Abstract
Suvorexant, a dual orexin receptor antagonist for the management of insomnia
INTRODUCTION
Insomnia is characterized subjectively and may consist of a variety of complaints, including difficulty falling asleep, difficulty maintaining sleep, or experiencing nonrestorative sleep. Despite a number of available treatments, insomnia is the most common medical complaint in general practice.1 It affects up to 30% of the adult population.2 It is also a major risk factor for anxiety disorder, substance abuse, and major depression, and it may lead to a decreased quality of life.2
A variety of treatment options are available for insomnia. The most common pharmacological interventions are benzodiazepines (BZDs) and the non-BZD gamma-amino-butyric acid (GABA)–acting hypnotics such as zolpidem (Ambien, Sanofi), eszopiclone (Lunesta, Sunovion), and zaleplon (Sonata, Pfizer).1 Other less frequently prescribed agents include sedating antidepressants, melatonin agonists, and antihistamines.2 Diminished efficacy and negative side effects limit the use of these treatment options for many patients.
Suvorexant (MK-4305, Merck), an orexin receptor antagonist (ORA), is the first in a new class of drugs in development for the treatment of insomnia. The tablets promote the natural transition from wakefulness to sleep by inhibiting the wakefulness-promoting orexin neurons of the arousal system.1 Suvorexant improves sleep onset and sleep maintenance. This unique alternative has a favorable tolerability and limited side-effect profile.2
CLINICAL PHARMACOLOGY
Composition
The molecular formula for suvorexant is C23H23ClN6O2. The molecular weight is approximately 450.932 g/mol.3 The chemical structure is shown in Figure 1.6
Figure 1.
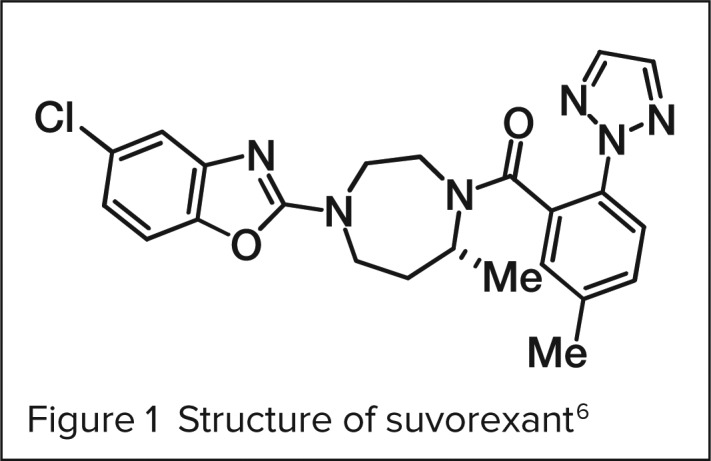
Structure of suvorexant 6
Mechanism of Action
Suvorexant is a potent dual orexin receptor antagonist that blocks both OX1R and OX2R. It promotes sleep through the binding inhibition of orexin A and B, neuropeptides that promote wakefulness. Roughly 70,000 orexin neurons are in the human brain, located in the perifornical lateral hypothalamus, which send signals throughout the brain and spinal cord.5
Pharmacodynamics and Pharmacokinetics6
Merck’s application to the FDA for approval included 32 studies that enrolled more than 900 subjects (both those who were healthy and those with insomnia). Suvorexant was effective and generally well tolerated. An advantage of suvorexant over previous insomnia therapies is the low potential for addiction or dependence. Studies demonstrated that plasma concentrations were higher in obese women than in men with a normal body mass index (BMI).2 However, there was no difference among patients with moderate hepatic or renal dysfunction, a desirable finding in the elderly population. Suvorexant, as with other hypnotic agents, should be avoided in individuals with severe hepatic impairment.6
Absorption. The onset of sleep occurred between 56 and 68 minutes after oral administration. Onset was most rapid in those who received 40 mg.7 Median peak plasma concentrations occur approximately two hours after administration and are not affected by food.
Distribution. Suvorexant has a volume of distribution of 105.9 L and is highly protein bound (99.5%).
Metabolism. The agent is primarily metabolized by the cytochrome P450 (CYP3A4) enzyme system, with some contribution from CYP2C19 into M9, an inactive metabolite.
