Abstract
Purpose.
Amblyopia is a developmental disorder that results in both monocular and binocular deficits. Although traditional treatment in clinical practice (i.e., refractive correction, or occlusion by patching and penalization of the fellow eye) is effective in restoring monocular visual acuity, there is little information on how binocular function, especially stereopsis, responds to traditional amblyopia treatment. We aim to evaluate the effects of perceptual learning on stereopsis in observers with amblyopia in the current study.
Methods.
Eleven observers (21.1 ± 5.1 years, six females) with anisometropic or ametropic amblyopia were trained to judge depth in 10 to 13 sessions. Red–green glasses were used to present three different texture anaglyphs with different disparities but a fixed exposure duration. Stereoacuity was assessed with the Fly Stereo Acuity Test and visual acuity was assessed with the Chinese Tumbling E Chart before and after training.
Results.
Averaged across observers, training significantly reduced disparity threshold from 776.7″ to 490.4″ (P < 0.01) and improved stereoacuity from 200.3″ to 81.6″ (P < 0.01). Interestingly, visual acuity also significantly improved from 0.44 to 0.35 logMAR (approximately 0.9 lines, P < 0.05) in the amblyopic eye after training. Moreover, the learning effects in two of the three retested observers were largely retained over a 5-month period.
Conclusions.
Perceptual learning is effective in improving stereo vision in observers with amblyopia. These results, together with previous evidence, suggest that structured monocular and binocular training might be necessary to fully recover degraded visual functions in amblyopia.
Keywords: perceptual learning, stereoacuity, amblyopia
Traditional treatment cannot fully normalize stereoacuity in amblyopia. This study identifies that perceptual learning is of potential in restoring stereoacuity in adults with anisometropic amblyopia.
Introduction
Amblyopia, defined as degradation of spatial vision in the absence of any detectable structural or pathologic ocular abnormalities, is a developmental disorder that is caused by early abnormal visual experiences, specifically a lack of coordinated and balanced registration between the images in the two eyes, most commonly due to uncorrected strabismus, anisometropia, or cataract-induced form deprivation.1,2 Amblyopia impacts not only monocular vision, such as visual acuity,3–5 vernier acuity,5–7 contrast sensitivity,3,8–10 spatial distortion,4,11,12 and spatial interactions,7,13,14 but also binocular functions, including binocular combination,15–18 interocular interaction,19–21 and stereopsis.22–24
Although early administrations of conventional refractive corrections, patching, Bangerter filters, or atropine penalization over the fellow eye are effective in restoring monocular visual acuity in young children with amblyopia, their effects on restoring binocular functions are mixed in young and older children with amblyopia.25–29 Several studies have reported that occlusion or refractive correction itself can induce improved stereoacuity only in a subset of amblyopic subjects.25,26,29 For example, Stewart et al.26 recently investigated changes of stereoacuity in patients receiving amblyopia treatments comprised by refractive adaptation and occlusion phases. They found that 38% of their patients who received refractive adaptation and 29% who received occlusion improved their stereoacuity by at least one octave. However, other studies reported failed attempts to normalize binocular functions in amblyopia.27,28 For example, Wallace et al.28 found that 248 children (3- to 13-years old) with anisometropic amblyopia showed subnormal stereoacuity after conventional treatment (patching or Bangerter filters), although their visual acuities improved to normal or near-normal levels. Scheiman et al.27 found that deficient binocular function cannot be improved by patching or application of atropine sulfate in the fellow eye among 7- to 12-year-old children.
In the present study, we attempted to improve degraded stereo vision in observers with anisometropic or ametropic amblyopia through perceptual learning. Although it is known that stereopsis is critical for human visual perception, such as perceiving the three-dimensional (3D) layout of our surroundings, reading, hand–eye coordination, and camouflaged object detection,30–32 attempts to directly improve stereopsis in amblyopia have been astonishingly scarce.
In the past decades, many studies found that training or practice of a specific visual task can improve performance of amblyopes in a variety of low-level visual tasks, including contrast detection with33 and without flankers,34,35 identification of contrast-defined and luminance-defined letters,36,37 and positional acuity.38,39 These effects of perceptual learning demonstrate substantial plasticity in the child and even adult brain.40 While significant perceptual learning of stereopsis has been well documented in adults with normal vision,41–44 improvement of stereopsis in amblyopes has been mostly evaluated following perceptual learning of other tasks.39,45–50 For example, Hess et al.51 found that, stereopsis was established after intensive training of dichoptic motion coherence discrimination in eight out of nine adult strabismic amblyopes. Li et al.39 also found that two amblyopic children, one with strabismus and the other with anisometropia, who had no gross stereopsis at the beginning of their study, demonstrated measurable stereopsis after intensive monocular training on a position-discrimination task.
Two recent studies employed paradigms that directly targeted stereoacuity to evaluate the potential of perceptual learning in restoring stereo vision in amblyopia. Ding and Levi52 trained one adult with anisometropic amblyopia and three adults with strabismic amblyopia, who were all stereoblind or stereoanomalous before training, to perform a stereo-depth judgment task with sine-wave gratings. After training, all subjects showed significant improvement in stereopsis in psychophysical tests although their monocular vernier acuities remained unchanged. Astle et al.53 performed a case study on two adult anisometropic amblyopes with initial monocular training (to improve visual acuity in the amblyopic eye) and then stereo training. They reported that 9 days of detecting depth in random dot stereograms (RDS) improved stereoacuity to the normal level in both subjects. Interestingly, the improvement in stereoacuity was established independently of visual acuity amelioration. These results, together with the reported failure to normalize binocular functions in amblyopia after treatment focusing on monocular vision,27,28 suggest that different treatments might be necessary to recover both stereoacuity and visual acuity in adult amblyopia.
