Abstract
The prognosis for patients with pancreatic cancer remains poor, but a widely recognized expert on pancreatic and biliary surgery at Massachusetts General Hospital and Harvard Medical School expresses optimism about improvements in the next decade.
P&T: Why have you made pancreatic cancer a focus of your career?
CF: It was not a deliberate decision early in my career. When I finished my surgical training, I focused on the pancreas. Since so many problems related to the pancreas have to do with cancer, by default I got more and more involved with pancreatic cancer. As I started to take care of patients with pancreatic cancer and interact with them and their families and did laboratory research, it became clear that this area needed a lot of work. I saw it as a challenge 25 years ago and I continue to see it as a challenge.
Some people jokingly say, “You picked the wrong girl to dance with at the party!” The results are not great or glamorous, but on the other hand the challenge to make the outcomes better for patients, to make their remaining time better, to try to unravel the mystery, energizes me.
Carlos Fernándezdel Castillo, MD
P&T: What is the most common form of pancreatic cancer?
CF: The most common form is ductal adenocarcinoma, which accounts for most of the 40,000 cases of pancreatic cancer annually (Figure 1). Ductal adenocarcinoma is also the most lethal form of pancreatic cancer. Among the rarer forms are neuroendocrine carcinoma, which was in the news a few years ago because that was the tumor that the late Apple CEO, Steve Jobs, had.1 Another less common form of pancreatic cancer is acinar cell carcinoma.
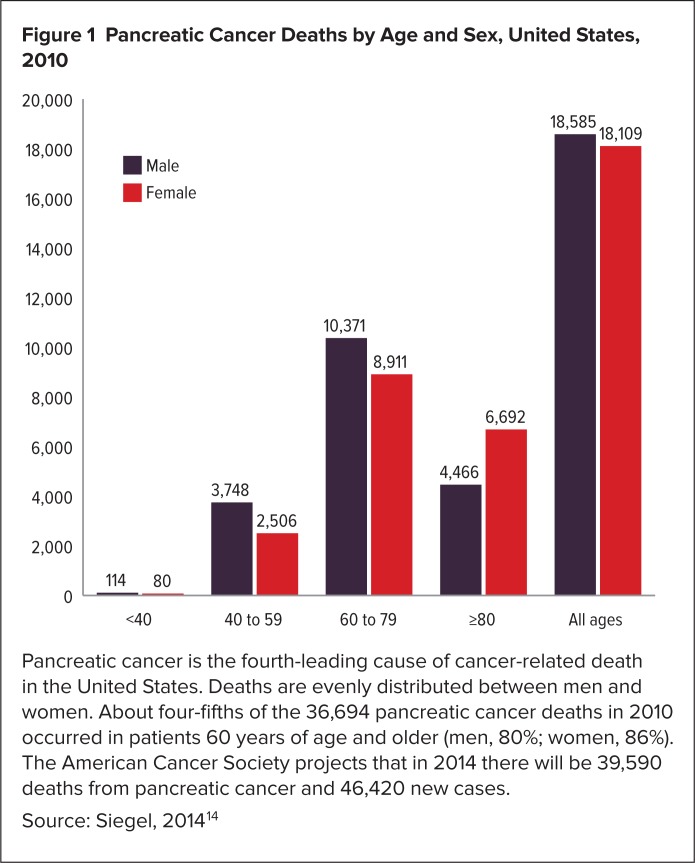
Figure 1.
Pancreatic Cancer Deaths by Age and Sex, United States, 2010
Pancreatic cancer is the fourth-leading cause of cancer-related death in the United States. Deaths are evenly distributed between men and women. About four-fifths of the 36,694 pancreatic cancer deaths in 2010 occurred in patients 60 years of age and older (men, 80%; women, 86%). The American Cancer Society projects that in 2014 there will be 39,590 deaths from pancreatic cancer and 46,420 new cases.
Source: Siegel, 2014 14
P&T: In general terms, how do the less common forms of pancreatic cancer differ from ductal adenocarcinoma in terms of diagnosis, prognosis, and management?
CF: Neuroendocrine and to some extent acinar carcinomas can be more indolent and have a slower progression, sometimes for many years or even a decade. They respond to different treatments, although surgery is usually the mainstay of treatment.
Neuroendocrine tumors sometimes produce excessive amounts of hormones such as gastrin, which stimulates the production of stomach acid. As a consequence, those patients can present with bleeding ulcers or diarrhea. Many neuroendocrine tumors are picked up incidentally. For example, during an MRI or CT scan for a kidney stone, a neuroendocrine tumor may be detected in the pancreas. Ductal adenocarcinoma rarely is picked up incidentally. The lesser common pancreatic tumors also share clinical presentation with ductal adenocarcinoma, so patients can present with jaundice, abdominal pain, or pancreatitis.
Acinar cell tumors are unique because they produce a lot of lipase and amylase. Sometimes the lipase produces areas of fat necrosis in the skin. A patient may present to a dermatologist complaining of skin nodules that are painful but then disappear. The dermatologist does a biopsy and finds dead fat cells in the skin, leading to the diagnosis of acinar cell carcinoma.
P&T: What are important recent advances in the treatment of pancreatic cancer?
CF: The most important recent advance is the finding that a chemotherapy combination called FOLFIRINOX is effective against this disease. A study done in France showed a marked improvement in the survival of patients with stage 4 pancreatic cancer.2 These are patients who are not curable and who used to have a very bad prognosis, with a survival of six months or less. This took their survival up to 11 months.
