Abstract
The chemokine receptor CCR4 has at least two natural agonist ligands, MDC (CCL22) and TARC (CCL17) which bind to the same orthosteric site with a similar affinity. Both ligands are known to evoke chemotaxis of CCR4-bearing T cells and also elicit CCR4 receptor internalization. A series of small molecule allosteric antagonists have been described which displace the agonist ligand, and inhibit chemotaxis. The aim of this study was to determine which cellular coupling pathways are involved in internalization, and if antagonists binding to the CCR4 receptor could themselves evoke receptor internalization. CCL22 binding coupled CCR4 efficiently to β-arrestin and stimulated GTPγS binding however CCL17 did not couple to β-arrestin and only partially stimulated GTPγS binding. CCL22 potently induced internalization of almost all cell surface CCR4, while CCL17 showed only weak effects. We describe four small molecule antagonists that were demonstrated to bind to two distinct allosteric sites on the CCR4 receptor, and while both classes inhibited agonist ligand binding and chemotaxis, one of the allosteric sites also evoked receptor internalization. Furthermore, we also characterize an N-terminally truncated version of CCL22 which acts as a competitive antagonist at the orthosteric site, and surprisingly also evokes receptor internalization without demonstrating any agonist activity. Collectively this study demonstrates that orthosteric and allosteric antagonists of the CCR4 receptor are capable of evoking receptor internalization, providing a novel strategy for drug discovery against this class of target.
Keywords: Chemokine, CCR4, MDC, TARC, CCL22, CCL17
1. Introduction
CCR4 is expressed on a variety of functionally distinct thymocytes including skin-homing T cells (Campbell et al., 1999), CD25+ T suppressor cells (Iellem et al., 2001) and T helper (Th)2 cells (Bonecchi et al., 1998), all of which have been shown to migrate to CCL22 and CCL17 released at sites of inflammation. CCR4+ T cells have been shown to be elevated in several types of allergic disease (Nouri-Aria et al., 2002; Yang et al., 2004) which increases further upon allergen challenge (Panina-Bordignon et al., 2001). Taken together, these findings have driven interest in CCR4 as a therapeutic target for the treatment of inflammatory diseases with a Th2 cell component, such as asthma (Gonzalo et al., 1999; Kawasaki et al., 2001) and atopic dermatitis (Reiss et al., 2001).
Consequently, considerable effort has gone into discovering small molecule CCR4 antagonists although only one molecule has made it to human clinical trials to date (Cahn et al., 2013). A subset of these small molecules have been shown to require intracellular access for their activity (Purandare and Somerville, 2006), resulting in the identification of an intracellular allosteric site on the receptor (referred to as ‘site-2’ in this study) (Andrews et al., 2008). In the same study, it was apparent that one compound (BMS-397, referred to as ‘Compound 2’ hereafter) did not bind to this intracellular allosteric site (Purandare, 2004) but a distinct site (referred to as ‘site-1’ in this study). Further work (Weston and Hall, 2008) confirmed that the interactions of these molecules with CCR4 indicate the presence of two allosteric binding sites on the receptor, both of which are spatially distinct from the orthosteric site to which CCL22 and CCL17 bind. Each of these small molecules demonstrated the ability to allosterically evoke agonist dissociation from the orthosteric site, antagonizing cellular functions such as chemotaxis and calcium mobilization. These molecular probes have been used in the present study to demonstrate biased signaling capacity of CCR4 receptors.
In addition to evoking chemotaxis, CCR4 ligands have also been shown to induce receptor internalization from the cell surface of human Th2 cells, resulting in a loss of functional responsiveness (Mariani et al., 2004; Sebastiani et al., 2005). GPCR internalization following agonist exposure is a component of receptor desensitization, however the role of receptor internalization in regulating the chemotactic response remains controversial.
In this study we expand the observation that the CCR4 agonists CCL22 and CCL17 can evoke receptor internalization by exploring the coupling of the CCR4 receptor to G-proteins and β-arrestin. Additionally, we confirm the presence of two allosteric binding sites and demonstrate that small molecules binding to ‘site-1’ are also capable of evoking receptor internalization while binders of ‘site-2’ do not. Lastly, we also characterize an N-terminally truncated version of CCL22 (MDC67) which acts as a competitive antagonist at the orthosteric site, and surprisingly also evokes receptor internalization without demonstrating any agonist activity. Based on these biased signaling observations we provide a novel strategy for drug discovery against this class of chemokine receptor.
2. Materials and methods
2.1. Reagents
NeuroProbe ChemoTx 3 µm-pore chemotaxis chambers were purchased from Receptor Technologies Ltd. (Leamington Spa, UK). Phycoerythrin (PE)-Mouse Anti-Human CCR4 (CD194) mAb, PE Mouse IgG Isotype control, Fluorescein Isothiocyanate (FITC) Mouse IgG Isotype Control, Purified mouse anti-human CD194 were purchased from BD Pharmingen (Beckton Dickenson UK Ltd., Oxford, UK). FITC-conjugated anti-human CD4, Alexa-fluor 647 Phalloidin and Alexa-488 conjugated rabbit anti-mouse was purchased from Invitrogen Ltd., (Paisley, UK). [125I]CCL-17 (specific activity 2200 Ci/mmol) was obtained from Perkin-Elmer LAS UK Ltd. (Beaconsfield, UK). [3H]compound 2 and [3H]compound 3 (specific activity 37 and 53 Ci/mmol respectively) were synthesized by GE Healthcare UK Ltd. (Little Chalfont, UK). The majority of the chemokines CCL22 and CCL17 were purchased from RandD Systems Europe Ltd. (Abingdon, UK). Truncated CCL22, (MDC67) was mostly supplied by Biological Reagents and Assay Development (BRandAD), GlaxoSmithKline. Other samples of CCL17 and MDC67 were purchased from PeproTech (London, UK) while some CCL22 was supplied by BRandAD, GlaxoSmithKline. In this study we used a range of protein and small molecule ligands of the CCR4 receptor to probe aspects of its function. Compound 1 and Compound 2 are representative of a class of lipophilic amines, and Compound 3 and Compound 4 are representative of a class of arylsulfonamides (Procopiou et al., 2012), all of which were synthesized by Respiratory Therapy Area Unit Medicinal Chemistry, GlaxoSmithKline (Supplementary data Fig. S1).
2.2. Cell lines
HUT78 cells were obtained from the European Collection of Cell Cultures (ECACC), Wiltshire, UK. Chinese Hamster Ovary (CHO) cells were obtained from the ECACC, Wiltshire, UK. CHO cells were transfected with CCR4 cDNA from human basophils (Power et al., 1995) in the pCIN4 vector; stable transfectants were selected and cultured in DMEM (Hams)-F12 media supplemented with 10% heat-inactivated FBS, 1% l-glutamine and 50 µg/ml G418. Human Peripheral Blood Mononuclear Cells (PBMCs) were obtained from the peripheral blood of healthy volunteers using a Percol gradient according to standard protocol (Ulmer et al., 1983). Acquisition of the blood samples was approved by Hertfordshire Research Ethics Committee and all donors gave written informed consent prior to donation. Prior to use in all experiments, cells were re-suspended in Assay Buffer [RPMI 1640 supplemented with 1% Bovine Serum Albumin and 1 mM HEPES].
2.3. Cell culture and membrane preparation
The cells were grown in Corning CellSTACKS© (Corning Inc., NY, USA) and membrane fragments were prepared from the CHO-CCR4 cells as previously described (Slack and Hall, 2012).
2.4. Radioligand binding studies
All [125I]-CCL17 and [3H]antagonist radioligand binding experiments were performed using a format previously described (Slack et al., 2011; Slack and Hall, 2012) but with minor modifications. Briefly, antagonist binding assays were carried out in 96-deep well plates at ambient room temperature (20–22 °C) in binding buffer (without BSA) with either [3H]compound 2 or [3H]compound 3, membranes and either vehicle or unlabeled antagonist. Non-specific binding (NSB) was determined for [3H]compound 2 and [3H]compound 3 by 10 μM unlabeled compounds 2 or 3 respectively. Binding was terminated by rapid vacuum filtration through a 48-well Brandel (Brandel Inc. Gaithersburg, MD, USA) harvester onto GF/B filter papers pre-soaked in 0.3% v/v poly-ethylenimine (for [3H]compound 2) or water (for [3H]compound 3). Samples were washed rapidly three times with ice cold distilled water and filters transferred into liquid scintillation (LS) vials containing 4 ml LS fluid (Ultima-Flo™ M, Perkin-Elmer LAS UK Ltd., Beaconsfield, UK). The amount of radioligand bound to receptor was measured by LS microscopy using a TriCarb 2900TR LS counter (Perkin-Elmer LAS UK Ltd., Beaconsfield, UK). For saturation binding, CCR4 membranes were incubated with increasing concentrations of [3H]compound 2 or [3H]compound 3 in the presence of vehicle or NSB for 2 h prior to filtration.
