; Kagami et al., 1995; Petitte et al., 1990; Thoraval et al., 1994). Both fresh and cryopreserved blastodermal cells were used for chimaera production, although fresh cells were more efficient (Etches et al., 1996; Kino et al., 1997).
Since long-term cultured cESC lines do not seem to be able readily to contribute to the germ line, and since lines of PGCs are difficult to establish, a method for reprogramming cESCs to acquire germline competence would be desirable. A recent study demonstrated that cESCs transfected with Cvh in an expression plasmid can colonise the embryonic gonad and express the germ cells' marker DAZL (Lavial et al., 2009). One study claims to have produced transgenic chicks using transdifferentiated chicken bone marrow cells transplanted into the testes (Heo et al., 2011).
Germ cell-based methods for transgenesis in the chick were described long ago, in a study using germinal crescent-derived PGCs along with a replication-deficient retroviral vector (Vick et al., 1993). Lentiviral vectors can also be used to introduce transgenes into gonadal-PGCs (Shin et al., 2008). Blood-derived PGCs have also been used successfully to generate transgenic chicks, using electroporation for gene transfer (van de Lavoir et al., 2006a). Methods that rely on PGCs for creating transgenic birds have been called “embryo-mediated system” (Han, 2009). A “testis-mediated system” has also been described (Lee et al., 2006); this method is said to be advantageous because it eliminates the need for PGC retrieval and reduces the time for the test cross (Han, 2009). However, a comparison between embryo-mediated and testis-mediated systems for obtaining high yields and efficient production of transgenic chicks has yet to be undertaken.
Thus, although cell-based methods do hold promise for targeted mutagenesis in the chick, this has yet to be successfully achieved. At present, lentiviral vector-mediated transgenesis appears to be the most efficient method for producing transgenic bird lines, but this does not allow targeted mutagenesis of endogenous loci.
Conclusions
Chick stem cells can be obtained from embryos and maintained in culture. They can be derived from different sources at various stages of embryonic development. They have been demonstrated to be pluripotent because they can form embryoid bodies, differentiate into cell types from all three embryonic germ layers and contribute to somatic and germline lineages in chimaeras. They are therefore comparable to mammalian stem cells, offering a model for studying stem cell biology as well as being a tool for many applications.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors' work was supported by the Medical Research Council (MRC) and a Ph.D. scholarship from the programme “Strategic Scholarships for Frontier Research Network” of Thailand's Commission on Higher Education (CHE-SFR), Royal Thai Government.
References
- Acloque H., Risson V., Birot A.M., Kunita R., Pain B., Samarut J. Identification of a new gene family specifically expressed in chicken embryonic stem cells and early embryo. Mech. Dev. 2001;103:79–91. doi: 10.1016/s0925-4773(01)00336-7. [DOI] [PubMed] [Google Scholar]
- Acloque H., Mey A., Birot A.M., Gruffat H., Pain B., Samarut J. Transcription factor cCP2 controls gene expression in chicken embryonic stem cells. Nucleic Acids Res. 2004;32:2259–2271. doi: 10.1093/nar/gkh545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akai J., Halley P.A., Storey K.G. FGF-dependent Notch signaling maintains the spinal cord stem zone. Genes Dev. 2005;19:2877–2887. doi: 10.1101/gad.357705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Theelen M., Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–109. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Garcia-Verdugo J.M., Mateo A.S., Merchant-Larios H. Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J. Neurosci. 1998;18:1020–1037. doi: 10.1523/JNEUROSCI.18-03-01020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Garcia-Verdugo J.M., Tramontin A.D. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Anzalone R., Corrao S., Lo Iacono M., Loria T., Corsello T., Cappello F., Di Stefano A., Giannuzzi P., Zummo G., Farina F., La Rocca G. Isolation and characterization of CD276 +/HLA-E + human subendocardial mesenchymal stem cells from chronic heart failure patients: analysis of differentiative potential and immunomodulatory markers expression. Stem Cells Dev. 2013;22:1–17. doi: 10.1089/scd.2012.0402. [DOI] [PubMed] [Google Scholar]
- Aramaki S., Sato F., Kato T., Soh T., Kato Y., Hattori M.A. Molecular cloning and expression of dead end homologue in chicken primordial germ cells. Cell Tissue Res. 2007;330:45–52. doi: 10.1007/s00441-007-0435-1. [DOI] [PubMed] [Google Scholar]
- Aubel P., Pain B. Chicken embryonic stem cells: establishment and characterization. Methods Mol. Biol. 2013;1074:137–150. doi: 10.1007/978-1-62703-628-3_11. [DOI] [PubMed] [Google Scholar]
- Bai C., Hou L., Li F., He X., Zhang M., Guan W. Isolation and biological characteristics of Beijing fatty chicken skeletal muscle satellite cells. Cell Commun. Adhes. 2012;19:69–77. doi: 10.3109/15419061.2012.743998. [DOI] [PubMed] [Google Scholar]
- Bai C., Hou L., Ma Y., Chen L., Zhang M., Guan W. Isolation and characterization of mesenchymal stem cells from chicken bone marrow. Cell Tissue Bank. 2013;14:437–451. doi: 10.1007/s10561-012-9347-8. [DOI] [PubMed] [Google Scholar]
- Bai C., Li X., Hou L., Zhang M., Guan W., Ma Y. Biological characterization of chicken mesenchymal stem/progenitor cells from umbilical cord Wharton's jelly. Mol. Cell. Biochem. 2013;376:95–102. doi: 10.1007/s11010-012-1553-y. [DOI] [PubMed] [Google Scholar]
- Bajada S., Mazakova I., Richardson J.B., Ashammakhi N. Updates on stem cells and their applications in regenerative medicine. J. Tissue Eng. Regen. Med. 2008;2:169–183. doi: 10.1002/term.83. [DOI] [PubMed] [Google Scholar]
- Barry F.P., Boynton R.E., Haynesworth S., Murphy J.M., Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105) Biochem. Biophys. Res. Commun. 1999;265:134–139. doi: 10.1006/bbrc.1999.1620. [DOI] [PubMed] [Google Scholar]
- Barry F., Boynton R., Murphy M., Haynesworth S., Zaia J. The SH-3 and SH-4 antibodies recognize distinct epitopes on CD73 from human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2001;289:519–524. doi: 10.1006/bbrc.2001.6013. [DOI] [PubMed] [Google Scholar]
- Berg C., Halldin K., Fridolfsson A.K., Brandt I., Brunstrom B. The avian egg as a test system for endocrine disrupters: effects of diethylstilbestrol and ethynylestradiol on sex organ development. Sci. Total. Environ. 1999;233:57–66. doi: 10.1016/s0048-9697(99)00179-5. [DOI] [PubMed] [Google Scholar]