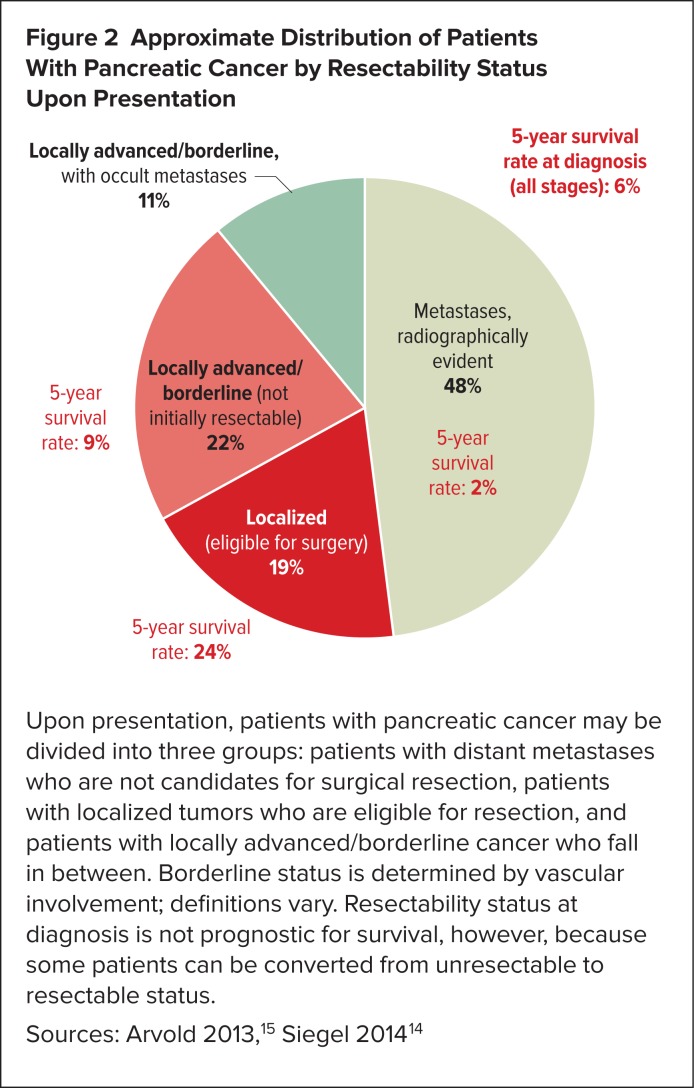
This finding has encouraged us to use FOLFIRINOX in patients who don’t have such advanced disease. Pancreatic cancer patients fall into one of three groups (Figure 2): patients who already have distant metastases (stage 4 disease), patients who are clearly operable because they have small tumors, and those patients in between, whose tumors are known as borderline or locally advanced tumors—that have not spread very far but are inoperable because they have involved some important blood vessels or other structures.
At the Massachusetts General Hospital Cancer Center, we have started using FOLFIRINOX in patients with locally advanced and borderline disease, and we have seen some dramatic results. Using this chemotherapy, oftentimes in combination with radiotherapy, has resulted in significant down-staging—a decrease in the size of the tumor and an increase in operability, so that we are able to take these patients into operations with clean margins (R0). This is a very significant recent advance in the care of patients with pancreatic cancer. Now, it remains to be seen if this will affect long-term cure rates and how long these patients will live, but as we speak we are seeing a gratifying thing that we have not seen in the last couple of decades. After FOLFIRINOX we are seeing very low levels of R1 or R2 resections relative to patients who had borderline tumors before or even had cleanly resectable tumors. This is encouraging news and speaks to the activity of this combination therapy.
In addition to FOLFIRINOX, another combination therapy that uses gemcitabine and albumin-bound paclitaxel (Abraxane; nab-paclitaxel) also has shown promising results. These are being tested all over the world in patients with all stages of pancreatic cancer.
A recent article described our initial experience at Massachusetts General Hospital with FOLFIRINOX.3 Now our experience is more than double that, and we continue to see these very encouraging results based on the pathology of specimens. We are in the process of writing an updated report and will present that data to the Society of Surgical Oncology soon.
Figure 2.
Approximate Distribution of Patients With Pancreatic Cancer by Resectability Status Upon Presentation
Upon presentation, patients with pancreatic cancer may be divided into three groups: patients with distant metastases who are not candidates for surgical resection, patients with localized tumors who are eligible for resection, and patients with locally advanced/borderline cancer who fall in between. Borderline status is determined by vascular involvement; definitions vary. Resectability status at diagnosis is not prognostic for survival, however, because some patients can be converted from unresectable to resectable status.
P&T: Above all else, what one thing would you like primary care physicians (PCPs) to know about pancreatic cancer?
CF: This is a very good and germane question. Because pancreatic cancer has had such bad outcomes, there is a perception in the medical community that this disease has a bad outcome all the time. On many occasions, patients’ initial impression is that the doctor has told them, “This is very bad news. Go prepare your will. You probably have six months to live.” That message is not accurate for all patients. It may be accurate for a subset of patients who have very advanced pancreatic cancer, but for some patients who don’t have advanced cancer there is hope of cure.
Even if the cure doesn’t happen, there is an opportunity for more than six months of life. The message for primary care doctors is, yes, this disease has a poor prognosis; yes, among all cancers this has one of the worst outcomes overall, but there are things that can be done and there are patients who can be cured. Primary care doctors will slowly warm to this message and, when appropriate, refer patients to specialized care to try to make the best of it.
P&T: What signs and symptoms should make a PCP suspect pancreatic cancer?
CF: Ninety percent of patients with pancreatic cancer present with either abdominal pain, jaundice, or a combination of the two. Whenever those appear in the right age group—patients in their 60s and older (Figure 1)—one should think of the possibility of pancreatic cancer. Other signs and symptoms can be more subtle, such as unexplained weight loss or an attack of pancreatitis in someone in this age group. Pancreatitis is most commonly caused by gallstones or excessive consumption of alcoholic beverages, but when we don’t have an explanation for pancreatitis we should think of the possibility of pancreatic cancer. Likewise, development of diabetes in a patient who otherwise shouldn’t be developing diabetes—someone who is thin, who doesn’t have a family history of diabetes but suddenly develops diabetes in their 60s or 70s—should raise the question of pancreatic cancer.
PCPs know about the typical signs and symptoms—pain and jaundice—and they diligently look for pancreatic cancer in the presence of unexplained weight loss. But the pancreatitis issue is not that clear to them. Sometimes in consultation we see patients who had an episode of unexplained pancreatitis, and no thought has been given to the possibility that it could be caused by a tumor.
P&T: How do gastroenterologists help with the diagnosis and management of pancreatic cancer?
