Abstract
The cornea is one of several orofacial structures requiring glandular secretion for proper lubrication. Glandular secretion is regulated through a neural reflex initiated by trigeminal primary afferent neurons innervating the corneal epithelium. Corneal sensory afferents must respond to irritating and potentially damaging stimuli, as well as drying that occurs with evaporation of the tear film, and the physiological properties of corneal afferents are consistent with these requirements. Polymodal neurons are sensitive to noxious mechanical, thermal and chemical stimuli, mechanoreceptive neurons are selectively activated by mechanical stimuli, and cool cells respond to innocuous cooling. The central terminations of corneal primary afferents are located within two regions of the spinal trigeminal nucleus. The more rostral region, located at the transition between the trigeminal subnucleus caudalis and interpolaris, represents a critical relay for the regulation of the lacrimation reflex. From this region, major control of lacrimation is carried through projections to preganglionic parasympathetic neurons located in or around the superior salivatory nucleus. Dry eye syndrome may be caused by a dysfunction in the tear secreting glands themselves or in the neuronal circuit regulating these glands. Furthermore, the dry eye condition itself may modify the properties of corneal afferents and affect their ability to regulate secretion, a possibility just now being explored.
Keywords: dry eye, trigeminal ganglion, trigeminal nucleus, cornea, lacrimation
1. Overview
The cornea requires constant secretion from multiple glands to provide lubrication, nourishment and a protective barrier to the external environment (Dartt, 2009). Although the secretory fluids from each gland vary with regards to composition, among the common constituents of glandular secretion are water, electrolytes, mucins, and other glycoproteins and proteins. Sensory innervation of the cornea is necessary to detect environmental stressors and, through brainstem circuits, regulate the flow of glandular secretion.
Neural innervation of the cornea is provided by the ophthalmic branch of the trigeminal nerve, the general sensory cranial nerve innervating the head and face. Glandular secretion to several cephalic structures, including the cornea, intraoral, and intranasal regions, are regulated through primary afferent projections to the spinal trigeminal nucleus (Vsp), where information is processed and relayed to preganglionic parasympathetic neurons located in and around the superior and inferior salivatory nuclei (Ishizuka and Murakami, 1986; Murakami et al., 1983; Murakami et al., 1982; Toth et al., 1999). The production of watery tears is through the activation of postganglionic parasympathetic neurons located in the pterygopalatine ganglion that terminate in the lacrimal gland (Dartt, 2009; Ruskell, 1971). In addition, conjunctival goblet cells, the main source of mucins, are innervated and regulated by the pterygopalatine ganglion (Dartt, 2004; Dartt et al., 1995; Kanno et al., 2003; Kessler and Dartt, 1994; Kessler et al., 1995). Preganglionic parasympathetic denervation decreases tear flow through diminished aqueous tear secretion as well as a reduction in goblet cell density (Toshida et al., 2007). Furthermore, the activation of corneal primary afferent neurons increases both aqueous and mucin secretion (Kessler et al., 1995; Yasui et al., 1997). Conjunctival and corneal epithelial cells can also modify tear composition through the secretion and absorption of electroytes and water. Fluid secretion from conjunctival epithelial cells appears to be increased primarily by sympathetic rather than parasympathetic agonists (Dartt, 2004; Kompella et al., 1996; Rios et al., 1999; Shi and Candia, 1995). Less is known regarding the neural regulation of meibomian glands, which are the main source of lipid content in tears. Meibomian glands receive parasympathetic innervation, however the significance of this innervation in their regulation remains unclear (LeDoux et al., 2001).
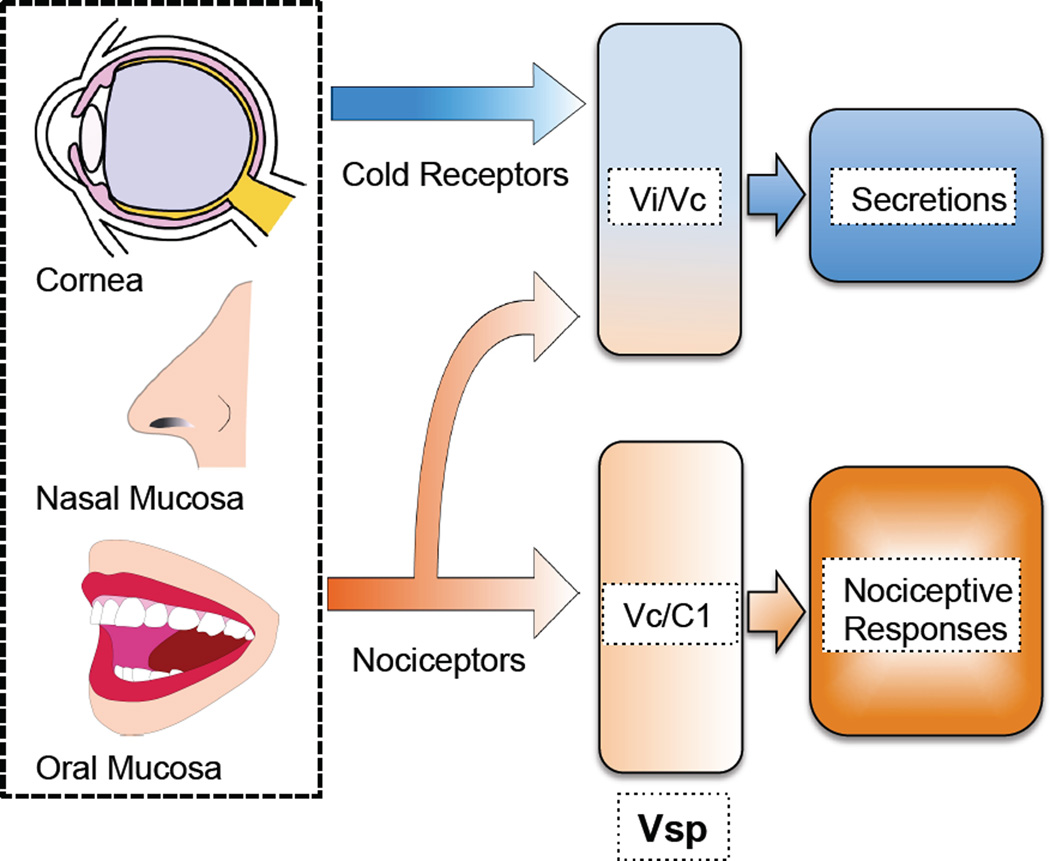
Primary afferent neurons innervating the cornea are able to sense whether the ocular surface is exposed to damaging or potentially damaging stimuli and evoke protective autonomic and somatic reflexes, including lacrimation and blinking, as well as irritating and painful sensations (Figure 1) (Belmonte et al., 2004b; Evinger et al., 2002; Pellegrini et al., 1995). The cornea, like other specialized tissues of the cephalic region innervated by the trigeminal nerve, is represented in multiple regions of Vsp that serve to carry out these different functions (Bereiter et al., 2000b). Lacrimation is regulated by a specialized region located in rostral Vsp, whereas other nociceptive specific functions can be attributed to more caudal regions (Bereiter et al., 2000b; Hirata et al., 2004; Meng et al., 1997).
Figure 1.
Activation of nociceptors innervating the cornea, nasal mucosa and oral mucosa induces secretion and nociceptive responses, including pain sensation. In contrast, cold receptors, activated by evaporative cooling of the ocular surface, increases secretion without evoking nociceptive responses. Cold and nociceptor evoked secretion is regulated by neurons located at the transition between subnucleus interpolaris and caudalis (Vi/Vc) in the spinal trigeminal nucleus (Vsp), whereas nociceptive responses are controlled by neurons located at Vc and the first cervical vertebra (C1).
