Abstract
Stress is known to trigger seizures in patients with epilepsy, highlighting the physiological stress response as a possible therapeutic target for epilepsy treatment. Nevertheless, little is currently known about how a genetic predisposition to epilepsy interacts with the stress response to influence seizure outcome. To address this question, we examined the effect of acute stress on seizure outcome in mice with mutations in the voltage-gated sodium channel (VGSC) gene Scn8a. Scn8a mutants display spontaneous spike-wave discharges (SWDs) characteristic of absence epilepsy. We saw that the baseline frequency of SWDs in Scn8a mutants correlates closely with the diurnal activity of the hypothalamic-pituitary-adrenal (HPA) axis, with a peak in seizure activity occurring at around the same time as the peak in corticosterone (1700h–1900h). A 20-minute acute restraint stress administered in the morning increases the frequency of spontaneous SWDs immediately following the stressor. Seizure frequency then returns to baseline levels within three hours after stressor exposure, but the subsequent evening peak in seizure frequency is delayed and broadened, changes that persist into the next evening and are accompanied by long-lasting changes in HPA axis activity. Scn8a mutants also show increased anxiety-like behavior in mildly stressful situations. A 20-minute acute restraint stress can also increase the severity and duration of chemically induced seizures in Scn8a mutants, changes that differ from wild-type littermates. Overall, our data show that a voltage-gated sodium channel mutation can alter the behavioral response to stress and can interact with the stress response to alter seizure outcome.
Keywords: Scn8a, Stress, Epilepsy, Anxiety, Absence, Seizures
Introduction
Seizures are paroxysmal events, suggesting the presence of endogenous or environmental triggering factors. Epilepsy patients self-report stress as being the most common trigger for seizure onset (Spatt et al., 1998; Haut et al., 2003; Nakken et al., 2005; Haut et al., 2007), an assertion further supported by controlled studies that establish a relationship between stress and increased seizure frequency (Feldman and Paul, 1976; Swinkels et al., 1998). In at least one study, the exacerbating effects of stress on seizure incidence was especially apparent in patients with typical absence seizures (Bosnjak et al., 2002), a subtype of epilepsy characterized by a brief loss of awareness associated with bursts of generalized synchronous spike-wave discharges (SWDs). Although stress and seizures are consistently linked, the physiological mechanisms that connect stress and seizure incidence or severity are not fully understood.
The acute stress response involves activation of the hypothalamic-pituitary-adrenal (HPA) axis, which results in the sequential release of corticotropin-releasing factor (CRF), adrenocorticotropic hormone (ACTH), and corticosterone (CORT). Receptors for both CRF and CORT can be found in numerous brain areas, such as the hippocampus and amygdala, suggesting these stress mediators can directly influence neuronal activity and excitability in limbic regions that are also important for seizure activity (Joels and Baram, 2009). In addition to stress, anxiety levels predict changes in seizure frequency (Thapar et al., 2009), and hyperemotionality may underlie the majority of behavioral problems in epilepsy (Frucht et al., 2000). Furthermore, patients with epilepsy have higher incidences of depression, anxiety, and even psychosis compared to the general population (Garcia-Morales et al., 2008). HPA axis function modulates activity in limbic regions, and dysfunction of the HPA axis is implicated in a number of affective disorders. Therefore, it has been hypothesized that the HPA axis could mediate both stress-induced seizures and behavioral alterations in seizure models (Heinrichs, 2010). Given the bidirectional relationship between hyperemotionality, stress, and epilepsy, a genetic mouse model of epilepsy would provide a unique opportunity for investigating the stress response and affective-like behaviors and may provide important insight into the pathophysiological effects of stress on seizure incidence and severity.