For competition binding displacement studies, membranes were mixed with [3H]compound 2 or [3H]compound 3 and increasing concentrations of unlabeled ligand for 2 h prior to filtration.
2.5. Beta-arrestin assay
The CCR4 cell line was obtained from DiscoveRx and grown in the manufacturer׳s suggested medium. Agonist induced coupling was detected as described (Demont et al., 2011).
2.6. GTPγS assay
CHO-CCR4 cell membranes (5 µg/ml) were mixed at a 1:1 ratio with 25 mg/ml WGA coupled PS imaging (Leadseeker) beads (Perkin-Elmer) before being incubated for 1 h at 4 °C. GDP was added to 384-well solid white plates (Nunc, FAC 4.4 μM) containing test compound. 35S-GTPγS (Perkin-Elmer) was diluted 1:1200 in assay buffer and 20 μl/well added to the plates before centrifugation at 1200 rpm for 30 s. After 3 h plates were read using Viewlux (Perkin-Elmer) with a 613/55(A09) emission filter.
2.7. Chemotaxis assay
HUT78 cell chemotaxis was measured using a transwell chemotaxis chambers. Cells were loaded with 7.5 µg/ml Calcein-AM, washed and re-suspended in assay buffer [RPMI 1640, 1% BSA, 1 mM HEPES] then incubated at 37 °C for 30 min with vehicle or antagonist. Chemokines were diluted in assay buffer, loaded into the lower wells of the chemotaxis chamber and cells (1×107 cells/ml) were placed above the filter and the whole chamber was incubated at 37 °C for 90 min. After incubation, the filter was removed and washed with 50 ml Dulbecco׳s Phosphate Buffered Saline (DPBS). The number of migrated HUT78 cells into the filter was measured immediately using a Safire Multiplate Reader (Tecan, UK).
PBMC T-cell actin polymerization.
The human biological samples were sourced ethically and their research use was in accord with the terms of the informed consents. PBMCs were isolated from human blood and stained with FITC-conjugated anti-human CD4 and PE-conjugated anti-CCR4 antibodies before incubation with antagonist or vehicle for 30 min at 37 °C. Cells were then incubated with agonist for 15 s. The assay was terminated by addition of 3% formaldehyde and the fixed cells stained with Alexa fluor-647 Phalloidin. Data is expressed as a fraction of the mean fluorescence intensity of CD4+CCR4+/CD4+CCR4− cells in the same sample.
2.8. Measurement of cell surface and intracellular CCR4 levels
Cells were re-suspended in assay buffer at a concentration of 1×106 cells/ml and incubated with vehicle or antagonist for 30 min at 37 °C. Cells were subsequently incubated with increasing concentrations of CCR4 agonists for 30 min at 37 °C, followed by incubation with PE-conjugated anti-CCR4 antibody at 4 °C for 60 min. PBMCs required additional incubation with FITC-conjugated anti-CD4 antibody to ensure only CD4+CCR4+ cells are measured. Un-stimulated cells were incubated with PE Mouse IgG Isotype control to give a basal value. The assay was terminated by the addition of 3% formaldehyde and CCR4 levels evaluated by flow cytometry on a FACSCanto (BD). CCR4+CD4+HUT78 cells were identified by their forward and side-scatter characteristics and the mean fluorescence intensity of the PE-CCR4 antibody conjugated cells measured. Receptor internalization was expressed as a percentage of total receptor surface expression calculated using control cells incubated with PE-CCR4 (100%) or matched PE-isotype control (0%). To ensure the calculated internalization was not a result of chemokine preventing antibody binding, cells were chilled to 4 °C and permeabilised with 0.5% Saponin prior to PE-CCR4 antibody incubation and FACS measurement.
2.9. Confocal microscopy
CHO-CCR4 cells (25,000/ml) were seeded on 13 mm glass cover slips in the bottom of 12 well plates and left to adhere for 48 h. Cells were washed 3 times and incubated in serum free media with or without addition of chemokine (100 nM final concentration) or drug (10µM final concentration) for 30 min at 4 °C or 37 °C. Cover slips were washed 3 times with ice-cold PBS followed by addition of ice cold fixative (4% PFA in PBS) for 15 min on ice followed by 15 min at room temperature. Cover slips were blocked for at least 30 min in PBS/1%FCS or PBS/1%FCS/0.2% Saponin. Staining with anti-CCR4 antibody (1/100) was performed for 1 h at room temperature followed by washing 3 times in PBS/1%FCS. Secondary antibody, rabbit anti-mouse Alexa488 (1/400 in PBS 1%FCS +/− saponin) was performed for 1 h at room temperature followed by washing 3 times in PBS. Coverslips were mounted in Mowiol 4–88+DAPI and viewed on a Zeiss LSM 5 Pa confocal microscope. For Transferrin endocytosis studies, 20 μg/ml Alexa-546 conjugated Transferrin (Invitrogen) and 100 nM MDC were incubated with CHO-CCR4 cells for 30 min at 4 °C in serum free medium containing 0.1% Bovine Serum Albumin (BSA). Cells were washed 3 times in ice cold serum free medium/0.1% BSA then warmed to 37 °C for 30 min prior to fixation, permeabilisation and staining with anti-CCR4 as described above.
2.10. Data analysis
Statistical analyses were performed using Graphpad Prism for Windows, version 5.04 (Graphpad Software, California, USA). All values are expressed as mean±S.E.M. To determine significance between two groups a Students unpaired t-test was applied. One-way ANOVA with either post-hoc Dunnett׳s or Bonferroni Multiple Comparison Tests were used to compare multiple data sets to control. P-values <0.05 were considered significant. IC50 values from competition binding displacement curves were converted to inhibition constant (Ki) values using the Cheng–Prusoff equation (Cheng and Prusoff, 1973). Curve fitting for concentration–response relationships was achieved using a 4-parameter logistic equation fit. In Fig. 6 the lower asymptote for TARC and MDC67 was constrained to the maximal response obtained.
Fig. 6.
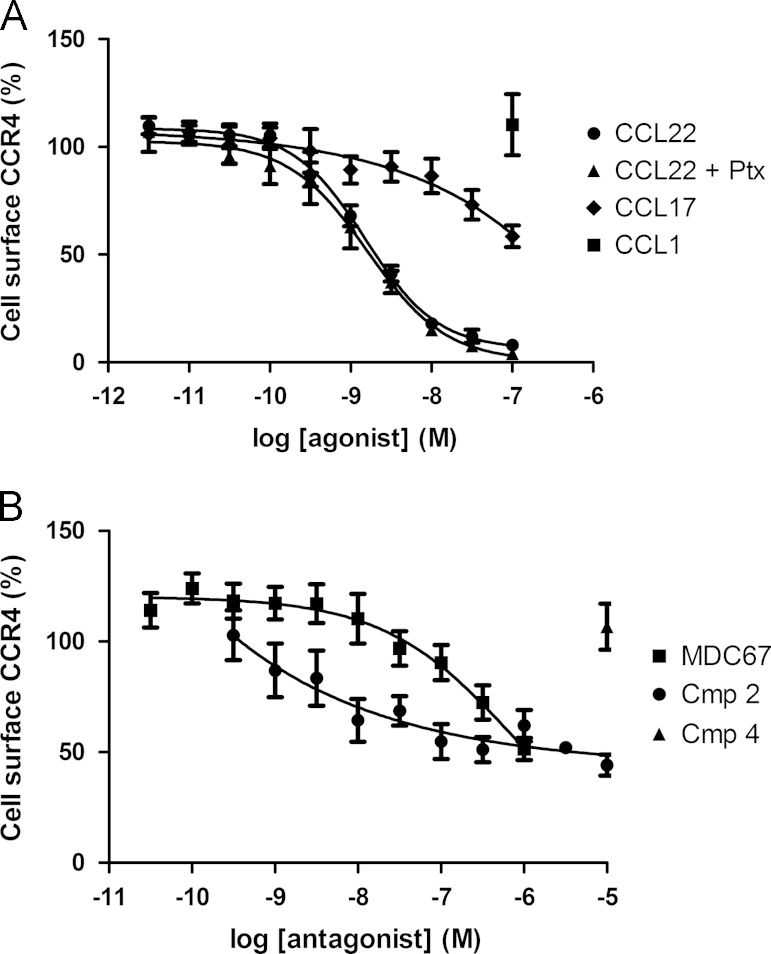
Agonist and antagonist evoked internalization of cell surface CCR4. Cell surface levels of CCR4 were determined in HUT78 cells using a PE-conjugated anti-CCR4 antibody. CCL22 evokes concentration-dependent internalization of HUT78 cell surface CCR4 receptors, which is unaffected by pretreatment with Pertussis toxin (panel A). CCL17 concentration-dependently evoked partial internalization of cell surface receptors, whereas the CCR8 agonist CCL1 (100 nM) had no effect (panel A). The antagonists MDC67 and Compound 2 also evoked partial internalization of cell surface receptors, whereas Compound 4 had no effect (panel B). Data shown are the mean±S.E.M.