- Bernardo M.E., Ball L.M., Cometa A.M., Roelofs H., Zecca M., Avanzini M.A., Bertaina A., Vinti L., Lankester A., Maccario R., Ringden O., Le Blanc K., Egeler R.M., Fibbe W.E., Locatelli F. Co-infusion of ex vivo-expanded, parental MSCs prevents life-threatening acute GVHD, but does not reduce the risk of graft failure in pediatric patients undergoing allogeneic umbilical cord blood transplantation. Bone Marrow Transplant. 2011;46:200–207. doi: 10.1038/bmt.2010.87. [DOI] [PubMed] [Google Scholar]
- Bertocchini F., Stern C.D. Gata2 provides an early anterior bias and uncovers a global positioning system for polarity in the amniote embryo. Development. 2012;139:4232–4238. doi: 10.1242/dev.081901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocchini F., Skromne I., Wolpert L., Stern C.D. Determination of embryonic polarity in a regulative system: evidence for endogenous inhibitors acting sequentially during primitive streak formation in the chick embryo. Development. 2004;131:3381–3390. doi: 10.1242/dev.01178. [DOI] [PubMed] [Google Scholar]
- Boast S., Stern C.D. Simple methods for generating neural, bone and endodermal cell types from chick embryonic stem cells. Stem Cell Res. 2012;10:20–28. doi: 10.1016/j.scr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Bonfield T.L., Caplan A.I. Adult mesenchymal stem cells: an innovative therapeutic for lung diseases. Discov. Med. 2010;9:337–345. [PubMed] [Google Scholar]
- Bonfield T.L., Koloze M., Lennon D.P., Zuchowski B., Yang S.E., Caplan A.I. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;299:L760–L770. doi: 10.1152/ajplung.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch P., Musgrave D.S., Lee J.Y., Cummins J., Shuler T., Ghivizzani T.C., Evans T., Robbins T.D., Huard J. Osteoprogenitor cells within skeletal muscle. J. Orthop. Res. 2000;18:933–944. doi: 10.1002/jor.1100180613. [DOI] [PubMed] [Google Scholar]
- Bosselman R.A., Hsu R.Y., Boggs T., Hu S., Bruszewski J., Ou S., Souza L., Kozar L., Martin F., Nicolson M. Replication-defective vectors of reticuloendotheliosis virus transduce exogenous genes into somatic stem cells of the unincubated chicken embryo. J. Virol. 1989;63:2680–2689. doi: 10.1128/jvi.63.6.2680-2689.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack A.S., Rando T.A. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell. 2012;10:504–514. doi: 10.1016/j.stem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder S.P., Caplan A.I. Osteogenic cell lineage analysis is facilitated by organ cultures of embryonic chick periosteum. Dev. Biol. 1990;141:319–329. doi: 10.1016/0012-1606(90)90388-y. [DOI] [PubMed] [Google Scholar]
- Bruder S.P., Fink D.J., Caplan A.I. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J. Cell. Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr. Opin. Genet. Dev. 2006;16:525–532. doi: 10.1016/j.gde.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Caplan A.I. The mesengenic process. Clin. Plast. Surg. 1994;21:429–435. [PubMed] [Google Scholar]
- Carraro G., Perin L., Sedrakyan S., Giuliani S., Tiozzo C., Lee J., Turcatel G., De Langhe S.P., Driscoll B., Bellusci S., Minoo P., Atala A., De Filippo R.E., Warburton D. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells. 2008;26:2902–2911. doi: 10.1634/stemcells.2008-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsience R.S., Clark M.E., Verrinder Gibbins A.M., Etches R.J. Germline chimeric chickens from dispersed donor blastodermal cells and compromised recipient embryos. Development. 1993;117:669–675. doi: 10.1242/dev.117.2.669. [DOI] [PubMed] [Google Scholar]
- Chang I.K., Tajima A., Chikamune T., Ohno T. Proliferation of chick primordial germ cells cultured on stroma cells from the germinal ridge. Cell Biol. Int. 1995;19:143–149. doi: 10.1006/cbir.1995.1055. [DOI] [PubMed] [Google Scholar]
- Chang I.K., Jeong D.K., Hong Y.H., Park T.S., Moon Y.K., Ohno T., Han J.Y. Production of germline chimeric chickens by transfer of cultured primordial germ cells. Cell Biol. Int. 1997;21:495–499. doi: 10.1006/cbir.1997.0173. [DOI] [PubMed] [Google Scholar]
- Chartoff E.H., Damez-Werno D., Sonntag K.C., Hassinger L., Kaufmann D.E., Peterson J., McPhie D., Cataldo A.M., Cohen B.M. Detection of intranasally delivered bone marrow-derived mesenchymal stromal cells in the lesioned mouse brain: a cautionary report. Stem Cells Int. 2011;2011:586586. doi: 10.4061/2011/586586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.W., Kim S., Kim T.M., Kim Y.M., Seo H.W., Park T.S., Jeong J.W., Song G., Han J.Y. Basic fibroblast growth factor activates MEK/ERK cell signaling pathway and stimulates the proliferation of chicken primordial germ cells. PLoS One. 2010;5:e12968. doi: 10.1371/journal.pone.0012968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M., Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- Chow K.S., Jun D., Helm K.M., Wagner D.H., Majka S.M. Isolation & characterization of Hoechst(low) CD45(negative) mouse lung mesenchymal stem cells. J. Vis. Exp. 2011:e3159. doi: 10.3791/3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier J.R., McInerney D., Schnell S., Maini P.K., Gavaghan D.J., Houston P., Stern C.D. A cell cycle model for somitogenesis: mathematical formulation and numerical simulation. J. Theor. Biol. 2000;207:305–316. doi: 10.1006/jtbi.2000.2172. [DOI] [PubMed] [Google Scholar]
- Cossu G., Biressi S. Satellite cells, myoblasts and other occasional myogenic progenitors: possible origin, phenotypic features and role in muscle regeneration. Semin. Cell Dev. Biol. 2005;16:623–631. doi: 10.1016/j.semcdb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- De Coppi P., Bartsch G., Jr., Siddiqui M.M., Xu T., Santos C.C., Perin L., Mostoslavsky G., Serre A.C., Snyder E.Y., Yoo J.J., Furth M.E., Soker S., Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- Delfino-Machin M., Lunn J.S., Breitkreuz D.N., Akai J., Storey K.G. Specification and maintenance of the spinal cord stem zone. Development. 2005;132:4273–4283. doi: 10.1242/dev.02009. [DOI] [PubMed] [Google Scholar]
- Diez del Corral R., Olivera-Martinez I., Goriely A., Gale E., Maden M., Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Elwan M.A., Sakuragawa N. Evidence for synthesis and release of catecholamines by human amniotic epithelial cells. Neuroreport. 1997;8:3435–3438. doi: 10.1097/00001756-199711100-00004. [DOI] [PubMed] [Google Scholar]
- Estes B.T., Wu A.W., Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006;54:1222–1232. doi: 10.1002/art.21779. [DOI] [PubMed] [Google Scholar]
- Etches R.J., Clark M.E., Toner A., Liu G., Gibbins A.M. Contributions to somatic and germline lineages of chicken blastodermal cells maintained in culture. Mol. Reprod. Dev. 1996;45:291–298. doi: 10.1002/(SICI)1098-2795(199611)45:3<291::AID-MRD5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Etches R.J., Clark M.E., Zajchowski L., Speksnijder G., Verrinder Gibbins A.M., Kino K., Pain B., Samarut J. Manipulation of blastodermal cells. Poult. Sci. 1997;76:1075–1083. doi: 10.1093/ps/76.8.1075. [DOI] [PubMed] [Google Scholar]
