Abstract
Background
Bullous pemphigoid is a cutaneous autoimmune blistering disorder. The etiology for what precipitates this disease is not entirely clear at this point, although it has been associated with certain medications.
Main observation
We describe the case of a 70-year-old male with a past medical history of diabetes type 2 who developed a diffuse eruption of bullae with skin biopsy positive for bullous pemphigoid. He had previously been prescribed sitagliptin 50 mg daily for at least one year prior to onset of his disease. The medication was discontinued and the patient was treated with first IV and then oral steroids with good clinical outcome.
There have been a few reports that have explored the relationship between DPP-IV inhibitors (gliptins) and bullous pemphigoid, including three case series and a report on sitagliptin associated allergic skin reactions submitted to the Adverse Event Reports System database of the FDA. According to the Naranjo ADR probability score there is a "possible" cause and effect relationship for this case.
Conclusion
The enzyme DPP-IV is ubiquitously expressed in almost every organ system, including the skin. The exact mechanism at this time is unknown but is believed to be multifactorial involving many aspects of the immune system. Our case and the findings from our literature review further demonstrate a link between dipeptidyl peptidase-IV inhibitors and the development of bullous pemphigoid.
Keywords: bullous pemphigoid, dipeptidyl peptidase-IV inhibitor
Introduction
Bullous pemphigoid is a cutaneous autoimmune blistering disorder against the hemidesmosome, a part of the basement membrane that attaches the epidermis to the dermis.[1] The etiology for what precipitates this disease is not entirely clear at this point, although it has been associated with certain medications. It most commonly occurs in the elderly, especially ages 70 years and over, and has increased risk for mortality as well as long term morbidity.[2]
Gliptins are a drug class that was first introduced into the market in 2006 to treat diabetes mellitus type 2. They work by competitively inhibiting the enzyme dipeptidyl peptidase IV (DPP-IV/CD26), which normally breaks down incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) that are released in response to a meal. By preventing GLP-1 and GIP inactivation, they are able to increase the secretion of insulin and suppress the release of glucagon by the pancreas.[3] Current medications on the market include sitagliptin, saxagliptin, linagliptin, and alogliptin in the United States, as well as vildagliptin in the UK. Vildagliptin was initially not approved by the FDA due to pre-clinical studies showing skin lesions in monkeys.[4] Since the release of gliptins into the market they have been linked to several skin reactions in humans, most serious of which is Steven Johnson syndrome.[5] The enzyme DDP-IV has been shown to be ubiquitously expressed in almost every organ system, including the skin.[6]
Case Report
We describe the case of a 70-year-old male with a past medical history of diabetes type 2, developmental delay, chronic iron deficiency anemia, and hypertension. He had chronic right-sided weakness secondary to a large left middle cerebral artery stroke one-year prior. He was up to date on routine cancer screening and had no history of malignancy or prior autoimmune disease. The patient had developed a diffuse eruption of bullae largely on his arms, neck, chest, and groin. Many of the bullae were still intact upon presentation to our facility two weeks following its onset. Lesions presented on both erythematous and normal appearing areas of skin. The patient also complained of significant urticaria and had excoriations on his extensor surfaces, however the urticaria had developed after the appearance of the lesions. There was no evidence of mucosal involvement. His home medications included acetaminophen, aspirin, citalopram, docusate sodium, levothyroxine, lisinopril, metformin, oxybutynin, and simvastatin. He had previously been on sitagliptin 50 mg daily for one year prior to onset of the rash, and it was discontinued by a physician four days before transfer to our facility. A complete blood count upon transfer consisted of a white blood count of 8.6 K/μL, hemoglobin 10.5 g/dL, hematocrit 31.6%, and platelets 281,000/mm3. His differential was significant for 9% eosinophils (normal range is 1-4%). Electrolytes and liver function tests were all within normal limits. His Hemoglobin A1C was 7.1%. A skin biopsy was performed and direct immunofluorescent staining revealed a linear staining pattern with complement C3 and IgG at the subepidermal basement membrane zone consistent with bullous pemphigoid. The patient was started on methylprednisolone 60 mg IV every eight hours for 3 days and was transitioned to oral prednisone 60 mg daily (the equivalent of 0.75 mg/kg/day of the patient’s body weight). The initial dosing of methylprednisolone was per the preference of the dermatologist. For the patient’s diabetes, he was continued on his metformin as well as initiated on subcutaneous insulin. There was significant improvement of the rash after 2-3 days and he was discharged on a prednisone. He had sustained remission and no recurrence of the lesions 3 months later at which point his prednisone had been tapered off.