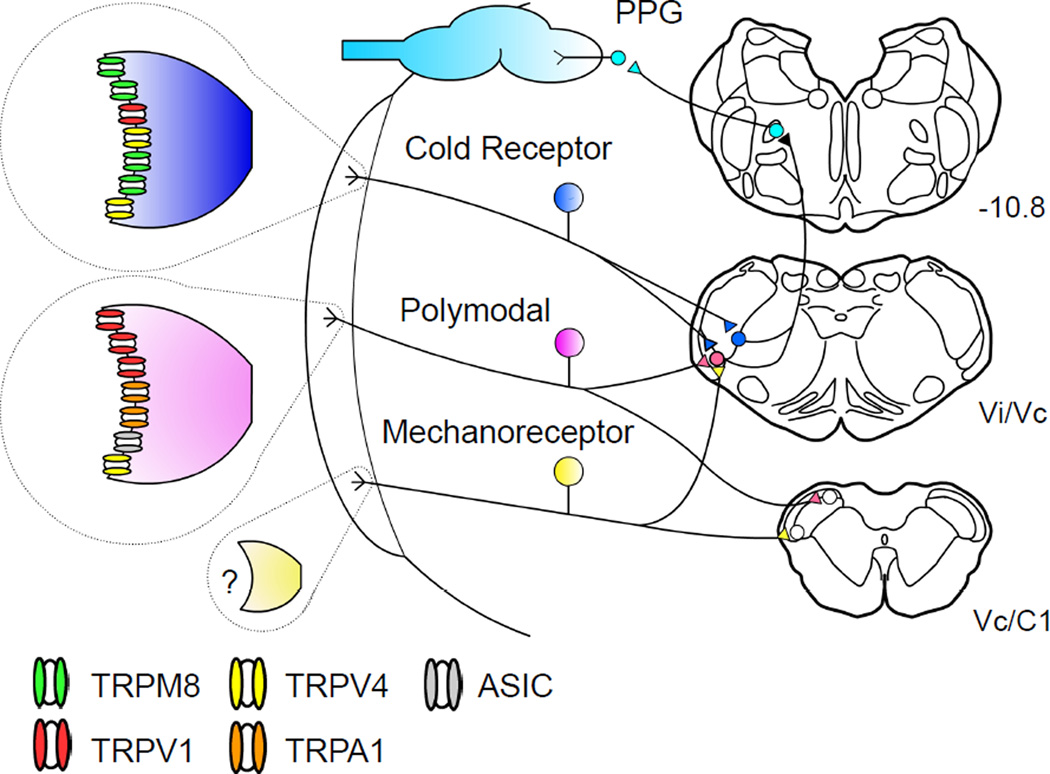
Increases in glandular secretion are often accompanied by pain or irritation and associated reflexes. However, most secretion, particularly that precipitated by the evaporation of water from the cornea, occurs without an accompanying conscious perception. Indeed evidence indicates that basal secretion and secretion evoked by noxious stimulation of the cornea are driven by unique, but possibly overlapping, brainstem reflex arcs (Figure 2) (Hirata and Meng, 2010; Hirata et al., 2004; Kurose and Meng, 2013a; Parra et al., 2010; Robbins et al., 2012).
Figure 2.
Model for neural control of lacrimation. Corneal primary afferent neurons express a range of membrane channels, which corresponds to their physiological characteristics. The primary afferent neurons innervating the cornea regulate secretion of basal tearing with a relay through the spinal trigeminal nucleus. It has been proposed that polymodal nociceptors express channels responding to noxious chemical, thermal, and mechanical stimulation, including TRPV1, TRPA1, TRPV4, and acid sensing ion channels (ASIC) channels. In contrast, cold receptors express TRPM8 channels, which are sensitive to innocuous cooling. The channels responsible for mechanical responses in mechanoreceptive neurons and hyperosmotic responses in cold receptors have yet to be identified. PPG: Pterygopalatine ganglion
Inadequate or altered tear film on the anterior ocular surface can result in dry eye syndrome (DES), a significant clinical problem affecting up to 38% of the population over the age of 50 (2007). Dry eye can result in sensations of dryness, grittyness, irritation, and burning pain, and in severe cases corneal lesions and infection can occur (2007). The causes of DES are not fully elucidated but may include inflammation and dysfunction of the lacrimal glands, resulting in aqueous tear deficiency, the meibomian glands, resulting in insufficient lipid content, or the conjunctival goblet cells, resulting in diminished mucin secretion (Javadi and Feizi, 2011; Mantelli and Argueso, 2008; Mathers, 2000; Sullivan et al., 1999). Alternatively, DES may be caused by an inability of sensory neurons in the tear reflex arc to properly regulate tearing (Dartt, 2009; Mathers, 2000; van Bijsterveld et al., 2003). In particular, dry eye associated with LASIK surgery, aging, and diabetes mellitus may involve a disruption in afferent drive, as dry eye symptoms may be accompanied by a decrease in corneal sensitivity (Ambrosio et al., 2008; De Paiva et al., 2006; Gallar et al., 2004; Konomi et al., 2008; Tavakoli et al., 2007).
The remaining sections will examine the properties of trigeminal primary afferent neurons innervating the cornea under normal and dry eye conditions, and examine the central pathways involved in controlling lacrimation. Dry eye induced alterations in the properties of corneal afferent neurons and the central processing of corneal input may have significant consequences for both the regulation of tearing and ocular pain. Understanding these changes are crucial to the development of novel interventions for the treatment of DES.
2. Cornea primary afferent neurons
The cornea is the most densely innervated tissue in the body and, based on their conduction velocity, is exclusively innervated by A-delta and C primary afferent fibers (Muller et al., 2003; Zander and Weddell, 1951). Afferent nerve bundles innervating the cornea enter the stroma from the periphery, branching to form a midstromal plexus (Marfurt et al., 2010). Continuing to branch, nerves penetrate Bowman’s layer to form the subbasal nerve plexus. In order to maintain the transparency of the cornea, the lightly myelinated A-delta corneal afferents lose their myelin sheaths as the nerve bundles enter the corneal limbus (Marfurt et al., 2010).
Corneal nerve endings originiating from the subbasal nerve plexus penetrate into all layers of the corneal epithelium and respond to multiple stimulus modalities, including mechanical, chemical and thermal stimuli (Belmonte et al., 1991; Belmonte and Giraldez, 1981; Gallar et al., 1993; Hirata and Meng, 2010; MacIver and Tanelian, 1993a; MacIver and Tanelian, 1993b; Parra et al., 2010; Robbins et al., 2012). Although irritation and pain are the primary, and possibly only, sensations evoked by stimulation of the cornea, not all sensory neurons that innervate the cornea should be considered nociceptors (Acosta et al., 2001b; Beuerman and Tanelian, 1979; Kenshalo, 1960). As evidence, innocuous stimulation of the cornea with mild cooling or menthol activates corneal afferents and induces tearing without causing irritation or pain (Parra et al., 2010; Robbins et al., 2012).
Similar to other primary afferent neurons, corneal afferents store and release the excitatory amino acid neurotransmitter glutamate (Hegarty et al., 2010). Approximately half of these neurons are peptidergic nocicptors containing substance P and/or calcitonin gene-related peptide (CGRP) (Felipe et al., 1999; Jones and Marfurt, 1998). In addition to their role in relaying sensory information to the central nervous system, corneal afferent neurons provide neurotrophic factors important for wound healing and the general health of the cornea. Both substance P, in combination with insulin-like growth factor-1, and CGRP have been shown to increase the rate of corneal wound healing (Mikulec and Tanelian, 1996; Nakamura et al., 1997). Furthermore, denervation of the cornea leads to corneal degeneration indicated by stromal thinning and perforation of the corneal epithelium, which may be due to a reduction in corneal epithelial stem cells (Ferrari et al., 2011; Ferrari et al., 2013; Ueno et al., 2012).
With nerves that terminate as free endings, the properties of corneal afferent neurons depend on the expression of channels that transduce mechanical, thermal and chemical stimuli into electrical potentials (Figure 2), although the importance of corneal epithelial cells in the responses properties of corneal afferent neurons should not be discounted (Pan et al., 2011b). In many instances, the transduction process involves the opening of transient receptor potential (TRP) channels (Huang et al., 2006). There are approximately 30 members in the mammalian TRP family, nine of which are thermosensitive cation channels (TRPV1-4, TRPM2-4, 8, and TRPA1) expressed on sensory primary afferent neurons. In the nerve terminals, opening of cation channels allows for the influx of sodium and calcium ions. This influx produces membrane depolarization, activation of voltage gated sodium channels, and the initiation of action potentials.
Of particular importance in nociceptive transmission, TRPV1 is opened by noxious heat stimulation (Caterina et al., 2000; Tominaga et al., 1998). Also physiologically relevant is the sensitivity of TRPV1 to low pH and capsaicin, the active ingredient in hot chili peppers. TRPV1 is not the only channel responsive to low pH, as acid sensing ion channels (ASICs) are also found on trigeminal sensory neurons, often co-expressed with TRPV1 (Ugawa et al., 2005a; Ugawa et al., 2005b). This combination of thermal and chemosensitivity found in TRPV1 channels is a property shared by TRPA1 and TRPM8 channels (Bandell et al., 2006; Bautista et al., 2007; de la Pena et al., 2005; Jordt et al., 2004; Madrid et al., 2006). TRPA1 channels are activated by noxious cold and allyl isothiocynate, the pungent chemical in mustard oil and wasabi, and TRPM8 channels are activated by non-noxious cooling and menthol. In addition, potassium channels such as members of the KCNK channel family may be involved in cold transduction (Thut et al., 2003). A reduction in potassium conductance would result in membrane depolarization and increased action potential generation.
Based primarily on in vivo electrophysiological recordings performed in cats, guinea pigs, and rats, corneal primary afferents fall into three main categories: mechanoreceptors, polymodal receptors, and cold receptors (Belmonte et al., 2004b). Corneal mechanoreceptors respond exclusively to mechanical stimulation, polymodal receptors are activated by noxious thermal and chemical stimuli as well as mechanical stimulation, and cold receptors respond to innocuous cooling. The overall composition of corneal afferents has been estimated as 70% polymodal nociceptors, 20% mechanoreceptors, and 10% cold receptors (Belmonte et al., 2004a). However, the true representation of these different afferent populations is unknown as these values depend on the sampling methods used in the various studies.