In the present study, we trained stereo depth perception in 11 observers with anisometropic amblyopia or amblyopia associated with high ametropia. High ametropia was defined as hypermetropia greater than 5 diopters (D) or astigmatism greater than 2 D in the absence of anisometropia or strabismus.54 Seven of the observers were novice and the other four received prior monocular contrast detection training. We used red–green glasses to present texture anaglyphs with different disparities but fixed exposure duration to the two eyes and trained subjects to detect stereo depth with feedback. Stereoacuity and visual acuity of both eyes were measured and compared before and after training. We focused on anisometropic or ametropic amblyopes because they are the predominant group, and other types of amblyopia (e.g., strabismic amblyopia), may be rather different in terms of the underlying mechanisms.6,51,55 Our aim was to evaluate the effects of our training method (e.g., anaglyphs made of textures and displayed with a fixed-duration) on stereo vision and visual acuity in adults with anisometropic or ametropic amblyopia.
Methods
Observers
The 11 participants in this experiment were 11- to 27-year-old (21.1 ± 5.1 years) observers with natural-occurring anisometropic or ametropic amblyopia. Among them, four observers (A1–A4) received monocular training prior to the study34; the other seven (A5–A11) were novice observers. All 11 observers wore glasses in their daily life (at least 1 year), five of which were prescribed by the third author of the paper (L-XF) and the other six by other doctors/experienced optometrists. Before they took part in the experiment, the third author (L-XF) carefully diagnosed them for potential ocular pathological defects and strabismus, and determined their refraction through mydriatic optometry (under cycloplegia). Corrective lens were prescribed based on their refraction and subjective trial of lens, if necessary. Subjects A3 and A6 were prescribed new glasses and wore the new glasses for at least 1 week before data collection. All other subjects wore their own glasses. The corneal light reflex test, cover–uncover test and alternate cover test were used to assess the patients' ocular alignment. Tropia or phoria was not found among these subjects. Detailed characteristics of these observers, including their sex, age, optical correction, and corrected visual acuity are listed in Table 1. None of the 11 observers had previous experience with stereograms made of anaglyph images and stereo training. All observers wore their corrective lenses during training.
Table 1.
Observer Characteristics
|
Observer |
Sex |
Age, y |
Treatment History |
Eye |
Correction |
Acuity, logMAR |
Exposure Time, s |
| A1* | F | 16 | Glasses for 1 y, no patching | AE (R) | +3.50 DS | 0.62 | 5 |
| DE (L) | −1.00 DS | −0.10 | |||||
| A2* | F | 17 | Glasses for 8 y, no patching | AE (L) | +7.00 DS/+1.5 DC × 90 | 0.66 | 2.5 |
| DE (R) | +1.25 DS | −0.22 | |||||
| A3* | M | 20 | Glasses for 10 y, no patching | AE (L) | +5.00 DS | 0.28 | 1 |
| DE (R) | −1.75 DS | −0.16 | |||||
| A4* | M | 11 | Glasses for 2 y, patching for 2 y | AE (L) | +3.00 DS/+2.00 DC × 90 | 0.26 | 2 |
| DE (R) | +3.00 DS/+2.00 DC × 85 | −0.10 | |||||
| A5 | M | 19 | Glasses for 4 y, no patching | AE (R) | +9.00 DS/+0.37 DC × 90 | 0.45 | 3 |
| DE (L) | +10.00 DS/+0.50 DC × 95 | 0.18 | |||||
| A6 | F | 27 | Glasses for 4 y, no patching | AE (R) | −3.50 DS | 0.38 | 5 |
| DE (L) | −2.50 DS | 0 | |||||
| A7 | M | 24 | Glasses for 12 y, patching duration unknown | AE (L) | 2.74 DS/+1.5 DC | 0.30 | 0.1 |
| DE (R) | 0 | −0.05 | |||||
| A8 | F | 21 | Glasses for 13 y, patching from age 8, duration unknown | AE (L) | +5.00 DS | 0.30 | 1 |
| DE (R) | +1.00 DS | −0.10 | |||||
| A9 | F | 25 | Glasses for 12 y, no patching | AE (R) | +4.00 DS/+0.50 DC × 90 | 0.72 | 0.3 |
| DE (L) | 0.50 DC × 90 | −0.10 | |||||
| A10 | F | 25 | Glasses for 3 y, no patching | AE (L) | +1.25 DS/+1.25 DC × 90 | 0.36 | 2 |
| DE (R) | 0 | −0.22 | |||||
| A11 | M | 27 | Glasses for 9 y, no patching | AE (R) | +4.50 DS | 0.48 | 0.5 |
| DE (L) | −2.75 DS | 0.08 |
Exposure Time: exposure duration of the two anaglyph images, derived from a pilot experiment (see Methods). Visual acuity was assessed with crowded Chinese Tumbling E chart. The stimuli were presented binocularly and the exposure time was the same for both eyes. AE, amblyopic eye; DE, dominant eye; L, left eye; R, right eye.
These four observers had monocular training experience before the stereo experiment.
We also trained five normal observers for 10 sessions with the same task and setups to assess whether training can also reduce disparity threshold in normal vision. The five observers went through the same screening examination as the amblyopic group did. They did not have any organic ocular disease and had normal or corrected-to-normal visual acuity and stereoacuity. Briefly, disparity threshold decreased from 914.0″ to 262.0″, a reduction of 74.0% (SE: ±16.5%) averaged across the observers. Please refer to the Supplementary Material for detailed characteristics of these observers, their improvements, and learning curves.