Figure 1.
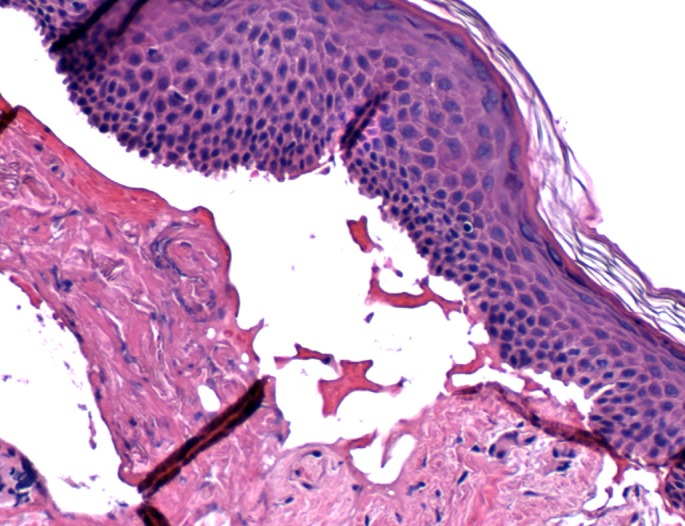
Represents a histopathologic view (H&E staining) of a subepidermal blister taken from skin biopsy of the patient. The entire epithelial layer is detaching from the dermis, known as "lifting off".
Figure 2.
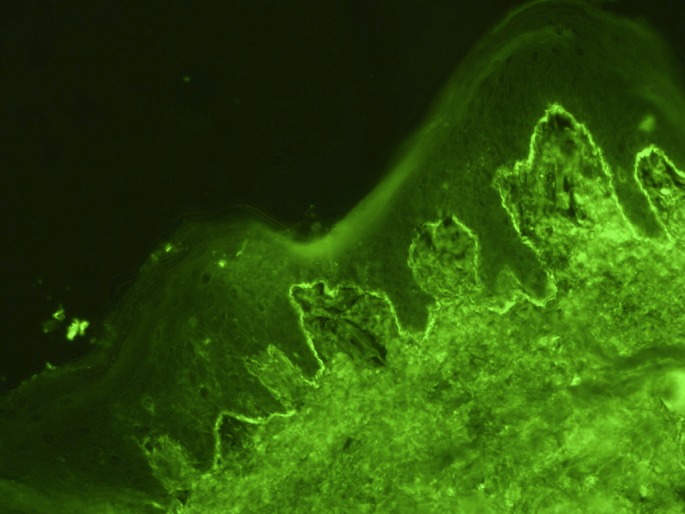
The image represents direct immunofluorescence microscopy of perilesional skin with evidence of linear deposition of IgG. A similar linear deposition was found on immunofluorescent staining of complement (C3).
Figure 3.
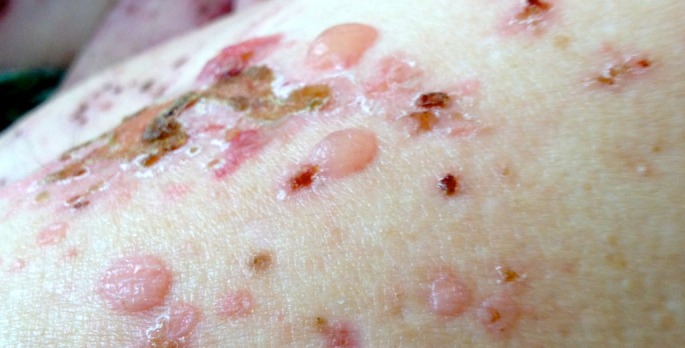
A close up image of bullae on the left upper arm of the patient. Many of the bullae were still intact on presentation, which helped differentiate the rash from the autoimmune cutaneous blistering disorder pemphigus vulgaris.