Polymodal nociceptors innervating the cornea express several different thermal and chemosensitive channels, most from the TRP family, that account for their physiological properties (Figure 2). Corneal polymodal nociceptors have been characterized that respond to noxious thermal stimulation and low pH, indicating the presence of TRPV1 and ASICs (Belmonte et al., 1991; Belmonte and Giraldez, 1981; Gallar et al., 1993). The response to acidity has been exploited with the use of CO2 stimulation in human and animal studies. CO2 pulses directed at the cornea activate polymodal corneal afferent neurons in animal studies, and produce irritation and lacrimation in humans (Acosta et al., 2004; Belmonte et al., 1999; Chen et al., 1995).
Unlike polymodal nociceptors, less is known regarding the transduction mechanism for corneal mechanoreceptive neurons (Figure 2). Several putative mechanotransduction molecules have been proposed for nociceptive neurons with free nerve endings, such as TRPA1, TRPV4, ASICs, and members of the KCNK family (Dubin and Patapoutian, 2010; Hu et al., 2006; Story and Gereau, 2006). However, the sensitivity of these channels to noxious chemicals, low pH, or thermal stimuli indicates that they are more likely to be relevant to mechanotransduction mechanisms in polymodal nociceptors rather than mechanoreceptive neurons. Additional candidates for mechanotransduction proteins include piezo proteins, recently identified as a unique class of cation channels, and epithelial sodium channels, ENaC (Garcia-Anoveros et al., 2001; Hao et al., 2013). Further study is needed to determine the role of these channels in corneal mechanotransduction.
Cold receptors represent a distinct population of corneal sensory neurons that respond to moderate, non-noxious reductions in temperature (Figure 2). Similar to cutaneous cold receptors, corneal cold receptors respond vigorously to cooling of the cornea and, when the cornea is warmed, activity is suppressed (Belmonte and Gallar, 2011; Brock et al., 2001; Carr and Brock, 2002; Carr et al., 2003; Gallar et al., 1993; Hirata and Meng, 2010; Madrid et al., 2006). In contrast to polymodal and mechanoreceptive neurons, cold sensitive neurons have relatively high ongoing activity at room temperature. Menthol, a TRPM8 channel agonist, is a potent activator of corneal cold receptors (Hirata and Meng, 2010; Kurose and Meng, 2013b; Madrid et al., 2006; Robbins et al., 2012).
In a property particularly relevant to the cornea, both polymodal and cold receptors are activated by hyperosmotic stimuli (Gallar et al., 1993; Hirata and Meng, 2010). The membrane channel responsible for the response to hyperosmotic stimuli is presently unknown, but may involve the expression of a TRPV1 splice variant (Pan et al., 2011a; Sharif Naeini et al., 2006). Thus, continuous evaporation of tears from the ocular surface causes cooling and increased tear osmolarity, ideal conditions for activating cold receptors. This sensitivity to mild cooling and hypertonicity would explain the previously described response of cold receptors to drying of the ocular surface (Hirata and Meng, 2010; Hirata et al., 2004).
Based on the properties of each subtype of corneal primary afferent neuron, it is possible to infer their distinct role in lacrimation. Mechanoreceptive neurons are often preferentially activated by movement tangential to the corneal surface rather than by punctate stimuli (MacIver and Tanelian, 1993a; MacIver and Tanelian, 1993b), suggesting that activation of mechanoreceptors in the cornea induces lacrimation and blinking to clear particulate matter from the eye (Acosta et al., 2004; Belmonte et al., 2004b). Polymodal nociceptors promote similar reflexes vital to protecting the corneal epithelium, typically in response to noxious thermal or chemical stimuli. This activation of mechanoreceptors and polymodal nociceptors by mechanical, chemical, and thermal stimuli induces glandular secretion while also producing the sensation of irritation or pain (Acosta et al., 2001a; Acosta et al., 2004; Belmonte et al., 2004b).
In contrast to mechanoreceptors and polymodal receptors, cold receptor activation appears to evoke secretion that is not accompanied by ocular pain. As evidence, cold receptors are the only known corneal primary afferent neuron with spontaneous activity at room temperature (Gallar et al., 1993). Diminished tearing observed after application of a topical analgesic to the corneal surface demonstrates the importance of this corneal afferent neural activity in tear production (Herreras et al., 1997). Furthermore, activation of cold receptors with cold room air increases lacrimation in human subjects (Parra et al., 2010). In animal studies, application of menthol to the ocular surface increases tearing in a TRPM8-dependent fashion, yet fails to elicit nociceptive behaviors (Robbins et al., 2012).
Each class of primary afferent neuron has the potential to induce tear secretion by responding to different environmental stimuli. Tear composition, however, may differ depending on the class of primary afferent neuron that is activated, a possibility that requires further study. For example, it is possible that activation of cold receptors increases lacrimal gland secretion, whereas activation of polymodal nociceptors may also induce mucin secretion from goblet cells (Kessler et al., 1995). Distinct central pathways processing corneal afferent information would be required in order for these divergent responses to occur.
3. Central Processing within the Spinal Trigeminal Nucleus
Trigeminal primary afferent neurons innervating the head and face carry sensory information to Vsp, which is divided into three primary subdivisions: subnucleus oralis (Vo), interpolaris (Vi), and caudalis (Vc), from rostral to caudal (Olszewski, 1950). The processing of sensory information in Vsp is unique from that of the spinal cord dorsal horn in that there are multiple representations of the same orofacial region in different subnuclei of Vsp (Bereiter et al., 2000a). As might be expected, multiple representations indicate a specialization of function amongst these regions (Figure 1).
Tract tracing studies of corneal primary afferent neurons have identified two discrete terminal fields located within Vsp in numerous animal species (Marfurt, 1981; Marfurt and Del Toro, 1987; Marfurt and Echtenkamp, 1988; Panneton and Burton, 1981). These two regions, one located at the transition between Vi and Vc (Vi/Vc) and the other located further caudally at the transition between Vc and the first cervical vertebra (Vc/C1), are also labelled with c-Fos protein following noxious stimulation of the cornea (Meng and Bereiter, 1996; Strassman and Vos, 1993). Neurons with corneal receptive fields have been characterized in each of these regions in the rat and noted differences in the receptive field properties have been described, a first indication that the Vi/Vc and Vc/C1 transition regions are functionally distinct (Hirata et al., 1999; Hirata et al., 2004; Meng et al., 1997; Meng et al., 1998).
The trigeminal subnucleus caudalis is often referred to as the medullary dorsal horn, emphasizing the similar anatomical and functional properties with the spinal cord dorsal horn (Dubner and Bennett, 1983; Gobel et al., 1977). As with the spinal cord dorsal horn, neurons in Vc process and relay nociceptive signals to brainstem and thalamic nuclei important in producing a wide array of nociceptive responses, including pain sensation and autonomic responses such as increased heart rate and blood pressure (Figure 1). Corneal units recorded at the Vc/C1 region typically posses nociceptive receptive fields that are contiguous with the cornea and have properties similar to other nociceptive neurons located in the medullary dorsal horn (Meng et al., 1997). They are excited by noxious heat, chemical (mustard oil), and acidic (CO2) stimuli, and project to the thalamus and parabrachial area, relays to sensory and autonomic output regions, respectively (Hirata et al., 1999; Hirata et al., 2000; Meng et al., 1997).
The properties exhibited by Vc/C1 cornea sensitive neurons suggest that this region performs a similar function in nociception as other medullary and spinal cord dorsal horn neurons (Sessle, 1999; Sessle, 2000). Additionally, the Vc/C1 region does not appear to directly regulate noxious stimulation-evoked tearing. In support of this view, direct activation of the Vc/C1 region with glutamate did not elicit tearing, and inactivation of the Vc/C1 transition region had no effect on tearing evoked by CO2 or bright light stimulation (Hirata et al., 2004; Okamoto et al., 2012). Instead, evidence has accumulated to indicate a critical role of the Vi/Vc transition region in regulating lacrimation.
Cornea sensitive neurons recorded at the Vi/Vc transition region possess several unique features that distinguish this region as one that is specialized for processing information unique to the cornea. Foremost among these features, many Vi/Vc neurons have excitatory receptive fields that include only the cornea (Hirata et al., 2004; Meng et al., 1997). In addition, compared to Vc/C1 cornea sensitive neurons, Vi/Vc neurons preferentially project to the superior salivatory nucleus (SSN) and the facial motor nucleus, regions involved in the efferent pathway for lacrimation and blinking (Figure 2) (Henriquez and Evinger, 2007; Hirata et al., 2004; Hirata et al., 2000; Toth et al., 1999). Presumably, emotional tears, a uniquely human response, are mediated by limbic projections to the SSN. Direct projections from the amygdala and hypothalamus to the SSN have been described in the cat and rat, respectively, providing a potential anatomical substrate for this response (Hosoya et al., 1983; Spencer et al., 1990; Takeuchi et al., 1991). In contrast to Vc/C1 corneal neurons, relatively few Vi/Vc neurons projected to thalamus and none could not be antidromically activated from the parabrachial area (Hirata et al., 1999; Hirata et al., 2000; Meng et al., 1997).