3. Results
3.1. Small molecule antagonists of CCR4 bind to two distinct allosteric sites
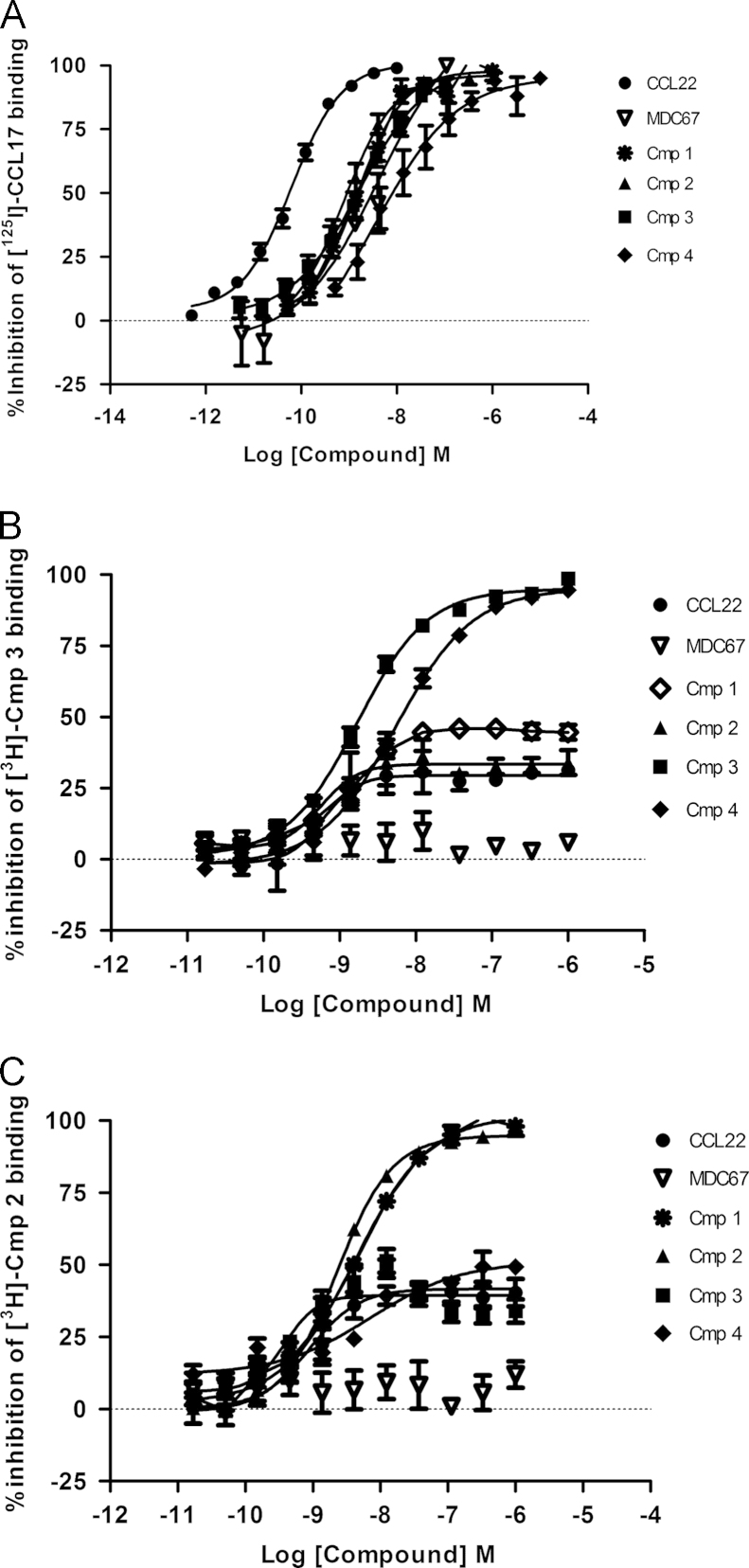
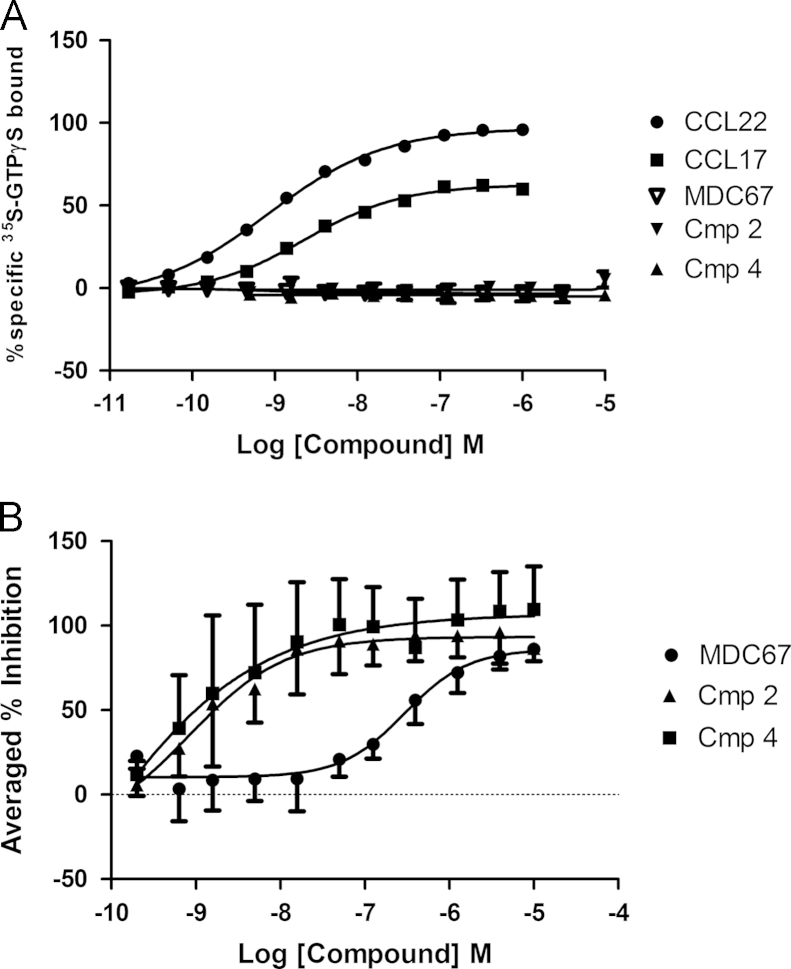
All of the four small molecule antagonists; Compound 1, Compound 2, Compound 3 and Compound 4 fully inhibited binding of 125I-CCL17 to CCR4 with pKis of 8.70±0.21, 9.10 ±0.09, 9.04±0.17 and 8.74±0.09 (n=8, Fig. 1A). Tritiated versions of Compound 2 and Compound 3 were synthesized and used in further radioligand binding studies. Unlabeled Compound 3 and Compound 4 fully competed binding of tritiated Compound 3, suggesting they are competitive at the same site (n=8, Fig. 1B). Conversely Compound 1, Compound 2 and CCL22 showed only partial competition and MDC67 showed none, suggesting they bound different sites on the receptor (n=8, Fig. 1B). This was confirmed using radiolabelled Compound 2, where unlabeled Compound 2 and Compound 1 were fully competitive with radiolabelled Compound 2, whereas Compound 3, Compound 4 and CCL22 are partial, and MDC67 has no effect (n=8, Fig. 1C).
Fig. 1.
Radiolabel binding studies reveal three distinct binding sites on the CCR4 receptor. CHO-CCR4 membranes were incubated with radiolabelled CCR4-ligand prior addition of displacing CCR4-ligands. CCL22 (MDC), MDC67 Compound 1, Compound 2, Compound 3 and Compound 4 all completely displaced radiolabelled CCL17 (TARC) (panel A). Compound 3 and Compound 4 completely displaced radiolabelled Compound 3, whereas Compound 1, Compound 2 and CCL22 (MDC) only partially displaced, and MDC67 had no effect (panel B). Radiolabelled Compound 2 was displaced completely by Compound 1 and Compound 2, but only partially displaced by Compound 3, Compound 4 and CCL22 (MDC) and not displaced by MDC67 (panel C). Data shown are the mean±S.E.M of at least three separate determinations.