Review of the literature
There have been four reports that have explored the relationship between dipeptidyl peptidase-IV inhibitors and bullous pemphigoid. The first case series looked at five patients who received a DDP-IV inhibitor (sitagliptin and vildagliptin) as well as metformin who developed bullous pemphigoid with an onset range of 2-13 months. These patients improved after withdrawal of the suspected agent and relatively mild therapeutic intervention.[7]
The second reported two cases of a DDP-IV inhibitor plus metformin causing bullous pemphigoid. Both patients were on vildagliptin, and developed bullous pemphigoid 1 and 3 months after combined therapy, respectively. They responded well to discontinuation of the therapy as well as treatment with steroids.[8]
In the third report, three more cases were reported associated with gliptin usage. Two of the cases were associated with sitagliptin and one with vildagliptin. One of the cases is described in detail as having a striking temporal relationship between starting the gliptin and the subsequent development of bullous pemphigoid, as well as significant improvement with drug cessation and topical corticosteroids.[9]
The fourth report was a study that looked at sitagliptin associated allergic reactions submitted to the Adverse Event Reports System database of the Food and Drug Administration from October 2006 to November 2008, which reported 26 cases of "serious skin reactions", two of which were Steven Johnson syndrome and two of which were toxic epidermal necrosis. The other 22 cases included a mix of dermatologic conditions that were described as "bullous, desquamative, blistering, exfoliative, urticarial, or exanthematous skin reactions". It was not defined what percentage of these represented bullous pemphigoid, but nevertheless emphasizes the relationship between serious skin reactions and gliptins.[5]
It should be noted that in almost all cases the patients were taking metformin concurrently with the DDP-IV inhibitor, as this is a common regimen for treatment of diabetes. However, metformin has been on the market for many years and to date the literature is devoid of metformin-induced cases of bullous pemphigoid.
Table 1. General characteristic of cases (in chronologic order).
| Author | Patient gender & age | Anti-diabetics | [Period before onset of BP] | Diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Attaway et al. (2013) |
M / 70 | Sitagliptin + metformin | 12 mo | DIF: Linear IgG and linear C3 BMZ pattern | Changed anti-hyperglycemic treatment on admission MP 60 mg q8hr IV x 3 days, then prednisone 0.75 mg/kg bw/day with tapering after stable remission |
Sustained remission after the sitagliptin was discontinued at 2 mo follow up |
| Aouidad et al. (2013) |
M / 61 | Vildagliptin + metformin | 6 mo | DIF: Linear IgG and linear C3 BMZ pattern Indirect IF positive | Topical corticosteroid (clobetasol propionate) on a 15-wk tapering regimen | Disappearance of the pruritus 1 wk after medication treatment halted Sustained remission at 2 mo follow up |
| M / 93 | Sitagliptin + gliclazide | 6 mo | Indirect IF negative * | Topical corticosteroid (clobetasol propionate) 2 months after first lesions appeared | Refractory disease under topical treatment and sitagliptin therapy for 10 months Partial remission 2 wk after stopping sitagliptin treatment |
|
| M / 76 | Sitagliptin + metformin | 5 mo | Indirect IF negative* | Topical corticosteroid (clobetasol propionate) | Delayed improvement of the eruption with topical treatment after 6 mo with persisting pruritus | |
| Skandalis et al. (2012) |
F / 78 | Vildagliptin + metformin | 13 mo | DIF: Linear IgG and linear C3 BMZ pattern Indirect IF positive |
MP: 0.5 mg∕kg bw∕day, followed by MTX: 0.1 mg ∕ kg bw ∕ week with MP tapering after induction of stable remission Delayed change of anti- hyperglycaemic treatment (after discharge) |