Elimination. Suvorexant is eliminated primarily via inactive metabolites in the feces; there is no renal elimination. The half-life is approximately 12.2 hours on average (range 8–19 hours). Steady-state plasma concentrations occur in about three days with daily administration.
CLINICAL EFFICACY
Sun et al.8
In a randomized, double-blind, placebo-controlled trial, researchers evaluated doses of suvorexant ranging from 10 to 100 mg. This four-week crossover polysomnography (PSG) study was concluded by an additional fifth week to gain pharmacokinetic information about suvorexant.
The trial enrolled 22 healthy young males between 18 and 45 years of age (mean, 29.6) with a mean weight of 80.2 kg; 19 subjects completed the study. Two subjects withdrew because of protocol deviations.
Participants received one of three doses of suvorexant (10 mg, 50 mg, or 100 mg) or placebo. Throughout the four-week study, subjects were evaluated in two eight-hour PSG recording sessions in a general laboratory setting.
The primary objective, to assess the efficacy of evening administration of suvorexant, was assessed according to sleep onset, or latency to persistent sleep (LPS); sleep maintenance, or waking after sleep onset (WASO); sleep efficiency (SE); and total sleep time (TST). The researchers also looked for evidence of next-day residual effects by administering the following tests 10 hours after administration: simple reaction time (SRT), choice reaction time (CRT), and digital symbol substitution tests (DSSTs).
Subjective assessments of next-day residual effects were measured via the Leeds Sleep Evaluation Questionnaire (LSEQ). During week 5 of the testing period, a 96-hour washout period occurred and blood samples were collected both before administration and throughout the night.
Statistically significant changes were noted for all doses. Patients receiving 50 mg and 100 mg showed a statistically significant decrease in both LPS and WASO as well as a corresponding increase in SE and TST. The 10-mg group showed a statistically significant decrease in WASO. Next-day residual side effects were dose-dependent. At doses of 100 mg, there was a statistically significant increase in reaction time as shown by SRT and CRT. However, this was not noted for the 10-mg and 50-mg groups. LSEQ responses revealed that subjects taking doses of 50 mg and 100 mg had more difficulty awakening and maintaining alertness compared with those taking placebo. Results from the fifth week of pharmacokinetic testing can be found in Table 1.
Table 1.
Effects of Suvorexant on Sleep Parameters as Measured by Polysomnography in Healthy Men
| Parameter | 10 mg (n = 5) | 50 mg (n = 7) | 100 mg (n = 7) |
|---|---|---|---|
| AUC0–∞ (μM * hour) | 6.69 | 10.87 | 29.76 |
| AUC0–24 (μM * hour) | 4.94 | 8.57 | 19.07 |
| Peak concentration (μM) | 0.44 | 0.87 | 2.12 |
| Time to peak concentration (hours) | 3.0 | 3.0 | 3.0 |
| Apparent terminal half-life (hours) | 9.0 | 10.8 | 13.1 |
AUC = area-under-the-curve concentration.
Adapted from Sun H, et al. Sleep 2013;36(2):259–267.8
Herring et al.9
The objective of the Herring clinical trial was to assess the utility of orexin receptor antagonism as a novel approach to treating insomnia. The patients were evaluated in a randomized, double-blind, placebo-controlled, two-period PSG study. Each treatment period was four weeks long, with a single-blind, one-week placebo washout period in between. The trial was conducted in 29 sites across the U.S. and in 12 sites in Japan. Men and women 18 to 64 years of age with primary insomnia, based on Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR) criteria, were enrolled.
Suvorexant 10-mg and 30-mg tablets, along with their corresponding placebos, were used to achieve doses of 10, 20, 40, and 80 mg per dose. Suvorexant improved sleep efficacy over four weeks in nonelderly adults with primary insomnia.
Efficacy was assessed primarily via PSG measurements. Visual scoring of PSG data was performed by blinded personnel. Morning and evening questionnaires, administered via an electronic patient diary, were used to record patients’ self-assessments of sleep. Adverse-event reports, vital signs, electrocardiograms, laboratory parameters, and physical examinations were obtained to assess patient tolerability and safety.
A total of 228 subjects completed the study, and 26 withdrew. Of the 254 subjects, 240 received at least one dose of placebo and 243 received at least one dose of suvorexant (62 patients received 10 mg, 61 patients received 20 mg, 59 patients received 40 mg, and 61 patients received 80 mg). Distribution of sex, race, age, and BMI was similar among the treatment regimens.