CF: Many symptoms that herald pancreatic cancer are gastrointestinal. Oftentimes patients presenting with jaundice, pancreatitis, diarrhea, or abdominal pain are referred to a gastroenterologist. There are many causes of jaundice, such as hepatitis, a gallstone blocking the bile duct, a tumor. The gastroenterologist does the workup to find the cause. Pancreatitis also has many causes, such as gallstones and alcohol abuse, and again the gastroenterologist often is the one who makes the diagnosis. Protracted diarrhea can be the initial manifestation of pancreatic cancer, and patients usually see a gastroenterologist for a workup of that. Abdominal pain is one of the most common manifestations of pancreatic cancer, and it is a common reason patients are referred to a gastroenterologist, perhaps because an ulcer or a problem with the colon or small intestine is suspected, but it turns out to be pancreatic cancer. When they examine these patients, gastroenterologists often can make a diagnosis using endoscopic ultrasound. Patients who present with jaundice often need relief of that jaundice, so a stent will be placed by the gastroenterologist via endoscopy.
Endoscopic ultrasound performed by a gastroenterologist also may be helpful for screening high-risk subpopulations.4 An advantage of endoscopic ultrasound is that it can identify small tumors that CT scans miss.
P&T: Which patients are considered to be at high risk of pancreatic cancer, and what kind of surveillance or screening is appropriate for them?
CF: This is an area in which we have yet to make a lot of progress. We know that people with familial pancreatic cancer, defined as having two first-degree relatives (e.g., a parent and a sibling) with a history of the disease, are at a much higher risk than the average person of developing pancreatic cancer. Those people need to be screened. The problem is we have not found an effective way to screen them. Most protocols employ MRI or endoscopic ultrasound, but the frequency with which imaging should be done and the changes seen on imaging that should prompt action are not clear. Even if we did MRIs every six months, the nature of the disease is such that some patients with early pancreatic cancer would be missed. Unfortunately, no blood test can substitute for imaging.
Other groups with a high risk of pancreatic cancer are people with a rare condition called hereditary pancreatitis and those with a BRCA2 mutation, which typically causes breast cancer but also increases risk for pancreatic cancer.
Intraductal papillary mucinous neoplasm (IPMN) is another entity that puts people at higher risk for developing pancreatic cancer and needs the attention of a specialist (a surgeon or gastroenterologist) familiar with the condition to follow it.5 IPMN is a lesion that can evolve into pancreatic cancer in some patients.6 We used to think IPMN was rare, but now we are detecting it with increasing frequency because we do so much more imaging, as patients are imaged with MRI or CT scans for other reasons. Today IPMN may be found in about 2% of the adult population, which is quite frequent. Although the majority of these little cysts on the pancreas will never amount to anything, some will progress. Once you see one, you are obligated to follow it.
P&T: In its guidelines for pancreatic ductal adenocarcinoma, the National Comprehensive Cancer Network (NCCN) recommends that decisions about diagnostic management and resectability be made via a multidisciplinary consultation at a high-volume center. Which disciplines should be represented?
CF: This is a good recommendation by the NCCN. Ideally, the multidisciplinary consultation should include a medical oncologist, a surgeon, a radiation oncologist, and if possible a gastrointestinal radiologist to help interpret the films, and, if appropriate, a cytopathologist.
In the typical consultation, the case is discussed after information has been received from the referring providers. Someone presents the case, including information about the patient’s presentation and workup and the patient’s medical history. Then, with the assistance of a gastrointestinal radiologist, we look at the images, typically a CT scan but perhaps an MRCP, an MRI, or an endoscopic ultrasound.
The conference may involve several surgeons if several patients are being discussed that day. If the patient has provided slides from a previous biopsy they will be read by a pathologist here at Mass General. We discuss the case and reach a consensus about whether or not the tumor is operable or whether it is already metastatic, and what would be the best option for treatment. Sometimes there are several options. For example, if the patient has a small, clearly resectable tumor that has not spread beyond the pancreas and if the patient has no other medical problems, we could go directly to surgery or we could offer the patient an opportunity to participate in a clinical trial looking at other ways to treat pancreatic cancer before proceeding to surgery.
P&T: The NCCN also recommends that surgical resection be done at an institution that performs a large number (15–20) of pancreatic resections annually. What benefits do patients receive when surgery is performed at a high-volume center?
CF: The surgery that is needed to take care of patients with pancreatic cancer is complex, especially when the tumor is located in the head of the pancreas. This often requires the Whipple procedure, which takes five to seven hours and historically has been associated with a high risk of complications. Patients cared for at a hospital performing a high volume of pancreatic resections have better outcomes, in terms of complication rates and overall survival.7–10
I’m not sure that 15 to 20 is a large number, however, because at Mass General we do more than 200 pancreatic resections per year, more than any other hospital in New England. This translates into better care for patients. It’s not just the expertise of the surgeon that matters but rather having a team—nurses, anesthesiologists, intensive care personnel if needed, the right radiologist. Yes, when surgery is indicated for pancreatic cancer, it is better to go to an institution that has a lot of experience doing it.
P&T: What tools and techniques are used to determine the patient’s resectability status?
CF: The most important images are provided by cross-linear imaging—a CT scan or an MRI. In some institutions, CT scans are replaced by a high-quality MRI. MRI can do most of the same things as a CT scan, but not all MRIs are done with the proper contrast or the proper timing. Oftentimes they can be complementary.
The best CT scan for assessing resectability is a dual-phase CT scan, often referred to as a pancreas protocol CT scan. This device scans twice, once as the contrast goes through the arteries and once as the contrast comes back through the portal vein into the liver. This lets us look carefully at the tumor, not just in terms of its size and location but also with respect to its relationship to the veins—the portal vein, the superior mesenteric vein—and the arteries, the hepatic artery, the celiac trunk, the superior mesenteric artery, and so forth. This technology also lets us look carefully at the liver for signs of metastasis, the liver being the most common site of metastasis in pancreatic cancer.