- Eyal-Giladi H., Kochav S. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev. Biol. 1976;49:321–337. doi: 10.1016/0012-1606(76)90178-0. [DOI] [PubMed] [Google Scholar]
- Fraser R.A., Carsience R.S., Clark M.E., Etches R.J., Gibbins A.M. Efficient incorporation of transfected blastodermal cells into chimeric chicken embryos. Int. J. Dev. Biol. 1993;37:381–385. [PubMed] [Google Scholar]
- Friedenstein A.J., Latzinik N.W., Grosheva A.G., Gorskaya U.F. Marrow microenvironment transfer by heterotopic transplantation of freshly isolated and cultured cells in porous sponges. Exp. Hematol. 1982;10:217–227. [PubMed] [Google Scholar]
- Friedenstein A.J., Chailakhyan R.K., Gerasimov U.V. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Fuchs J.R., Kaviani A., Oh J.T., LaVan D., Udagawa T., Jennings R.W., Wilson J.M., Fauza D.O. Diaphragmatic reconstruction with autologous tendon engineered from mesenchymal amniocytes. J. Pediatr. Surg. 2004;39:834–838. doi: 10.1016/j.jpedsurg.2004.02.014. (discussion 834–838) [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Ukeshima A., Kiyofuji R. The origin, migration and morphology of the primordial germ cells in the chick embryo. Anat. Rec. 1976;185:139–145. doi: 10.1002/ar.1091850203. [DOI] [PubMed] [Google Scholar]
- Gao Y., Pu Y., Wang D., Hou L., Guan W., Ma Y. Isolation and biological characterization of chicken amnion epithelial cells. Eur. J. Histochem. 2012;56:e33. doi: 10.4081/ejh.2012.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles J.R., Olson L.M., Johnson P.A. Characterization of ovarian surface epithelial cells from the hen: a unique model for ovarian cancer. Exp. Biol. Med. (Maywood) 2006;231:1718–1725. doi: 10.1177/153537020623101108. [DOI] [PubMed] [Google Scholar]
- Ginsburg M. Primordial germ cell development in avians. Poult. Sci. 1997;76:91–95. doi: 10.1093/ps/76.1.91. [DOI] [PubMed] [Google Scholar]
- Ginsburg M., Eyal-Giladi H. Temporal and spatial aspects of the gradual migration of primordial germ cells from the epiblast into the germinal crescent in the avian embryo. J. Embryol. Exp. Morpholog. 1986;95:53–71. [PubMed] [Google Scholar]
- Ginsburg M., Eyal-Giladi H. Primordial germ cells of the young chick blastoderm originate from the central zone of the area pellucida irrespective of the embryo-forming process. Development. 1987;101:209–219. doi: 10.1242/dev.101.2.209. [DOI] [PubMed] [Google Scholar]
- Giuliani M., Fleury M., Vernochet A., Ketroussi F., Clay D., Azzarone B., Lataillade J.J., Durrbach A. Long-lasting inhibitory effects of fetal liver mesenchymal stem cells on T-lymphocyte proliferation. PLoS One. 2011;6:e19988. doi: 10.1371/journal.pone.0019988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S.A., Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc. Natl. Acad. Sci. U. S. A. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X., Sun Z., Cui D., Xu X., Zhu H., Wang L., Qian W., Han X. Isolation and characterization of lung resident mesenchymal stem cells capable of differentiating into alveolar epithelial type II cells. Cell Biol. Int. 2012 doi: 10.1002/cbin.10240. [DOI] [PubMed] [Google Scholar]
- Ha J.Y., Park T.S., Hong Y.H., Jeong D.K., Kim J.N., Kim K.D., Lim J.M. Production of germline chimeras by transfer of chicken gonadal primordial germ cells maintained in vitro for an extended period. Theriogenology. 2002;58:1531–1539. doi: 10.1016/s0093-691x(02)01061-0. [DOI] [PubMed] [Google Scholar]
- Halevy O., Piestun Y., Rozenboim I., Yablonka-Reuveni Z. In ovo exposure to monochromatic green light promotes skeletal muscle cell proliferation and affects myofiber growth in posthatch chicks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1062–R1070. doi: 10.1152/ajpregu.00378.2005. [DOI] [PubMed] [Google Scholar]
- Halldin K. Impact of endocrine disrupting chemicals on reproduction in Japanese quail. Domest. Anim. Endocrinol. 2005;29:420–429. doi: 10.1016/j.domaniend.2005.02.036. [DOI] [PubMed] [Google Scholar]
- Halldin K., Axelsson J., Brunstrom B. Effects of endocrine modulators on sexual differentiation and reproductive function in male Japanese quail. Brain Res. Bull. 2005;65:211–218. doi: 10.1016/j.brainresbull.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Han J.Y. Germ cells and transgenesis in chickens. Comp. Immunol. Microbiol. Infect. Dis. 2009;32:61–80. doi: 10.1016/j.cimid.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Heo Y.T., Lee S.H., Yang J.H., Kim T., Lee H.T. Bone marrow cell-mediated production of transgenic chickens. Lab. Invest. 2011;91:1229–1240. doi: 10.1038/labinvest.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H., Tategaki A., Yamashita Y., Hisamatsu H., Ogawa M., Noguchi T., Aosasa M., Kawashima T., Akita S., Nishimichi N., Mitsui N., Furusawa S., Matsuda H. Chicken leukemia inhibitory factor maintains chicken embryonic stem cells in the undifferentiated state. J. Biol. Chem. 2004;279:24514–24520. doi: 10.1074/jbc.M313231200. [DOI] [PubMed] [Google Scholar]
- Horiuchi H., Furusawa S., Matsuda H. Maintenance of chicken embryonic stem cells in vitro. Methods Mol. Biol. 2006;329:17–34. doi: 10.1385/1-59745-037-5:17. [DOI] [PubMed] [Google Scholar]
- Hunter C.V., Tiley L.S., Sang H.M. Developments in transgenic technology: applications for medicine. Trends Mol. Med. 2005;11:293–298. doi: 10.1016/j.molmed.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Imamura M., Okuno H., Tomioka I., Kawamura Y., Lin Z.Y., Nakajima R., Akamatsu W., Okano H.J., Matsuzaki Y., Sasaki E., Okano H. Derivation of induced pluripotent stem cells by retroviral gene transduction in mammalian species. Methods Mol. Biol. 2012;925:21–48. doi: 10.1007/978-1-62703-011-3_2. [DOI] [PubMed] [Google Scholar]
- Inada S., Hattori M.A., Fujihara N., Morohashi K. In vivo gene transfer into the blastoderm of early developmental stage of chicken. Reprod. Nutr. Dev. 1997;37:13–20. doi: 10.1051/rnd:19970102. [DOI] [PubMed] [Google Scholar]
- Intarapat S., Stern C.D. Sexually dimorphic and sex-independent left–right asymmetries in chicken embryonic gonads. PLoS One. 2013;8:e69893. doi: 10.1371/journal.pone.0069893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiai M., Uchida E., Takata M. Establishment of the DNA repair-defective mutants in DT40 cells. Methods Mol. Biol. 2012;920:39–49. doi: 10.1007/978-1-61779-998-3_4. [DOI] [PubMed] [Google Scholar]
- Jaiswal N., Haynesworth S.E., Caplan A.I., Bruder S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- Javazon E.H., Beggs K.J., Flake A.W. Mesenchymal stem cells: paradoxes of passaging. Exp. Hematol. 2004;32:414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Jung J.G., Kim D.K., Park T.S., Lee S.D., Lim J.M., Han J.Y. Development of novel markers for the characterization of chicken primordial germ cells. Stem Cells. 2005;23:689–698. doi: 10.1634/stemcells.2004-0208. [DOI] [PubMed] [Google Scholar]