3.2. Antagonism of the CCR4 receptor inhibits increases in F-actin content
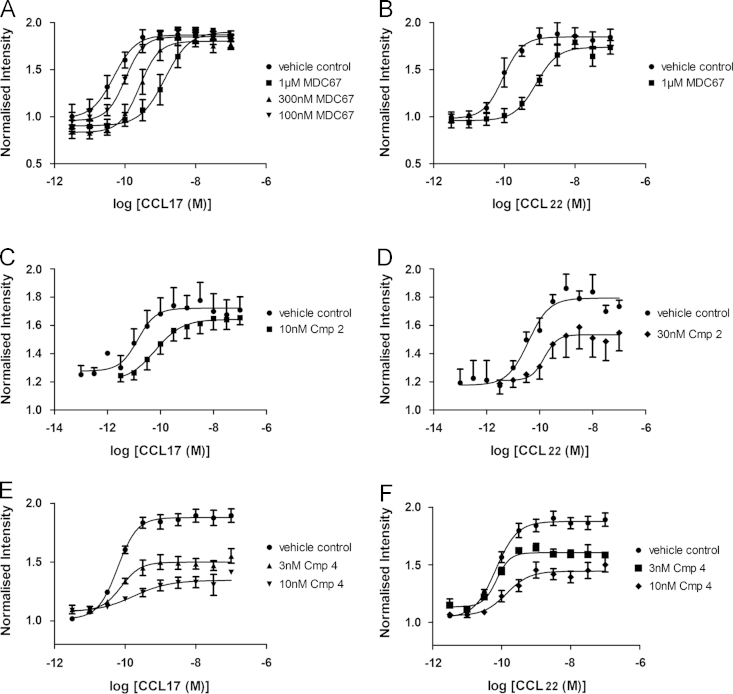
CCL17 (Fig. 2A) and CCL22 (Fig. 2B) evoked a concentration-dependent increase in the cellular F-actin content of CCR4+CD4+ peripheral blood mononuclear cells (PBMCs) (CCL17 pEC50=10.3±0.25; CCL22 pEC50=9.9±0.11, n=4). Fig. 2A and B shows that MDC67 evoked a parallel rightward shift in the concentration–response curve to both CCL17 and CCL22 without reducing the maximal response (pA2 of MDC67 vs CCL17=7.43±0.04, n=4; and vs CCL22 pA2=7.65±0.07, n=4). Importantly, MDC67 caused no observable change in cellular F-actin content at concentrations up to 1 µM exposure (n=4, Supplementary data Fig. S2). Fig. 2C and D suggest Compound 2 is an insurmountable antagonist with a pA2 of 8.0±0.2 against CCL22, whereas this compound was a surmountable antagonist for CCL17 (pA2=8.56±0.14, Fig. 2C). Similarly, as shown in Fig. 2E and F, Compound 4 is an insurmountable antagonist of CCL17 (pA2=8.21±0.09) and CCL22 (pA2=8.02±0.21).
Fig. 2.
Activation of CCR4 receptors evokes actin polymerization, which is inhibited by antagonists of the CCR4 receptor. Human CD4+CCR4+ T cells were challenged with CCL22 (MDC) or CCL17 (TARC) for 15 s and increases in the F-actin content were determined as described. Increasing concentrations of MDC67 evoked parallel rightward shifts in the concentration–response to CCL17 (panel A), and CCL22 (panel B). Compound 2 evoked a rightward shift concentration–response to CCL17 (panel C), and CCL22 (panel D) accompanied with a reduction in the maximal response. Compound 4 also evoked rightward shifts in the concentration–response to CCL17 (panel E), and CCL22 (panel F) accompanied with a reduction in the maximal response.
3.3. Antagonism of the CCR4 receptor also inhibits cellular chemotaxis
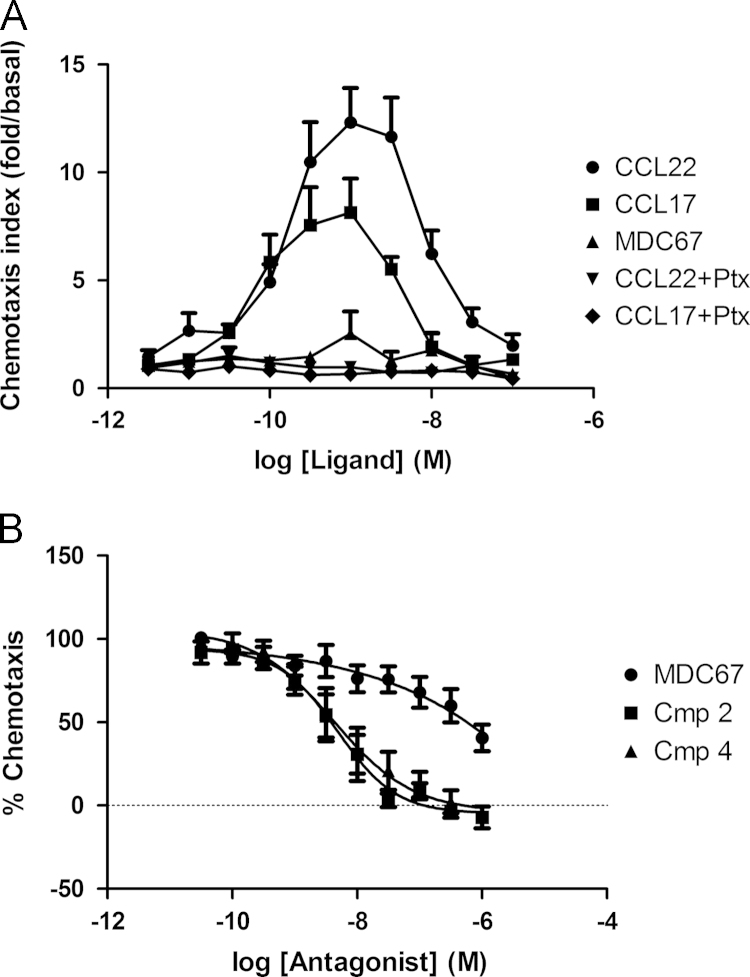
CCL22 and CCL17 both evoked chemotaxis of HUT78 cells and generated typical bell-shaped concentration–response curves, and as a control a 24 h pre-treatment with pertussis toxin (Ptx) completely abolished chemotaxis (Fig. 3A). The concentration–response peaked at 1 nM of agonist, after which, chemotaxis declined, hence pEC50׳s were calculated using the rising phase only; (pEC50 CCL22=9.79±0.13, n=11, pEC50 CCL17=10.20±0.19, n=5). CCL22 trends to greater efficacy than CCL17, however results did not reach significance (difference in peak chemotaxis; CCL22=12.31±1.6, n=11: CCL17=8.14±1.6, n=5 respectively, P=0.13). Importantly, MDC67 displays no agonist activity in evoking chemotaxis (n=5), similar to a Ptx treated control. Compound 2 and Compound 4 induced concentration-dependent inhibition of the chemotactic response to 1 nM CCL22 (Fig. 3B, pIC50=8.31±0.14, and 8.38±0.21, respectively, n=5). Although we were unable to use sufficiently high concentrations of MDC67 to produce complete inhibition of chemotaxis, from this partial inhibition, a pIC50 value of 6.76±0.16 (n=5) was calculated.
Fig. 3.
Activation of CCR4 induces chemotaxis of HUT78 cells. HUT78 cells were challenged with CCL22 (MDC) or CCL17 (TARC) in a transwell chemotaxis chamber system. CCL22 and CCL17 both evoked a concentration-dependent chemotaxis of HUT78 cells, which was completely inhibited by pretreatment with Pertussis toxin (Ptx), whereas MDC67 displays no agonist activity (panel A). Compound 2 and Compound 4 completely inhibit HUT78 cell chemotaxis to 1 nM CCL22, whereas MDC67 evokes partial inhibition of chemotaxis over the concentration range used (panel B). Data shown are the mean±S.E.M.