The research protocol was approved by the ethics committee of the Institute of Psychology, Chinese Academy of Sciences (Beijing, China) and all research activities adhered to the tenets of the Declaration of Helsinki. Informed written consent was obtained from all observers before the experiment.
Apparatus and Stimuli
The experiments were controlled by a desktop computer running Visual C++ (Microsoft, Inc., Redmond, WA, USA). The stimuli were presented on a SONY G520 color monitor (P22 phosphor; Sony, Tokyo, Japan) driven by the internal graphics card of the computer with a spatial resolution of 1600 × 1200 pixels and a refresh rate of 75 Hz. At a viewing distance of 100 cm, each pixel subtends 41.3″. Observers wore red–green anaglyph glasses which only passed red patterns in the stimuli to the left eye and green patterns to the right eye.
The stimuli used in this experiment were three different textures (18.36° × 2.75°; Fig. 1A). We started with black and white textures with pixel gray levels at 150 ± 46.43, 113 ± 52.74, 94 ± 57.41 (mean ± SD). To generate anaglyphs, we removed the blue component of the textures in a pixel-wise fashion, dissected the remaining texture into the red and green components, and shifted the red component relative to its green counterpart according to the desired disparity.
Figure 1.
(A) Three textures used in the experiment. (B) Schematic illustration of stereo training task. In this example, the red component of the upper texture was shifted to right relative to its green counterpart to create an uncrossed disparity; the lower texture has zero disparity. The correct response is to press the down key in the keyboard to indicate the lower texture is the nearer one.
In a given trial, one texture was selected randomly and displayed in two locations, one above and one below the fixation point (radius = 0.11°; Fig. 1B). Images in the two locations were both red–green anaglyphed but disparity, either crossed or uncrossed, was only endowed to the anaglyph in one of the two locations. The fixation dot and one of the two images (zero-disparity) were referred as the zero planes. Each anaglyph was trimmed to eliminate edges that contain information from only one eye when the two anaglyphs are combined in binocular vision. Observers were asked to indicate which one of the two textures appeared to be nearer and respond with the up or down key on the keyboard. During training, a brief tone followed each correct response. The response also initiated the next trial. Three textures were presented randomly with the constraint that no more than three consecutive trials used the same texture.
Design and Procedure
The experiment consisted of pretraining assessment, stereopsis training at one exposure duration and posttraining assessment. We also conducted a follow-up test of stereoacuity and visual acuity in three observers 5 months after posttraining assessment.
In both pre- and posttraining assessments, stereoacuity and visual acuity were measured for all observers. Stereoacuity was assessed with the Fly Stereo Acuity Test. Two versions of the Titmus Fly Test were used, one with 10 circles ranging from 800 to 40 arcsecs (Stereo Fly SO-001; Stereo optical Co., Inc., Chicago, IL, USA) for observers A1, A2, A3, A5, and A6, and the other with 10 circles ranging from 400 to 20 arcsecs (Fly Stereo Acuity Test; Vision Assessment Corporation, Elk Grove Village, IL, USA) for the other six subjects. We administered the tests according to the manufacture's guidance and lessons learned from the literature. In a bright room (without direct lighting on the testing material), subjects looked directly at the test material at a viewing distance of 40 cm (strictly tape measured). Subjects were first made familiar with the task using the easiest fly of 3000″ and then reported which circle was out of the plane of the other three (zero plane) in turn (easy–hard). Lighting level was kept almost the same across tests. We confirmed that subjects relied on stereopsis to accomplish the test by rotating the testing material by 90° in three subjects. Visual acuity was assessed with the Chinese Tumbling E Chart,56 which has 14 lines, with the size of the optotypes ranging from 1 to −0.3 logMAR and changing by 0.1 log unit from line to line. Subjects were required to report the orientation (the opening) of the letter “E.” Visual acuity is defined as the logMAR associated with 75% correct identification. The order of tests in the pre- and posttraining measurements was counterbalanced.
Before training, we conducted a pilot experiment to determine the suitable exposure duration for each observer. We varied the exposure duration of the three anaglyphs from 10, 5, 4, 3, 2.5, 2, 1.5, 1, 0.5, 0.3, to 0.1 second in a descending order and obtained rough estimates of the disparity thresholds with approximately 50 trials in each condition. We then chose the exposure duration that corresponded to a disparity threshold around 0.23° (20 pixels) as the display duration during training. The disparity of 0.23° was chosen because the stereo task was demanding but still accomplishable in that condition, leaving enough room for subjects to improve. The display duration for each observer was listed in Table 1. The same task was used in the pilot and training experiments.
Stereo training took an average of 11 sessions (ranged from 10–13, 1 session/d; 8–60 min/session), during which the exposure time was fixed while disparity was changed based on observers' performance. Each training session consisted of three 80-trial blocks. In each block, disparity threshold was measured by a two-down, one-up staircase procedure in which two consecutive correct responses resulted in a reduction of disparity [Dn+1 = 0.9 Dn] and one wrong response resulted in an increase in disparity [Dn+1 = 1.1 Dn], converging to a performance level of 70.7% correct. All disparities were expressed in units of pixels and rounded to their closest integer values. A reversal resulted when the staircase changed its direction (changing from increasing to decreasing disparity or vice versa). According to standard psychophysical practice, the first three (if there were an odd number of total reversals) or four (if even) reflections were discarded and the average of the remaining reversals were taken as the threshold. The starting disparity was set at 20 pixels (0.23°) for the first session. It was set as the threshold of the previous session in subsequent sessions.