In addition, we selectively use laparoscopy to inspect the abdominal and pelvic cavities because pancreatic cancer has a proclivity to send small metastases that you cannot see by MRI, CT scan, or ultrasound into the liver or peritoneum. Laparoscopy can identify these small 2–3-mm metastases. We don’t use laparoscopy all the time, because in some patients, such as those with a tumor in the head of the pancreas, the yield is very low. But if the cancer is in the body or tail of the pancreas, the yield can be as high as 46%. In those patients we almost always do a laparoscopy to make sure the patient is resectable and does not have metastatic disease.
P&T: What clinical characteristics define patients with borderline pancreatic cancer?
CF: The majority of pancreatic cancers occur in the head, uncinate process, and neck of the pancreas (Figure 3). The portal vein is an important vascular structure lying next to the head of the pancreas, so it is not uncommon for a tumor to involve this vein to various degrees. A borderline resectable pancreatic cancer is one in which there is typically some involvement of the portal vein and occasionally some involvement of nearby arteries. It is called borderline because there are techniques that allow us to remove the vein and put it back together, but we also know if we operate on these patients we will have a higher percentage of positive margins, which is not good for the patient. Our goal as surgeons is to have negative margins all around.
If 25% of the circumference of the portal vein is involved, the majority of the time we will be able to operate. But if, for example, 50% or 75% of the perimeter is involved, the likelihood of resection will be low. There are many definitions of borderline. In some, any involvement of the vein will be classified as borderline resectable. Other definitions reserve borderline for involvement of more than 50% of the vein’s circumference.
Figure 3.
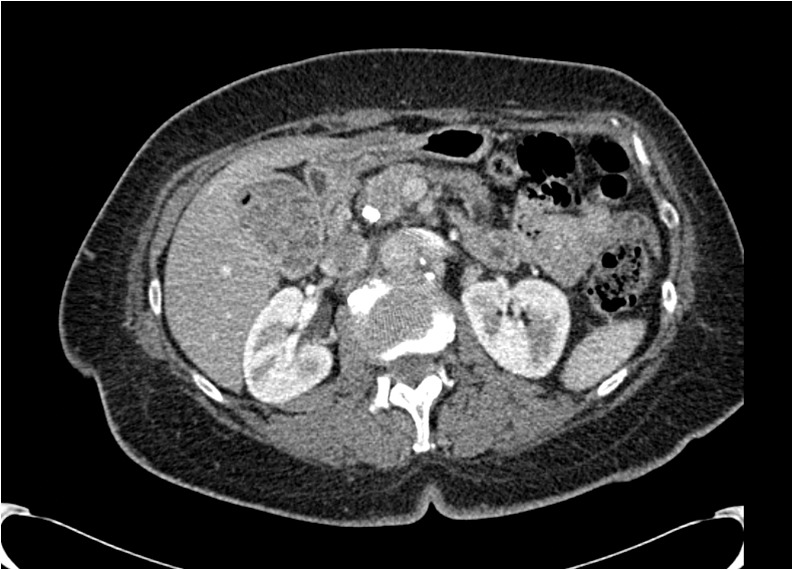
A borderline resectable tumor located in the head of the pancreas. The tumor is in contact with approximately 50% of the circumference of the superior mesenteric vein (indicated by the arrow).
P&T: What factors determine when adjuvant or neoadjuvant therapy is used and the kind(s) of therapies employed?
CF: Adjuvant therapy refers to treatment given after surgery. For the most part, adjuvant therapy is indicated for all patients who have pancreatic cancer and underwent an operation, because data show improvement by giving chemotherapy. In the United States, many institutions also offer radiotherapy in addition to chemotherapy. That is not the case in Europe or in all places in the U.S. and Canada.
Neoadjuvant therapy refers to treatment given before the operation. In general, neoadjuvant therapy is reserved for patients with borderline resectable tumors or locally advanced disease, in which there is hope that shrinkage of the tumor will allow for the possibility of an operation (Figure 4). Usually neoadjuvant therapy consists of chemotherapy, and some but not all institutions add radiotherapy.
Figure 4.
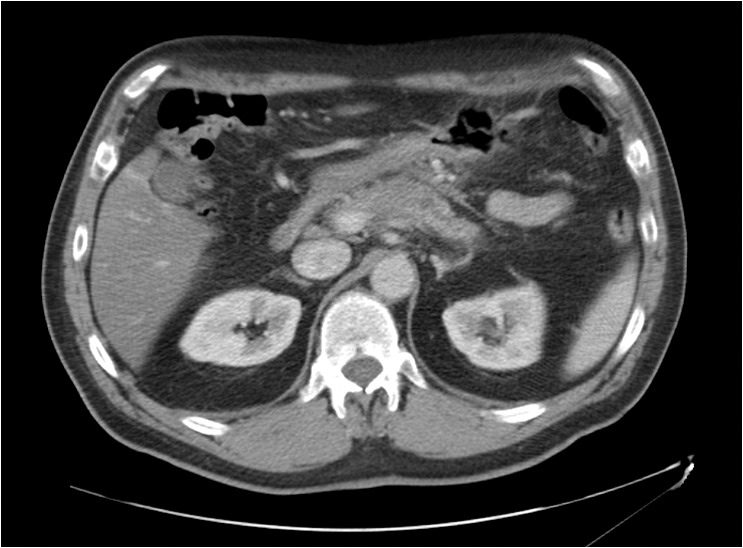
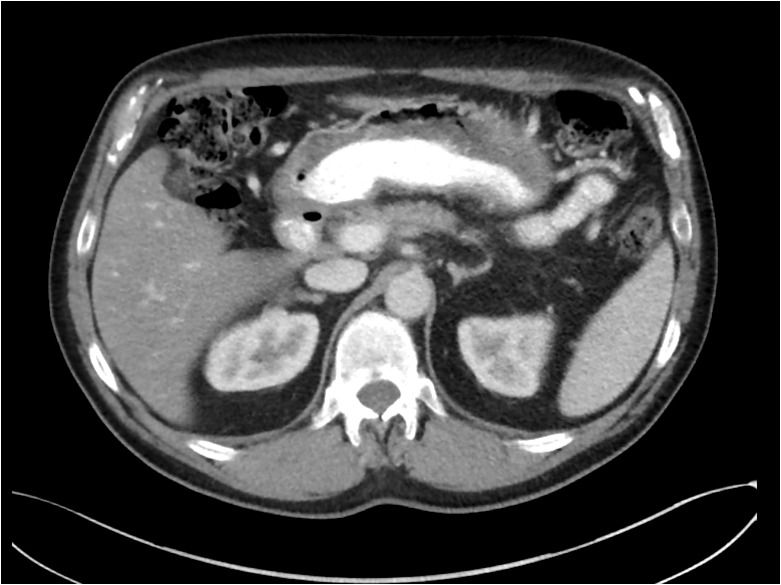
This example of neoadjuvant therapy shows the 3.5-cm tumor (indicated by the arrow) before treatment, left, and seven months later after treatment with FOLFIRINOX and radiation, right. After treatment, the tumor is no longer visible. CA 19-9, the tumor marker, had normalized. The patient underwent a distal pancreatectomy with extensive dissection of the hepatic artery and celiac trunk. Pathology found extensive fibrosis and a single 2-mm focus of adenocarcinoma within the specimen.