3.4. CCR4 receptors couple to G-proteins and β-arrestin
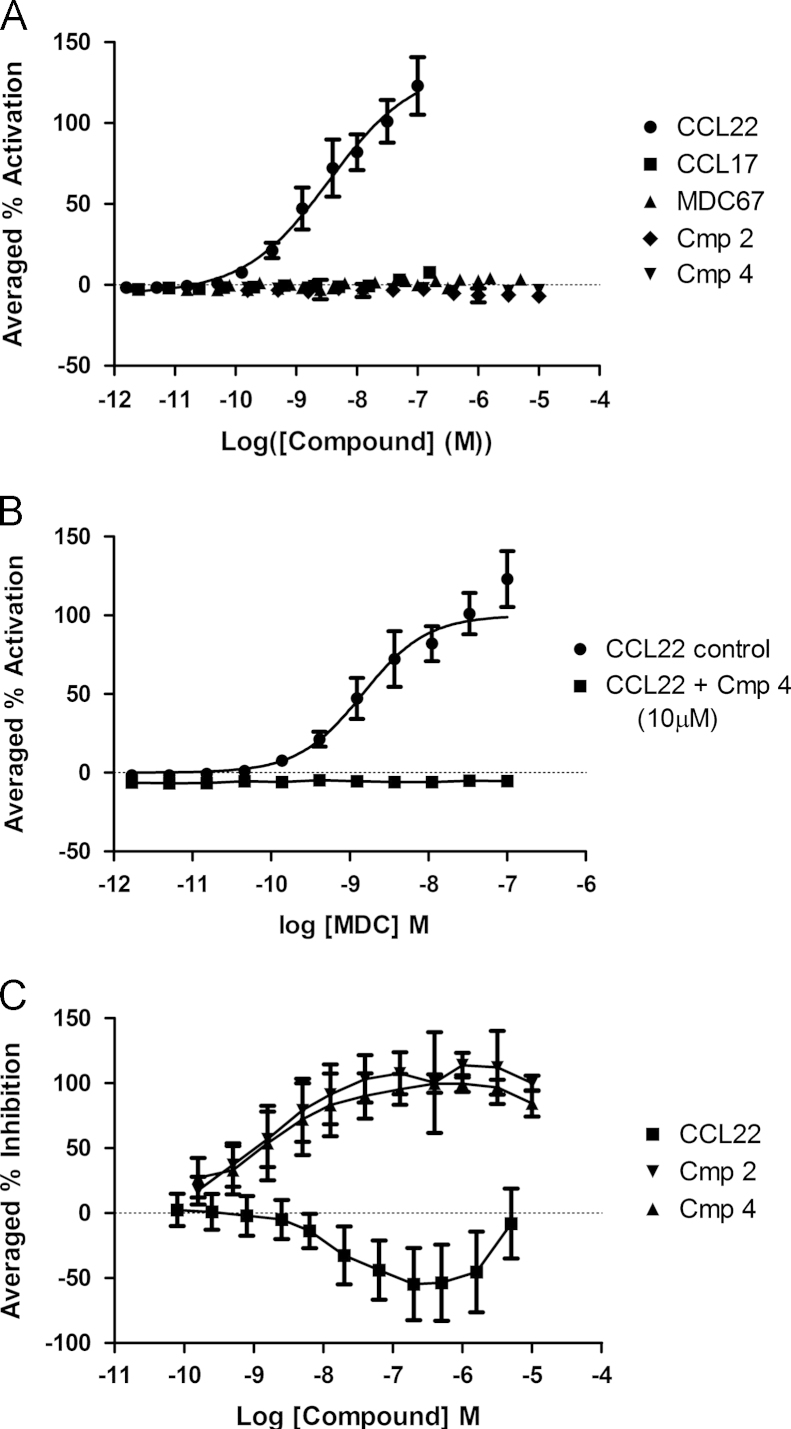
CCL22 induced a concentration-responsive coupling of CCR4 to β-arrestin, whereas none of the other agents, including CCL17 showed any detectable activity (n=4, Fig. 4A). Compound 4 (10 µM) could fully antagonize this CCL22-induced coupling of CCR4 to β-arrestin (n=4, Fig. 4B). Compound 4 and Compound 2 concentration-dependently inhibited CCL22 (7.2 nM) induced CCR4 coupling to β-arrestin (pIC50׳s=8.9±0.7; 8.72±0.5, n=16) whereas MDC67 was not antagonistic in this assay format, and surprisingly showed some stimulation of CCL22 induced coupling (n=4, Fig. 4C).
Fig. 4.
CCR4 ligands couple differentially to β-arrestin. β-arrestin coupling was assessed using an enzyme complementation assay. CCL22 induced a concentration–response coupling of CCR4 to β-arrestin, whereas none of the other tested ligands showed any activity (panel A). Compound 4 (10 µM) fully antagonized the CCL22-induced coupling of CCR4 to β-arrestin (panel B). Similarly, concentration–responses of Compound 4 and Compound 2 fully inhibited CCL22 (7.2 nM) induced CCR4 coupling to β-arrestin, whereas MDC67 showed some stimulation of coupling (panel C).
Both CCL17 and CCL22 induce concentration-dependent increases in [35S]GTPγS membrane binding, although the maximum response to CCL17 was lower than that to CCL22 (n=4, Fig. 5A). Conversely, Compounds 2 and 4 and MDC67 were completely inactive (n=4, Fig. 5A). Furthermore, MDC67, Compounds 2 and 4 all act as antagonists against CCL22-evoked GTPγS binding (n=4, Fig. 5B).
Fig. 5.
CCR4 ligands couple differently to heterotrimeric G-protein. Membranes were prepared from CHO-CCR4 cells and 35S-GTPγS binding determined. CCL22 displays full agonist behavior whereas CCL17 is a partial agonist, and Compound 2, Compound 4 and MDC67 are completely inactive (panel A). MDC67, Compound 2 and Compound 4 all fully inhibit CCL22-evoked 35S-GTPγS binding (panel B). Data shown are the mean±S.E.M.
3.5. The orthosteric and one of the allosteric sites are capable of evoking CCR4 receptor internalization by binding of antagonist molecules
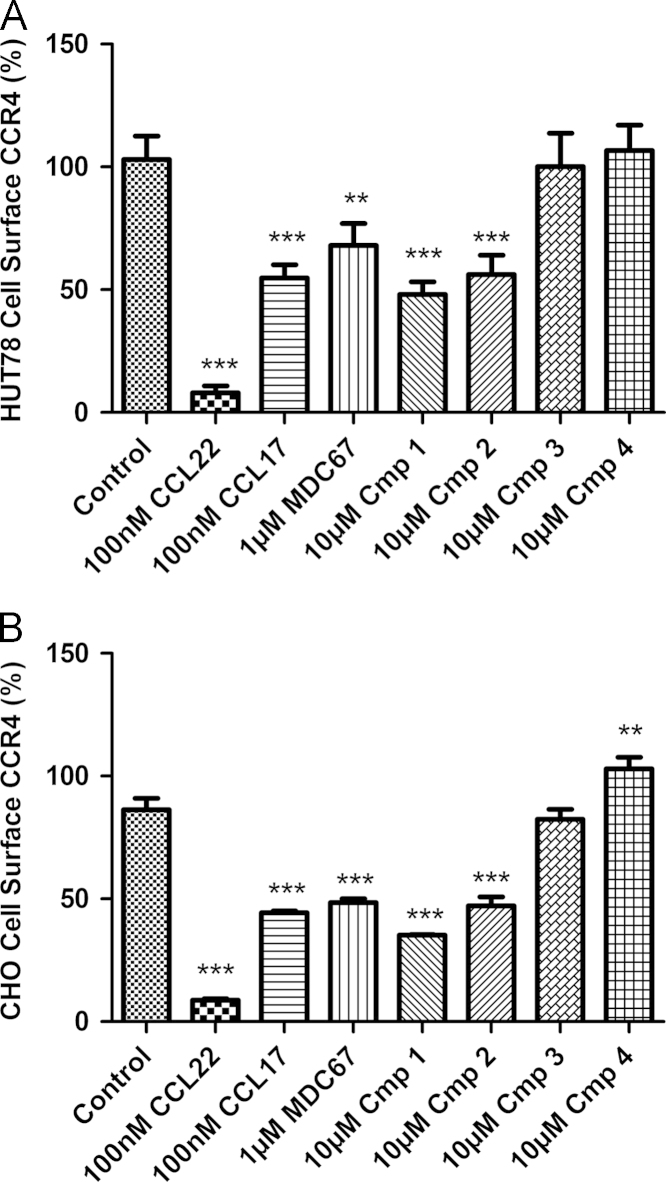
Confirming previous observations, CCL22 and CCL17 evoked concentration-dependent reduction in cell surface expression of CCR4 receptors on HUT78 cells (pEC50׳s of 8.77±0.08 (n=26) and 7.98±0.23 (n=8) respectively). CCL22 evoked almost complete receptor internalization and loss of cell surface expression whereas, CCL17 was markedly less potent and over the concentration ranges we were able to test approximately 50% of the receptor remained on the cell surface (Fig. 6A). Although Ptx treatment completely abolished chemotaxis (Fig. 3A, n=3) it had no effect on CCL22 induced receptor internalization. As expected, a CCR8 agonist, I309 had no impact on CCR4 cell surface expression (n=8, Fig. 6A).
The lipophilic amines (Compound 1 and Compound 2) which bind to the ‘site-1’ allosteric site also evoked concentration-dependent reduction in cell surface expression of CCR4 receptors (pEC50׳s of 6.4±0.2 (n=4) and 8.0±0.2 (n=8) respectively, Supplementary Table 1) whereas conversely the arylsulfonamides (Compound 3 and Compound 4), which bind to the second allosteric site, “site-2”, induced no detectable internalization in three different cell types (n=4–5, Supplementary Table 1).
Surprisingly, MDC67, despite being an antagonist of other responses, also induced receptor internalization equivalent in amount to CCL17 over the concentration range tested (pEC50=7.01±0.2, n=6). Fig. 6B displays the concentration–response of Compound 2 and MDC67, and the highest concentration of Compound 4 tested. Fig. 7 shows the comparative CCR4 internalization data for all ligands against HUT78 and also CHO-CCR4 cells at the highest concentrations tested.