The Vi/Vc region contains a heterogeneous population of neurons, with at least four broad classes of cornea-responsive neurons that have been characterized based on their responses to mechanical, thermal, and acidic conditions. The first category responds only to mechanical stimulation, indicating selective input from mechanoreceptive primary afferent neurons (Meng et al., 1997). A second category responds to mechanical stimulation, innocuous cooling, and hyperosmotic stimulation, suggesting input from cold receptors and possibly mechanoreceptors (Kurose and Meng, 2013a). A third group is sensitive to mechanical stimulation, innocuous cooling, hyperosmotic stimulation, noxious heat, and low pH. This combination of responses indicates a convergence of input from all three categories of corneal primary afferent neurons (Kurose and Meng, 2013a). The final class of neuron is activated by mechanical, noxious heat, and low pH, likely receiving selective input from polymodal receptors (Hirata et al., 1999; Hirata et al., 2000; Meng et al., 1997). These labelled lines of transmission through the Vi/Vc region may reflect distinct pathways involved in the regulation of tearing and blinking.
Given their receptive field properties and projection targets, it has been proposed that cornea sensitive neurons at the Vi/Vc region are involved in the regulation of tearing, blinking, and other homeostatic functions unique to the cornea (Henriquez and Evinger, 2007; Hirata et al., 2004; Pellegrini et al., 1995). In support of this idea, glutamate microinjections into the Vi/Vc region have been shown to increase lacrimation (Hirata et al., 2004). Furthermore, tearing elicited by CO2 and bright light stimulation was blocked by prior inactivation of the Vi/Vc transition region (Hirata et al., 2004; Okamoto et al., 2012). These results demonstrate the importance of the Vi/Vc region in noxious stimulation evoked tearing. This region may also represent a critical relay in tearing induced by simple evaporation of the tear film, as suggested by the select class of neurons activated by innocuous cooling (Figure 2) (Kurose and Meng, 2013a).
In summary, the cornea, like other specialized tissues of the cephalic region innervated by the trigeminal nerve, has its own requirements for health. The available evidence indicates that the Vi/Vc transition region is unique in its ability to regulate lacrimal gland secretion and blinking. As illustrated in Figure 1, we propose that this region is also involved in the regulation of intranasal and intraoral secretion. The convergence of input from the cornea, intranasal mucosa, and intraoral region onto individual neurons in the Vi/Vc transition region may explain why stimulation of one region can affect secretion in another (Drummond, 1995; Gupta et al., 1997; Nicolodi, 1994; Philip et al., 1994; Pramanik and Ghising, 2009; Togias et al., 1990). Despite the progress that has been made in understanding the neural regulation of secretion, relatively little is known regarding the function of neurons under pathophysiological conditions such as dry eye.
4. Properties of corneal sensory neurons in dry eye
In vivo confocal microscopy in humans has allowed for the examination of the subbasal nerve plexus in dry eye patients (Cruzat et al., 2010). Results have not been entirely consistent with regards to differences in the overall density of the subbasal plexus, which may be the result of the varying causes and stages of dry eye in these studies. However, an increase in subbasal nerve tortuosities and bead-like formations has been consistently described (Benitez-Del-Castillo et al., 2007; Erdelyi et al., 2007; Tuisku et al., 2008; Tuominen et al., 2003; Villani et al., 2007; Zhang et al., 2005). The correlation between these morphological differences and potential sensory changes in dry eye is still unclear. An examination of the excitability of nerve endings in dry eye would allow for these morphological alterations to be associated with potential functional changes.
Animal models of dry eye typically use tear levels and corneal fluorescein staining as end points for evaluating the potential therapeutic effects of various interventions (Barabino et al., 2004; Barabino and Dana, 2004; Fabiani et al., 2009; Fujihara et al., 2001; Higuchi et al., 2010; Lin et al., 2011; Zhu et al., 2009; Zhu et al., 2012). However, improvements in the condition of the corneal epithelium may not be indicative of changes in the properties of corneal sensory neurons, and it could be argued that the most relevant outcome to dry eye treatment is an overall improvement in irritation and pain. Therefore, the assessment of potential therapeutics for treating dry eye requires an understanding of how the dry eye condition itself may affect corneal sensory processing.
The effect lacrimal gland excision on the properties of corneal primary afferent cold receptors has recently been explored in the rat (Kurose and Meng, 2013b). In this study, unilateral removal of the exorbital and infraorbital lacrimal glands resulted in diminished tear production and elevated fluorescein staining over an 8-week observation period. In addition, spontaneous blink rates on the side ipsilateral to lacrimal gland excision were elevated. These findings are consistent with previous studies that have found increased fluorescein staining and spontaneous blinking after removal of the exorbital lacrimal gland (Fujihara et al., 2001; Higuchi et al., 2012; Higuchi et al., 2010; Kaminer et al., 2011). The increase in blinking, while referred to as “spontaneous” (Kaminer et al., 2011), likely requires the activation of corneal primary afferent neurons. In preliminary studies, application of a topical anaesthetic to the cornea caused a notable reduction in the spontaneous blink rate in dry eye animals (Meng et al., 2013). While the class of primary afferent neuron driving these spontaneous blinks in the dry eye condition are still unknown, it may be indicative of ongoing irritation or pain.
Cold receptors recorded 8 weeks following lacrimal gland excision had warmer activation temperature thresholds and an increase in cold-evoked activity (Kurose and Meng, 2013b). In addition, cold receptors demonstrated an increased sensitivity to menthol, suggesting an upregulation of TRPM8 receptors. This type of cold receptor sensitization in dry eye would most likely lead to an increase in afferent drive for both tearing and blinking. These results also run counter to the cornea and lacrimal gland feedback model proposed by Mathers (Mathers, 2000), in which it was hypothesized that dry eye produces a decrease in the sensitivity of corneal primary afferent neurons. The effect of dry eye on the properties of mechanoreceptive and polymodal neurons has not been examined, yet sensitization of these neurons would be consistent with studies that have reported significant hyperesthesia in individuals symptomatic for ocular dryness (Chen and Simpson, 2011; De Paiva and Pflugfelder, 2004; Situ et al., 2008a; Situ et al., 2008b; Tuisku et al., 2008)(but see (Benitez-Del-Castillo et al., 2007; Bourcier et al., 2005; Stapleton et al., 2006)).
The sensitization of corneal afferents in dry eye may involve inflammatory mediators, which have been shown to sensitize polymodal nociceptors innervating other tissues (Schaible et al., 2011). Under dry eye conditions, increased prostaglandins and inflammatory cytokines such as IL1-beta and TNF-alpha increase in corneal tissue, and could potentially sensitize corneal polymodal receptors (LaMotte et al., 1992; Liang et al., 2001; Lin et al., 2011; Massingale et al., 2009; Shim et al., 2012; Stevenson et al., 2012; Szolcsanyi, 1987; Zhu et al., 2009). However, similar factors have been shown to desensitize cold receptors (Linte et al., 2007; Zhang et al., 2012). This area of research requires further study, since any effect of dry eye on corneal primary afferent neurons would alter their ability to regulate lacrimation.
Dry eye may also affect the function of trigeminal brainstem neurons that regulate secretion. The properties of Vsp cornea-responsive neurons in the dry eye condition have not been explored, yet studies indicate neurons located at the Vi/Vc and Vc/C1 transition regions respond differently to repeated noxious stimulation and ocular inflammation or injury. At the Vc/C1 transition region, noxious heat applied repeatedly to the cornea caused an increase in neuronal responses, whereas Vi/Vc neurons desensitized to the same stimulus (Meng et al., 1997).
These early results are consistent with more recent studies characterizing cornea-responsive neurons following ocular inflammation (Bereiter et al., 2005; Tashiro et al., 2010). In an animal model of photokeratitis, ultraviolet irradiation of the eye resulted in the sensitization of neurons recorded at the Vc/C1 but not Vi/Vc transition region (Tashiro et al., 2010). The sensitization of Vc/C1 neurons correlated with an increase in hypertonic saline evoked eye wipe behavior. Furthermore, only Vc/C1 neurons were sensitized to CO2 stimulation one-week after endotoxin-induced uveitis (Bereiter et al., 2005). The enhanced neural activity correlated with an increase in CO2-induced blinking. At this same time-point, however, there was no change in CO2-induced lacrimation. Taken together, these results indicate that differences in central processing of corneal input at the Vi/Vc and Vc/C1 transition regions can result in distinct functional consequences with respect to lacrimation and sensation. In dry eye, even though ocular conditions may sensitize primary afferent neurons, the ability of Vi/Vc neurons to stimulate secretion may still be impaired.
5. Future Directions
At present, the neural regulation of tearing under normal conditions is relatively well characterized. Noxious mechanical, thermal, and chemical stimuli activate corneal primary afferent polymodal receptors and mechanoreceptors, whereas innocuous cooling, such as that which occurs during minute-to-minute evaporation of the tear film, increases corneal cold receptor activity. These primary afferent neurons project to the Vi/Vc transition region in the brainstem, a critical relay in the lacrimation circuit. At the Vi/Vc region, select labelled-lines remain as distinct categories of second-order neurons, although convergence of several different classes of primary afferent neurons is also apparent. Corneal input at the Vi/Vc region is processed and modified before information is sent to pre-ganglionic parasympathetic neurons located in the salivatory nuclei.