P&T: After resection and adjunctive therapy, how do you monitor the patient’s response?
CF: CA 19-9 is a marker that has been used in pancreatic cancer for many decades. It is elevated in a large percentage of patients with pancreatic cancer, but it is not elevated in all patients because some patients do not genetically express CA 19-9. Patients with small tumors sometimes can have normal CA 19-9 levels, and some healthy patients can have elevations of the tumor marker, so it is not a good tool for screening. However, if a patient with pancreatic cancer has elevated CA 19-9 that normalizes after the operation, CA 19-9 often can be used as a marker of disease activity. It is a relatively inexpensive test. Carcinoembryonic antigen (CEA), which is not as widely used, also can indicate disease progression.
For patients with a neuroendocrine tumor, chromogranin A (CgA) can be a useful marker, and you can measure the hormones gastrin or glucagon. For acinar cell tumors, you can measure lipase. There also are clinical manifestations, so the patient is examined and their general well-being, weight, the presence or absence of pain, a palpable tumor in their abdomen or more distant, for example, spread to lymph nodes.
In addition, oncologists and surgeons in general use CT scans after surgery every three or six months for the first couple of years to monitor for the development of local recurrence or distant metastases (Figure 5).
Figure 5.
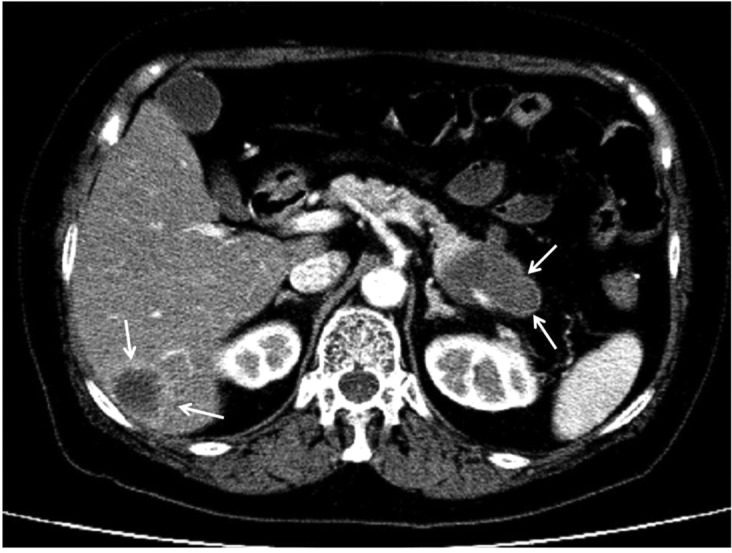
A tumor in the tail of the pancreas (indicated by arrows) with liver metastases.
P&T: What are the greatest unmet needs in pancreatic cancer?
CF: There are needs at many levels. We must remember that pancreatic cancer is the fourth-leading cause of cancer deaths in this country. Even though it is not a very common cancer, compared with breast, prostate, lung, or colon, the high lethality of pancreatic cancer takes it to the top five causes of cancer death. By the time we diagnosis it, in most patients the tumor is not operable because it is metastatic, and the prognosis is grim. So we need to provide better palliation of symptoms for patients with this disease and to better identify the patients likely to benefit from chemotherapy. If a patient already has stage 4 pancreatic cancer, the chances that he or she will be alive in one year are very low. If you subject them to chemotherapy, some patients do very well and are happy to have received chemotherapy, but other patients do not do well and suffer a lot from its effects. Identifying which patients will benefit, or not, from chemotherapy is an important challenge.
Another unmet need comes from understanding that pancreatic cancers are not all the same. We used to think of pancreatic cancer as one disease in which everyone has a very bad prognosis, but within pancreatic cancer there is a lot of heterogeneity. Patients can have different patterns of mutations and perhaps respond to different types of treatment and have different prognoses. Developing an individualized approach is an unmet need in pancreatic cancer, unlike a lot of other cancers such as breast, prostate, or lung cancer, where we have a lot of strategies to identify subgroups.
We also have unmet needs in understanding adjuvant therapy. In the U.S. we traditionally have used radiation in addition to chemotherapy. We believe this makes a difference, but does it make a difference for all patients? Are different types of radiation better? For example, in addition to the traditional radiation using photons, now we also have the proton beam, which may be better and may be associated with fewer side effects. We’re doing groundbreaking work with proton-beam therapy at Mass General. It has been used in many patients and we are beginning to see promising long-term results.
Other new approaches include the CyberKnife, the NanoKnife, and intraoperative radiation therapy (IORT). At Mass General, we have a strong program in IORT. We use it to sterilize the tumor bed after resection of tumors that were initially inoperable but responded to chemotherapy and external radiation. We recently published one of the largest pancreatic cancer experiences with this modality and have several long-term survivors.11 But we need clinical trials to address these approaches, because these treatments are expensive. We need trials to identify the patients who are likely to benefit from them.
Personalized medicine is not yet a reality in pancreatic cancer the way it is in some other cancers, but I believe it will become so in the next few years. One of our oncologists recently looked at patterns of mutations in over 100 patients with pancreatic cancer and, sure enough, different patterns of mutations are associated with different survival rates. (See Table 1 for common mutations associated with pancreatic cancer.) This will help us to triage patients in a better way and perhaps offer them better treatments. An abstract about these data was recently submitted to the American Society of Clinical Oncology (ASCO).