Fig. 7.
The ability of CCR4 ligands to evoke internalization of cell surface CCR4 is dependent on their site of binding. Cell surface levels of CCR4 were determined in HUT78 cells (panel A) and CHO-CCR4 cells (panel B) using a PE-conjugated anti-CCR4 antibody. Cells were incubated for 30 min with CCR4 agonists CCL22 and CCL17, and the antagonists MDC67, Compound 1, Compound 2, Compound 3 and Compound 4, before having cell surface CCR4 levels assessed. One-way ANOVA with post-hoc Dunnett׳s Multiple Comparison Test was used to compare data sets to control; ⁎denotes P<0.05, ⁎⁎ denotes P<0.01, and ⁎⁎⁎ denotes P<0.001. Data shown are the mean±S.E.M.
To ensure the apparent internalization was not a result of chemokine preventing antibody binding, cells were permeabilised with 0.5% Saponin prior to PE-CCR4 antibody incubation and FACS measurement to ensure that the total number of receptors detected per cell was not changed by addition of chemokine (Supplementary data Fig. S3). In all cell types explored addition of saponin gives an indication of total cellular CCR4 content, which was unaffected by 100 nM MDC.
In summary; CCL22 induces close to complete CCR4 internalization and loss of cell surface receptors, whereas CCL17 induces only partial internalization at the concentrations tested. Similarly, the competitive orthosteric antagonist MDC67 had a similar profile to CCL17 while the allosteric ‘site-1’ antagonists clearly evoke partial internalization, whereas the allosteric ‘site-2’ antagonists do not induce internalization.
3.6. The internalization of cell surface CCR4 receptors was confirmed using confocal microscopy
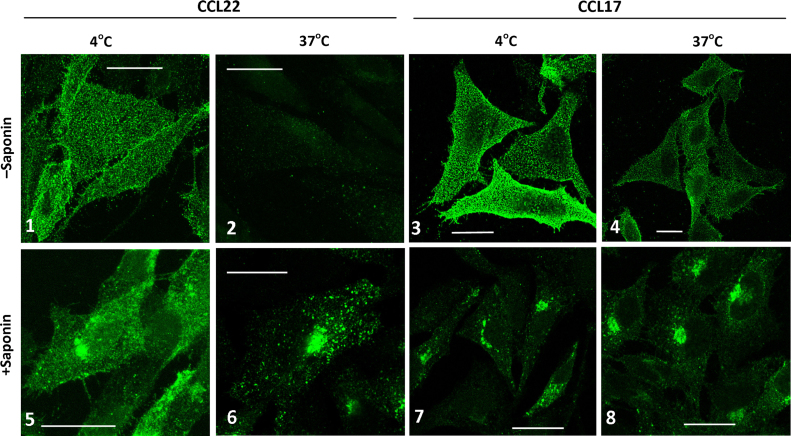
Fig. 8 shows examples of receptor staining following addition of 100 nM CCL22 or CCL17 to CHO-CCR4 cells. CCL22 (panel 1) or CCL17 (panel 3) was incubated with cells at 4 °C for 30 min prior to fixation or fixation and permeabilisation (panels 5 and 7). Blocking endocytosis at 4 °C reveals abundant cell surface CCR4 expression in fine punctate structures on the plasma membrane (panels 1 and 3) and significant juxtanuclear intracellular staining when cells are permeabilised prior to staining with anti-CCR4 antibody (panels 5 and 7). However, incubation of cells with CCL22 at 37 °C for 30 min clearly confirms that almost no CCR4 remains on the cell surface and all the receptor appears in intracellular vesicular structures (compare panel 2 with 6).
Fig. 8.
Localization of CCR4 in CHO-CCR4 cells following agonist addition. CHO-CCR4 cells were treated with 100 nM CCL22 (panels 1, 2, 5, and 6) or 100 nM CCL17 (panels 3, 4, 7, and 8) for 30 min at either 4 °C (panels 1, 3, 5, and 7) or 37 °C (panels 2, 4, 6, and 8). Following fixation, cells were left unpermeabilised (panels 1–4) or permeabilised with 0.2% saponin (panels 5–8). CCR4 was stained with anti-CCR4 followed by Alexa488-rabbit anti-mouse antibody, imaged by confocal microscopy and single 0.37 µm optical sections are shown. Scale bar=20 µm.
In comparison incubation of cells with CCL17 at 37 °C for 30 min induced relatively little receptor redistribution from the plasma membrane to intracellular vesicles (compare panel 4 with 8). Controls slides were processed by omitting the primary anti-CCR4 antibody or by omitting addition of ligands (Supplementary data Fig. S4).
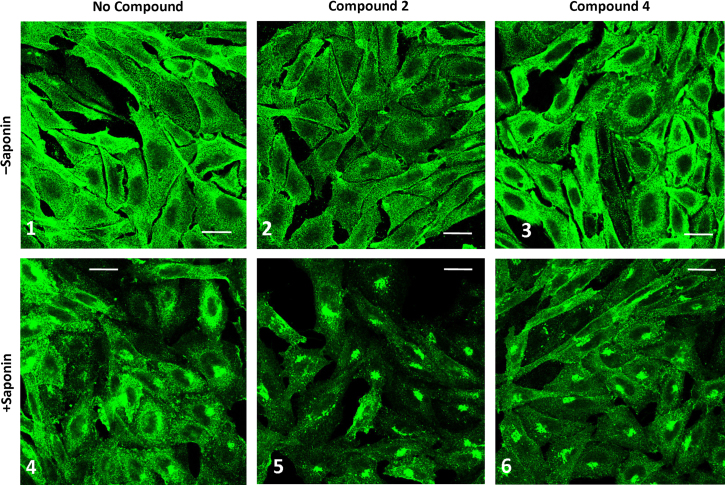
Fig. 9 shows CHO-CCR4 cells incubated with or without Compound 2 or Compound 4 for 30 min at 37 °C followed by fixation (panels 1–3) or fixation and permeabilization (panels 4–6). CHO-CCR4 cells without ligand treatment showed abundant punctate cell surface expression of CCR4 (panel 1) and some juxtanuclear intracellular staining following permeabilisation (panel 4). Treatment of cells with Compound 2 resulted in a discernible reduction in cell surface CCR4 staining (panel 2) and increased intracellular accumulation (panel 5). However treatment of cells with Compound 4 did not appear to either reduce cell surface expression of CCR4 or enhance its intracellular accumulation.
Fig. 9.
Localization of CCR4 in CHO-CCR4 cells following antagonist addition. CHO-CCR4 cells were incubated with no addition (panels 1 and 4) or with Compound 2 (panels 2 and 5) or Compound 4 (panels 3 and 6) for 30 min at 37 °C followed by fixation (panels 1–3) or fixation and permeabilisation with 0.2% Saponin (panels 4–6). CCR4 was stained with anti-CCR4 followed by Alexa488-rabbit anti-mouse antibody, imaged by confocal microscopy and single 0.37 µm optical sections are shown. Scale bar=20 µm.
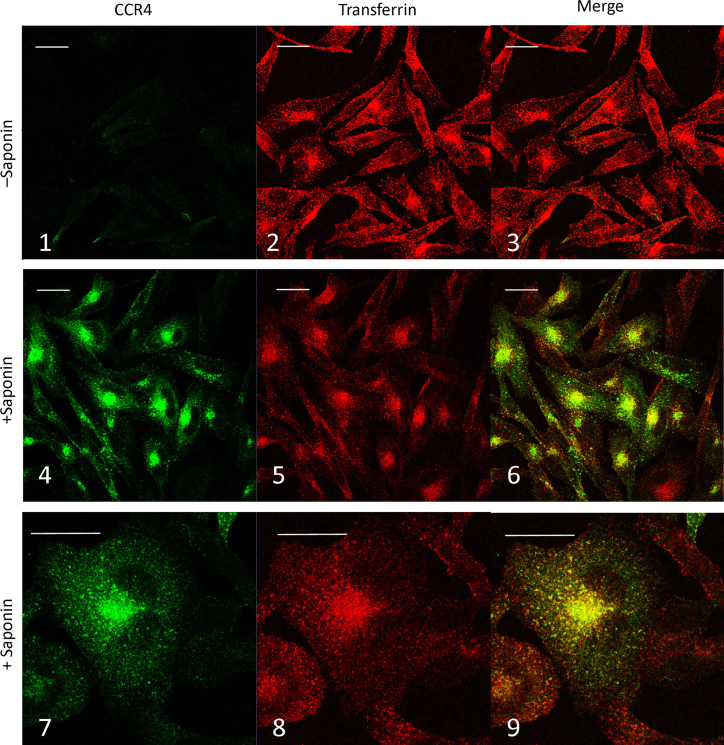
The juxtanuclear intracellular staining of internalized CCR4 was studied by co-incubating CHO-CCR4 cells with 100 nM CCL22 and Alexa-546 conjugated transferrin. Cells were incubated at 4 °C for 30 min, washed in ice cold media then warmed to 37 °C for 30 min prior to fixation (Fig. 10). As shown previously, incubation with CCL22 removed almost all CCR4 from the cell surface (panel 1) and the receptor appears intracellularly in a juxtanuclear staining pattern (panel 4) which largely overlaps with the intracellular accumulation of transferrin (panels 5 and 6). At higher magnification (panels 7–9) it is clear that this overlap of staining is substantial, nevertheless it is not complete and there are numerous individual green stained vesicles and red stained vesicles.