- Jung J.G., Lee Y.M., Park T.S., Park S.H., Lim J.M., Han J.Y. Identification, culture, and characterization of germline stem cell-like cells in chicken testes. Biol. Reprod. 2007;76:173–182. doi: 10.1095/biolreprod.106.056275. [DOI] [PubMed] [Google Scholar]
- Kagami H., Clark M.E., Verrinder Gibbins A.M., Etches R.J. Sexual differentiation of chimeric chickens containing ZZ and ZW cells in the germline. Mol. Reprod. Dev. 1995;42:379–387. doi: 10.1002/mrd.1080420403. [DOI] [PubMed] [Google Scholar]
- Karagenc L., Petitte J.N. Soluble factors and the emergence of chick primordial germ cells in vitro. Poult. Sci. 2000;79:80–85. doi: 10.1093/ps/79.1.80. [DOI] [PubMed] [Google Scholar]
- Karagenc L., Cinnamon Y., Ginsburg M., Petitte J.N. Origin of primordial germ cells in the prestreak chick embryo. Dev. Genet. 1996;19:290–301. doi: 10.1002/(SICI)1520-6408(1996)19:4<290::AID-DVG2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Kastrinaki M.C., Andreakou I., Charbord P., Papadaki H.A. Isolation of human bone marrow mesenchymal stem cells using different membrane markers: comparison of colony/cloning efficiency, differentiation potential, and molecular profile. Tissue Eng. Part C Methods. 2008;14:333–339. doi: 10.1089/ten.tec.2008.0173. [DOI] [PubMed] [Google Scholar]
- Kern S., Eichler H., Stoeve J., Kluter H., Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Kharazi A., Levy M.L., Visperas M.C., Lin C.M. Chicken embryonic brain: an in vivo model for verifying neural stem cell potency. J. Neurosurg. 2013 doi: 10.3171/2013.1.JNS12698. DOI: 10.3171/2013.3171.JNS12698, (March 1) [DOI] [PubMed] [Google Scholar]
- Khatri M., Sharma J.M. Susceptibility of chicken mesenchymal stem cells to infectious bursal disease virus. J. Virol. Methods. 2009;160:197–199. doi: 10.1016/j.jviromet.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Khatri M., O'Brien T.D., Sharma J.M. Isolation and differentiation of chicken mesenchymal stem cells from bone marrow. Stem Cells Dev. 2009;18:1485–1492. doi: 10.1089/scd.2008.0223. [DOI] [PubMed] [Google Scholar]
- Khatri M., O'Brien T.D., Goyal S.M., Sharma J.M. Isolation and characterization of chicken lung mesenchymal stromal cells and their susceptibility to avian influenza virus. Dev. Comp. Immunol. 2010;34:474–479. doi: 10.1016/j.dci.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.H., Yun T.W., Rengaraj D., Lee S.I., Lim S.M., Seo H.W., Park T.S., Han J.Y. Conserved functional characteristics of the PIWI family members in chicken germ cell lineage. Theriogenology. 2012;78:1948–1959. doi: 10.1016/j.theriogenology.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Kino K., Pain B., Leibo S.P., Cochran M., Clark M.E., Etches R.J. Production of chicken chimeras from injection of frozen–thawed blastodermal cells. Poult. Sci. 1997;76:753–760. doi: 10.1093/ps/76.5.753. [DOI] [PubMed] [Google Scholar]
- Knowles B.B., Aden D.P., Solter D. Monoclonal antibody detecting a stage-specific embryonic antigen (SSEA-1) on preimplantation mouse embryos and teratocarcinoma cells. Curr. Top. Microbiol. Immunol. 1978;81:51–53. doi: 10.1007/978-3-642-67448-8_8. [DOI] [PubMed] [Google Scholar]
- Kuwana T., Rogulska T. Migratory mechanisms of chick primordial germ cells toward gonadal anlage. Cell. Mol. Biol. 1999;45:725–736. [PubMed] [Google Scholar]
- Lavial F., Pain B. Chicken embryonic stem cells as a non-mammalian embryonic stem cell model. Dev. Growth Differ. 2010;52:101–114. doi: 10.1111/j.1440-169X.2009.01152.x. [DOI] [PubMed] [Google Scholar]
- Lavial F., Acloque H., Bertocchini F., Macleod D.J., Boast S., Bachelard E., Montillet G., Thenot S., Sang H.M., Stern C.D., Samarut J., Pain B. The Oct4 homologue PouV and Nanog regulate pluripotency in chicken embryonic stem cells. Development. 2007;134:3549–3563. doi: 10.1242/dev.006569. [DOI] [PubMed] [Google Scholar]
- Lavial F., Acloque H., Bachelard E., Nieto M.A., Samarut J., Pain B. Ectopic expression of Cvh (Chicken Vasa homologue) mediates the reprogramming of chicken embryonic stem cells to a germ cell fate. Dev. Biol. 2009;330:73–82. doi: 10.1016/j.ydbio.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M.E., Remberger M., Dini G., Egeler R.M., Bacigalupo A., Fibbe W., Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Qu-Petersen Z., Cao B., Kimura S., Jankowski R., Cummins J., Usas A., Gates C., Robbins P., Wernig A., Huard J. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J. Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee O.K., Kuo T.K., Chen W.M., Lee K.D., Hsieh S.L., Chen T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- Lee Y.M., Jung J.G., Kim J.N., Park T.S., Kim T.M., Shin S.S., Kang D.K., Lim J.M., Han J.Y. A testis-mediated germline chimera production based on transfer of chicken testicular cells directly into heterologous testes. Biol. Reprod. 2006;75:380–386. doi: 10.1095/biolreprod.106.052084. [DOI] [PubMed] [Google Scholar]
- Lee C.C., Christensen J.E., Yoder M.C., Tarantal A.F. Clonal analysis and hierarchy of human bone marrow mesenchymal stem and progenitor cells. Exp. Hematol. 2010;38:46–54. doi: 10.1016/j.exphem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Sun G., Sun H., Xu Q., Gao B., Zhou G., Zhao W., Wu X., Bao W., Yu F., Wang K., Chen G. Efficient generation of transgenic chickens using the spermatogonial stem cells in vivo and ex vivo transfection. Sci. China C Life Sci. 2008;51:734–742. doi: 10.1007/s11427-008-0100-2. [DOI] [PubMed] [Google Scholar]
- Li B., Wang X.Y., Tian Z., Xiao X.J., Xu Q., Wei C.X., F.Y., Sun H.C., Chen G.H. Directional differentiation of chicken spermatogonial stem cells in vitro. Cytotherapy. 2010;12:326–331. doi: 10.3109/14653240903518155. [DOI] [PubMed] [Google Scholar]
- Li B.C., Tian Z.Q., Sun M., Xu Q., Wang X.Y., Qin Y.R., Xu F., Gao B., Wang K.H., Sun H.C., Chen G.H. Directional differentiation of chicken primordial germ cells into adipocytes, neuron-like cells, and osteoblasts. Mol. Reprod. Dev. 2010;77:795–801. doi: 10.1002/mrd.21224. [DOI] [PubMed] [Google Scholar]
- Lillico S.G., McGrew M.J., Sherman A., Sang H.M. Transgenic chickens as bioreactors for protein-based drugs. Drug Discov. Today. 2005;10:191–196. doi: 10.1016/S1359-6446(04)03317-3. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhang C., Tang X., Zeng W., Mi Y. Stimulating effects of androgen on proliferation of cultured ovarian germ cells through androgenic and estrogenic actions in embryonic chickens. Domest. Anim. Endocrinol. 2005;28:451–462. doi: 10.1016/j.domaniend.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhang C., Zeng W. Estrogenic and antioxidant effects of a phytoestrogen daidzein on ovarian germ cells in embryonic chickens. Domest. Anim. Endocrinol. 2006;31:258–268. doi: 10.1016/j.domaniend.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Liu L., He P., Cai K., Zhang Y., Li J., Cao F., Ding Z., Zhang N. Lentivirus-mediated expression of MxA in chicken spermatogonial stem cells. Reprod. Domest. Anim. 2010;45:e131–e137. doi: 10.1111/j.1439-0531.2009.01534.x. [DOI] [PubMed] [Google Scholar]