Table 1.
Common Genetic Mutations in Pancreatic Adenocarcinoma
| Gene | Protein | Result of Somatic Mutation | Prevalence of Alteration in Ductal Adenocarcinoma | Comment |
|---|---|---|---|---|
| CDKN2A | p16 | Cell cycle dysregulation | 95% | Normal gene is important tumor suppressor gene |
| KRAS2 | Kras | Increase in proliferation and survival signaling | 90% | Normal protein is small GTPase involved in signaling pathway triggered by growth factor receptors; oncogene is activated by early-occurring point mutation |
| TP53 | p53 | Dysregulation of DNA repair, apoptosis | 50%–75% | Normal protein plays key role in cellular stress response; loss of gene function through mutation promotes pancreatic neoplasia |
| SMAD4 (DPC4) | Smad4 (Dpc4) | Aberrant TGF-β signaling | 50% | Normal protein mediates TGF-β signaling; SMAD4 is tumor suppressor gene; mutations are associated with poor prognosis and greater metastatic disease |
Source: Data from Hidalgo, 201013
P&T: Do you encourage patients with pancreatic cancer to enroll in clinical trials?
CF: I do, very strongly. Survival rates for pancreatic cancer today really are no different than they were 30 years ago. We’ve made little progress. If it were not for clinical trials, however, we would not know that FOLFIRINOX is effective. Without clinical trials, we will not advance the field. Here at Mass General we have numerous clinical trials open for patients with pancreatic cancer at all stages—resectable, locally advanced, and meta-static. We support these trials because we firmly believe this is the only way to make progress.
I also encourage patients to enroll in clinical trials because there is a little more attention to their care. So much attention is given to making sure they are following the trial protocol and monitoring their side effects that patients get the benefit of greater surveillance.
I am very honest and transparent with my patients about the pros and cons of these trials. Some patients are averse to being part of a clinical trial. They have suspicions and fears. Sometimes those suspicions and fears can be allayed, but sometimes they cannot. Sometimes patients cannot be part of a clinical trial because they live far away and to be part of the trial they would have to live closer to Boston. Sometimes they are not eligible for clinical trials because of other medical issues or because they’ve been dealing with another cancer. But in general we encourage patients to participate in clinical trials because we believe it is the right thing to do for the patient and for the disease. (See Table 2 for examples of clinical trials currently recruiting patients with ductal adenocarcinoma.)
Table 2.
Selected Clinical Trials Currently Recruiting Patients With Pancreatic Ductal Adenocarcinoma
| NCT Number / Phase (N) | Condition | Experimental Arm(s) | Selected Outcome Measures | Start Date / Primary Completion Date | Sponsor (PI) / Collaborators |
|---|---|---|---|---|---|
| NCT01494155 /phase 2 (50) | Resectable disease | Neoadjuvant hydroxy-chloroquine and proton beam RT followed by adjuvant gemcitabine | PFS, OS | Dec 2011 / Dec 2014 | Massachusetts General Hospital (Theodore Hong, MD) |
| NCT01821729 / phase 2 (32) | Locally advanced, unresectable disease | FOLFIRINOX, losartan, proton beam radiation therapy | Feasibility of combining FOLFIRINOX and losartan; OS, PFS | July 2013 / July 2015 | Massachusetts General Hospital (Theodore Hong, MD) / National Cancer Institute |
| NCT01621243 / phase 1/2 (180) | Metastatic disease | Nab-paclitaxel, gemcitabine, placebo vs nab-paclitaxel, gemcitabine, M402 | OS, PFS | May 2012 / Jan 2015 | Momenta Pharmaceuticals |
| NCT01839487 / phase 2 (132) | Metastatic disease | PEGPH20, nab-paclitaxel, gemcitabine vs nab-paclitaxel + gemcitabine | PFS, OS, ORR | April 2013 / Sept 2015 | Halozyme Therapeutics |
| NCT01431794 /Phase 1/2 (52) | Borderline resectable | Gemcitabine, nab-paclitaxel LDE225 vs gemcitabine, nab-paclitaxel | Resection rate, OS, OTR | Sept 2011 / Dec 2015 | Sidney Kimmel Comprehensive Cancer Center (Ana De Jesus-Acosta, MD) / Novartis |
| NCT02042378 /Phase 2 (100) | Ductal adenocarcinoma with deleterious BRCA mutation | Rucaparib | ORR, OS, DOR | April 2014 / Aug 2016 | Clovis Oncology |
DOR, duration of response; ORR, overall response rate; OS, overall survival; OTR, objective tumor response; PI, principal investigator; PFS, progression-free survival; RT, radiation therapy
Source: www.clinicaltrials.gov
P&T: Which trials are you following with the greatest interest?
CF: Mass General was the first institution to use proton-beam therapy in pancreatic cancer, and one of our most exciting trials uses the proton beam for patients with resectable pancreatic cancer. We’re giving the treatment before the operation. We’re eagerly awaiting long-term results. We have some other upcoming trials using FOLFIRINOX and gemcitabine/nab-paclitaxel in patients with resectable pancreatic cancer, in the hopes of finding better ways to treat this disease.
P&T: How intense is the pain associated with pancreatic cancer and its treatments, and how is the pain managed?
CF: A number of patients with pancreatic cancer present with pain. The pain can be quite severe. Unfortunately, in many patients with pancreatic cancer surgery is not an option, but as the disease evolves the pain can be quite a problem. It has to be given priority in the management of the patient. It can be managed with oral pain medications; sometimes narcotics are required.
Another way to manage pain is to do blocks of the celiac nerve plexus. If the patient happens to undergo an operation, for example, and is found not to be operable, then the surgeon should make a block of the celiac plexus with alcohol. There is good evidence that this is useful for patients with pancreatic cancer. If an operation is not being done, the blocks can be done under guidance with CT scan or endoscopic ultrasound.
P&T: When is it appropriate for the patient to begin thinking about palliative care? From what you have observed over the years, is palliative care used often enough and soon enough?
