Abstract
Background
Mucous membrane pemphigoid (MMP) is a subepithelial autoimmune mucocutaneous disease. It most frequently affects the oral mucosa, followed by ocular and nasal mucosa, nasopharyngeal, anogenital, skin, laryngeal and esophageal mucosa.
Main observation
Two half-sisters developed mucous membrane pemphigoid at approximately the same age. The older sister presented with primarily mucosal disease, while the younger had a more cutaneous disease. The histopathology demonstrated a subepithelial blister and direct immunofluorescence showed deposition of IgG and C3 at the basement membrane zone of perilesional tissues in both sisters. Antibodies to human β4 integrin were present in the sera of both patients and correlated with disease activity. Both sisters carried the same HLADQβ1* 0301 allele.
Conclusions
This is the first case of mucous membrane pemphigoid occurring in two half-sisters. Perhaps, it is the low incidence of mucous membrane pemphigoid that may account for the lack of reports on familial cases of the disease.
Keywords: basement membrane zone, dapsone, dysphagia, family, IVIG, pemphigoid, tacrolimus
Introduction
Mucous membrane pemphigoid (MMP) is a subepithelial autoimmune mucocutaneous disease. It most frequently affects the oral mucosa, followed by ocular and nasal mucosa, nasopharyngeal, anogenital, skin, laryngeal and esophageal mucosa.[1] Due to the natural course of the disease, lesions may manifest as intact blisters, erosions or pseudomembranes.[2] Conjunctival lesions typically begin in one eye and may involve the other eye. The development of symblepharon is not uncommon in ocular pemphigoid.[3] Ocular involvement can result in blindness secondary to corneal scarring.[4] Histology shows a subepithelial vesicle, and on direct immunofluorescence (DIF), continuous deposition of IgG, IgA or complement components along the basement membrane zone (BMZ) is observed.[1] MMP targets various autoantigens including α6-integrin,[5] β4-integrin,[6] laminin[5,7] an unidentified 168-kDa mucosa antigen,[8] BPAg1 and BPAg2.[6] The association of HLA-DQβ1*0301 allele may enhance the susceptibility to developing MMP.[9,10] We report two half-sisters with differing clinical profiles of MMP that had autoantibodies to β4-integrin on serological studies.
Case Report
In 1991, a 50-year-old Caucasian female complained of painful erosions in her mouth. Biopsy demonstrated a subepithelial blister. Deposition of IgG and complement at the BMZ was seen on DIF. Salt-slit skin showed that the antibody bound to the roof of the blister. Six years of intermittent systemic corticosteroids therapy did not control her disease. Dapsone produced marginal improvement and was discontinued due to hemolytic anemia. She then complained of dysphagia and erosive and crusted lesions in the nasal mucosa on the lateral sides in both nostrils. The presence of several erosions in the upper one-third of the esophagus on endoscopy confirmed esophageal MMP. She was then treated with oral tacrolimus as an immunosuppressive agent.
The disease progressed to involve the larynx, pharynx, vagina and conjunctiva [Fig. 1]. Early ocular involvement was confirmed by a conjunctival biopsy. Due to the progressive course and lack of response to conventional therapy, IVIg was initiated in June 1997. She was treated according to a published protocol.[11] Disease stabilization was achieved and clinical lesions resolved without scarring. IVIg was discontinued in December 2001. Patient is in complete remission without any systemic therapy. This clinical remission has been sustained for over ten years.
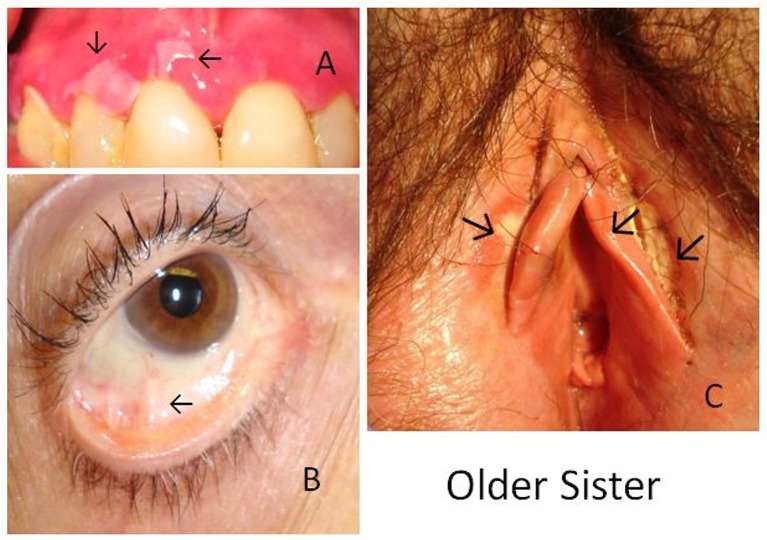
Figure 1.