Statistical Analysis
Pre- and posttraining disparity threshold, visual acuity and Titmus stereoacuity were compared using paired t-tests. For each observer, the percent improvement for all the three measures (disparity threshold, visual acuity and stereoacuity measured with Titmus Fly test) was calculated as:
 |
For each observer, the magnitude of improvement for stereoacuity measured with the Titmus Fly test was also calculated as:
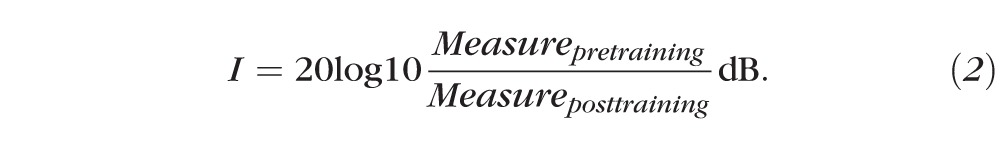 |
The learning curve (i.e., disparity threshold as a function of training session [in log unit] for each observer and the group average) was fit with a linear function:
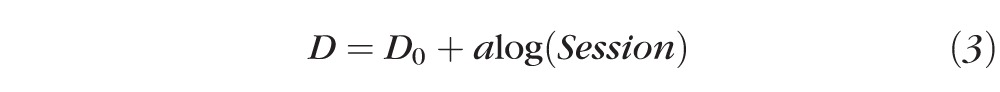 |
where D denotes disparity threshold and a is the slope of the learning curve.
Results
Stereo Training
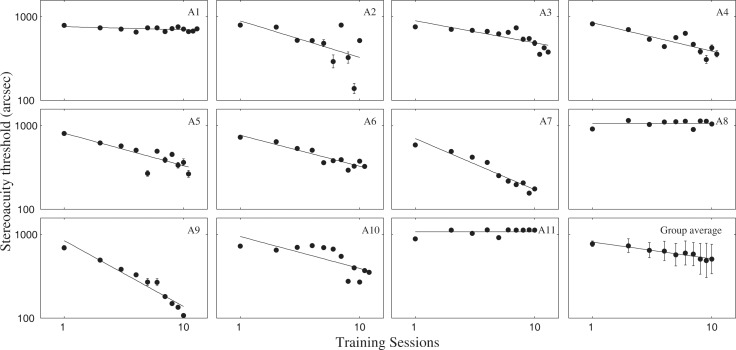
As illustrated in Figure 2, disparity threshold decreased significantly in 9 of 11 amblyopic observers over training sessions. Averaged across observers, disparity threshold decreased from 776.7″ to 490.4″, a reduction of 36.9% (SE: ±10.8%; t[10] = 3.493, P < 0.01). The average slope of the learning curve was −290.2″ per log unit of training session (P < 0.001). Specifically, the four observers with prior monocular training (A1–A4) improved from 781.9″ to 457.5″, with an average reduction of 41.5% (±12.7%); the other seven novices improved from 773.7″ to 509.2″, with an average reduction of 34.2% (±16.1%). There was no significant difference in terms of the magnitude of improvement in the two subgroups (P > 0.5).
Figure 2.
Individual and group average learning curves. Stereo training significantly reduced disparity threshold for 9 of 11 amblyopic observers over 10 to 13 training sessions. The group data were computed by averaging across the 10 common sessions of the 11 observers. Data were fitted with a Log-linear function. Error bars: ±1 SE.
Stereoacuity and Visual Acuity Tests
Training improved Titmus stereoacuity from 200.3″ to 81.6″, an average reduction of 59.3% (t[10] = 4.264, P < 0.01, or 7.80 dB; Table 2). The improvement was 50% (SE: ±10.8%) for the four observers with prior monocular training (210″–105″, or 6.02 dB), and 64.9% (SE: ±5.5%) for the seven without monocular training (194.7″–68.3″, or 9.10 dB), without significant difference in the magnitude of improvement (P > 0.1). A previous study57 indicated that the appropriate criterion for normal stereopsis is stereoacuity less than 40″. According to this criterion, observers 4, 7, 8, and 9 achieved normal stereoacuity following training.
Table 2.
Pre- and Posttraining Titmus Stereoacuity
|
Observer |
Pre |
Post |
Improvement, % |
Retention* |
|
|
Retest |
% |
||||
| A1† | 400″ | 200″ | 50.0 | 300″ | 66.7 |
| A2† | 140″ | 80″ | 42.9 | 80″ | 100 |
| A3† | 140″ | 100″ | 28.6 | 67″ | 148 |
| A4† | 100″ | 20″ | 80 | ||
| A5 | 200″ | 100″ | 50.0 | ||
| A6 | 400″ | 200″ | 50.0 | ||
| A7 | 100″ | 25″ | 75 | ||
| A8 | 63″ | 20″ | 68.3 | ||
| A9 | 100″ | 20″ | 80 | ||
| A10 | 160″ | 63″ | 60.6 | ||
| A11 | 400″ | 50″ | 87.5 | ||
Retention after 5 months for three retested observers.
These four observers had previous monocular training experience.
Training also significantly improved visual acuity in the amblyopic eyes (0.44–0.35 logMAR, ∼0.9 lines, or 18.8% on average, t[10] = 3.089, P < 0.05) but not in the fellow eyes (average −0.05%, t[10] = 0.667, P > 0.5) relative to their prestereo training visual acuity (Table 3). The improvement was 13.2% (0.46–0.40 logMAR, ∼0.6 lines, SE: ±2.1%) for the four observers with prior monocular training and 22.0% (0.43–0.32 logMAR, ∼1.1 lines, SE: ±6.4%) for the seven without prior monocular training, without significant difference in the magnitude of improvement (P > 0.1). No observer improved to normal visual acuity after training.
Table 3.
