Abstract
TAML activators promise a novel water treatment approach by efficiently catalysing peroxide-based degradation of chemicals of high concern at environmental concentrations. Green design ethics demands an exploration of TAML toxicity. Exposure to high concentrations of certain activators caused adverse effects in zebrafish. At typical TAML operational concentrations, development was not perturbed.
Introduction
Endocrine disruptors (EDs) are of tectonic significance to sustainability. EDs typically display non-monotonic dose-response behavior and at low doses are often able to impair cells and animals by altering the hormonal control of development.1 Certain active pharmaceutical ingredients, pesticides, flame retardants, polymer consituents including monomers and additives, and other everyday chemicals are now known to be EDs. Three strategies are apparent for coping with EDs: regulate, replace, remediate. The latter two fall into the province of green chemistry.
Recently, the ability of chemists to design against EDs took a major step forward upon the publication in this journal of the Tiered Protocol for Endocrine Disruption (TiPED).2 The five-tier TiPED is an essential instrument for combating EDs. It gives chemists a living road map for evaluating the endocrine disrupting behavior of chemicals to the highest standards of contemporary science. It can both enable the avoidance of new EDs and guide the development of replacements for existing EDs. However, some EDs are of such surmised high value that it is hard to imagine society would willingly relinquish them. For example, birth control chemicals such as 17α-ethinylestradiol (EE2) bring perceived benefits that include the long-term sustainability advantages of population control. Unfortunately, EE2 is found today in natural waters where this powerful ED can feminize fish at low parts per trillion.3 Thus, while the first order call of green chemistry is for chemical products and processes that are safe by design, EE2 underscores that ecotoxicity often derives from precisely the same chemical properties that endow a molecule with enticing technical performance. In these cases, the sustainability challenge to chemists translates into finding a green treatment process for post factum application as an essential second implement for minimizing risks associated with those EDs that society deems necessary.
TAML activators (Fig. 1) are emblematic of developing green approaches to water treatment. These miniaturized peroxidase replicas are known to catalyse peroxide processes under mild conditions giving the rapid, oxidative decomposition of water-borne contaminants of concern among which are many persistent EDs. Examples include, inter alia, pentachlorophenol, 2,4,6-trichlorophenol, thiophosphate pesticides, the drug Sertraline, and the estrogens 17α and β-estradiol, estrone, and EE2.4–7
Fig. 1.
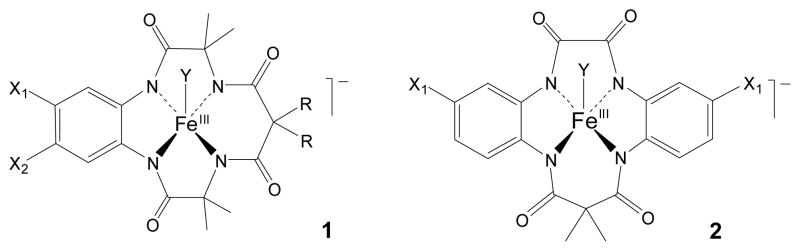
First (1) and second (2) generation TAML activators used in this study (Y=H2O). 1a: X1=X2= H, R= CH3; 1b: X1=X2= Cl, R= F; 1c: X1= NO2, X2= H, R= F; 2a: X1=X2= H; 2b: X1=X2= Cl; 2c: X1= CN, X2= H; 2d: X1=NO2, X2= H.
Given the goal of green water treatment, we consider it essential that TAML activators, their degradation products, and all other aspects of the catalysed processes should be examined for and made free of endocrine disruption by design. To this end, initial work on three TAML activators in collaboration with Bruce Blumberg has yielded negative estrogen, androgen, and thyroid receptor activation in cellular assays (TiPED Tier 2). In this study, we extend the search for potential low dose toxicity endpoints associated just with exposures to TAML catalysts (and not yet the decomposition products or process solutions) via in vivo zebrafish assays (TiPED Tier 4). The zebrafish is an emerging model for the assessment of developmental toxicity and human health.8–10 Utilizing this model for Tier 4 tests yields expanded opportunities for exploring a diversity of toxicity endpoints which may or may not result from ED processes.