The coprimary efficacy endpoints were sleep efficiency on night 1 and at the end of week 4. Secondary endpoints were sleep maintenance (WASO) and sleep onset (LPS). Results showed a significant (P < 0.01) dose-related improvement compared with placebo for the coprimary endpoints. Dose-related effects were also observed for maintenance (WASO) and sleep induction (LPS). Suvorexant was generally well tolerated.
INDICATION
The proposed indication for suvorexant is the treatment of insomnia (difficulty with sleep onset or sleep maintenance) in adults 18 years of age and older.6
SAFETY PROFILE
Contraindications
There are currently no contraindications to the use of suvorexant.
Adverse Drug Reactions
As with other insomnia medications, suvorexant has the potential to produce drowsiness the next day that might interfere with daily activities. This effect is observed with doses higher than 40 mg and increases proportionally with dosage increases.9 A more detailed list of adverse reactions can be found in Table 2.
Table 2.
Adverse Drug Events Associated With Suvorexant and Placebo
| Adverse Event | Placebo | 10 mg | 20 mg | 40 mg | 80 mg |
|---|---|---|---|---|---|
| ≥1 Adverse event | 20.1% | 17.7% | 19.7% | 30.5% | 36.1% |
| ≥1 Drug-related event | 6.8% | 4.8% | 6.6% | 20.3% | 23% |
| Somnolence | 0.4% | 1.6% | 4.9% | 10.2% | 11.5% |
| Sedation | 0.4% | 0 | 0 | 0 | 3.3% |
| Muscle weakness | 0 | 0 | 0 | 3.4% | 1.6% |
| Abnormal dreams | 0.8% | 1.6% | 0 | 0 | 4.9% |
| Headache | 2.4% | 0 | 1.6% | 5.1% | 4.9% |
From Herring WJ, et al. Neurology 2012;79(23):2265–2274.9
When abuse potential of single doses of suvorexant was compared with single doses of zolpidem in healthy female and male users of recreational polydrugs, suvorexant scores on the Drug Liking Visual Analogue Scale were 22.84, 21.99, and 20.44 with 40 mg, 80 mg, and 150 mg, respectively. Scores in the zolpidem groups were similar—24.86 (15 mg) and 28.08 (30 mg).2 Abrupt discontinuation of suvorexant after chronic use does not result in rebound insomnia or withdrawal effects.6
Drug Interactions
Patients should avoid taking other CYP3A medications while they are taking suvorexant. Potent CYP3A inhibitors such as fluconazole (Diflucan, Pfizer) increase plasma concentrations, placing patients well above the desired therapeutic threshold. Moderate inhibitors such as diltiazem (Cardizem, AbbVie) can be used safely, especially in the elderly when lower doses (e.g., 10 mg) of suvorexant are administered.6 CYP3A inducers, such as rifampin (Rifadin, Sanofi), result in significantly reduced suvorexant plasma concentrations. Suvorexant is a mild inhibitor of CYP3A, but when administered with CYP3A substrates, including oral contraceptives and warfarin, it had minimal effects.6
Special Populations
A double-blind, three-period, single-dose crossover study compared the effects of suvorexant (30 mg), zolpidem (5 mg), and placebo on balance and memory during middle-of-the-night awakenings in 12 healthy subjects 65 years of age and older.6 Suvorexant therapy led to significantly impaired balance more than placebo at 1.5 hours but not at four and eight hours. However, this impairment was less than that observed with zolpidem. The effects on memory were not statistically significantly different in comparisons of suvorexant and zolpidem. The safety profile of suvorexant was favorable in adults younger than 65 years of age (up to 40 mg) and in patients 65 years of age and older (up to 30 mg).6
DOSAGE AND ADMINISTRATION
Although the original recommended dose was 40 mg, this dose was associated with safety concerns. As a result, the recommended initial daily dose is 10 mg. Doses may be titrated to a maximum of 40 mg daily, and escalation is advised only in those patients who can tolerate lower doses with no adverse effects.