Sustained remission at 384 mg prednisone equivalent cumulative dose Refractory disease with slow tapering of MP |
| F / 80 | Sitagliptin + metformin | 4 mo | DIF: Linear BMZ C3 pattern Indirect IF positive |
MP: 0.5 mg/kg bw/day, followed by MTX: 0.1 mg/kg bw∕week MP tapering after induction of stable remission Delayed change of anti- hyperglycaemic treatment (before discharge) |
Sustained remission induced at 115 mg prednisone equivalent cumulative dose Rash reduction of MP at 4 mg/day within 6 weeks |
|
| F / 72 | Vildagliptin + metformin | 8 mo | DIF: negative Indirect IF positive |
Change of anti-hyperglycaemic treatment on admission Topical mometasone furoate 0.1% cream treatment only |
Relapsed course under systemic corticosteroid pulses during a 4 mo period before admission Sustained remission within the first wk (6 days) after withdrawal of gliptin with topical treatment only |
|
| M / 67 | Vildagliptin + metformin | 10 mo | DIF: Linear IgG and linear BMZ C3 pattern Indirect IF negative |
Change of anti-hyperglycemic treatment on admission MP: 0.5 mg/kg bw/day MP tapering after induction of stable remission |
Relapsed course under interrupted glucocorticoid treatment courses during a 6 mo period prior to admit Sustained remission 5 days after withdrawal of gliptin under treatment with MP (at cumulative dose 192 mg prednisone equivalent) MP tapering to 4 mg/day without relapse |
|
| M / 75 | Vildagliptin + metformin | 2 mo | DIF: Linear IgG and linear BMZ C3 pattern Indirect IF positive |
Changed anti-hyperglycemic treatment on admission MP: 0.5 mg/kg bw/day MTX: 0.1 mg/kg bw/week MP tapering after induction of stable remission |
Gliptin treatment withdrawal 1 week before onset of anti-BP treatment with partial remission without anti-BP treatment at admission Sustained BP remission under quickly tapered MP schedule (complete remission at 115 mg cumulative prednisone equivalent dose) |
|
| Pasmatzi et al. (2011) # |
F / 59 | Vildagliptin + metformin | 2 mo | DIF positive | 0.5 mg/kg/day methylprednisolone on an 8-week tapering scheme | Complete remission was achieved 10 weeks after the vildagliptin / metformin was discontinued |
| M / 67 | Vildagliptin + metformin | 2 mo | DIF positive | 200 mg/day doxycycline for a period of 4 weeks | Complete remission was achieved 8 weeks after the vildagliptin / metformin was discontinued | |
| * It was implied but not explicitly stated that a skin biopsy was performed with linear IgG and BMZ C3 pattern. # It was stated that histologic and immunofluorescence patterns from skin biopsy were consistent with BP but the exact studies done were not commented on. | ||||||
Table 2. Summary of data.*.
| Number of patients | 11 |
| DPP-IV inhibitor | 64% Vildagliptin (7/11) 36% Sitagliptin (4/11) |
| Sex (M/F) | 64% M (7/11) 36% F (4/11) |
| Average age (yrs) | 72 (+/- 11) |
| Period before onset of Bullous pemphigoid (in months) | 6 (+/- 8) |
| Treatment (topical vs oral steroid vs other) | Topical: 55% (6/11) Oral: 36% (4/11) Other: 9% (1/11) |
| Outcome (Sustained or not) |
Sustained: 55% (6/11) Refractory: 45% (5/11) Refractory until gliptin stopped: 27% (3/11) |
| *The average is taken to be the median +/- the inter quartile range. | |
Discussion
The gliptins are a relatively new class of drug and have been known to cause adverse skin reactions as reviewed above. According to the Naranjo ADR probability scale, this patient’s use of sitagliptin suggests that this is a "possible" reaction.[10] The patient did have other additional risk factors that have been independently associated with risk for bullous pemphigoid. According to a 2007 prospective case control study this patient’s other risk factors included his age, low mini-mental state examination (severe cognitive impairment), and history of prior stroke. It should be noted that gliptins were introduced into the market in 2006 and would have not been included in this study.[11] The other medications that the patient had been prescribed have not been strongly associated with drug induced bullous pemphigoid. Classic drugs associated with drug-induced BP include spironolactone, chloroquine, furosemide, and penicillin antibiotics.[1,12,20]