Results and Discussion
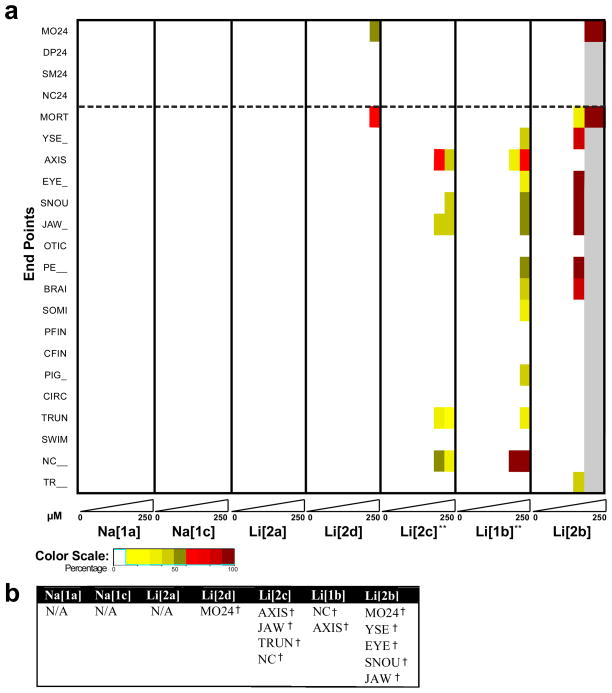
The embryonic zebrafish model was used to assess the developmental toxicity of seven TAML activators (1a-c, 2a-d). Dechorionated embryos were statically exposed to seven concentrations of each activator (0 – 250 μM) and evaluated for developmental malformations at 24 and 120 hours post fertilization (hpf). Only embryos that survived were accounted for in malformation statistics.
At 24 hpf, embryos were evaluated for mortality (MO24), developmental progression (DP24), spontaneous movement (SM24), and notochord distortion (NC24). In addition, embryos were screened at 120 hpf for 18 endpoints. Individual embryos were scored for mortality (MORT), then euthanized and evaluated for abnormal morphology. The embryos that survived were evaluated for fifteen morphological malformations: yolk sac edema (YSE), bent body axis (AXIS), eye (EYE), snout (SNOU), jaw (JAW), otic (OTIC), pericardial edema (PE), brain (BRAI), somite (SOMI), pectoral fin (PFIN), caudal fin (CFIN), circulation (CIRC), pigmentation (PIG), trunk length (TRUN), swim bladder (SWIM), notochord distortion (NC), and alterations in touch response (TR).
Lower concentration exposure (0.08, 0.4, and 2μM)
All of the TAML activators induced toxic responses that were similar to background incidences within this concentration.
Higher concentration exposure (10, 50, and 250μM)
Most of the observed mortality resulted from higher concentration exposure and occurred within the first 24 hpf. Only two activators induced prominent (>30% incidence) embryo lethality: Li[2b] at 50 and 250 μM (100% mortality at 24 hpf) and Li[2d] at 250 μM (50% mortality at 24 hpf for a total of 90% mortality at 120 hpf).
Four activators, Na[1a], Na[1c], Li[2a], Li[2d], induced low incidences of morphological malformations. Only two TAML activators were observed to cause notochord distortion (Fig. 3b): Li[1b] and Li[2c] at 50 and 250 μM. This malformation was not observed in any of the controls (Fig. 3a) and did not result from exposure to any of the other five activators. Li[1b]also induced prominent malformations of the body axis, snout and jaw, as well as pericardial edema, but only at 250 μM. No malformation data could be gathered for Li[2b] at 50 and 250 μM (100% mortality). However at 10 μM, this activator caused 100% incidence of malformations of the eye, snout, and jaw as well as pericardial edema in addition to a 75% incidence of yolk sac edema and brain malformations. In addition to impacts on the body axis discussed above, Li[2c]induced prominent developmental malformations of the jaw at 50 and 250 μM and of the body axis, jaw and trunk at 50 μM. Touch response malformations resulted only from exposure to Li[2b] at 10 μM.
Fig. 3.
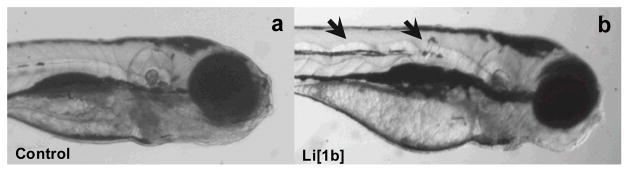
Representative image of a 120 hpf embryonic zebrafish (a) control, (b) following exposure to 250μMLi[1b] where the arrows highlight the notochord malformation.