- Liu K., Ji G., Mao J., Liu M., Wang L., Chen C., Liu L. Generation of porcine-induced pluripotent stem cells by using OCT4 and KLF4 porcine factors. Cell. Reprogram. 2012;14:505–513. doi: 10.1089/cell.2012.0047. [DOI] [PubMed] [Google Scholar]
- Lu Y., West F.D., Jordan B.J., Mumaw J.L., Jordan E.T., Gallegos-Cardenas A., Beckstead R.B., Stice S.L. Avian-induced pluripotent stem cells derived using human reprogramming factors. Stem Cells Dev. 2012;21:394–403. doi: 10.1089/scd.2011.0499. [DOI] [PubMed] [Google Scholar]
- Macdonald J., Glover J.D., Taylor L., Sang H.M., McGrew M.J. Characterisation and germline transmission of cultured avian primordial germ cells. PLoS One. 2010;5:e15518. doi: 10.1371/journal.pone.0015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald J., Taylor L., Sherman A., Kawakami K., Takahashi Y., Sang H.M., McGrew M.J. Efficient genetic modification and germ-line transmission of primordial germ cells using piggyBac and Tol2 transposons. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E1466–E1472. doi: 10.1073/pnas.1118715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A., Lu D., Lu M., Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. (discussion 702-693) [DOI] [PubMed] [Google Scholar]
- Marzullo G. Production of chick chimaeras. Nature. 1970;225:72–73. doi: 10.1038/225072a0. [DOI] [PubMed] [Google Scholar]
- McGrew M.J., Sherman A., Ellard F.M., Lillico S.G., Gilhooley H.J., Kingsman A.J., Mitrophanous K.A., Sang H. Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Rep. 2004;5:728–733. doi: 10.1038/sj.embor.7400171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew M.J., Sherman A., Lillico S.G., Ellard F.M., Radcliffe P.A., Gilhooley H.J., Mitrophanous K.A., Cambray N., Wilson V., Sang H. Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development. 2008;135:2289–2299. doi: 10.1242/dev.022020. [DOI] [PubMed] [Google Scholar]
- Montarras D., Morgan J., Collins C., Relaix F., Zaffran S., Cumano A., Partridge T., Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Mu R., Bian Y.C., Pu Y.B., Li X.C., Wang F.L., Guan W.J. Isolation and biological characterization of mesenchymal stem cells from Beijing fatty chicken fetal liver. Yi Chuan. 2013;35:365–372. doi: 10.3724/sp.j.1005.2013.00365. [DOI] [PubMed] [Google Scholar]
- Naito M., Agata K., Otsuka K., Kino K., Ohta M., Hirose K., Perry M.M., Eguchi G. Embryonic expression of beta-actin-lacZ hybrid gene injected into the fertilized ovum of the domestic fowl. Int. J. Dev. Biol. 1991;35:69–75. [PubMed] [Google Scholar]
- Naito M., Sasaki E., Ohtaki M., Sakurai M. Introduction of exogenous DNA into somatic and germ cells of chickens by microinjection into the germinal disc of fertilized ova. Mol. Reprod. Dev. 1994;37:167–171. doi: 10.1002/mrd.1080370207. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yamamoto Y., Usui F., Mushika T., Ono T., Setioko A.R., Takeda K., Nirasawa K., Kagami H., Tagami T. Migration and proliferation of primordial germ cells in the early chicken embryo. Poult. Sci. 2007;86:2182–2193. doi: 10.1093/ps/86.10.2182. [DOI] [PubMed] [Google Scholar]
- Neuhuber B., Timothy Himes B., Shumsky J.S., Gallo G., Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Nichols J., Chambers I., Smith A. Derivation of germline competent embryonic stem cells with a combination of interleukin-6 and soluble interleukin-6 receptor. Exp. Cell Res. 1994;215:237–239. doi: 10.1006/excr.1994.1338. [DOI] [PubMed] [Google Scholar]
- Nicolas J.F., Mathis L., Bonnerot C., Saurin W. Evidence in the mouse for self-renewing stem cells in the formation of a segmented longitudinal structure, the myotome. Development. 1996;122:2933–2946. doi: 10.1242/dev.122.9.2933. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P.D., Sutasurya L.A. Cambridge University Press; 1979. Primordial Germ Cells in the Chordates. [Google Scholar]
- Ninagawa N., Murakami R., Isobe E., Tanaka Y., Nakagawa H., Torihashi S. Mesenchymal stem cells originating from ES cells show high telomerase activity and therapeutic benefits. Differentiation. 2011;82:153–164. doi: 10.1016/j.diff.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Neuronal replacement in adulthood. Ann. N. Y. Acad. Sci. 1985;457:143–161. doi: 10.1111/j.1749-6632.1985.tb20803.x. [DOI] [PubMed] [Google Scholar]
- Okita K., Yamanaka S. Induction of pluripotency by defined factors. Exp. Cell Res. 2010;316:2565–2570. doi: 10.1016/j.yexcr.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Okita K., Nakagawa M., Hyenjong H., Ichisaka T., Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Okita K., Yamakawa T., Matsumura Y., Sato Y., Amano N., Watanabe A., Goshima N., Yamanaka S. An Efficient non-viral method to generate integration-free human iPS cells from cord blood and peripheral blood cells. Stem Cells. 2013;31:458–466. doi: 10.1002/stem.1293. [DOI] [PubMed] [Google Scholar]
- Owen M., Friedenstein A.J. Stromal stem cells: marrow-derived osteogenic precursors. CIBA Found. Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- Pain B., Clark M.E., Shen M., Nakazawa H., Sakurai M., Samarut J., Etches R.J. Long-term in vitro culture and characterisation of avian embryonic stem cells with multiple morphogenetic potentialities. Development. 1996;122:2339–2348. doi: 10.1242/dev.122.8.2339. [DOI] [PubMed] [Google Scholar]
- Palmeirim I., Rodrigues S., Dale J.K., Maroto M. Development on time. Adv. Exp. Med. Biol. 2008;641:62–71. doi: 10.1007/978-0-387-09794-7_5. [DOI] [PubMed] [Google Scholar]
- Park T.S., Han J.Y. Derivation and characterization of pluripotent embryonic germ cells in chicken. Mol. Reprod. Dev. 2000;56:475–482. doi: 10.1002/1098-2795(200008)56:4<475::AID-MRD5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Park T.S., Jeong D.K., Kim J.N., Song G.H., Hong Y.H., Lim J.M., Han J.Y. Improved germline transmission in chicken chimeras produced by transplantation of gonadal primordial germ cells into recipient embryos. Biol. Reprod. 2003;68:1657–1662. doi: 10.1095/biolreprod.102.006825. [DOI] [PubMed] [Google Scholar]
- Paton J.A., Nottebohm F.N. Neurons generated in the adult brain are recruited into functional circuits. Science. 1984;225:1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- Perin L., Giuliani S., Jin D., Sedrakyan S., Carraro G., Habibian R., Warburton D., Atala A., De Filippo R.E. Renal differentiation of amniotic fluid stem cells. Cell Prolif. 2007;40:936–948. doi: 10.1111/j.1365-2184.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitte J.N., Clark M.E., Liu G., Verrinder Gibbins A.M., Etches R.J. Production of somatic and germline chimeras in the chicken by transfer of early blastodermal cells. Development. 1990;108:185–189. doi: 10.1242/dev.108.1.185. [DOI] [PubMed] [Google Scholar]