Pre- and Posttraining Visual Acuity
|
Observer |
Eye |
Visual Acuity, logMAR |
||||||
|
Initial |
Monocular Training |
Stereo Training |
Retention* |
|||||
|
Post |
Imp, % |
Post |
Imp, % |
Retest |
% |
|||
| A1† | AE | 0.85 | 0.62 | 40.8 | 0.57 | 11.9 | 0.54 | 105.7 |
| DE | 0.08 | −0.10 | 33.3 | −0.16 | 12.5 | −0.16 | 100 | |
| A2† | AE | 0.85 | 0.66 | 35.2 | 0.56 | 21.7 | 0.57 | 97.3 |
| DE | −0.16 | −0.22 | 14.3 | −0.16 | −16.7 | −0.10 | 87.5 | |
| A3† | AE | 0.58 | 0.28 | 50.0 | 0.23 | 10.5 | 0.20 | 106.2 |
| DE | −0.05 | −0.16 | 22.2 | −0.16 | 0 | −0.16 | 100 | |
| A4† | AE | 0.34 | 0.26 | 18.2 | 0.23 | 5.6 | ||
| DE | −0.05 | −0.10 | 11.1 | −0.10 | 0 | |||
| A5 | AE | 0.45 | 0.28 | 32.1 | ||||
| DE | 0.18 | 0.11 | 13.3 | |||||
| A6 | AE | 0.38 | 0.30 | 16.7 | ||||
| DE | 0 | 0.04 | −10 | |||||
| A7 | AE | 0.30 | 0.28 | 5 | ||||
| DE | −0.05 | −0.05 | 0 | |||||
| A8 | AE | 0.30 | 0.26 | 10 | ||||
| DE | −0.10 | −0.10 | 0 | |||||
| A9 | AE | 0.72 | 0.38 | 54.7 | ||||
| DE | −0.10 | −0.16 | 12.5 | |||||
| A10 | AE | 0.36 | 0.26 | 21.7 | ||||
| DE | −0.22 | −0.22 | 0 | |||||
| A11 | AE | 0.48 | 0.48 | 0 | ||||
| DE | 0.08 | 0.08 | 0 | |||||
The visual acuity was assessed by crowded Chinese Tumbling E chart. Imp, improvement.
Retention after 5 months for three retested observers.
These four observers had monocular training experience before the stereo experiment.
To examine long-term retention of the training effects on stereoacuity and visual acuity, three observers (A1–A3) were retested 5 months after posttraining assessment for stereoacuity and visual acuity. Stereoacuity deteriorated from 200 to 300 arcsecs for A1, remained at 80 arcsecs for A2, and improved from 100 arcsec to 67 arcsec for A3. On average, the three observers retained 104.9% of training results in stereoacuity (Table 2) and 103.1% of visual acuity improvements in the amblyopic eyes (Table 3).
Correlation Between Different Measures
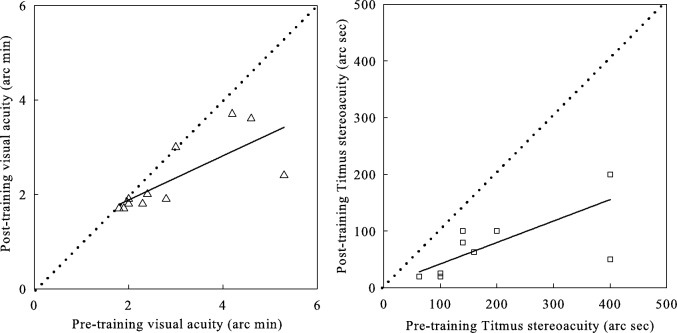
In Figure 3, we plotted pretraining measures of visual acuity and Titmus stereopsis versus posttraining counterparts. Almost all data points are below the identity line, which indicates significant improvement. The best-fitting linear regression line for visual acuity (r2 = 0.56, P < 0.01) has a slope of 0.47, suggesting greater visual acuity improvements for observers with worse initial acuities, consistent with previous reports.35 The best-fitting linear regression for Titmus stereoacuity (r2 = 0.57, P < 0.01) has a slope of 0.38, indicating the worse the initial stereoacuity, the greater the improvement.
Figure 3.
Posttraining measures of visual acuity and Titmus stereopsis versus pretraining counterparts. The dashed line is the identity line (slope = 1; i.e., no improvement).
We also performed Pearson's correlation analysis between the improvements on disparity threshold, Titmus stereoacuity, and visual acuity in the amblyopic eyes. Neither correlation between the improvements on Titmus stereoacuity and disparity threshold (P > 0.5) nor between the improvements on Titmus stereoacuity and visual acuity in the amblyopic eyes (P > 0.5) was significant. We also did not find significant correlation between the improvements on disparity threshold and visual acuity in the amblyopic eyes (P = 0.094; R = 0.529).
Discussion
In the current study, we demonstrated that 10 to 13 brief sessions of stereo training using textures stimuli at a fixed exposure time significantly reduced disparity threshold in 9 of 11 amblyopic observers. Training also significantly improved stereoacuity measured with the Titmus Fly Test and visual acuity in all observers, including the two who did not show improvement in the disparity threshold. Moreover, improvements in stereoacuity for two of the three retested observers were largely retained for at least 5 months, suggesting that stereo training induced genuine improvement of stereopsis in those observers.