LC50 values (the concentrations of compounds causing mortality in 50% of the sample) and/or EC50 values (the concentrations of compounds eliciting adverse effects including mortality and any malformations in 50% of the sample) were computed (Table 1). Na[1a], Na[1c], and Li[2a] did not cause a 50% incidence of malformations or mortalities therefore an LC50 or EC50 was not computable. LC50 values could be computed for only two of the seven TAML activators. Li[2b] was more toxic than Li[2d] with LC50 values of 26.7 and 235 μM, respectively. EC50 values for Li[2d], Li[2c], Li[1b], and Li[2b] are 245, 25.6, 32, and 4.5 μM. It is noteworthy that both EC50 and LC50 values for these four activators are greater than those concentrations typically employed in TAML processes (<2 μM).
Table 1.
LC50 and EC50 values generated by the Probit analysis of the data collected over 6 exposure concentrations.
| Compound | LC50 (μM) | EC50 (μM) |
|---|---|---|
| Na[1a] | - | - |
| Na[1c] | - | - |
| Li[2a] | - | - |
| Li[2d] | 235 | 245 |
| Li[2c] | - | 25.6 |
| Li[1b] | - | 32 |
| Li[2b] | 26.7 | 4.5 |
- Signifies that the Probit analysis was not applicable.
Nitro- and chloro-aromatics
Nitro- and chloro-aromatics are often associated with toxicity that is also structure dependent. Nitro groups appear to induce minimal toxicity compared with chloro groups in the studied series and the observed toxicities are markedly structure dependent. We consider that this emphasizes the importance of the TiPED approach for unraveling subtle structure-activity toxicity relationships. Indeed, the two aromatic chlorine substituents make 1b significantly more toxic than the singly nitrated 1c. The most extensively chlorinated catalyst 2b is clearly the most toxic of the entire tested set with an LC50 of 26.7 μM and an EC50 of 4.5 μM, ~20 fold more toxic than Li[2d]. This study suggests a tentative toxicity ordering for the aromatic substituents of Cl > CN > NO2. However, more structures with these substituents would need to be analysed to assess whether this represents general functional group effects or specific molecular manifestations of toxicity for TAML activators.
Conclusions
The results of this work clearly show that TAML activators are not toxic to zebrafish at concentrations ≤2 μM. Since water treatment is likely to employ TAML activator concentrations at the lower end of the 0.01 – 1 μM range, these results are encouraging. As Fig. 2a illustrates, catalyst structural features determine higher dose adverse effects making it clear that an interplay between TAML catalyst design and zebrafish toxicity feedback has potential for guiding the green design of these singular peroxidase mimics. This work highlights the vital need for interdisciplinary collaborations between chemists and toxicologists and emphasizes the need for the TiPED analytical design guide. By both utilizing an in vivo assessment platform and being able to precisely control the structural features of TAML activators, we are developing a collaborative platform for informing the design of homogenous green oxidation catalysts.
Fig. 2.
Biological responses of embryonic zebrafish after exposure to TAML catalysts. A) Heatmap depicting the response of embryos exposed to each activator at 6 different concentrations. At each concentration, four endpoints were evaluated at 24 hpf and 18 endpoints at 120 hpf. The grey bars shown for activator 2bindicate that at those concentrations (50, 250 μM), no viable embryos were available to evaluate the 17 remaining endpoints (those other than mortality). **Denotes compounds that induced notochord distortions. B) A table showing statistical significance and dose-dependent morphological malformations determined by logistic regression and denoted with an †(p<0.001).
Experimental
General procedure for sample preparation
A sample of each TAML activator (10 mg) was dissolved in ultrapure water (1 mL) and diluted with the appropriate volume of embryo medium (EM) to give a 5 mM working solution. Standard EM consisted of 15 mM NaCl, 0.5 mM KCl, 1mM CaCl2, 1mM MgSO4, 0.15 mM KH2PO4, 0.05 mM Na2HPO4 and 0.7 mM NaHCO3.13 Serial dilutions of the working solution were made less than 1 hour prior to loading the embryos into each well of a 96 well plate.
General procedure for the toxicity assessment
Waterborne exposure: At 4 hours post fertilization (hpf), the chorions were enzymatically removed as described in Truong et al. (2011).14 At 6 hpf, the embryos were transferred into individual wells of a 96-well plate containing 100 μL of a prepared solution. Exposure plates were sealed to prevent evaporation and wrapped with aluminum foil to reduce light exposure. Embryos (n=12, two replicates per concentration of TAML) were exposed to [TAML] of 0.08, 0.4, 2, 10, 50, and 250 μM, as well as both a negative control ([TAML] = 0 μM) and a positive control ([trimethyltin chloride] = 5 μM) to examine the embryonic responsiveness.