A de novo gain-of-function point mutation in SCN8A was recently identified in a individual who exhibited generalized seizures and sudden unexplained death in epilepsy (SUDEP) (Veeramah et al., 2012). The SCN8A gene encodes the α-subunit of the Nav1.6 voltage-gated sodium channel (VGSC). VGSCs are transmembrane complexes that facilitate the initiation and propagation of transient depolarizing currents and electrical signaling between cells in the nervous system. Nav1.6 is the most widely expressed VGSC in the central nervous system, including areas involved in the stress response such as the hippocampus and hypothalamus (Schaller et al., 1995; Krzemien et al., 2000). Nav1.6 plays a major role in the transmission of subthreshold currents, namely persistent current and resurgent current (Raman et al., 1997), and the electrophysiological properties of Nav1.6 make these channels especially suited for the sustained repetitive firing of neurons (Van Wart and Matthews, 2006), a key feature in many neuronal circuits. In addition to its role in seizure activity, a two-bp deletion in SCN8A was found to co-segregate with motor and cognitive deficits in a small pedigree comprising six affected members (Trudeau et al., 2006). Furthermore, findings from two association studies point to possible roles for SCN8A in emotional instability, suicide, and bipolar disorder (Wasserman et al., 2005; Wang et al., 2008).
Today there are several mouse models of Scn8a dysfunction. Null mutants, such as the Scn8amed mouse, are the most severe, with homozygous Scn8amed/med mice showing muscle weakness, progressive paralysis, and premature death (Burgess et al., 1995). In contrast, heterozygous Scn8amed/+ mice exhibit normal gross motor behavior and a normal lifespan. Interestingly, the Scn8amed/+ mice have increased resistance to chemically induced seizures (Martin et al., 2007), but exhibit frequent SWDs characteristic of absence epilepsy (Supplemental Figure 1A) (Papale et al., 2009). Other Scn8a mutants, such as Scn8amed-jo and Scn8a8J, also display spontaneous SWDs (Papale et al., 2009), and greater resistance to chemically induced seizures and hippocampal kindling have been seen in Scn8amed-jo and Scn8atg mutants, respectively (Martin et al., 2007; Blumenfeld et al., 2009). Increases in both anxiety-like and depressive-like behaviors have been reported in the Scn8atg mutant (McKinney et al., 2008), while our lab previously found only minor changes in anxiety-like behavior in the Scn8amed-jo mutant (Papale et al., 2010). Scn8a mutants, therefore, provide the opportunity to investigate the consequences of altered sodium channel function on the stress response and behavior, as well as the interaction between stress and spontaneous and induced seizures.
The goal of this study was to investigate the interaction between acute stress and epilepsy using the Scn8amed/+ mouse to answer these four questions: (i) Does acute stress alter the frequency of spontaneous absence seizures? (ii) Does acute stress affect seizure thresholds? (iii) Does sodium channel dysfunction affect HPA axis function? and (iv) Does sodium channel dysfunction alter anxiety-like or depressive-like behaviors?
Methods and Materials
Subjects
Scn8amed/+ mice were purchased from The Jackson Laboratories (Bar Harbor, ME) and maintained on the C3HeB/FeJ background. Genotyping was performed on tail DNA using The Jackson Laboratory protocol. Male mice, 3–4 months old, were used for all experiments. Wild-type (WT) littermates were used as controls for all experiments. Mice were group-housed after weaning in ventilated cages under uniform conditions with a 12/12 h light/dark cycle with lights on at 0700h and lights off at 1900h. Food and water were available ad libitum. All behavioral tests and all stressors were administered between the hours of 0800h and 1100h to minimize variation due to circadian factors and were performed in rooms with lighting of approximately 1,000 lux. All experiments were approved by the Emory University Institutional Animal Care and Use Committee (IACUC).
Acute stressor
Mice were placed into an adequately ventilated clear polypropylene restrainer (50-mL conical tubes measuring 9.7 cm in length with an internal diameter of 2.8 cm) for 20 min. Breathing was carefully observed to ensure the animals were not compressed.