MMP in older sister. Photographs demonstrate MMP lesions as A) desquamation of the gum, B) early conjunctival involvement and C) erosion of the labia.
In 2007, the patient’s younger half-sister, at age 44, noted recurrent blistering lesions on her face and scalp. The lesions were intact blisters varying in size from 3 mm to 7 mm in diameter. Some lesions were erosions while others were covered with skin debris and thick crusts. Development of new lesions on her foot, nose, and mouth prompted evaluation by a dermatologist. The lesion on her foot was 2.5 cm in diameter and had a thick adherent scab with an underlying erosion. The lesions in the oral cavity consisted of intense erythema of the upper and lower gingiva and erosions on the hard palate [Fig. 2]. Histology demonstrated a subepidermal blister and DIF showed deposition of IgG and C3 at the BMZ. Saltsplit skin demonstrated binding of the antibody to the roof of the blister. Systemic corticosteroids were avoided based on a history of palpitations and hypertension. The patient had an excellent response to dapsone 100 mg daily. She remained on this therapy for 22 months. Dapsone was gradually tapered and discontinued by December 2009. Patient has been in remission for three and a half years without any scarring or recurrence and without any systemic therapy.
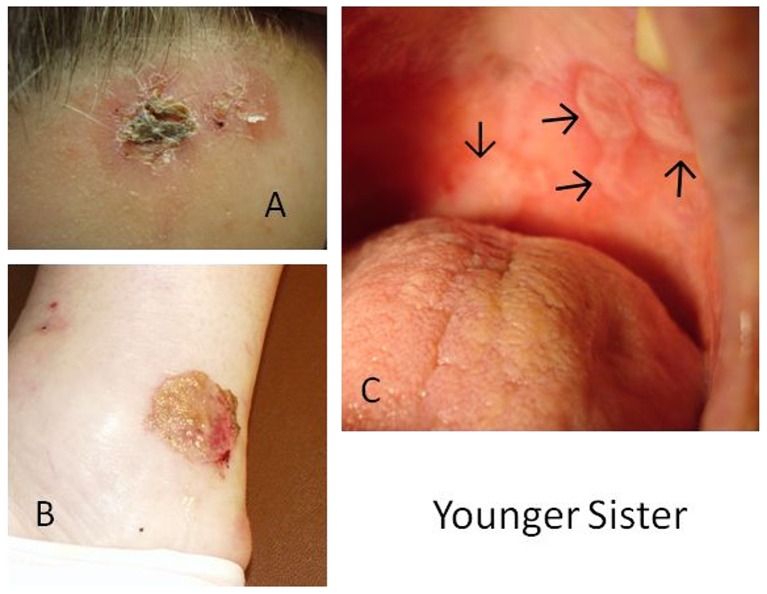
Figure 2.
MMP in younger sister. Photographs demonstrate MMP lesions as A) a flaccid blister on forehead with crust formation as it heals, B) ruptured blister with thick crust formation above the medial malleolus of the right foot, and C) erosive lesions and denuded epithelium of the hard palate.
At time of diagnosis in both sisters, antibodies against β4-integrin subunits were detected by an immunoblot assay described earlier.[12] Antibodies to β4-integrin subunits also correlated with acute clinical flares, but the titer level was not detectable during remission. In the older sister, antibodies to BPAg1 and BPAg2 were detected by ELISA and the levels did not correlate with clinical disease. On periodic and sporadic testing, the older sister has antibodies to BPAg2 (BP180) in absence of any clinical disease. The younger sister did not have any demonstrable antibodies to BPAg1 and BPAg2 on repeated testing during the diagnosis and clinical follow-ups.