Acknowledgments
The Oregon State authors would like to thank the staff at Sinnhuber Aquatic Research laboratory for the embryos. These studies were partially supported by National Institute of Environmental Health Sciences (NIEHS), R01 ES016896, P30 ES000210, P42 ES016465, and F31 ES019445. Carnegie Mellon authors thank the Heinz Endowments for generous support and SK thanks the R. K. Mellon Foundation for a Presidential Fellowship.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/b000000x/
Footnotes should appear here. These might include comments relevant to but not central to the matter under discussion, limited experimental and spectral data, and crystallographic data.
Contributor Information
Lisa Truong, Email: tc1u@andrew.cmu.edu.
Robert L. Tanguay, Email: Robert.Tanguay@oregonstate.edu.
Notes and references
- 1.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, David JR, Jr, Lee D-H, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Endocrine Reviews. 2012:33. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schug TT, Abagyan R, Blumberg B, Collins TJ, Crews D, Defur PL, Dickerson SM, Edwards TM, Gore AC, Guillette LJ, Hayes T, Heindel JJ, Moores A, Patisaul HB, Tai TL, Thayer KA, Vandenberg LN, Warner JC, Watson CS, vom Saal FS, O’Brien KP, Myers JP. Green Chem. 2013;15:181–198. doi: 10.1039/C2GC35055F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW. Proc Natl Acad Sci. 2007:8897–8901. doi: 10.1073/pnas.0609568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta SS, Stadler MJ, Noser CA, Ghosh A, Steinhoff B, Lenoir D, Horwitz CP, Schramm KW, Collins TJ. Science. 2002;296:326–328. doi: 10.1126/science.1069297. [DOI] [PubMed] [Google Scholar]
- 5.Chanda A, Khetan SK, Bannerjee D, Ghosh A, Collins TJ. J Am Chem Soc. 2006;128:12058–12059. doi: 10.1021/ja064017e. [DOI] [PubMed] [Google Scholar]
- 6.Shen LQ, Beach ES, Xiang Y, Tshudy DJ, Khanina N, Horwitz CP, Bier ME, Collins TJ. Environ Sci Technol. 2011;45:7882–7887. doi: 10.1021/es201392k. [DOI] [PubMed] [Google Scholar]
- 7.Shappel NW, Vrabel MA, Madsen PJ, Harrington G, Billey LO, Hakk H, Larsen GL, Beach ES, Horwitz CP, Ro K, Hunt PG, Collins TJ. Environ Sci Technol. 2008;42:1296–1300. doi: 10.1021/es7022863. [DOI] [PubMed] [Google Scholar]
- 8.McCollum CW, Ducharme NA, Bondesson M, Gustafsson JA. Birth defects research Part C, Embryo today: reviews. 2011;93:67–114. doi: 10.1002/bdrc.20210. [DOI] [PubMed] [Google Scholar]
- 9.Truong L, Tilton SC, Zaikova T, Richman E, Waters KM, Hutchison JE, Tanguay RL. Nanotoxicology. 2013;7:192–201. doi: 10.3109/17435390.2011.648225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eimon PM, Rubinstein AL. Expert opinion on drug metabolism & toxicology. 2009;5:393–401. doi: 10.1517/17425250902882128. [DOI] [PubMed] [Google Scholar]
- 11.Jin EJ, Thibaudeau G. Biochimica et biophysica acta. 1999;1449:93–99. doi: 10.1016/s0167-4889(98)00176-1. [DOI] [PubMed] [Google Scholar]
- 12.Stachel SE, Grunwald DJ, Myers PZ. Development (Cambridge, England) 1993;117:1261–1274. doi: 10.1242/dev.117.4.1261. [DOI] [PubMed] [Google Scholar]
- 13.Westerfield M. The Zebrafish Book. University of Oregon Press; Eugene, OR: 1999. [Google Scholar]
- 14.Truong L, Moody IS, Stankus DP, Nason JA, Lonergan MC, Tanguay RL. Archives of Toxicology. 2011;85:787–798. doi: 10.1007/s00204-010-0627-4. [DOI] [PMC free article] [PubMed] [Google Scholar]