While the basic circuitry involved in the neural regulation of glandular secretion has been described, several unanswered questions remain. In particular, relatively little is understood regarding the peripheral and central changes that occur under pathophysiological conditions such as dry eye. Modifications in the properties of cornea-responsive neurons could have a severe impact on the regulation of tearing. Already, dry eye induced by lacrimal gland excision has been shown to sensitize corneal cold receptors (Kurose and Meng, 2013b). Understanding the mechanisms underlying these changes may prove important in developing novel treatments for dry eye. In such an approach, increasing the sensitivity of cold receptors could enhance tearing elicited by evaporative cooling.
In addition to the sensitization of cold receptors observed under dry eye conditions, it is now clear that cold receptors are also susceptible to desensitization (Kurose and Meng, 2013b; Robbins et al., 2012). Following low concentrations of menthol, cold-evoked neuronal activity is increased, yet after high concentrations of menthol these same neurons will desensitize. Lacrimal gland excision also increased the propensity for menthol to desensitize cold receptors (Kurose and Meng, 2013b). The process of desensitization is unknown, but may involve changes in membrane phospholipids (Daniels et al., 2009; Rohacs et al., 2005; Yudin et al., 2011; Yudin and Rohacs, 2012). Thus, while it may be advantageous to activate cold receptors to increase lacrimation, the potential to desensitize these neurons also must be considered.
Modifying cold receptor activity may be a useful strategy in promoting tearing without inducing irritation or pain (Parra et al., 2010; Robbins et al., 2012). However, the development of better treatments for irritation and pain often associated with dry eye and other ocular conditions will require a greater understanding of alterations that occur in mechanoreceptive and polymodal neurons under these conditions. Importantly, it should be recognized that changes in the properties of primary afferent neurons are not necessarily reflected in central neurons that regulate lacrimation. The effect of dry eye on the processing of corneal input at the Vi/Vc transition region has not been explored, yet modifications in the properties of these neurons would have a severe impact on tear secretion.
Novel therapies must address both ocular pain, produced by activation of polymodal receptors, and insufficient tearing, which may be caused by inadequate cold receptor activity. In order to assess the potential efficacy of novel therapies for dry eye and ocular pain, it is essential to determine their effect on the properties of corneal afferent neurons in the dry eye condition.
Highlights.
Tearing is regulated through a neural reflex initiated by trigeminal primary afferent neurons.
The properties of corneal sensory afferents are consistent with their ability to sense potentially damaging stimuli and drying that occurs with evaporation of the tear film.
Projections from a specialized region in the spinal trigeminal nucleus to preganglionic parasympathetic neurons located in or around the superior salivatory nucleus control secretions.
The dry eye condition may modify the properties of corneal primary afferent and second-order neurons, affecting their ability to regulate secretions.
Acknowledgements
Funding was provided by the National Eye Institute R01EY021230 to I.D.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:179–193. doi: 10.1016/s1542-0124(12)70086-1. 2007. [DOI] [PubMed] [Google Scholar]
- Acosta MC, Belmonte C, Gallar J. Sensory experiences in humans and single-unit activity in cats evoked by polymodal stimulation of the cornea. J Physiol. 2001a;534:511–525. doi: 10.1111/j.1469-7793.2001.t01-1-00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta MC, Peral A, Luna C, Pintor J, Belmonte C, Gallar J. Tear secretion induced by selective stimulation of corneal and conjunctival sensory nerve fibers. Invest Ophthalmol Vis Sci. 2004;45:2333–2336. doi: 10.1167/iovs.03-1366. [DOI] [PubMed] [Google Scholar]
- Acosta MC, Tan ME, Belmonte C, Gallar J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 2001b;42:2063–2067. [PubMed] [Google Scholar]
- Ambrosio R, Jr, Tervo T, Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: pathophysiology and strategies for prevention and treatment. J Refract Surg. 2008;24:396–407. doi: 10.3928/1081597X-20080401-14. [DOI] [PubMed] [Google Scholar]
- Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci. 2006;9:493–500. doi: 10.1038/nn1665. [DOI] [PubMed] [Google Scholar]
- Barabino S, Chen W, Dana MR. Tear film and ocular surface tests in animal models of dry eye: uses and limitations. Exp Eye Res. 2004;79:613–621. doi: 10.1016/j.exer.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Barabino S, Dana MR. Animal models of dry eye: a critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci. 2004;45:1641–1646. doi: 10.1167/iovs.03-1055. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004a;78:513–525. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Acosta MC, Schmelz M, Gallar J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Invest Ophthalmol Vis Sci. 1999;40:513–519. [PubMed] [Google Scholar]
- Belmonte C, Aracil A, Acosta MC, Luna C, Gallar J. Nerves and sensations from the eye surface. Ocul Surf. 2004b;2:248–253. doi: 10.1016/s1542-0124(12)70112-x. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Gallar J. Cold thermoreceptors, unexpected players in tear production and ocular dryness sensations. Invest Ophthalmol Vis Sci. 2011;52:3888–3892. doi: 10.1167/iovs.09-5119. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Gallar J, Pozo MA, Rebollo I. Excitation by irritant chemical substances of sensory afferent units in the cat's cornea. J. Physiol. 1991;437:709–725. doi: 10.1113/jphysiol.1991.sp018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C, Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J. Physiol. 1981;321:355–368. doi: 10.1113/jphysiol.1981.sp013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Del-Castillo JM, Acosta MC, Wassfi MA, Diaz-Valle D, Gegundez JA, Fernandez C, Garcia-Sanchez J. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48:173–181. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Hirata H, Hu JW. Trigeminal subnucleus caudalis: beyond homologies with the spinal dorsal horn. Pain. 2000a;88:221–224. doi: 10.1016/S0304-3959(00)00434-6. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Hirata H, Hu JW. Trigeminal subnucleus caudalis: beyond homologies with the spinal dorsal horn. Pain. 2000b;88:221–224. doi: 10.1016/S0304-3959(00)00434-6. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Okamoto K, Tashiro A, Hirata H. Endotoxin-induced uveitis causes long-term changes in trigeminal subnucleus caudalis neurons. J Neurophysiol. 2005;94:3815–3825. doi: 10.1152/jn.00616.2005. [DOI] [PubMed] [Google Scholar]
- Beuerman RW, Tanelian DL. Corneal pain evoked by thermal stimulation. Pain. 1979;7:1–14. doi: 10.1016/0304-3959(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Bourcier T, Acosta MC, Borderie V, Borras F, Gallar J, Bury T, Laroche L, Belmonte C. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005;46:2341–2345. doi: 10.1167/iovs.04-1426. [DOI] [PubMed] [Google Scholar]
- Brock JA, Pianova S, Belmonte C. Differences between nerve terminal impulses of polymodal nociceptors and cold sensory receptors of the guinea-pig cornea. J Physiol. 2001;533:493–501. doi: 10.1111/j.1469-7793.2001.0493a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RW, Brock JA. Electrophysiology of corneal cold receptor nerve terminals. Adv Exp Med Biol. 2002;508:19–23. doi: 10.1007/978-1-4615-0713-0_3. [DOI] [PubMed] [Google Scholar]
- Carr RW, Pianova S, Fernandez J, Fallon JB, Belmonte C, Brock JA. Effects of heating and cooling on nerve terminal impulses recorded from cold-sensitive receptors in the guinea-pig cornea. J Gen Physiol. 2003;121:427–439. doi: 10.1085/jgp.200308814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chen J, Simpson TL. A role of corneal mechanical adaptation in contact lens-related dry eye symptoms. Invest Ophthalmol Vis Sci. 2011;52:1200–1205. doi: 10.1167/iovs.10-5349. [DOI] [PubMed] [Google Scholar]
- Chen X, Gallar J, Pozo MA, Baeza M, Belmonte C. CO2 stimulation of the cornea: a comparison between human sensation and nerve activity in polymodal nociceptive afferents of the cat. Eur J Neurosci. 1995;7:1154–1163. doi: 10.1111/j.1460-9568.1995.tb01105.x. [DOI] [PubMed] [Google Scholar]
- Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25:171–177. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem. 2009;284:1570–1582. doi: 10.1074/jbc.M807270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA. Control of mucin production by ocular surface epithelial cells. Exp Eye Res. 2004;78:173–185. doi: 10.1016/j.exer.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–177. doi: 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA, McCarthy DM, Mercer HJ, Kessler TL, Chung EH, Zieske JD. Localization of nerves adjacent to goblet cells in rat conjunctiva. Curr Eye Res. 1995;14:993–1000. doi: 10.3109/02713689508998520. [DOI] [PubMed] [Google Scholar]
- de la Pena E, Malkia A, Cabedo H, Belmonte C, Viana F. The contribution of TRPM8 channels to cold sensing in mammalian neurones. J Physiol. 2005;567:415–426. doi: 10.1113/jphysiol.2005.086546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paiva CS, Chen Z, Koch DD, Hamill MB, Manuel FK, Hassan SS, Wilhelmus KR, Pflugfelder SC. The incidence and risk factors for developing dry eye after myopic LASIK. Am J Ophthalmol. 2006;141:438–445. doi: 10.1016/j.ajo.2005.10.006. [DOI] [PubMed] [Google Scholar]
- De Paiva CS, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol. 2004;137:109–115. doi: 10.1016/s0002-9394(03)00897-3. [DOI] [PubMed] [Google Scholar]
- Drummond PD. Lacrimation and cutaneous vasodilatation in the face induced by painful stimulation of the nasal ala and upper lip. J Auton Nerv Syst. 1995;51:109–116. doi: 10.1016/0165-1838(94)00121-y. [DOI] [PubMed] [Google Scholar]
- Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120:3760–3772. doi: 10.1172/JCI42843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubner R, Bennett GJ. Spinal and trigeminal mechanisms of nociception. Annu. Rev. Neurosci. 1983;6:381–34138. doi: 10.1146/annurev.ne.06.030183.002121. [DOI] [PubMed] [Google Scholar]
- Erdelyi B, Kraak R, Zhivov A, Guthoff R, Nemeth J. In vivo confocal laser scanning microscopy of the cornea in dry eye. Graefes Arch Clin Exp Ophthalmol. 2007;245:39–44. doi: 10.1007/s00417-006-0375-6. [DOI] [PubMed] [Google Scholar]
- Evinger C, Bao JB, Powers AS, Kassem IS, Schicatano EJ, Henriquez VM, Peshori KR. Dry eye blinking, and blepharospasm. Mov Disord. 2002;17(Suppl 2):S75–S78. doi: 10.1002/mds.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani C, Barabino S, Rashid S, Dana MR. Corneal epithelial proliferation and thickness in a mouse model of dry eye. Exp Eye Res. 2009 doi: 10.1016/j.exer.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felipe CD, Gonzalez GG, Gallar J, Belmonte C. Quantification and immunocytochemical characteristics of trigeminal ganglion neurons projecting to the cornea: effect of corneal wounding. Eur J Pain. 1999;3:31–39. doi: 10.1053/eujp.1998.0100. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Chauhan SK, Ueno H, Nallasamy N, Gandolfi S, Borges L, Dana R. A novel mouse model for neurotrophic keratopathy: trigeminal nerve stereotactic electrolysis through the brain. Invest Ophthalmol Vis Sci. 2011;52:2532–2539. doi: 10.1167/iovs.10-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Ueno H, Bignami F, Rama P, Dana R. Trigeminal stereotactic electrolysis induces dry eye in mice. Acta Ophthalmol. 2013;91:e162–e163. doi: 10.1111/j.1755-3768.2012.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara T, Murakami T, Fujita H, Nakamura M, Nakata K. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001;42:96–100. [PubMed] [Google Scholar]
- Gallar J, Acosta MC, Moilanen JA, Holopainen JM, Belmonte C, Tervo TM. Recovery of corneal sensitivity to mechanical and chemical stimulation after laser in situ keratomileusis. J Refract Surg. 2004;20:229–235. doi: 10.3928/1081-597X-20040501-06. [DOI] [PubMed] [Google Scholar]
- Gallar J, Pozo MA, Tuckett RP, Belmonte C. Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation of the cat's cornea. J. Physiol. 1993;468:609–622. doi: 10.1113/jphysiol.1993.sp019791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Anoveros J, Samad TA, Zuvela-Jelaska L, Woolf CJ, Corey DP. Transport and localization of the DEG/ENaC ion channel BNaC1alpha to peripheral mechanosensory terminals of dorsal root ganglia neurons. J Neurosci. 2001;21:2678–2686. doi: 10.1523/JNEUROSCI.21-08-02678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel S, Falls WM, Hockfield S. The division of the dorsal and ventral horns of the mammalian caudal medulla into eight layers using anatomical criteria. In: Anderson DJ, Matthews B, editors. Pain in the Trigeminal Region. Amsterdam: Elsevier; 1977. pp. 443–453. [Google Scholar]
- Gupta A, Heigle T, Pflugfelder SC. Nasolacrimal stimulation of aqueous tear production. Cornea. 1997;16:645–648. [PubMed] [Google Scholar]
- Hao J, Ruel J, Coste B, Roudaut Y, Crest M, Delmas P. Piezo-electrically driven mechanical stimulation of sensory neurons. Methods Mol Biol. 2013;998:159–170. doi: 10.1007/978-1-62703-351-0_12. [DOI] [PubMed] [Google Scholar]
- Hegarty DM, Tonsfeldt K, Hermes SM, Helfand H, Aicher SA. Differential localization of vesicular glutamate transporters and peptides in corneal afferents to trigeminal nucleus caudalis. J Comp Neurol. 2010;518:3557–3569. doi: 10.1002/cne.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez VM, Evinger C. The three-neuron corneal reflex circuit and modulation of second-order corneal responsive neurons. Exp Brain Res. 2007;179:691–702. doi: 10.1007/s00221-006-0826-7. [DOI] [PubMed] [Google Scholar]
- Herreras JM, Perez S, Perez H, Calonge M, Pastor JC. Influence of topical anesthesia on tests diagnostic of blepharitis-associated dry eye syndrome. Ocul Immunol Inflamm. 1997;5:33–41. doi: 10.3109/09273949709085048. [DOI] [PubMed] [Google Scholar]
- Higuchi A, Inoue H, Kawakita T, Ogishima T, Tsubota K. Selenium Compound Protects Corneal Epithelium against Oxidative Stress. PLoS One. 2012;7:e45612. doi: 10.1371/journal.pone.0045612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi A, Takahashi K, Hirashima M, Kawakita T, Tsubota K. Selenoprotein P controls oxidative stress in cornea. PLoS One. 2010;5:e9911. doi: 10.1371/journal.pone.0009911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Hu JW, Bereiter DA. Responses of medullary dorsal horn neurons to corneal stimulation by CO(2) pulses in the rat. J Neurophysiol. 1999;82:2092–2107. doi: 10.1152/jn.1999.82.5.2092. [DOI] [PubMed] [Google Scholar]
- Hirata H, Meng ID. Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: implications for dry eye disease. Invest Ophthalmol Vis Sci. 2010;51:3969–3976. doi: 10.1167/iovs.09-4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Okamoto K, Tashiro A, Bereiter DA. A novel class of neurons at the trigeminal subnucleus interpolaris/caudalis transition region monitors ocular surface fluid status and modulates tear production. J Neurosci. 2004;24:4224–4232. doi: 10.1523/JNEUROSCI.0381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Takeshita S, Hu JW, Bereiter DA. Cornea-responsive medullary dorsal horn neurons: modulation by local opioids and projections to thalamus and brain stem. J Neurophysiol. 2000;84:1050–1061. doi: 10.1152/jn.2000.84.2.1050. [DOI] [PubMed] [Google Scholar]
- Hosoya Y, Matsushita M, Sugiura Y. A direct hypothalamic projection to the superior salivatory nucleus neurons in the rat. A study using anterograde autoradiographic and retrograde HRP methods. Brain research. 1983;266:329–333. doi: 10.1016/0006-8993(83)90664-9. [DOI] [PubMed] [Google Scholar]
- Hu J, Milenkovic N, Lewin GR. The high threshold mechanotransducer: a status report. Pain. 2006;120:3–7. doi: 10.1016/j.pain.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang X, McNaughton PA. Modulation of temperature-sensitive TRP channels. Semin Cell Dev Biol. 2006;17:638–645. doi: 10.1016/j.semcdb.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Ishizuka K, Murakami T. Responses of inferior salivatory neurons to stimulation of trigeminal sensory branches. Exp Neurol. 1986;91:269–276. doi: 10.1016/0014-4886(86)90067-1. [DOI] [PubMed] [Google Scholar]
- Javadi MA, Feizi S. Dry eye syndrome. J Ophthalmic Vis Res. 2011;6:192–198. [PMC free article] [PubMed] [Google Scholar]
- Jones MA, Marfurt CF. Peptidergic innervation of the rat cornea. Exp Eye Res. 1998;66:421–435. doi: 10.1006/exer.1997.0446. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Kaminer J, Powers AS, Horn KG, Hui C, Evinger C. Characterizing the spontaneous blink generator: an animal model. J Neurosci. 2011;31:11256–11267. doi: 10.1523/JNEUROSCI.6218-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno H, Horikawa Y, Hodges RR, Zoukhri D, Shatos MA, Rios JD, Dartt DA. Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am J Physiol Cell Physiol. 2003;284:C988–C998. doi: 10.1152/ajpcell.00582.2001. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR. Comparison of thermal sensitivity of the forehead lip conjunctiva and cornea. J. Appl. Physiol. 1960;15:987–991. doi: 10.1152/jappl.1960.15.6.987. [DOI] [PubMed] [Google Scholar]