CF: That’s a very good question. Unfortunately, most patients with pancreatic cancer eventually die from their disease. The disease will progress despite their having had an operation or chemotherapy and radiation therapy. Once the disease progresses, there are not many options. There may be additional chemotherapy that can be given or clinical trials in which the patient can participate, but eventually the patient will succumb. The point at which the coat should be hung on palliative care becomes a decision for the patient and the oncologist. I think we should be using palliative care more often and perhaps sooner in some patients. Being under the care of a team that has expertise in palliative care has enormous benefits, not just for the patients but for the families, in terms of controlling symptoms and making something better out of a bad situation.
P&T: Which risk factors for pancreatic cancer are modifiable, and how should PCPs address them?
CF: There is only one strong modifiable risk factor, smoking. Of all the risk factors for pancreatic cancer, smoking is the one that has been firmly established, aside from a strong family history or genetic abnormalities. Of course, smoking is a modifiable risk factor. If you smoke, you are more likely to have pancreatic cancer, and if you smoke more, your risk increases. We also know—and this is good news—that if you stop smoking your risk will start to come down and actually level off and be the same as the rest of the population about 15 years after you stop.12 If a PCP has a patient who smokes, it’s not just lung cancer or cancer of the larynx, kidney, or bladder that’s worrisome but also cancer of the pancreas, aside from all the effects of smoking on the heart and lungs. Patients with hereditary pancreatitis, who have a high risk of pancreatic cancer, have a markedly higher risk of pancreatic cancer if they smoke, and they also are more likely to develop pancreatic cancer earlier in life.
Other data show that diets high in saturated fat and high in protein can increase risk of pancreatic cancer. Although obesity is not as strong a risk factor as smoking, it is something PCPs can make their patients think about, not just for pancreatic cancer but for many other diseases, including other cancers.
P&T: Is there anything else you would like readers of P&T to know about pancreatic cancer?
CF: Pancreatic cancer remains a deadly disease in which we have made relatively little progress. There is great hope that our understanding of the genomics and epigenetics of pancreatic cancer will give us insights to find new and better drugs. We need to keep our sight on the horizon. We hope that over the next decade we will make progress in pancreatic cancer as we have in other common cancers.
GlOSSARY
- Abraxane
Celgene’s branded nab-paclitaxel, a microtubule inhibitor. Initial Food and Drug Administration approval: 2005. Indicated as first-line treatment for metastatic adenocarcinoma of the pancreas, in combination with gemcitabine. Administered via intravenous infusion.
- Acinar cell carcinoma
a rare exocrine cell malignancy of the pancreas, typically arising in the head of the pancreas and associated with increased serum lipase and subcutaneous fat necrosis.
- Adenocarcinoma
a tumor originating in glandular epithelium; about 95% of pancreatic cancers are ductal adenocarcinoma.
- Adjuvant therapy
chemotherapy, radiation therapy, or both, provided after surgery. Contrast with neoadjuvant therapy.
- Borderline resectable
tumors that are technically resectable but which present little chance of achieving clear margins upon resection. Neoadjuvant chemotherapy may convert some patients to resectable status. Definitions of borderline resectable vary.
- CA 19-9
cancer antigen 19-9, carbohydrate antigen 19-9. A marker often used postoperatively for prognosis and surveil-lance. It is not pancreatic cancer-specific and thus not useful for screening and diagnosis.
- Camptosar
Pfizer’s branded irinotecan.
- Capecitabine
Hoffman La Roche’s Xeloda. Initial FDA approval: 1998. In vivo, capecitabine is converted by enzymes to 5-fluorouracil.
- CEA
carcinoembyronic antigen. A high CEA level in a person recently treated for pancreatic cancer (among other cancers) may indicate return of the cancer. However, higher-than-normal CEA levels alone cannot diagnose a new cancer; CEA cannot be used to screen for cancer.
- CgA
chromogranin A. Peptide serving as a useful marker in diagnosis of neuroendocrine tumors.
- CT
computed tomography.
- CyberKnife
a robotic radiosurgery system designed for noninvasive treatment of inoperable or surgically complex tumors; received FDA clearance in 2001 to treat tumors anywhere in the body. About one week prior to use of the CyberKnife, three to five tiny gold seeds are inserted, in a short outpatient procedure, into the pancreatic tumor under CT guidance to serve as reference points during treatment. Then the patient is fitted for a body cradle to facilitate consistent positioning and a vest that generates data enabling the robot to follow the position of the tumor as the patient breathes.
- ECOG
Eastern Cooperative Oncology Group.
- ECOG Performance Status
functional status of cancer patients, as assessed by a 6-point scale: 0 = asymptomatic and fully active; 1 = symptomatic but completely ambulatory, with restrictions in physically strenuous activity but able to execute light or sedentary work; 2 = in bed less than 50% during the day, capable of self-care but not of performing any work; 3 = in bed more than 50% during the day, capable of limited self-care; 4 = completely disabled, unable to perform any self-care, totally confined to bed or chair; 5 = death.
- Fluorouracil
component of FOLFIRINOX. Anabolic metabolism of fluorouracil is believed to block methylation reaction of deoxyuridylic acid to thymidylic acid, creating a thymine deficit that impairs DNA synthesis and results in cell death. Cells that are growing rapidly take up fluorouracil more rapidly than other cells.
- FOLFIRINOX
Four-drug chemotherapy combination: leucovorin (folinic acid), fluorouracil, irinotecan, and oxaliplatin. In patients with good ECOG performance status, FOLFIRINOX showed better overall survival benefit than gemcitabine alone.
- Gemcitabine
Gemzar. Gemcitabine kills cells undergoing DNA synthesis and blocks the progression of cells through the G1/S-phase boundary. Became reference regimen for advanced pancreatic cancer after randomized trial showed overall survival benefit versus fluorouracil (Burris, 1997). Initial FDA approval: 1996. Indicated as first-line treatment for patients with locally advanced (nonresectable stage 2 or 3) or metastatic (stage 4) adenocarcinoma of the pancreas. Indicated for patients previously treated with 5-FU.
- Gemzar
Lilly’s branded gemcitabine. Initial FDA approval: May 15, 1996.