Ten of the 11 observers showed improvement on visual acuity in the amblyopic eyes after stereo training, but the magnitude of the improvement did not significantly correlate with the magnitude of improvement on disparity threshold (P = 0.094). Similarly, Hess et al.45 found binocular fusion training resulted in significant improvements in stereoacuity as well as Snellen acuity in the amblyopic eye, but the magnitudes of the improvements were not correlated. In their study, the training task of the nine strabismic amblyopic subjects was dichoptic motion detection near coherence thresholds. In the current study, the 11 anisometropic or ametropic amblyopic observers were directly trained to judge depth by manipulating the disparity of texture stimuli. Mechanisms underlying strabismic and anisometropic amblyopia are thought to be different.6,51 It would be interesting to apply both training paradigms in different types of amblyopia.
Astle et al.53 reported that their two observers did not show improvements in visual acuity, although their stereo vision reached normal level. They attributed the lack of correlation between the magnitudes of improvements in visual acuity and stereoacuity to the subjects' extensive monocular training experience before stereo training. In the current study, 4 of the 11 observers underwent a monocular contrast detection task over 10,000 trials prior to stereo training.34 It is interesting to note that we still found some improvements in visual acuity in those subjects (0.05, 0.1, 0.05, and 0.03 logMAR), comparable to the improvements in the other seven subjects without prior monocular training experience (0.17, 0.08, 0.02, 0.04, 0.34, 0.1, and 0 logMAR), although there was no significant correlation between the magnitudes of improvements in visual acuity and disparity threshold in the 11 subjects. It would be useful to compare different combinations of monocular and stereo training tasks/paradigms in a large sample of subjects to test if recovery of visual acuity and stereoacuity is truly independent.
Two observers (A8 and A11) showed no change in stereoacuity threshold during training, but they showed a very large improvement in performance on the Titmus test. We note that the two tests differed significantly with each other in many ways and the results of the two measurements may not correlate with each other. The Titmus test used polarized broad-band circles that can be viewed freely and our psychophysical tests used texture red/green anaglyphs that were displayed with fixed exposure duration. And the viewing distance was 40 and 100 cm, respectively. We performed a Pearson's correlation analysis between the pretest Titmus stereoacuity and pretest disparity threshold and the posttest Titmus stereoacuity and posttest disparity threshold. Indeed neither correlation was significant (P > 0.1).
In previous studies, we found that for normal observers, both eyes contributed almost equally in binocular combination.16,17 For amblyopic observers, stimulus of equal contrast was weighted much less in the amblyopic eye relative to the fellow eye. The effective contrast of the amblyopic eye in binocular combination was equal to approximately 11% to 28% of the same contrast presented to the fellow eye.16 Stereo disparity threshold was on the other hand found to depend not only on image contrasts in the two eyes but also the ratio between them.58–61 It would be interesting to match the effective contrasts in the fellow and amblyopic eyes in assessing stereoacuity. Future stereo training studies should also consider the effects of interocular suppression on stereo performance in amblyopia.
We have previously shown that the binocular deficits in contrast and phase perception in anisometropic amblyopia were jointly determined by the attenuation of the signal from the amblyopic eye and a disproportionally stronger inhibition from the fellow eye to the amblyopic eye based on the multipathway, contrast-gain control model (MCM) of binocular vision.17 We later demonstrated that subject's disparity threshold can also be understood within the MCM framework, indicating both signal attenuation and interocular inhibition contributed to stereo information computation.59 In this scenario, the observation of improved stereoacuity might reflect an enhancement of the signal in the amblyopic eye or a decrease of interocular suppression from the fellow eye to the amblyopic eye. On the other hand, Sowden et al.41 has suggested that the improvements in stereoacuity in normal observers might not occur at an early level of visual processing. All these possibilities need to be further evaluated.
In summary, we found that training significantly improved stereoacuity in amblyopic observers but the improvement in stereoacuity and visual acuity was not significantly correlated with each other. Our results, together with others,27,28,45,52,53 suggested that perceptual learning may be valuable in improving stereoacuity in observers with anisometropic amblyopia, and recovery of monocular visual acuity and stereo vision in amblyopia may need separate monocular and binocular treatments.
Acknowledgments
The authors thank Yifeng Zhou of University of Science and Technology of China for help with subject recruitment and part of the experiments, and Zhe Wang for assistance with programming.
Supported by grants from the Knowledge Innovation Program of the Chinese Academy of Sciences (Y3CX102003, C-BH), Institute of Psychology (Y1CX201006, C-BH), the Scientific Foundation of Institute of Psychology, Chinese Academy of Sciences (Y3CX182005, JX), the National Eye Institute (EY021553, Z-LL), National Natural Science Foundation of China (81300796, L-XF) and Natural Science Foundation of Anhui Province (Grant 1208085MH156, L-XF).