Discussion
In this manuscript, the authors describe what may possibly be the first reported case of MMP occurring in two halfsisters who share a common mother but have different fathers. They both developed MMP at approximately the same age. The younger sister had more cutaneous disease while the older sister had primarily mucosal disease. It has been shown that titers of autoantibodies to β4 integrin correlate with clinical severity and decrease as disease improves and become undetectable when patients go into remission.[13] The same observation was made from serological studies of both sisters. Experiments using explants of normal human conjunctival and oral mucosa have demonstrated that human β4-integrin is a possible target antigen recognized by the sera from patients with MMP and may produce subepidermal vesicles.[5,14]
Antibodies to BPAg1 and BPAg2 by ELISA in the older sister did not correlate with disease activity. This observation has been previously reported.[15]
The clinical course was longer and recalcitrant to treatment in the older sister. She eventually responded to IVIg and has remained in clinical remission for over 10 years. Studies have shown that IVIG has the capacity to produce long-term sustained clinical remissions in MMP.[13,16,17] The younger sister had a shorter duration of disease and a rapid and complete response to dapsone therapy. Several studies have demonstrated the benefits of dapsone in MMP.[1,18]
Several studies have demonstrated that mucous membrane pemphigoid and bullous pemphigoid are in linkage disequilibrium with HLA-DQβ1*0301.[9,10]Since both sisters carried the HLA-DQβ1*0301 allele, it is tempting to speculate that the presence of this particular allele may have provided an enhanced susceptibility. In one report on two monozygotic twins both carrying the HLA-DQβ1*0301 allele, only one developed biopsy proven ocular pemphigoid, while the other did not.[19]
The autoantibodies that target BMZ proteins may be either IgG or IgA and thus, MMP is considered a humoral immunemediated disease. Loss of immunological tolerance to proteins in the BMZ results in the production of autoantibodies. Different investigators have used different technologies to demonstrate that these antibodies against BMZ proteins recognize BPAg1, BPAg2, α6, β4, laminin 332, and possibly other yet undiscovered molecules.[2] The severity or the clinical course of the disease cannot be predicted by the antibody- binding profile of any patient’s sera. Clinical followup is the only true mechanism by which prognosis is determined.
Conclusion
MMP is an exceedingly rare disease. The reported incidence is 2 cases per million population in Germany and 1.3 cases per million in France.[4] Perhaps, it is the low incidence of MMP that may account for the lack of reports on familial cases in MMP.
References
- Chan LS, Ahmed AR, Anhalt GJ, Bernauer W, Cooper KD, Elder MJ, Fine JD, Foster CS, Ghohestani R, Hashimoto T, Hoang-Xuan T, Kirtschig G, Korman NJ, Lightman S, Lozada-Nur F, Marinkovich MP, Mondino BJ, Prost-Squarcioni C, Rogers RS 3rd, Setterfield JF, West DP, Wojnarowska F, Woodley DT, Yancey KB, Zillikens D, Zone JJ. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138:370–379. doi: 10.1001/archderm.138.3.370. [DOI] [PubMed] [Google Scholar]
- Xu HH, Werth VP, Parisi E, Sollecito TP. Mucous membrane pemphigoid. Dent Clin North Am. 2013;57:611–630. doi: 10.1016/j.cden.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruch-Gerharz D, Hertl M, Ruzicka T. Mucous membrane pemphigoid: clinical aspects, immunopathological features and therapy. Eur J Dermatol. 2007;17:191–200. doi: 10.1684/ejd.2007.0148. [DOI] [PubMed] [Google Scholar]
- Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- Bhol KC, Dans MJ, Simmons RK, Foster CS, Giancotti FG, Ahmed AR. The autoantibodies to alpha 6 beta 4 integrin of patients affected by ocular cicatricial pemphigoid recognize predominantly epitopes within the large cytoplasmic domain of human beta 4. J Immunol. 2000;165:2824–2829. doi: 10.4049/jimmunol.165.5.2824. [DOI] [PubMed] [Google Scholar]
- Leverkus M, Bhol K, Hirako Y, Pas H, Sitaru C, Baier G, Bröcker EB, Jonkman MF, Ahmed AR, Zillikens D. Cicatricial pemphigoid with circulating autoantibodies to beta4 integrin, bullous pemphigoid 180 and bullous pemphigoid 230. Br J Dermatol. 2001;145:998–1004. doi: 10.1046/j.1365-2133.2001.04543.x. [DOI] [PubMed] [Google Scholar]
- Lazarova Z, Yee C, Lazar J, Yancey KB. IgG autoantibodies in patients with anti-epiligrin cicatricial pemphigoid recognize the G domain of the laminin 5 alpha-subunit. Clin Immunol. 2001;101:100–105. doi: 10.1006/clim.2001.5091. [DOI] [PubMed] [Google Scholar]
- Ghohestani RF, Nicolas JF, Rousselle P, Claudy AL. dentification of a 168-kDa mucosal antigen in a subset of patients with cicatricial pemphigoid. J Invest Dermatol. 1996;107:136–139. doi: 10.1111/1523-1747.ep12298424. [DOI] [PubMed] [Google Scholar]
- Ahmed AR, Foster S, Zaltas M, Notani G, Awdeh Z, Alper CA, Yunis EJ. Association of DQw7 (DQβ1*0301) with ocular cicatricial pemphigoid. Proc Natl Acad Sci USA. 1991;88:11579–11582. doi: 10.1073/pnas.88.24.11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis JJ, Mobini N, Yunis EJ, Alper CA, Deulofeut R, Rodriguez A, Foster CS, Marcus-Bagley D, Good RA, Ahmed AR. Common major histocompatibility complex class II markers in clinical variants of cicatricial pemphigoid. Proc Natl Acad Sci USA. 1994;91:7747–7751. doi: 10.1073/pnas.91.16.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed AR, Dahl MV. Consensus statement on the use of intravenous immunoglobulin therapy in the treatment of autoimmune mucocutaneous blistering diseases. Arch Dermatol. 2003;139:1051–1059. doi: 10.1001/archderm.139.8.1051. [DOI] [PubMed] [Google Scholar]
- Rashid KA, Gürcan HM, Ahmed AR. Antigen specificity in subsets of mucous membrane pemphigoid. J Invest Dermatol. 2006;126:2631–2636. doi: 10.1038/sj.jid.5700465. [DOI] [PubMed] [Google Scholar]
- Letko E, Bhol K, Foster CS, Ahmed AR. nfluence of intravenous immunoglobulin therapy on serum levels of anti-beta 4 antibodies in ocular cicatricial pemphigoid. A correlation with disease activity. A preliminary study. Curr Eye Res. 2000;21:646–654. [PubMed] [Google Scholar]
- Kumari S, Bhol KC, Simmons RK, Razzaque MS, Letko E, Foster CS, Ahmed AR. Identification of ocular cicatricial pemphigoid antibody binding site(s) in human beta4 integrin. Invest Ophthalmol Vis Sci. 2001;42:379–385. [PubMed] [Google Scholar]
- Yeh SW, Usman AQ, Ahmed AR. Profile of autoantibody to basement membrane zone proteins in patients with mucous membrane pemphigoid: long-term follow up and influence of therapy. Clin Immunol. 2004;112:268–272. doi: 10.1016/j.clim.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Sami N, Bhol KC, Ahmed AR. Intravenous immunoglobulin therapy in patients with multiple mucosal involvement in mucous membrane pemphigoid. Clin Immunol. 2002;102:59–67. doi: 10.1006/clim.2001.5150. [DOI] [PubMed] [Google Scholar]
- Ahmed AR. Use of intravenous immunoglobulin therapy in autoimmune blistering diseases. Int Immunopharmacol. 2006;6:557–578. doi: 10.1016/j.intimp.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Gürcan HM, Ahmed AR. Efficacy of dapsone in the treatment of pemphigus and pemphigoid. Am J Clin Dermatol. 2009;10:383–396. doi: 10.2165/11310740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Bhol K, Udell I, Haider N, Yunis JJ, Mohimen A, Neuman R, Grasso C, Ahmed AR, Foster S. Ocular cicatricial pemphigoid. A case of monozygotic twins discordant for the disease. Arch Ophthalmol. 1995;113:202–207. doi: 10.1001/archopht.1995.01100020086034. [DOI] [PubMed] [Google Scholar]