- Hepatic veins
return blood from liver to heart. Contrast with hepatic portal vein.
- IORT
intraoperative radiation therapy.
- IPMN
intraductal papillary mucinous neoplasm.
- Irinotecan
Pfizer’s Camptosar. Inhibits topoisomerase 1 (via its active metabolite SN-38), which prevents DNA from unwinding. Received accelerated FDA approval in 1996 and full approval in 1998. A component of FOLFIRINOX. Has synergistic activity when administered before fluorouracil and leucovorin. Diarrhea and extreme immunosuppression are most important adverse events.
- LDE225
investigational Novartis once-daily oral blocker of Hedgehog pathway (involved in control of cell growth) being investigated in combination with gemcitabine.
- Leucovorin
folinic acid, a component of FOLFIRINOX. Enhances effect of 5-fluorouracil by inhibiting thymidylate synthase.
- Losartan
angiotensin-receptor blocker (ARB) being investigated in combination with FOLFIRINOX plus proton beam radiation therapy versus FOLFIRINOX plus proton beam.
- M402
investigational Momenta product, re-engineered from heparin to have lower blood-thinning activity while retaining antitumor activity shown in prior animal and human studies.
- MRCP
magnetic resonance cholangiopancreatography.
- Nab-paclitaxel
Abraxane. Albumin-bound form of paclitaxel, a microtubule inhibitor that promotes assembly of microtubules from tubulin dimers and stabilizes microtubules by preventing depolymerization. This stability inhibits normal dynamic reorganization of the microtubule network required for interphase and mitotic cellular functions. Paclitaxel induces abnormal “bundles” of microtubules throughout the cell cycle and multiple asters of microtubules during mitosis.
- NanoKnife
minimally invasive procedure employing irreversible electroporation (IRE, the use of low-energy direct- current electrical pulses to open permanent pores in cell membranes); the NanoKnife Ablation System has FDA clearance for surgical ablation of soft tissue. Guided by ultra-sound or CT imaging, an interventional radiologist places up to six probes in and around the target; a generator delivers a series of short electrical pulses between the probes to kill tumor cells.
- Oxaliplatin
component of FOLFIRINOX. Has clinical activity against pancreatic cancer only when combined with fluorouracil.
- PEGPH20
PEGylated recombinant human hyaluronidase, an investigational Halozyme product being studied in metastatic pancreatic cancer. Hyaluronidase enhances tissue permeability to increase dispersion and absorption of other injected drugs.
- Portal vein
conducts blood from GI tract and spleen to liver. Formed by confluence of superior mesenteric vein (SMV) and splenic vein. Not a true vein (because it conducts blood to capillary beds in artery rather than directly to heart). Supplies liver with about 75% of its blood.
- R0, R1, R2
residual tumor classification system indicating absence or presence of residual tumor after treatment. In general, R0 indicates negative surgical margin (potentially curative surgery); R1, microscopic residual tumor; R2, macroscopic residual tumor; definitions vary.
- Rucaparib
also known as CO-338, PF 01367338, AG 14699. An oral small-molecule inhibitor of poly-adenosine diphosphate (ADP) ribose polymerase (PARP), which inhibits a DNA repair pathway, base excision repair (BER). PARP inhibitors have been shown to kill tumors with a defect in BRCA1 or BRCA2.
- SMV-PV
superior mesenteric vein–portal vein.
- Splenic vein
large vein running behind pancreas and below splenic artery. Its juncture with superior mesenteric vein forms hepatic portal vein.
- Superior mesenteric vein (SMV)
drains blood from small intestine (jejunum and ileum), with tributaries draining small intestine, large intestine, stomach, pancreas, and appendix. Terminates behind neck of pancreas, where SMV combines with splenic vein to form hepatic portal vein. SMV lies to right of superior mesenteric artery, originating from abdominal aorta.
- Uncinate process
an extension of lower half of head of pancreas, lying between superior mesenteric vein and artery and aorta.
REFERENCES
- 1.Harmon K. The puzzle of pancreatic cancer: how Steve Jobs did not beat the odds—but Nobel winner Ralph Steinman did. Sci Am. 2011 Oct 7; Available at: http://www.scientificamerican.com/article/pancreatic-cancer-type-jobs. Accessed March 3, 2014. [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, et al. PRODIGE Intergroup FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Faris JE, Blaszkowsky LS, McDermott S, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 2013;18(5):543–548. doi: 10.1634/theoncologist.2012-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helmstaedter L, Riemann JF. Pancreatic cancer—EUS and early diagnosis. Langenbecks Arch Surg. 2008;393(6):923–927. doi: 10.1007/s00423-007-0275-1. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International Association of Pancreatology International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Fernández-del Castillo C, Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2010;139(3):708–713. doi: 10.1053/j.gastro.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg. 1995;130(3):295–299. doi: 10.1001/archsurg.1995.01430030065013. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann J, Michalski CW, Martignoni ME, et al. Pancreatic resection for pancreatic cancer. HPB (Oxford) 2006;8(5):346–351. doi: 10.1080/13651820600803981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Bentrem DJ, Ko CY, et al. Multimodality therapy for pancreatic cancer in the U.S.: utilization, outcomes, and the effect of hospital volume. Cancer. 2007;110(6):1227–1234. doi: 10.1002/cncr.22916. [DOI] [PubMed] [Google Scholar]
- 10.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 11.Cai S, Hong TS, Goldberg SI, et al. Updated long-term outcomes and prognostic factors for patients with unresectable locally advanced pancreatic cancer treated with intraoperative radio-therapy at the Massachusetts General Hospital, 1978 to 2010. Cancer. 2013;119(23):4196–4204. doi: 10.1002/cncr.28329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4) Ann Oncol. 2012;23(7):1880–1888. doi: 10.1093/annonc/mdr541. Erratum in: Ann Oncol 2012;23(10):2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 14.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 15.Arvold ND, Ryan DP, Niemierko A, et al. Long-term outcomes of neoadjuvant chemotherapy before chemoradiation for locally advanced pancreatic cancer. Cancer. 2012;118(12):3026–3035. doi: 10.1002/cncr.26633. [DOI] [PubMed] [Google Scholar]