While an association has been established between the dipeptidyl peptidase-IV inhibitors and bullous pemphigoid, the exact etiology has not been clearly defined. Dipeptidyl peptidase IV, also known as CD26, is a cell surface glycoprotein with intrinsic enzyme activity that is ubiquitously expressed throughout the body including the skin, but is also expressed in the brain, heart, intestine, kidney, lung, and lymphocytes. Decreased levels of DPP-IV enzyme has been found to correlate with disease severity of autoimmune diseases such as: Rheumatoid arthritis, systemic lupus erythematous, inflammatory bowel disease, granulomatosis with polyangiitis (formerly Wegener’s), and eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss).[13]
Inhibition of DPP-IV by gliptins could promote eosinophil activation in the skin by a CCL11/exotaxin-mediated mechanism.[7,14] Eosinophil activation is known to contribute significantly to blister formation in bullous pemphigoid.[1,2,14] Decreased levels of DPP-IV enzyme has also been associated with increased levels of transforming growth factor beta-1 (TGF beta 1) in T cells leading to its extracellular secretion.[11] TGF beta 1 has previously been demonstrated to be elevated in bullous pemphigoid in the serum and not the blister fluid[16,19]; it's role in the serum has been postulated as a part of the Th3 regulatory component of the autoimmune reaction that inhibits further production of inflammatory cytokines like interferon gamma and interleukin 2.[15-17] The DPP-IV receptor, on the other hand, is felt to play an important role in the Th1 component of immunity.[13] Bullous pemphigoid has previously demonstrated a combination of Th1, Th2, and Th3 mediated immunity through the measurement of its various cytokines. The gliptin induced inhibition of the DDP-IV/CD26 mediated Th1 component of immunity, as well as the gliptin-induced increase in TGF beta 1 through Th3 regulatory mechanisms could promote a more balanced Th1, Th2, and Th3 cytokine profile, which is what bullous pemphigoid has consistently been shown to demonstrate.[17-18]
Conclusion
Our case and the findings from our literature review further demonstrate a link between dipeptidyl peptidase-IV inhibitors and the development of bullous pemphigoid. The exact mechanism at this time is unknown but is believed to be multifactorial involving many aspects of the immune system. Due to the morbidity and mortality associated with bullous pemphigoid, this link can help providers make more informed decisions on their own patients’ medical regimens and avoid adverse drug reactions in at risk populations.
References
- Kasperkiewicz M, Zillikens D. The pathophysiology of bullous pemphigoid. Clin Rev Allergy Immunol. 2007;33:67–77. doi: 10.1007/s12016-007-0030-y. [DOI] [PubMed] [Google Scholar]
- Culton DA. Chapter 56. Bullous Pemphigoid. In: Fitzpatrick's Dermatology in General Medicine (Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K), 8th edn. McGraw-Hill; 2012. [Google Scholar]
- Herman GA, Bergman A, Liu F, Stevens C, Wang AQ, Zeng W, Chen L, Snyder K, Hilliard D, Tanen M, Tanaka W, Meehan AG, Lasseter K, Dilzer S, Blum R, Wagner JA. Pharmacokinetics and pharmacodynamic effects of the oral DPP-4 inhibitor sitagliptin in middle-aged obese subjects. J Clin Pharmacol. 2006;46:876–886. doi: 10.1177/0091270006289850. [DOI] [PubMed] [Google Scholar]
- European Medical Agency. EMA monkey study report. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human /000807/WC500030597.pdf. Accessed September 2, 2013.