- Petitte J.N., Karagenc L., Ginsburg M. The origin of the avian germ line and transgenesis in birds. Poult. Sci. 1997;76:1084–1092. doi: 10.1093/ps/76.8.1084. [DOI] [PubMed] [Google Scholar]
- Petitte J.N., Liu G., Yang Z. Avian pluripotent stem cells. Mech. Dev. 2004;121:1159–1168. doi: 10.1016/j.mod.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Picard C.A., Marcelle C. Two distinct muscle progenitor populations coexist throughout amniote development. Dev. Biol. 2013;373:141–148. doi: 10.1016/j.ydbio.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Pittenger M.F., Martin B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Poynter G., Lansford R. Generating transgenic quail using lentiviruses. Methods Cell Biol. 2008;87:281–293. doi: 10.1016/S0091-679X(08)00215-X. [DOI] [PubMed] [Google Scholar]
- Poynter G., Huss D., Lansford R. Generation of high-titer lentivirus for the production of transgenic quail. Cold Spring Harb. Protoc. 2009 doi: 10.1101/pdb.prot5117. [DOI] [PubMed] [Google Scholar]
- Poynter G., Huss D., Lansford R. Injection of lentivirus into stage-X blastoderm for the production of transgenic quail. Cold Spring Harb. Protoc. 2009 doi: 10.1101/pdb.prot5118. [DOI] [PubMed] [Google Scholar]
- Poynter G., Huss D., Lansford R. Japanese quail: an efficient animal model for the production of transgenic avians. Cold Spring Harb. Protoc. 2009 doi: 10.1101/pdb.emo112. [DOI] [PubMed] [Google Scholar]
- Primmett D.R., Norris W.E., Carlson G.J., Keynes R.J., Stern C.D. Periodic segmental anomalies induced by heat shock in the chick embryo are associated with the cell cycle. Development. 1989;105:119–130. doi: 10.1242/dev.105.1.119. [DOI] [PubMed] [Google Scholar]
- Prockop D.J. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol. Ther. 2009;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusa A.R., Marton E., Rosner M., Bernaschek G., Hengstschlager M. Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum. Reprod. 2003;18:1489–1493. doi: 10.1093/humrep/deg279. [DOI] [PubMed] [Google Scholar]
- Qu X., Liu T., Song K., Li X., Ge D. Induced pluripotent stem cells generated from human adipose-derived stem cells using a non-viral polycistronic plasmid in feeder-free conditions. PLoS One. 2012;7:e48161. doi: 10.1371/journal.pone.0048161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese J.S., Koc O.N., Gerson S.L. Human mesenchymal stem cells provide stromal support for efficient CD34 + transduction. J. Hematother. Stem Cell Res. 1999;8:515–523. doi: 10.1089/152581699319966. [DOI] [PubMed] [Google Scholar]
- Relaix F., Rocancourt D., Mansouri A., Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Rengaraj D., Zheng Y.H., Kang K.S., Park K.J., Lee B.R., Lee S.I., Choi J.W., Han J.Y. Conserved expression pattern of chicken DAZL in primordial germ cells and germ-line cells. Theriogenology. 2010;74:765–776. doi: 10.1016/j.theriogenology.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Reynolds B.A., Rietze R.L. Neural stem cells and neurospheres—re-evaluating the relationship. Nat. Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- Riordan N.H., Ichim T.E., Min W.P., Wang H., Solano F., Lara F., Alfaro M., Rodriguez J.P., Harman R.J., Patel A.N., Murphy M.P., Lee R.R., Minev B. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J. Transl. Med. 2009;7:29. doi: 10.1186/1479-5876-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A., Allegrucci C., Alberio R. Modulation of pluripotency in the porcine embryo and iPS cells. PLoS One. 2012;7:e49079. doi: 10.1371/journal.pone.0049079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanoff A.L. Macmillan; New York: 1960. The avian Embryo: Structural and Functional Development; p. 1305. [Google Scholar]
- Romanov Y.A., Darevskaya A.N., Merzlikina N.V., Buravkova L.B. Mesenchymal stem cells from human bone marrow and adipose tissue: isolation, characterization, and differentiation potentialities. Bull. Exp. Biol. Med. 2005;140:138–143. doi: 10.1007/s10517-005-0430-z. [DOI] [PubMed] [Google Scholar]
- Rudnicki M.A., Le Grand F., McKinnell I., Kuang S. The molecular regulation of muscle stem cell function. Cold Spring Harb. Symp. Quant. Biol. 2008;73:323–331. doi: 10.1101/sqb.2008.73.064. [DOI] [PubMed] [Google Scholar]
- Saito T., Dennis J.E., Lennon D.P., Young R.G., Caplan A.I. Myogenic expression of mesenchymal stem cells within myotubes of mdx mice in vitro and in vivo. Tissue Eng. 1995;1:327–343. doi: 10.1089/ten.1995.1.327. [DOI] [PubMed] [Google Scholar]
- Sang H. Prospects for transgenesis in the chick. Mech. Dev. 2004;121:1179–1186. doi: 10.1016/j.mod.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Sato Y., Lansford R. Transgenesis and imaging in birds, and available transgenic reporter lines. Dev. Growth Differ. 2013;55:406–421. doi: 10.1111/dgd.12058. [DOI] [PubMed] [Google Scholar]
- Sato Y., Poynter G., Huss D., Filla M.B., Czirok A., Rongish B.J., Little C.D., Fraser S.E., Lansford R. Dynamic analysis of vascular morphogenesis using transgenic quail embryos. PLoS One. 2010;5:e12674. doi: 10.1371/journal.pone.0012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C.E., Wynn S.L., Sesay A., Cruz C., Cheung M., Gomez Gaviro M.V., Booth S., Gao B., Cheah K.S., Lovell-Badge R., Briscoe J. SOX9 induces and maintains neural stem cells. Nat. Neurosci. 2010;13:1181–1189. doi: 10.1038/nn.2646. [DOI] [PubMed] [Google Scholar]
- Seidl A.H., Sanchez J.T., Schecterson L., Tabor K.M., Wang Y., Kashima D.T., Poynter G., Huss D., Fraser S.E., Lansford R., Rubel E.W. Transgenic quail as a model for research in the avian nervous system: a comparative study of the auditory brainstem. J. Comp. Neurol. 2013;521:5–23. doi: 10.1002/cne.23187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selleck M.A., Stern C.D. Fate mapping and cell lineage analysis of Hensen's node in the chick embryo. Development. 1991;112:615–626. doi: 10.1242/dev.112.2.615. [DOI] [PubMed] [Google Scholar]
- Selleck M.A., Stern C.D. Evidence for stem cells in the mesoderm of Hensen's node and their role in embryonic pattern formation. In: Bellairs R., Sanders E.J., Lash J.W., editors. Formation and Differentiation of Early Embryonic Mesoderm. Plenum Press; New York: 1992. pp. 23–31. [Google Scholar]
- Shevinsky L.H., Knowles B.B., Damjanov I., Solter D. Monoclonal antibody to murine embryos defines a stage-specific embryonic antigen expressed on mouse embryos and human teratocarcinoma cells. Cell. 1982;30:697–705. doi: 10.1016/0092-8674(82)90274-4. [DOI] [PubMed] [Google Scholar]
- Shin S.S., Kim T.M., Kim S.Y., Kim T.W., Seo H.W., Lee S.K., Kwon S.C., Lee G.S., Kim H., Lim J.M., Han J.Y. Generation of transgenic quail through germ cell-mediated germline transmission. FASEB J. 2008;22:2435–2444. doi: 10.1096/fj.07-101485. [DOI] [PubMed] [Google Scholar]