Disclosure: J. Xi, None; W.-L. Jia, None; L.-X. Feng, None; Z.-L. Lu, None; C.-B. Huang, None
References
- 1. Ciuffreda KJ, Levi DM, Selenow A. Amblyopia: Basic and Clinical Aspects. Boston: Butterworth-Heinemann; 1991. [Google Scholar]
- 2. McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. J Vis. 2003; 3: 380–405 [DOI] [PubMed] [Google Scholar]
- 3. Gstalder RJ, Green DG. Laser interferometric acuity in amblyopia. J Pediatr Ophthalmol. 1971; 8: 251–256 [Google Scholar]
- 4. Hess RF, Campbell FW, Greenhalgh T. On the nature of the neural abnormality in human amblyopia; neural aberrations and neural sensitivity loss. Eur J Physiol. 1978; 377: 201–207 [DOI] [PubMed] [Google Scholar]
- 5. Levi DM, Klein S. Hyperacuity and amblyopia. Nature. 1982; 298: 268–270 [DOI] [PubMed] [Google Scholar]
- 6. Levi DM, Klein S. Differences in vernier discrimination for grating between strabismic and anisometropic amblyopes. Invest Ophthalmol Vis Sci. 1982; 23: 398–407 [PubMed] [Google Scholar]
- 7. Levi DM, Klein SA. Vernier acuity, crowding and amblyopia. Vision Res. 1985; 25: 979–991 [DOI] [PubMed] [Google Scholar]
- 8. Bradley A, Freeman RD. Contrast sensitivity in anisometropic amblyopia. Invest Ophthalmol Vis Sci. 1981; 21: 467–476 [PubMed] [Google Scholar]
- 9. Hess RF, Howell ER. The threshold contrast sensitivity function in strabismic amblyopia: evidence for a two type classification. Vision Res. 1977; 17: 1049–1055 [DOI] [PubMed] [Google Scholar]
- 10. Levi M, Haworth RS. Spatiotemporal interactions in anisometropic and strabismic amblyopia. Invest Ophthalmol Vis Sci. 1977; 16: 90–95 [PubMed] [Google Scholar]
- 11. Bedell HD, Flom MC. Monocular spatial distortion in strabismic amblyopia. Invest Ophthalmol Vis Sci. 1981; 20: 263–268 [PubMed] [Google Scholar]
- 12. Sireteanu R, Lagreze WD, Constantinescu DH. Distortions in two-dimensional visual space perception in strabismic observers. Vision Res. 1993; 33: 677–690 [DOI] [PubMed] [Google Scholar]
- 13. Levi DM, Hariharan S, Klein SA. Suppressive and facilitatory spatial interactions in amblyopic vision. Vision Res. 2002; 42: 1379–1394 [DOI] [PubMed] [Google Scholar]
- 14. Polat URI, Sagi DOV, Norcia AM. Abnormal long-range spatial interactions in amblyopia. Vision Res. 1997; 37: 737–744 [DOI] [PubMed] [Google Scholar]
- 15. Baker DH, Meese TS, Mansouri B, Hess RF. Binocular summation of contrast remains intact in strabismic amblyopia. Invest Ophthalmol Vis Sci. 2007; 48: 5332–5338 [DOI] [PubMed] [Google Scholar]
- 16. Huang CB, Zhou JW, Lu ZL, Feng LX, Zhou YF. Binocular combination in anisometropic amblyopia. J Vis. 2009; 9: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang CB, Zhou JW, Lu ZL, Zhou YF. Deficient binocular combination reveals mechanisms of anisometropic amblyopia: signal attenuation and interocular inhibition. J Vis. 2011; 11: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lema SA, Blake R. Binocular summation in normal and stereoblind humans. Vision Res. 1977; 17: 691–695 [DOI] [PubMed] [Google Scholar]
- 19. Harrad RA, Hess RF. Binocular integration of contrast information in amblyopia. Vision Res. 1992; 32: 2135–2150 [DOI] [PubMed] [Google Scholar]
- 20. Levi DM, Harwerth RS, Manny RE. Suprathreshold spatial frequency detection and binocular interaction in strabismic and anisometropic amblyopia. Invest Ophthalmol Vis Sci. 1979; 18: 714–725 [PubMed] [Google Scholar]
- 21. Levi DM, Harwerth RS, Smith EL. Binocular interactions in normal and anomalous binocular vision. Doc Ophthalmol. 1980; 49: 303–324 [DOI] [PubMed] [Google Scholar]
- 22. Walraven J, Janzen P. TNO stereopsis test as an aid to the prevention of amblyopia. Ophthalmic Physiol Opt. 1993; 13: 350–356 [DOI] [PubMed] [Google Scholar]
- 23. Cooper J, Feldman J. Random-dot-stereogram performance by strabismic, amblyopic, and ocular-pathology patients in an operant-discrimination task. Am J Optom Physiol Opt. 1978; 55: 599–609 [DOI] [PubMed] [Google Scholar]
- 24. Wood IC, Fox JA, Stephenson MG. Contrast threshold of random dot stereograms in anisometropic amblyopia: a clinical investigation. Br J Ophthalmol. 1978; 62: 34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee SY, Isenberg SJ. The relationship between stereopsis and visual acuity after occlusion therapy for amblyopia. Ophthalmology. 2003; 110: 2088–2092 [DOI] [PubMed] [Google Scholar]
- 26. Stewart CE, Wallace MP, Stephens DA, Fielder AR, Moseley MJ. The effect of amblyopia treatment on stereoacuity. J AAPOS. 2013; 17: 166–173 [DOI] [PubMed] [Google Scholar]
- 27. Scheiman MM, Hertle RW, Beck RW, et al. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch Ophthalmol. 2005; 123: 437–447 [DOI] [PubMed] [Google Scholar]
- 28. Wallace DK, Lazar EL, Melia M, et al. Stereoacuity in children with anisometropic amblyopia. J Am Assoc Pediatr Ophthalmol Strabismus. 2011; 15: 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richardson SR, Wright CM, Hrisos S, Buck D, Clarke MP. Stereoacuity in unilateral visual impairment detected at preschool screening: outcomes from a randomized controlled trial. Invest Ophthalmol Vis Sci. 2005; 46: 150–154 [DOI] [PubMed] [Google Scholar]
- 30. Jones RK, Lee DN. Why two eyes are better than one: the two views of binocular vision. J Exp Psychol Hum Percep Perform. 1981; 7: 30–40 [DOI] [PubMed] [Google Scholar]