EEG analysis of spontaneous seizure activity
Under isoflurane anesthesia, mice were surgically implanted with electroencephalogram (EEG) and electromyography (EMG) electrodes for seizure monitoring as previously described (Papale et al., 2010). Following 7 d of recovery, the animals were singly housed in Plexiglas boxes (20×20×30 cm3), and a microconnector was attached to a series of bioelectric amplifiers (Stellate Harmonie software, Natus Medical Inc., California, USA) via a small counterbalanced commutator (Dragonfly Research, West Virginia, USA). Following 24 h of habituation, continuous EEG and EMG data were collected for a 48-h baseline period. The amplified signals were digitally acquired, collected, and processed by Stellate. Data were manually analyzed for the number and duration of spike-wave discharges (SWDs), the electroencephalographic feature of absence epilepsy. SWDs were defined by a rhythmic oscillation with a burst frequency of 7–9 Hz lasting at least 0.5 s, with an amplitude at least twice that of the background. To examine circadian variation, SWDs were grouped into 2-h intervals and averaged within each interval over day 1 and day 2 of the baseline recordings. Following the baseline recording, the seizure response to acute restraint stress was determined in a subset of animals by placing animals into modified restraint tubes (50-mL conical plastic tube with a 1.5×1.5 cm2 hole cut out for EEG headcap placement) for 20 min. Continuous EEG and EMG data were collected for the 20-min restraint period, as well as for the 24-h period following restraint (post-restraint, PR). To examine the response to stress, SWDs were analyzed in 2-h intervals for the 24-h PR period. The first 3-h PR period was also analyzed by further sub-dividing SWD occurrence into 20-min bins. The total time the animals spent struggling while in the tube was determined by the presence of EMG activity during the 20-min restraint period. A separate group of 8 Scn8amed/+ mutants was used to determine how long post-stress changes in SWD activity persisted. These animals were implanted with EEG electrodes and were recorded for 48 h of baseline, followed by a 20-min restraint stress and 5 d PR.
Chemiconvulsant seizure induction
Mice were brought into the procedure room 2 h prior to the experiment for acclimation. Half of the mice were subjected to an acute 20-min restraint stress just prior to injection, while the other half served as unstressed controls. Mice were injected intraperitoneally (IP) with either 5 mg/kg picrotoxin (Sigma) or 30 mg/kg kainic acid (Sigma). Both drugs were dissolved in 0.9% saline, and all mice were injected at a volume of 10 mL/kg. Picrotoxin-injected mice were observed for 1 h, and kainic acid-injected mice were observed for 2 h post-injection. Mice were scored for seizure latencies and progression according to modified Racine scales (Racine, 1972) described as follows. Picrotoxin is a noncompetitive GABAA receptor antagonist that induces seizures by reducing overall inhibition (Velisek, 2006). Picrotoxin-induced seizure activity was scored based on the following criteria recommended for seizures induced by disruption of the GABAergic system (Veliskova, 2006): 0) No behavioral response, 0.5) Abnormal behavior (freezing, staring, orientation problems), 1) Isolated myoclonic jerks, 2) Atypical clonic seizure, 3) Fully developed bilateral forelimb clonus, 3.5) Forelimb clonus with a tonic component and twist of the body, 4) Generalized tonic-clonic seizure (GTCS) with a suppressed tonic phase, and 5) Fully developed GTCS with hindlimb extension. Kainic acid is a specific agonist for the glutamatergic kainate receptor and induces seizures by increasing excitability in limbic regions (Velisek, 2006). Kainic acid-induced seizure activity was scored based on the following recommended criteria (Veliskova, 2006): 0) No behavioral response, 1) Head nodding and/or staring with mouth clonus, 2) Automatisms (excessive washing, sniffing, rearing, scratching, circling, orientation problems), 3) Unilateral forelimb clonus, 4) Bilateral forelimb clonus, 5) Bilateral forelimb clonus with rearing and falling, 6) GTCS.
Diurnal corticosterone profile
Mice were single-housed a week prior to the experiment. Blood was collected at 0700h and 1900h in order to coincide with the expected nadir and peak of circulating corticosterone (CORT) levels. In a separate group of animals, the effect of a morning stressor on evening CORT levels was measured. Animals were single-housed for one week prior to the experiment. During the first experimental week, blood was collected at 1900h to establish baseline 1900h CORT levels. A week later, blood was drawn from each animal between 0830h and 0840h for a basal measurement prior to a 20-min restraint stress, and then again at 1900h for a post-stress evening CORT measurement. For all CORT measurements, blood was obtained within 1 min of cage disturbance. Blood was collected from the facial vein into Microvette CB 300 Z tubes (Starstedt) and then allowed to clot for 1 h at room temperature. The serum was then separated by centrifugation at 5600 rpm for 15 min at 4°C and stored at −80°C until analysis. Serum CORT levels for the diurnal blood draws were assayed using a commercial radioimmunoassay (RIA) kit (MP Biomedicals) according to the manufacturer’s instructions.