- Kessler TL, Dartt DA. Neural stimulation of conjunctival goblet cell mucous secretion in rats. Adv Exp Med Biol. 1994;350:393–398. doi: 10.1007/978-1-4615-2417-5_68. [DOI] [PubMed] [Google Scholar]
- Kessler TL, Mercer HJ, Zieske JD, McCarthy DM, Dartt DA. Stimulation of goblet cell mucous secretion by activation of nerves in rat conjunctiva. Curr Eye Res. 1995;14:985–992. doi: 10.3109/02713689508998519. [DOI] [PubMed] [Google Scholar]
- Kompella UB, Kim KJ, Shiue MH, Lee VH. Cyclic AMP modulation of active ion transport in the pigmented rabbit conjunctiva. J Ocul Pharmacol Ther. 1996;12:281–287. doi: 10.1089/jop.1996.12.281. [DOI] [PubMed] [Google Scholar]
- Konomi K, Chen LL, Tarko RS, Scally A, Schaumberg DA, Azar D, Dartt DA. Preoperative characteristics and a potential mechanism of chronic dry eye after LASIK. Invest Ophthalmol Vis Sci. 2008;49:168–174. doi: 10.1167/iovs.07-0337. [DOI] [PubMed] [Google Scholar]
- Kurose M, Meng ID. Corneal dry-responsive neurons in the spinal trigeminal nucleus respond to innocuous cooling in the rat. Journal of neurophysiology. 2013a;109:2517–2522. doi: 10.1152/jn.00889.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose M, Meng ID. Dry eye modifies the thermal and menthol responses in rat corneal primary afferent cool cells. Journal of neurophysiology. 2013b doi: 10.1152/jn.00222.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux MS, Zhou Q, Murphy RB, Greene ML, Ryan P. Parasympathetic innervation of the meibomian glands in rats. Invest Ophthalmol Vis Sci. 2001;42:2434–2441. [PubMed] [Google Scholar]
- Liang YF, Haake B, Reeh PW. Sustained sensitization and recruitment of rat cutaneous nociceptors by bradykinin and a novel theory of its excitatory action. J Physiol. 2001;532:229–239. doi: 10.1111/j.1469-7793.2001.0229g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Liu X, Zhou T, Wang Y, Bai L, He H, Liu Z. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol Vis. 2011;17:257–264. [PMC free article] [PubMed] [Google Scholar]
- Linte RM, Ciobanu C, Reid G, Babes A. Desensitization of cold- and menthol-sensitive rat dorsal root ganglion neurones by inflammatory mediators. Exp Brain Res. 2007;178:89–98. doi: 10.1007/s00221-006-0712-3. [DOI] [PubMed] [Google Scholar]
- MacIver MB, Tanelian DL. Free nerve ending terminal morphology Is fiber type specific for A∂ and C fiber innervating rabbit corneal epithelium. J. Neurophysiol. 1993a;69:1779–1783. doi: 10.1152/jn.1993.69.5.1779. [DOI] [PubMed] [Google Scholar]
- MacIver MB, Tanelian DL. Structural and functional specialization of A-d and C fiber free nerve endings innervating rabbit corneal epithelium. J. Neurosci. 1993b;13:4511–4524. doi: 10.1523/JNEUROSCI.13-10-04511.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R, Donovan-Rodriguez T, Meseguer V, Acosta MC, Belmonte C, Viana F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J Neurosci. 2006;26:12512–12525. doi: 10.1523/JNEUROSCI.3752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008;8:477–483. doi: 10.1097/ACI.0b013e32830e6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt CF. The central projections of trigeminal primary afferent neurons in the cat as determined by the tranganglionic transport of horseradish peroxidase. J Comp Neurol. 1981;203:785–798. doi: 10.1002/cne.902030414. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90:478–492. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Del Toro DR. Corneal sensory pathway in the rat: a horseradish peroxidase tracing study. J. Comp. Neurol. 1987;261:450–459. doi: 10.1002/cne.902610309. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Echtenkamp SF. Central projections and trigeminal ganglion location of corneal afferent neurons in the monkey, Macaca fascicularis. J Comp Neurol. 1988;272:370–382. doi: 10.1002/cne.902720307. [DOI] [PubMed] [Google Scholar]
- Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28:1023–1027. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- Mathers WD. Why the eye becomes dry: a cornea and lacrimal gland feedback model. Clao J. 2000;26:159–165. [PubMed] [Google Scholar]
- Meng ID, Barton ST, Twaite AL. Lacrimal gland removal increases primary afferent driven spontaneous blinking and produces ocular hyperalgesia in the rat. Invest Ophthalmol Vis Sci. 2013;54:906. [Google Scholar]
- Meng ID, Bereiter DA. Differential distribution of Fos-like immunoreactivity in the spinal trigeminal nucleus after noxious and innocuous thermal and chemical stimulation of rat cornea. Neuroscience. 1996;72:243–254. doi: 10.1016/0306-4522(95)00541-2. [DOI] [PubMed] [Google Scholar]
- Meng ID, Hu JW, Benetti AP, Bereiter DA. Encoding of corneal input in two distinct regions of the spinal trigeminal nucleus in the rat: cutaneous receptive field properties, responses to thermal and chemical stimulation, modulation by diffuse noxious inhibitory controls, and projections to the parabrachial area. J Neurophysiol. 1997;77:43–56. doi: 10.1152/jn.1997.77.1.43. [DOI] [PubMed] [Google Scholar]
- Meng ID, Hu JW, Bereiter DA. Differential effects of morphine on corneal-responsive neurons in rostral versus caudal regions of spinal trigeminal nucleus in the rat. J Neurophysiol. 1998;79:2593–2602. doi: 10.1152/jn.1998.79.5.2593. [DOI] [PubMed] [Google Scholar]
- Mikulec AA, Tanelian DL. CGRP increases the rate of corneal re-epithelialization in an in vitro whole mount preparation. J Ocul Pharmacol Ther. 1996;12:417–423. doi: 10.1089/jop.1996.12.417. [DOI] [PubMed] [Google Scholar]
- Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Murakami T, Ishizuka K, Yoshihara M, Uchiyama M. Reflex responses of single salivatory neurons to stimulation of trigeminal sensory branches in the cat. Brain Research. 1983;280:233–237. doi: 10.1016/0006-8993(83)90053-7. [DOI] [PubMed] [Google Scholar]
- Murakami T, Yoshihara M, Ishizuka KI, Uchiyama M. Antidromic responses and reflex activity of single salivatory neurons in the cat. Exp Neurol. 1982;76:218–224. doi: 10.1016/0014-4886(82)90113-3. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ofuji K, Chikama T, Nishida T. Combined effects of substance P and insulin-like growth factor-1 on corneal epithelial wound closure of rabbit in vivo. Curr Eye Res. 1997;16:275–278. doi: 10.1076/ceyr.16.3.275.15409. [DOI] [PubMed] [Google Scholar]
- Nicolodi M. Nostril capsaicin application as a model of trigeminal primary sensory neuronal activation. Cephalalgia. 1994;14:134–138. doi: 10.1046/j.1468-2982.1994.1402134.x. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Tashiro A, Thompson R, Nishida Y, Bereiter DA. Trigeminal interpolaris/caudalis transition neurons mediate reflex lacrimation evoked by bright light in the rat. Eur J Neurosci. 2012;36:3492–3499. doi: 10.1111/j.1460-9568.2012.08272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski J. On the Anatomical and Functional Organization of the Trigeminal Nucleus. J. Comp. Neurol. 1950;92:40l–44l43. doi: 10.1002/cne.900920305. [DOI] [PubMed] [Google Scholar]
- Pan Z, Wang Z, Yang H, Zhang F, Reinach PS. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2011a;52:485–493. doi: 10.1167/iovs.10-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Yang H, Reinach PS. Transient receptor potential (TRP) gene superfamily encoding cation channels. Hum Genomics. 2011b;5:108–116. doi: 10.1186/1479-7364-5-2-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneton WM, Burton H. Corneal and periocular representation within the trigeminal sensory complex in the cat studied with transganglionic transport of horseradish peroxidase. J Comp Neurol. 1981;199:327–344. doi: 10.1002/cne.901990303. [DOI] [PubMed] [Google Scholar]
- Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, Gallar J, Dhaka A, Viana F, Belmonte C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010;16:1396–1399. doi: 10.1038/nm.2264. [DOI] [PubMed] [Google Scholar]
- Pellegrini JJ, Horn AK, Evinger C. The trigeminally evoked blink reflexINeuronal circuits. Exp Brain Res. 1995;107:166–180. doi: 10.1007/BF00230039. [DOI] [PubMed] [Google Scholar]
- Philip G, Baroody FM, Proud D, Naclerio RM, Togias AG. The human nasal response to capsaicin. J Allergy Clin Immunol. 1994;94:1035–1045. doi: 10.1016/0091-6749(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Pramanik T, Ghising R. Salivation induced better lacrimal gland function in dry eyes. Nepal Med Coll J. 2009;11:258–260. [PubMed] [Google Scholar]
- Rios JD, Zoukhri D, Rawe IM, Hodges RR, Zieske JD, Dartt DA. Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Invest Ophthalmol Vis Sci. 1999;40:1102–1111. [PubMed] [Google Scholar]