Fig. 10.
Localization of CCR4 and endocytosed transferrin. CHO-CCR4 cells were incubated with 100 nM CCL22 and Alexa-546 conjugated Transferrin for 30 min at 4 °C prior to washing and warming to 37 °C for 30 min. Following fixation and permeabilisation, CCR4 was stained with anti-CCR4 followed by Alexa488-rabbit anti-mouse antibody, imaged by confocal microscopy and single 0.37 µm optical sections are shown. Scale bar=20 µm.
4. Discussion
CCR4 is a promising drug target for the treatment of allergic disorders, however to date only one antagonist has made it to the clinic (Cahn et al., 2013), and there is a need for novel approaches to targeting chemokine receptors. In this study we demonstrate that there are two allosteric binding sites on CCR4, only one of which evokes receptor internalization. Targeting receptor internalization rather than conventional allosteric or orthosteric antagonism may provide a novel strategy for drug discovery against chemokine receptors.
Using a panel of CCR4 ligands we first characterized them for receptor binding and functional assays. Using 125I-CCL17 in CCR4 binding studies we confirmed that CCL22 was able to fully compete binding. We also demonstrated that the N-terminal truncation of CCL22 is also able to fully compete with 125I-CCL17 binding to CCR4 but with a 66-fold lower affinity than native CCL22. Moreover, MDC67 clearly acted as a competitive antagonist in the actin polymerization assay, and inhibited ligand binding, chemotaxis and GTPγS binding while displaying no agonist activity in any of these assays.
This was a surprising result as it has previously been suggested that MDC67 acts as a full agonist for monocyte chemotaxis, and could evoke HUT78 cell chemotaxis at high concentrations (Pal et al., 1997; Struyf et al., 1998). In those studies MDC67 did not induce a Ca2+ response in cells and it also failed to inhibit a CCL22 or CCL17 induced response suggesting a complex mode of action.
Four small molecules were used in the current study to fully characterize the allosteric binding sites, and all fully inhibited binding of 125I-CCL17 to CCR4. A competitive ligand will inhibit the binding of a radioligand to NSB whereas an allosteric antagonist will inhibit binding to a level dependent on its cooperativity factor as described (Christopoulos and Kenakin, 2002). Radiolabelled Compound 3 was only fully displaced by cold Compound 3 and Compound 4, while radiolabelled Compound 2 was only fully displaced by cold Compound 2 and Compound 1. Therefore, we conclude from these binding studies that CCL22, CCL17 and MDC67 all bind to the same orthosteric site whereas Compound 4 and Compound 3 bind to a second site, shown previously to be on the intracellular surface of CCR4 (Andrews et al., 2008). This study also concludes that Compound 1 and Compound 2 bind to a third distinct novel allosteric site, supporting previous observations (Weston and Hall, 2008).
Using actin polymerization as a functional response to agonist we showed that Compound 2 and Compound 4 appear as insurmountable antagonists, consistent with previous observations (Andrews et al., 2008). Both compounds also fully inhibited chemotaxis, CCR4 coupling to GTPγS and β-arrestin and were devoid of agonist activity.
To further our understanding of CCR4 receptor coupling we studied the effects of the natural ligands on receptor coupling using a combination of β-arrestin and GTPγS binding. We first examined β-arrestin binding to CCR4 and found CCL22 but not CCL17 was able to induce coupling. Consistent with their antagonist activity, Compound 4 and Compound 2 completely abolished CCL22 coupling to β-arrestin whereas MDC67 did not block coupling and indeed may have stimulated it, suggesting a potential third binding site for this ligand. To quantify heterotrimeric G protein coupling, we measured GTPγS binding to CHO-CCR4 membranes following ligand stimulation and again found significant differences between CCL22 and CCL17. Compound 2 and Compound 4 were unable to stimulate GTPγS binding and were able to fully antagonize CCL22 induced coupling, as was MDC67. These are the first studies to show differences in CCL22 and CCL17 coupling to downstream effectors through CCR4.
Having shown that CCL22 and CCL17 are capable of biased signaling, we examined their effects on receptor internalization. In our study, CCL22 was able to induce almost complete CCR4 cell surface downregulation in both cell types examined, with CCL17 only able to internalize about ~50% of cell surface CCR4 over the concentration ranges we were able to test. Importantly, our confocal microscopy confirmed the data generated using flow cytometry, and also reveals ligand induced accumulation of CCR4 in juxtanuclear endosomes that show a substantial, but not complete, overlap with vesicles containing endocytosed transferrin. Since CCL22 couples CCR4 to β-arrestin one would expect endocytosis via clathrin coated pits for which transferrin endocytosis is a marker.
Careful measurement of the concentration response curves for the two natural ligands shows that the actin polymerization and chemotaxis responses are closely concordant with one another and between the two agonists, whereas induction of CCR4 downregulation requires a 10 fold higher concentration of CCL22 and a 100 fold higher concentration of CCL17.
The maximal chemotaxis response to CCL22 and CCL17 occurs at a concentration of 1 nM consequently at this concentration about 50% of CCR4 will still be on the surface following stimulation by CCL22 and ~100% following stimulation with CCL17. Over the 1 - 10 nM CCL17 concentration range, chemotaxis falls away to almost zero, whereas there is almost no reduction in cell surface CCR4 expression. Consequently, it is unlikely that receptor downregulation alone is the cause of the cessation of chemotaxis in response to CCL17. The opposite is true for CCL22 where the CCR4 downregulation concentration response curve and the descending phase of the chemotaxis curve show concordance. Consequently, “stop-go” responses to CCL22 and CCL17 may be controlled differently suggesting these chemokines may not have completely identical or redundant functions.
We conclude from several lines of evidence presented here that internalization and the chemotactic responses are not necessarily linked as has been suggested by receptor mutagenesis experiments. The observation that chemotaxis is completely inhibited by a 24 h pre-treatment with Ptx whereas endocytosis is not affected at all also suggests these pathways are coupled independently. Furthermore, the similarity in potency between chemotaxis and actin polymerization assays and the large separation with potency in endocytosis further supports the notion of two distinctly coupled pathways.
Our study also demonstrates that this biased signaling can be achieved by antagonist molecules binding to one of the two allosteric sites. Using two distinct small molecule classes of CCR4 antagonists, we have shown that the lipophilic amines (Compounds 1 and 2) which bind allosteric ‘site-1’ appear able to drive receptor internalization whereas the arylsulphonamides (Compounds 3 and 4) which bind to allosteric ‘site-2’ are not. While small molecule antagonists for the CCR4 receptor have been described previously, their ability to evoke receptor internalization is novel. Recently a novel CCR4 antagonist K777 has been described which also demonstrated the ability of allosteric antagonist evoked receptor internalization (Sato et al., 2013). Collectively these studies provide a unique opportunity for drug discovery targeting this process, rather than simply relying on the antagonist׳s ability to displace the natural agonist molecules.
Having demonstrated that a number of CCR4 antagonists are able to induce receptor internalization, the next question is how does this mechanism of antagonism compare with and interact with the competitive or allosteric effects of these compounds? The effects of reducing surface receptor density should be similar to the effects of an irreversible antagonist, since both mechanisms reduce the number of accessible receptor binding sites for an agonist, at least on intact cells. However, in assays in broken cell preparations, such as the [35S]GTPγS binding assay, where receptor internalization is prevented, an internalizing antagonist would not have the additional effects discussed below. Hence this mechanism could explain a discrepancy between the effects of an antagonist in intact vs broken cell preparations. Also, assuming that internalized receptors are unable to couple to signaling pathways then receptor internalization will also reduce constitutive signaling and, hence, appear like inverse agonism in intact cell systems. Using a recently published modification of the operational model of agonist activity (Slack and Hall, 2012; Hall, 2013) suggests that the contribution of this effect is no greater than a 3-fold shift in the concentration–response curves to the agonists, but will be dependent on the degree of internalization.
5. Conclusions
In summary, using a panel of agonists or orthosteric and allosteric antagonists to CCR4 we have been able to reveal biased functions of this receptor both in terms of β-arrestin coupling and GTPγS binding and in terms of ligand mediated receptor internalization. CCL22, CCL17 and MDC67 all competitively bind to the orthosteric site; Compound 1 and Compound 2 bind to ‘site-1’ which is believed to be extracellular and couples to internalization, whereas Compound 3 and Compound 4 bind to the second intracellular ‘site-2’ allosteric site.