- Shiue Y.L., Tailiu J.J., Liou J.F., Lu H.T., Tai C., Shiau J.W., Chen L.R. Establishment of the long-term in vitro culture system for chicken primordial germ cells. Reprod. Domest. Anim. 2009;44:55–61. doi: 10.1111/j.1439-0531.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- Sirotkin A.V., Grossmann R. Leptin directly controls proliferation, apoptosis and secretory activity of cultured chicken ovarian cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007;148:422–429. doi: 10.1016/j.cbpa.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Smith A.G., Hooper M.L. Buffalo rat liver cells produce a diffusible activity which inhibits the differentiation of murine embryonal carcinoma and embryonic stem cells. Dev. Biol. 1987;121:1–9. doi: 10.1016/0012-1606(87)90132-1. [DOI] [PubMed] [Google Scholar]
- Smith C.A., Sinclair A.H. Sex determination in the chicken embryo. J. Exp. Zool. 2001;290:691–699. doi: 10.1002/jez.1119. [DOI] [PubMed] [Google Scholar]
- Smith C.A., Sinclair A.H. Sex determination: insights from the chicken. Bioessays. 2004;26:120–132. doi: 10.1002/bies.10400. [DOI] [PubMed] [Google Scholar]
- Solchaga L.A., Penick K., Porter J.D., Goldberg V.M., Caplan A.I., Welter J.F. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J. Cell. Physiol. 2005;203:398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- Solter D., Knowles B.B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1) Proc. Natl. Acad. Sci. U. S. A. 1978;75:5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A.G., Rozelle S.S., Sullivan S., Mills J.A., Park S.M., Smith B.W., Iyer A.M., French D.L., Kotton D.N., Gadue P., Murphy G.J., Mostoslavsky G. Generation of human induced pluripotent stem cells from peripheral blood using the STEMCCA lentiviral vector. J. Vis. Exp. 2012;68:e4327. doi: 10.3791/4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino A., Ferracin M., Castelli G., Biffoni M., Tomaselli G., Baiocchi M., Fatica A., Negrini M., Peschle C., Valtieri M. Isolation and characterization of CD146 + multipotent mesenchymal stromal cells. Exp. Hematol. 2008;36:1035–1046. doi: 10.1016/j.exphem.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Spratt N.T., Haas H. Integrative mechanisms in development of the early chick blastoderm. I. Regulative potentiality of separated parts. J. Exp. Zool. 1960;145:97–137. [Google Scholar]
- Steigman S.A., Ahmed A., Shanti R.M., Tuan R.S., Valim C., Fauza D.O. Sternal repair with bone grafts engineered from amniotic mesenchymal stem cells. J. Pediatr. Surg. 2009;44:1120–1126. doi: 10.1016/j.jpedsurg.2009.02.038. (discussion 1126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern C.D. Chick stem cells. Curr. Top. Microbiol. Immunol. 1996;212:195–206. doi: 10.1007/978-3-642-80057-3_16. [DOI] [PubMed] [Google Scholar]
- Stern C.D. The chick embryo—past, present and future as a model system in developmental biology. Mech. Dev. 2004;121:1011–1013. doi: 10.1016/j.mod.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Stern C.D. Gastrulation in the chick. In: Stern C.D., editor. Gastrulation: From Cells to Embryo. Cold Spring Harbor Press; New York: 2004. pp. 219–232. [Google Scholar]
- Stern C.D. The chick; a great model system becomes even greater. Dev. Cell. 2005;8:9–17. doi: 10.1016/j.devcel.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Stern C.D., Hatada Y., Selleck M.A., Storey K.G. Relationships between mesoderm induction and the embryonic axes in chick and frog embryos. Dev. Suppl. 1992;151–156 [PubMed] [Google Scholar]
- Stern C.D., Charite J., Deschamps J., Duboule D., Durston A.J., Kmita M., Nicolas J.F., Palmeirim I., Smith J.C., Wolpert L. Head-tail patterning of the vertebrate embryo: one, two or many unresolved problems? Int. J. Dev. Biol. 2006;50:3–15. doi: 10.1387/ijdb.052095cs. [DOI] [PubMed] [Google Scholar]
- Suraeva N.M., Vorotelyak E.A., Prokofiev M.I., Samoilov A.V., Vasiliev A.V., Terskikh V.V., Baryshnikov A.Y. Isolation, characteristics, and long-term culturing of chicken gonadal primordial germ cells and blastodermal cells. Dokl. Biol. Sci. 2008;423:461–463. doi: 10.1134/s0012496608060276. [DOI] [PubMed] [Google Scholar]
- Tagirov M., Golovan S. The effect of busulfan treatment on endogenous spermatogonial stem cells in immature roosters. Poult. Sci. 2012;91:1680–1685. doi: 10.3382/ps.2011-02014. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Okita K., Nakagawa M., Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takata M., Yamamoto K., Matsushita N., Kitao H., Hirano S., Ishiai M. The Fanconi anemia pathway promotes homologous recombination repair in DT40 cell line. Subcell. Biochem. 2006;40:295–311. doi: 10.1007/978-1-4020-4896-8_17. [DOI] [PubMed] [Google Scholar]
- Takata M., Ishiai M., Kitao H. The Fanconi anemia pathway: insights from somatic cell genetics using DT40 cell line. Mutat. Res. 2009;668:92–102. doi: 10.1016/j.mrfmmm.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Thoraval P., Lasserre F., Coudert F., Dambrine G. Somatic and germline chicken chimeras obtained from brown and white Leghorns by transfer of early blastodermal cells. Poult. Sci. 1994;73:1897–1905. doi: 10.3382/ps.0731897. [DOI] [PubMed] [Google Scholar]
- Toma C., Pittenger M.F., Cahill K.S., Byrne B.J., Kessler P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- Trivedi P., Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp. Hematol. 2008;36:350–359. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsagias N., Koliakos I., Karagiannis V., Eleftheriadou M., Koliakos G.G. Isolation of mesenchymal stem cells using the total length of umbilical cord for transplantation purposes. Transfus. Med. 2011;21:253–261. doi: 10.1111/j.1365-3148.2011.01076.x. [DOI] [PubMed] [Google Scholar]
- Tsunekawa N., Naito M., Sakai Y., Nishida T., Noce T. Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development. 2000;127:2741–2750. doi: 10.1242/dev.127.12.2741. [DOI] [PubMed] [Google Scholar]
- van de Lavoir M.C., Mather-Love C. Avian embryonic stem cells. Methods Enzymol. 2006;418:38–64. doi: 10.1016/S0076-6879(06)18003-9. [DOI] [PubMed] [Google Scholar]
- van de Lavoir M.C., Diamond J.H., Leighton P.A., Mather-Love C., Heyer B.S., Bradshaw R., Kerchner A., Hooi L.T., Gessaro T.M., Swanberg S.E., Delany M.E., Etches R.J. Germline transmission of genetically modified primordial germ cells. Nature. 2006;441:766–769. doi: 10.1038/nature04831. [DOI] [PubMed] [Google Scholar]
- van de Lavoir M.C., Mather-Love C., Leighton P., Diamond J.H., Heyer B.S., Roberts R., Zhu L., Winters-Digiacinto P., Kerchner A., Gessaro T., Swanberg S., Delany M.E., Etches R.J. High-grade transgenic somatic chimeras from chicken embryonic stem cells. Mech. Dev. 2006;123:31–41. doi: 10.1016/j.mod.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Vasa M., Fichtlscherer S., Aicher A., Adler K., Urbich C., Martin H., Zeiher A.M., Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- Velazquez P.N., Peralta I., Bobes R.J., Romano M.C. Insulin stimulates proliferation but not 17beta-estradiol production in cultured chick embryo ovarian cells. Poult. Sci. 2006;85:100–105. doi: 10.1093/ps/85.1.100. [DOI] [PubMed] [Google Scholar]