P&T COMMITTEE CONSIDERATIONS
Comparisons With Other Insomnia Medications
Currently, the market for insomnia medications is dominated by benzodiazepines, such as lorazepam or temazepam. Nonbenzodiazepines such as zolpidem and eszopiclone are widely prescribed as well.10 Both classes exert effects on GABA and have more global inhibitory effects in the brain. This effect results in amnesia, next-day limited sedation, and rebound insomnia. In addition, both drug classes can be habit-forming and have the potential to promote dependence. For these reasons, these agents should be used intermittently or for the short term in treating insomnia. This guideline is often ignored in clinical practice, however. Clinicians often prescribe these agents outside of these parameters.
Suvorexant’s mechanism of action differs from that of the benzodiazepines and nonbenzodiazepines; it has no effect on GABA. Instead of promoting sleep, it inactivates wakefulness.5 Adverse effects commonly observed with benzodiazepines and nonbenzodiazepines are virtually eliminated. As a result, suvorexant can be used daily on a long-term basis with no risk of rebound insomnia or physical dependence.
Limitations
In a study of more than 9,000 subjects 65 years of age and older by the National Institute on Aging, 80% reported sleep disorders.1 This group is the most likely to seek pharmacological treatment for sleep problems with suvorexant; it is also the group most sensitive to its adverse effects.
Cost
At the time of writing, suvorexant had not yet received Food and Drug Administration approval. No cost data are available.
Efficacy
Low-dose (20-mg) and high-dose (40-mg) suvorexant in individuals younger than age 65 years both proved superior to placebo in the following categories: waking after sleep onset (WASO), latency to persistent sleep (LPS), mean subjective total sleep time (sTSTm), mean subjective waking after sleep onset (sWASOm), and mean subjective time to sleep onset (sTSOm). The data suggest that suvorexant effectively decreases time to onset of sleep and increases maintenance of sleep. In clinical trials, no evidence of rebound insomnia was observed after a minimum of three months and a maximum of 12 months.6
CONCLUSION
Although benzodiazepines and nonbenzodiazepines are effective for insomnia, their adverse-effect profiles and recommended limitations on long-term use may prompt patients and clinicians to seek other options. Patients who experience both sleep onset and sleep maintenance insomnia may be particularly challenging to treat. The recent discovery of orexins and their receptors has led to the development of new therapy targets. Suvorexant is an effective orexin receptor antagonist with a unique clinical profile. Evidence suggests that this medication offers a sustained benefit for patients with symptoms of chronic insomnia.
REFERENCES
- 1.Dopp J, Phillips B. Sleep disorders. In: DiPiro J, Talbert R, Yee G, et al., editors. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York: McGraw-Hill; 2011. pp. 621–623. [Google Scholar]
- 2.Briefing Materials from Peripheral and Central Nervous System Advisory Committee 2013 Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PeripheralandCentralNervousSystem-DrugsAdvisoryCommittee/UCM352969.pdf. Accessed August 12, 2013. [Google Scholar]
- 3.Osborne R. First-in-class insomnia drug on the brink of approval nod. Nat Rev Drug Discov. 2013;12(7):492–493. doi: 10.1038/nrd4067. [DOI] [PubMed] [Google Scholar]
- 4.Suvorexant Available at: www.chemspider.com/Chemical-Structure.24662178.html. Accessed August 12, 2013. [Google Scholar]
- 5.Mieda M, Sakurai T. Orexin (hypocretin) receptor agonists and antagonists for treatment of sleep disorders. Rationale for development and current status. CNS Drugs. 2013;27(2):83–90. doi: 10.1007/s40263-012-0036-8. [DOI] [PubMed] [Google Scholar]
- 6. FDA Advisory Committee Meeting, Briefing Document. Suvorexant Tablets Insomnia Indication, NDA 204569, May 22, 2013. Available at: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PeripheralandCentralNervousSystem-DrugsAdvisoryCommittee/UCM352970.pdf. Accessed August 14, 2013.
- 7.Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant—a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1–2):52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- 8.Sun H, Kennedy WP, Wilbraham D, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36(2):259–267. doi: 10.5665/sleep.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265–2274. doi: 10.1212/WNL.0b013e31827688ee. [DOI] [PubMed] [Google Scholar]
- 10.Scammell TE, Winrow CJ. Orexin receptors: Pharmacology and therapeutic opportunities. In: Cho AK, editor. Annual Review of Pharmacology and Toxicology. Palo Alto California: Annual Reviews; 2011. pp. 243–266. [DOI] [PMC free article] [PubMed] [Google Scholar]