- 31. Sheedy JE, Bailey IL, Buri M, Bass E. Binocular vs. monocular task performance. Am J Optom Physiol Opt. 1986; 63: 839–846 [DOI] [PubMed] [Google Scholar]
- 32. Suttle CM, Melmoth DR, Finlay AL, Sloper JJ, Grant S. Eye–hand coordination skills in children with and without amblyopia. Invest Ophthalmol Vis Sci. 2011; 52: 1851–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Polat U, Ma-Naim T, Belkin M, Sagi D. Improving vision in adult amblyopia by perceptual learning. Proc Natl Acad Sci U S A. 2004; 101: 6692–6697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang C-B, Zhou Y-F, Lu Z-L. Broad bandwidth of perceptual learning in the visual system of adults with anisometropic amblyopia. Proc Natl Acad Sci U S A. 2008; 105: 4068–4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou Y-F, Huang C-B, Xu P-J, et al. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vision Res. 2006; 46: 739–750 [DOI] [PubMed] [Google Scholar]
- 36. Chung STL, Li RW, Levi DM. Identification of contrast-defined letters benefits from perceptual learning in adults with amblyopia. Vision Res. 2006; 46: 3853–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chung STL, Li RW, Levi DM. Learning to identify near-threshold luminance-defined and contrast-defined letters in observers with amblyopia. Vision Res. 2008; 48: 2739–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li RW, Levi DM. Characterizing the mechanisms of improvement for position discrimination in adult amblyopia. J Vis. 2004; 4: 476–487 [DOI] [PubMed] [Google Scholar]
- 39. Li RW, Provost A, Levi DM. Extended perceptual learning results in substantial recovery of positional acuity and visual acuity in juvenile amblyopia. Invest Ophthalmol Vis Sci. 2007; 48: 5046–5051 [DOI] [PubMed] [Google Scholar]
- 40. Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001; 31: 681–697 [DOI] [PubMed] [Google Scholar]
- 41. Sowden P, Davies I, Rose D, Kaye M. Perceptual learning of stereoacuity. Perception. 1996; 25: 1043–1052 [DOI] [PubMed] [Google Scholar]
- 42. Fendick M, Westheimer G. Effects of practice and the separation of test targets on foveal and peripheral stereoacuity. Vision Res. 1983; 23: 145–150 [DOI] [PubMed] [Google Scholar]
- 43. Ramachandran VS, Braddick O. Orientation-specific learning in stereopsis. Perception. 1973; 2: 371–376 [DOI] [PubMed] [Google Scholar]
- 44. O'Toole AJ, Daniel K. Learning to see random-dot stereograms. Perception. 1992; 21: 227–243 [DOI] [PubMed] [Google Scholar]
- 45. Hess RF, Mansouri B, Thompson B. A new binocular approach to the treatment of amblyopia in adults well beyond the critical period of visual development. Restor Neurol Neurosci. 2010; 28: 793–802 [DOI] [PubMed] [Google Scholar]
- 46. Hess RF, Mansouri B, Thompson B. A binocular approach to treating amblyopia: antisuppression therapy. Optom Vis Sci. 2010; 87: 697–704 [DOI] [PubMed] [Google Scholar]
- 47. Hess RF, Mansouri B, Thompson B. Restoration of binocular vision in amblyopia. Strabismus. 2011; 19: 110–118 [DOI] [PubMed] [Google Scholar]
- 48. Black JM, Hess RF, Cooperstock JR, To L, Thompson B. The measurement and treatment of suppression in amblyopia. J Vis Exp. 2012; 70: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hess RF, Thompson B, Black JM, et al. An iPod treatment of amblyopia: an updated binocular approach. Optometry. 2012; 83: 87–94 [PubMed] [Google Scholar]
- 50. Knox PJ, Simmers AJ, Gray LS, Cleary M. An exploratory study: prolonged periods of binocular stimulation can provide an effective treatment for childhood amblyopia. Invest Ophthalmol Vis Sci. 2012; 53: 817–824 [DOI] [PubMed] [Google Scholar]
- 51. Hess RF, Pointer JS. Differences in the neural basis of human amblyopia: The distribution of the anomaly across the visual field. Vision Res. 1985; 25: 1577–1594 [DOI] [PubMed] [Google Scholar]
- 52. Ding J, Levi DM. Recovery of stereopsis through perceptual learning in human adults with abnormal binocular vision. Proc Natl Acad Sci U S A. 2011; 108: E733–E741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Astle AT, McGraw PV, Webb BS. Recovery of stereo acuity in adults with amblyopia. BMJ Case Rep. 2011; 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cavazos H, Haase W, Meyer E. Ametropic amblyopia. Strabismus. 1993; 1: 63–67 [DOI] [PubMed] [Google Scholar]
- 55. Bonne YS, Sagi D, Polat U. Local and non-local deficits in amblyopia: acuity and spatial interactions. Vision Res. 2004; 44: 3099–3110 [DOI] [PubMed] [Google Scholar]
- 56. Mou T. Logarithmic visual acuity chart and five-score recording. Chinese J Ophthalmol. 1966; 13: 96–106 [Google Scholar]
- 57. Fielder AR, Moseley MJ. Does stereopsis matter in humans? Eye. 1996; 10: 233–238 [DOI] [PubMed] [Google Scholar]
- 58. Halpern DL, Blake RR. How contrast affects stereoacuity. Perception. 1988; 17: 483–495 [DOI] [PubMed] [Google Scholar]
- 59. Hou F, Huang C-B, Liang J, Zhou Y-F, Lu Z-L. Contrast gain control in stereo depth and cyclopean contrast perception. J Vis. 2013; 13: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Legge GE, Gu Y. Stereopsis and contrast. Vision Res. 1989; 29: 989–1004 [DOI] [PubMed] [Google Scholar]
- 61. Schor C, Heckmann T. Interocular differences in contrast and spatial frequency: effects on stereopsis and fusion. Vision Res. 1989; 29: 837–847 [DOI] [PubMed] [Google Scholar]