Collectively this study demonstrates that orthosteric and allosteric antagonists of the CCR4 receptor are capable of evoking receptor internalization, providing a novel strategy for drug discovery against this class of target.
Acknowledgments
Elements of this study were funded by the following grants; Wellcome Trust Grant number 083567/Z/07/Z for the Centre for Respiratory Infection MRC Grant G1000758 for the MRC-Asthma UK Centre in Allergic Mechanisms of Asthma MRC/GSK Alliance Programme Grant G1100238 Mechanisms of Interplay Between Allergy and Viruses in Asthma.
Appendix A. Supplementary materials
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ejphar.2014.02.007.
Appendix A. Supplementary materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
References
- Andrews G., Jones C., Wreggett K.A. An intracellular allosteric site for a specific class of antagonists of the CC chemokine G protein-coupled receptors CCR4 and CCR5. Mol. Pharmacol. 2008;73:855–867. doi: 10.1124/mol.107.039321. [DOI] [PubMed] [Google Scholar]
- Bonecchi R., Bianchi G., Bordignon P.P., D׳Ambrosio D., Lang R., Borsatti A., Sozzani S., Allavena P., Gray P.A., Mantovani A., Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn A., Hodgson S., Wilson R., Robertson. J., Watson J., Beerahee M., Hughes S.C., Young G., Graves R., Hall D., van Marle S., Solari R. Safety, tolerability, pharmacokinetics and pharmacodynamics of GSK2239633, a CC-chemokine receptor 4 antagonist, in healthy male subjects: results from an open-label and from a randomised study. BMC Pharmacol. Toxicol. 2013;14:14. doi: 10.1186/2050-6511-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.J., Haraldsen G., Pan J., Rottman J., Qin S., Ponath P., Andrew D.P., Warnke R., Ruffing N., Kassam N., Wu L., Butcher. E.C. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Christopoulos A., Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- Demont E.H., Andrews B.I., Bit R.A., Campbell C.A., Cooke J.W.B., Deeks N., Desai S., Dowell S.J., Gaskin P., Gray J.R.J., Haynes A., Holmes D.S., Kumar U., Morse M.A., Osborne G.J., Panchal T., Patel B., Perboni A., Taylor S., Watson R., Witherington J., Willis R. Discovery of a selective S1P1 receptor agonist efficacious at low oral dose and devoid of effects on heart rate. ACS Med. Chem. Lett. 2011;2:444–449. doi: 10.1021/ml2000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo J.A., Pan Y., Lloyd C.M., Jia G.Q., Yu G., Dussault B., Powers C.A., Proudfoot A.E., Coyle A.J., Gearing D., Gutierrez-Ramos J.C. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J. Immunol. 1999;163:403–411. [PubMed] [Google Scholar]
- Hall D.A. Application of receptor theory to allosteric modulation of receptors. Prog. Mol. Biol. Transl. Sci. 2013;115:217–290. doi: 10.1016/B978-0-12-394587-7.00006-3. [DOI] [PubMed] [Google Scholar]
- Iellem A., Mariani M., Lang R., Recalde H., Panina-Bordignon P., Sinigaglia F., D׳Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki S., Takizawa H., Yoneyama H., Nakayama T., Fujisawa R., Izumizaki M., Imai T., Yoshie O., Homma I., Yamamoto K., Matsushima K. Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J. Immunol. 2001;166:2055–2062. doi: 10.4049/jimmunol.166.3.2055. [DOI] [PubMed] [Google Scholar]
- Mariani M., Lang R., Binda E., Panina-Bordignon P., D׳Ambrosio D. Dominance of CCL22 over CCL17 in induction of chemokine receptor CCR4 desensitization and internalization on human Th2 cells. Eur. J. Immunol. 2004;34:231–240. doi: 10.1002/eji.200324429. [DOI] [PubMed] [Google Scholar]
- Nouri-Aria K.T., Wilson D., Francis J.N., Jopling L.A., Jacobson M.R., Hodge M.R., Andrew D.P., Till S.J., Varga E.M., Williams T.,J., Pease J.E., Lloyd C.M., Sabroe I., Durham S.R. CCR4 in human allergen-induced late responses in the skin and lung. Eur. J. Immunol. 2002;32:1933–1938. doi: 10.1002/1521-4141(200207)32:7<1933::AID-IMMU1933>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Pal R., Garzino-Demo A., Markham P.D., Burns J., Brown M., Gallo R.C., DeVico A.L. Inhibition of HIV-1 infection by the b-chemokine MDC. Science. 1997;278:695–698. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P., Papi A., Mariani M., Di Lucia P., Casoni G., Bellettato C., Buonsanti C., Miotto D., Mapp C., Villa A., Arrigoni G., Fabbri L.M., Sinigaglia F. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J. Clin. Invest. 2001;107:1357–1364. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C.A., Meyer A., Nemeth K., Bacon K.B., Hoogewerf A.J., Proudfoot A.E., Wells T.N. Molecular cloning and functional expression of a novel CC chemokine receptor cDNA from a human basophilic cell line. J. Biol. Chem. 1995;270:19495–19500. doi: 10.1074/jbc.270.33.19495. [DOI] [PubMed] [Google Scholar]
- Procopiou P.A., Ford A.J., Graves R.H., Hall D.A., Hodgson S.T., Lacroix Y.M., Needham D., Slack R.J. Lead optimisation of the N1 substituent of a novel series of indazole arylsulfonamides as CCR4 antagonists and identification of a candidate for clinical investigation. Bioorg. Med. Chem. Lett. 2012;22:2730–2733. doi: 10.1016/j.bmcl.2012.02.104. [DOI] [PubMed] [Google Scholar]
- Purandare A.V., 2004. World patent WO2004020584. March 11.
- Purandare A.V., Somerville J.E. Antagonists of CCR4 as immunomodulatory agents. Curr. Top. Med. Chem. 2006;6:1335–1344. doi: 10.2174/15680266106061335. [DOI] [PubMed] [Google Scholar]
- Reiss Y., Proudfoot A.E., Power C.A., Campbell J.J., Butcher G. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J. Exp. Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Iwase M., Miyama M., Komai M., Ohshima E., Asai A., Yano H., Miki I. Internalization of CCR4 and inhibition of chemotaxis by K777, a potent and selective CCR4 antagonist. Pharmacology. 2013;91:305–313. doi: 10.1159/000350390. [DOI] [PubMed] [Google Scholar]
- Sebastiani S., Danelon G., Gerber B., Uguccioni M. CCL22-induced responses are powerfully enhanced by synergy inducing chemokines via CCR4: evidence for the involvement of first beta-strand of chemokine. Eur. J. Immunol. 2005;35:746–756. doi: 10.1002/eji.200525800. [DOI] [PubMed] [Google Scholar]
- Slack R.J., Russell L.J., Hall D.A., Luttmann M.A., Ford A.J., Saunders K.A., Hodgson S.T., Connor H.E., Browning C., Clark K.L. Pharmacological characterization of GSK1004723, a novel, long-acting antagonist at histamine H(1) and H(3) receptors. Br. J. Pharmacol. 2011;164:1627–1641. doi: 10.1111/j.1476-5381.2011.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack R.J., Hall D.A. Development of operational models of receptor activation including constitutive receptor activity and their use to determine the efficacy of the chemokine CCL17 at the CC chemokine receptor CCR4. Br. J. Pharmacol. 2012;166:1774–1792. doi: 10.1111/j.1476-5381.2012.01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyf S., Proost. P., Sozzani S., Mantovani A., Wuyts A., De Clercq E., Schols D., Van Damme J. Enhanced anti-HIV-1 activity and altered chemotactic potency of NH2-terminally processed macrophage-derived chemokine (MDC) imply an additional MDC receptor. J. Immunol. 1998;161:2672–2675. [PubMed] [Google Scholar]
- Ulmer A.J., Scholz W., Ernst M., Brandt E., Flad H.-D. Isolation and subfractionation of human peripheral blood mononuclear cells (PBMC) by density gradient centrifugation on Percoll. Immunobiology. 1983;166:238–250. doi: 10.1016/S0171-2985(84)80042-X. [DOI] [PubMed] [Google Scholar]
- Weston C., Hall D. Functional studies in primary human T cells indicate a second allosteric regulatory site on CCR4. Proc. Br. Pharm. Soc. 2008;6:066P. [Google Scholar]
- Yang P.T., Kasai H., Zhao L.J., Xiao W.G., Tanabe F., Ito M. Increased CCR4 expression on circulating CD4(+) T cells in ankylosing spondylitis, rheumatoid arthritis and systemic lupus erythematosus. Clin. Exp. Immunol. 2004;138:342–347. doi: 10.1111/j.1365-2249.2004.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data