- Desai S, Brinker A, Swann J, Iyasu S. Sitagliptin-associated drug allergy: review of spontaneous adverse event reports. Arch Intern Med. 2010;170:1169–1171. doi: 10.1001/archinternmed.2010.188. [DOI] [PubMed] [Google Scholar]
- Thielitz A, Ansorge S, Bank U, Tager M, Wrenger S, Gollnick H, Reinhold D. The ectopeptidases dipeptidyl peptidase IV (DP IV) and aminopeptidase N (APN) and their related enzyme as possible targets in the treatment of skin diseases. Front Biosci. 2008;13:2364–2375. doi: 10.2741/2850. [DOI] [PubMed] [Google Scholar]
- Skandalis K, Spirova M, Gaitanis G, Tsartsarakis A, Bassukas ID. Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol Venereol. 2012;26:249–253. doi: 10.1111/j.1468-3083.2011.04062.x. [DOI] [PubMed] [Google Scholar]
- Pasmatzi E, Monastirli A, Habeos J, Georgiou S, Tsambaos D. Dipeptidyl peptidase-4 inhibitors cause bullous pemphigoid in diabetic patients: report of two cases. Diabetes Care. 2011;34:e133. doi: 10.2337/dc11-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouidad I, Fite C, Marinho E, Deschamps L, Crickx B, Descamps V. A case report of bullous pemphigoid induced by dipeptidyl peptidase-4 inhibitors. JAMA Dermatol. 2013;149:243–245. doi: 10.1001/jamadermatol.2013.1073. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- Bastuji-Garin S, Joly P, Lemordant P, Sparsa A, Bedane C, Delaporte E, Roujeau JC, Bernard P, Guillaume JC, Ingen-Housz-Oro S, Maillard H, Pauwels C, Picard-Dahan C, Dutronc Y, Richard MA. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol. 2011;131:637–643. doi: 10.1038/jid.2010.301. [DOI] [PubMed] [Google Scholar]
- Fellner MJ. Drug-induced bullous pemphigoid. Clin Dermatol. 1993;11:515–520. doi: 10.1016/0738-081x(93)90159-a. [DOI] [PubMed] [Google Scholar]
- Ohnuma K, Hosono O, Dang NH, Morimoto C. Dipeptidyl peptidase in autoimmune pathophysiology. Adv Clin Chem. 2011;53:51–84. doi: 10.1016/b978-0-12-385855-9.00003-5. [DOI] [PubMed] [Google Scholar]
- Forssmann U, Stoetzer C, Stephan M, Kruschinski C, Skripuletz T, Schade J, Schmiedl A, Pabst R, Wagner L, Hoffmann T, Kehlen A, Escher SE, Forssmann WG, Elsner J, von Hörsten S. Inhibition of CD26/ dipeptidyl peptidase IV enhances CCL11/eotaxin-mediated recruitment of eosinophils in vivo. J Immunol. 2008;181:1120–1127. doi: 10.4049/jimmunol.181.2.1120. [DOI] [PubMed] [Google Scholar]
- Caproni M, Calzolari A, Salvatore E, Giomi B, Volpi W, D'Agata A, Santucci M, Fabbri P. Cytokine profile and supposed contribution to scarring in cicatricial pemphigoid. J Oral Pathol Med. 2003;32:34–40. doi: 10.1034/j.1600-0714.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Ameglio F, D'Auria L, Bonifati C, Ferraro C, Mastroianni A, Giacalone B. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: relationships with disease intensity. Br J Dermatol. 1998;138:611–614. doi: 10.1046/j.1365-2133.1998.02169.x. [DOI] [PubMed] [Google Scholar]
- D'Auria L, Cordiali Fei P, Ameglio F. Cytokines and bullous pemphigoid. Eur Cytokine Netw. 1999;10:123–134. [PubMed] [Google Scholar]
- Giomi B, Caproni M, Calzolari A, Bianchi B, Fabbri P. Th1, Th2 and Th3 cytokines in the pathogenesis of bullous pemphigoid. J Dermatol Sci. 2002;30:116–128. doi: 10.1016/s0923-1811(02)00067-1. [DOI] [PubMed] [Google Scholar]
- Giacalone B, D'Auria L, Bonifati C, Ferraro C, Riccardi E, Mussi A, D'Agosto G, Cordiali-Fei P, Ameglio F. Decreased interleukin-7 and transforming growth factor-beta1 levels in blister fluids as compared to the respective serum levels in patients with bullous pemphigoid. Opposite behavior of TNF-alpha, interleukin-4 and interleukin-10. Exp Dermatol. 1998;7:157–161. doi: 10.1111/j.1600-0625.1998.tb00317.x. [DOI] [PubMed] [Google Scholar]
- Bastuji-Garin S, Joly P, Picard-Dahan C, Bernard P, Vaillant L, Pauwels C, Salagnac V, Lok C, Roujeau JC. Drugs associated with bullous pemphigoid. A case-control study. Arch Dermatol. 1996;132:272–276. [PubMed] [Google Scholar]