- Robbins A, Kurose M, Winterson BJ, Meng ID. Menthol Activation of Corneal Cool Cells Induces TRPM8-Mediated Lacrimation but Not Nociceptive Responses in Rodents. Invest Ophthalmol Vis Sci. 2012;53:7034–7042. doi: 10.1167/iovs.12-10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. The distribution of autonomic post-ganglionic nerve fibres to the lacrimal gland in monkeys. J Anat. 1971;109:229–242. [PMC free article] [PubMed] [Google Scholar]
- Schaible HG, Ebersberger A, Natura G. Update on peripheral mechanisms of pain: beyond prostaglandins and cytokines. Arthritis Res Ther. 2011;13:210. doi: 10.1186/ar3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessle BJ. Neural mechanisms and pathways in craniofacial pain. Can J Neurol Sci. 1999;26(Suppl 3):S7–S11. doi: 10.1017/s0317167100000135. [DOI] [PubMed] [Google Scholar]
- Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- Sharif Naeini R, Witty MF, Seguela P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci. 2006;9:93–98. doi: 10.1038/nn1614. [DOI] [PubMed] [Google Scholar]
- Shi XP, Candia OA. Active sodium and chloride transport across the isolated rabbit conjunctiva. Curr Eye Res. 1995;14:927–935. doi: 10.3109/02713689508995132. [DOI] [PubMed] [Google Scholar]
- Shim J, Park C, Lee HS, Park MS, Lim HT, Chauhan S, Dana R, Lee H, Lee HK. Change in prostaglandin expression levels and synthesizing activities in dry eye disease. Ophthalmology. 2012;119:2211–2219. doi: 10.1016/j.ophtha.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Situ P, Simpson TL, Fonn D, Jones LW. Conjunctival and corneal pneumatic sensitivity is associated with signs and symptoms of ocular dryness. Invest Ophthalmol Vis Sci. 2008a;49:2971–2976. doi: 10.1167/iovs.08-1734. [DOI] [PubMed] [Google Scholar]
- Situ P, Simpson TL, Jones LW, Fonn D. Conjunctival and corneal hyperesthesia in subjects with dryness symptoms. Optom Vis Sci. 2008b;85:867–872. doi: 10.1097/OPX.0b013e3181852788. [DOI] [PubMed] [Google Scholar]
- Spencer SE, Sawyer WB, Wada H, Platt KB, Loewy AD. CNS projections to the pterygopalatine parasympathetic preganglionic neurons in the rat: a retrograde transneuronal viral cell body labeling study. Brain research. 1990;534:149–169. doi: 10.1016/0006-8993(90)90125-u. [DOI] [PubMed] [Google Scholar]
- Stapleton F, Hayward KB, Bachand N, Trong PH, Teh DW, Deng KM, Yang EI, Kelly SL, Lette M, Robinson D. Evaluation of corneal sensitivity to mechanical and chemical stimuli after LASIK: a pilot study. Eye Contact Lens. 2006;32:88–93. doi: 10.1097/01.icl.0000174757.49938.82. [DOI] [PubMed] [Google Scholar]
- Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130:90–100. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Gereau RWt. Numbing the senses: role of TRPA1 in mechanical and cold sensation. Neuron. 2006;50:177–180. doi: 10.1016/j.neuron.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Vos BP. Somatotopic and laminar organization of fos-like immunoreactivity in the medullary and upper cervical dorsal horn induced by noxious facial stimulation in the rat. J Comp Neurol. 1993;331:495–516. doi: 10.1002/cne.903310406. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, Krenzer KL, Sullivan BD, Tolls DB, Toda I, Dana MR. Does androgen insufficiency cause lacrimal gland inflammation and aqueous tear deficiency? Invest Ophthalmol Vis Sci. 1999;40:1261–1265. [PubMed] [Google Scholar]
- Szolcsanyi J. Selective responsiveness of polymodal nociceptors of the rabbit ear to capsaicin, bradykinin and ultra-violet irradiation. J Physiol. 1987;388:9–23. doi: 10.1113/jphysiol.1987.sp016598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y, Fukui Y, Ichiyama M, Miyoshi S, Nishimura Y. Direct amygdaloid projections to the superior salivatory nucleus: a light and electron microscopic study in the cat. Brain Res Bull. 1991;27:85–92. doi: 10.1016/0361-9230(91)90285-r. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Chang Z, Bereiter DA. Behavioral and neurophysiological correlates of nociception in an animal model of photokeratitis. Neuroscience. 2010;169:455–462. doi: 10.1016/j.neuroscience.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Malik RA. Corneal sensitivity is reduced and relates to the severity of neuropathy in patients with diabetes. Diabetes Care. 2007;30:1895–1897. doi: 10.2337/dc07-0175. [DOI] [PubMed] [Google Scholar]
- Thut PD, Wrigley D, Gold MS. Cold transduction in rat trigeminal ganglia neurons in vitro. Neuroscience. 2003;119:1071–1083. doi: 10.1016/s0306-4522(03)00225-2. [DOI] [PubMed] [Google Scholar]
- Togias A, Lykens K, Kagey-Sobotka A, Eggleston PA, Proud D, Lichtenstein LM, Naclerio RM. Studies on the relationships between sensitivity to cold, dry air hyperosmolal solutions, and histamine in the adult nose. Am Rev Respir Dis. 1990;141:1428–1433. doi: 10.1164/ajrccm/141.6.1428. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Toshida H, Nguyen DH, Beuerman RW, Murakami A. Evaluation of novel dry eye model: preganglionic parasympathetic denervation in rabbit. Invest Ophthalmol Vis Sci. 2007;48:4468–4475. doi: 10.1167/iovs.06-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth IE, Boldogkoi Z, Medveczky I, Palkovits M. Lacrimal preganglionic neurons form a subdivision of the superior salivatory nucleus of rat: transneuronal labelling by pseudorabies virus. J Auton Nerv Syst. 1999;77:45–54. doi: 10.1016/s0165-1838(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjogren's syndrome. Exp Eye Res. 2008;86:879–885. doi: 10.1016/j.exer.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Tuominen IS, Konttinen YT, Vesaluoma MH, Moilanen JA, Helinto M, Tervo TM. Corneal innervation and morphology in primary Sjogren's syndrome. Invest Ophthalmol Vis Sci. 2003;44:2545–2549. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- Ueno H, Ferrari G, Hattori T, Saban DR, Katikireddy KR, Chauhan SK, Dana R. Dependence of corneal stem/progenitor cells on ocular surface innervation. Invest Ophthalmol Vis Sci. 2012;53:867–872. doi: 10.1167/iovs.11-8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa S, Ueda T, Yamamura H, Nagao M, Shimada S. Coexpression of vanilloid receptor subtype-1 and acid-sensing ion channel genes in the human trigeminal ganglion neurons. Chem Senses. 2005a;30(Suppl 1):i195. doi: 10.1093/chemse/bjh181. [DOI] [PubMed] [Google Scholar]
- Ugawa S, Ueda T, Yamamura H, Shimada S. In situ hybridization evidence for the coexistence of ASIC and TRPV1 within rat single sensory neurons. Brain Res Mol Brain Res. 2005b;136:125–133. doi: 10.1016/j.molbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- van Bijsterveld OP, Kruize AA, Bleys RL. Central nervous system mechanisms in Sjogren's syndrome. Br J Ophthalmol. 2003;87:128–130. doi: 10.1136/bjo.87.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani E, Galimberti D, Viola F, Mapelli C, Ratiglia R. The cornea in Sjogren's syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007;48:2017–2022. doi: 10.1167/iovs.06-1129. [DOI] [PubMed] [Google Scholar]
- Yasui T, Karita K, Izumi H, Tamai M. Correlation between vasodilatation and secretion in the lacrimal gland elicited by stimulation of the cornea and facial nerve root of the cat. Invest Ophthalmol Vis Sci. 1997;38:2476–2482. [PubMed] [Google Scholar]
- Yudin Y, Lukacs V, Cao C, Rohacs T. Decrease in phosphatidylinositol 4,5-bisphosphate levels mediates desensitization of the cold sensor TRPM8 channels. J Physiol. 2011;589:6007–6027. doi: 10.1113/jphysiol.2011.220228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin Y, Rohacs T. Regulation of TRPM8 channel activity. Mol Cell Endocrinol. 2012;353:68–74. doi: 10.1016/j.mce.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander E, Weddell G. Observations on the innervation of the cornea. J. Anat. 1951;85:68–99. [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Chen J, Luo L, Xiao Q, Sun M, Liu Z. Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal microscopy. Cornea. 2005;24:818–824. doi: 10.1097/01.ico.0000154402.01710.95. [DOI] [PubMed] [Google Scholar]
- Zhang X, Mak S, Li L, Parra A, Denlinger B, Belmonte C, McNaughton PA. Direct inhibition of the cold-activated TRPM8 ion channel by Galphaq. Nat Cell Biol. 2012;14:851–858. doi: 10.1038/ncb2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Shen J, Zhang C, Park CY, Kohanim S, Yew M, Parker JS, Chuck RS. Inflammatory cytokine expression on the ocular surface in the Botulium toxin B induced murine dry eye model. Mol Vis. 2009;15:250–258. [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Zhang C, Chuck RS. Topical steroid and non-steroidal anti-inflammatory drugs inhibit inflammatory cytokine expression on the ocular surface in the botulinum toxin B-induced murine dry eye model. Mol Vis. 2012;18:1803–1812. [PMC free article] [PubMed] [Google Scholar]