- Vick L., Li Y., Simkiss K. Transgenic birds from transformed primordial germ cells. Proc. Biol. Sci. 1993;251:179–182. doi: 10.1098/rspb.1993.0026. [DOI] [PubMed] [Google Scholar]
- Vija L., Farge D., Gautier J.F., Vexiau P., Dumitrache C., Bourgarit A., Verrecchia F., Larghero J. Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes Metab. 2009;35:85–93. doi: 10.1016/j.diabet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Wakitani S., Goto T., Pineda S.J., Young R.G., Mansour J.M., Caplan A.I., Goldberg V.M. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J. Bone Joint. Surg. Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- Wakitani S., Saito T., Caplan A.I. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- Wang J., Jiao F., Pan X.H., Xie S.Y., Li Z.L., Niu X.H., Du L.X. Directed differentiation of chick embryonic germ cells into neural cells using retinoic acid induction in vitro. J. Neurosci. Methods. 2009;177:168–176. doi: 10.1016/j.jneumeth.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Wang L., Tran I., Seshareddy K., Weiss M.L., Detamore M.S. A comparison of human bone marrow-derived mesenchymal stem cells and human umbilical cord-derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Eng. Part A. 2009;15:2259–2266. doi: 10.1089/ten.tea.2008.0393. [DOI] [PubMed] [Google Scholar]
- Weiss M.L., Troyer D.L. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2:155–162. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West F.D., Terlouw S.L., Kwon D.J., Mumaw J.L., Dhara S.K., Hasneen K., Dobrinsky J.R., Stice S.L. Porcine induced pluripotent stem cells produce chimeric offspring. Stem Cells Dev. 2010;19:1211–1220. doi: 10.1089/scd.2009.0458. [DOI] [PubMed] [Google Scholar]
- Whalley K., Gogel S., Lange S., Ferretti P. Changes in progenitor populations and ongoing neurogenesis in the regenerating chick spinal cord. Dev. Biol. 2009;332:234–245. doi: 10.1016/j.ydbio.2009.05.569. [DOI] [PubMed] [Google Scholar]
- Wiedemann A., Hemmer K., Bernemann I., Gohring G., Pogozhykh O., Figueiredo C., Glage S., Schambach A., Schwamborn J.C., Blasczyk R., Muller T. Induced pluripotent stem cells generated from adult bone marrow-derived cells of the nonhuman primate (Callithrix jacchus) using a novel quad-cistronic and excisable lentiviral vector. Cell. Reprogram. 2012;14:485–496. doi: 10.1089/cell.2012.0036. [DOI] [PubMed] [Google Scholar]
- Wilson V., Olivera-Martinez I., Storey K.G. Stem cells, signals and vertebrate body axis extension. Development. 2009;136:1591–1604. doi: 10.1242/dev.021246. [DOI] [PubMed] [Google Scholar]
- Wu Y., Ge C., Zeng W., Zhang C. Induced multilineage differentiation of chicken embryonic germ cells via embryoid body formation. Stem Cells Dev. 2010;19:195–202. doi: 10.1089/scd.2008.0383. [DOI] [PubMed] [Google Scholar]
- Wunderlich S., Haase A., Merkert S., Beier J., Schwanke K., Schambach A., Glage S., Gohring G., Curnow E.C., Martin U. Induction of pluripotent stem cells from a cynomolgus monkey using a polycistronic simian immunodeficiency virus-based vector, differentiation toward functional cardiomyocytes, and generation of stably expressing reporter lines. Cell. Reprogram. 2012;14:471–484. doi: 10.1089/cell.2012.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M., Zhang C. Estrogenic and toxic effects of polychlorinated biphenyls on cultured ovarian germ cells of embryonic chickens. Reprod. Toxicol. 2004;19:79–86. doi: 10.1016/j.reprotox.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. Development and postnatal regulation of adult myoblasts. Microsc. Res. Tech. 1995;30:366–380. doi: 10.1002/jemt.1070300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Usui F., Nakamura Y., Ito Y., Tagami T., Nirasawa K., Matsubara Y., Ono T., Kagami H. A novel method to isolate primordial germ cells and its use for the generation of germline chimeras in chicken. Biol. Reprod. 2007;77:115–119. doi: 10.1095/biolreprod.107.061200. [DOI] [PubMed] [Google Scholar]
- Yamanaka S. Pluripotency and nuclear reprogramming. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2008;363:2079–2087. doi: 10.1098/rstb.2008.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J.U., Barthel T.S., Nishimura K., Solchaga L., Caplan A.I., Goldberg V.M., Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J. Bone Joint. Surg. Am. 1998;80:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Yoshimura K., Shigeura T., Matsumoto D., Sato T., Takaki Y., Aiba-Kojima E., Sato K., Inoue K., Nagase T., Koshima I., Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- Young R.G., Butler D.L., Weber W., Caplan A.I., Gordon S.L., Fink D.J. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J. Orthop. Res. 1998;16:406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., Slukvin I.I., Thomson J.A. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yu J., Hu K., Smuga-Otto K., Tian S., Stewart R., Slukvin I.I., Thomson J.A. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Ding L.J., Sun G.B., Sun P.X., He X.H., Ni L.G., Li B.C. Transgenic sperm produced by electrotransfection and allogeneic transplantation of chicken fetal spermatogonial stem cells. Mol. Reprod. Dev. 2010;77:340–347. doi: 10.1002/mrd.21147. [DOI] [PubMed] [Google Scholar]
- Zhang X., Hirai M., Cantero S., Ciubotariu R., Dobrila L., Hirsh A., Igura K., Satoh H., Yokomi I., Nishimura T., Yamaguchi S., Yoshimura K., Rubinstein P., Takahashi T.A. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J. Cell. Biochem. 2011;112:1206–1218. doi: 10.1002/jcb.23042. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yang H., Zhang Z., Shi Q., Wang D., Zheng M., Li B., Song J. Isolation of chicken embryonic stem cell and preparation of chicken chimeric model. Mol. Biol. Rep. 2013;40:2149–2156. doi: 10.1007/s11033-012-2274-8. [DOI] [PubMed] [Google Scholar]
- Zhong B., Trobridge G.D., Zhang X., Watts K.L., Ramakrishnan A., Wohlfahrt M., Adair J.E., Kiem H.P. Efficient generation of nonhuman primate induced pluripotent stem cells. Stem Cells Dev. 2011;20:795–807. doi: 10.1089/scd.2010.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Benda C., Dunzinger S., Huang Y., Ho J.C., Yang J., Wang Y., Zhang Y., Zhuang Q., Li Y., Bao X., Tse H.F., Grillari J., Grillari-Voglauer R., Pei D., Esteban M.A. Generation of human induced pluripotent stem cells from urine samples. Nat. Protoc. 2012;7:2080–2089. doi: 10.1038/nprot.2012.115. [DOI] [PubMed] [Google Scholar]
- Zsebo K.M., Williams D.A., Geissler E.N., Broudy V.C., Martin F.H., Atkins H.L., Hsu R.Y., Birkett N.C., Okino K.H., Murdock D.C. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]