Corticosterone response to acute stress
Mice were subjected to a 20-min acute restraint stress. At six different time points (once before the stressor (baseline) and at 5, 30, 45, 60, and 120 min following the 20-min restraint stress) one group of animals was anesthetized with isoflurane, decapitated, and their trunk blood was collected into Microvette CB 300 Z tubes (Starstedt). The blood was allowed to clot for 1 h at room temperature, and then separated by centrifugation at 5600 rpm for 15 min at 4°C and stored at −80°C until analysis. Measurement of the serum CORT levels in response to an acute stressor was conducted by the Yerkes National Primate Research Center Biomarkers Core Lab (Atlanta, GA) using a commercially prepared kit (Siemens).
Predator odor stress
Predator odor exposure occurred in an enclosed transparent PVC box (30x30x30 cm3) fitted with a small shelf to hold a single piece of filter paper. Synthetic fox feces odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT, Contech, Victoria, Canada), was diluted 1:10 in ethanol. The mice were single-housed for one week prior to testing. Animals were brought into a procedural holding room 2 h prior for acclimation. Animals were transported one at a time to the testing room. On the first day, animals were placed into the control box, and 35 μL of vehicle (ethanol) was placed on a small piece of filter paper. The filter paper was placed on the ledge inside the box, the box was closed, and the animal was observed and videotaped for 15 min. Forty-eight h later, the animal was placed into the odor box, and 35 μL of diluted TMT was placed on the filter paper. The animal was again observed and videotaped for 15 min. After an additional 48-h period, the animal was returned to the odor box, and 35 μL of vehicle was again placed on the filter paper to determine if repeated exposure to the same environment affected freezing levels. The animal was observed and videotaped a third time. Videotapes were later scored by an observer blind to genotype and condition. Mice were scored for total freezing time (defined as the lack of movement, except for respiration and heart beat) and number of rearing occurrences.
Novelty stress
The mice were single-housed for one week prior to testing. Animals were brought into the procedure room 2 h prior to testing for acclimation. To measure response to novelty, animals were placed into a new standard mouse cage (30×17 cm2) containing only corncob bedding. Animals were observed and videotaped for 15 min. Videotapes were later scored by an observer blind to genotype. Mice were scored for grooming and exploratory behaviors (rearing and stretch-attends) by counting the number of individual occurrences of these behaviors.
Forced swim stress
The forced swim apparatus is composed of a 4-L glass beaker (24 cm height × 18 cm diameter) filled three quarters full with water. Water temperature was maintained at 25 ± 1.5°C. Animals were brought into the procedure room 2 h prior to testing for acclimation. Mice were placed individually into the cylinder for 10 min and videotaped. Videotapes were later scored by an observer blind to genotype. Latency to first immobility and the total durations of immobility and struggling during the test period were scored. A mouse was judged to be immobile when making only those movements necessary to keep its head above water. Struggling was defined as vigorous movements with forepaws breaking the surface of the water.
Statistics
Data are reported as mean ± standard error of the mean (SEM). A two-tailed t-test was used to compare WT and Scn8amed/+ mice for the total numbers of SWD, average durations of SWD, total time spent in SWD activity, absolute change in SWD frequency following restraint stress, average latency to first SWD following stress, baseline CORT prior to stress, amount of struggle, amount of grooming, number of stretch-attends, and number of rears. Parametric data sets with 2 or more groups/factors to be compared were analyzed using two-way analysis of variance (ANOVA). In experiments where all of the groups/conditions contained different sets of animals and there was only one measurement per animal (CORT response to acute stress; induced seizure thresholds) a two-way ANOVA was used. In experiments where there were multiple data values from a single animal (multiple time points in the 24-h rhythm of SWD; 0700h and 1900h in the diurnal CORT profile; baseline SWD and SWD response to acute stress over multiple time points; predator odor response over three test days) a two-way repeated measures ANOVA (rANOVA) was used. Following the ANOVA analyses, the Tukey pairwise comparison test was used to further distinguish among groups. Nonparametric data (Racine scores) were analyzed using the Mann-Whitney Rank Sum test. Dichotomous data (presence/absence of GTCS) were analyzed using Fisher’s Exact test. All results were considered statistically significant if p<0.05.
Results
Scn8amed/+ mice have frequent spontaneous SWDs
The Scn8amed line is maintained on a C3HeB/FeJ background, and wild-type (WT) animals from this background strain exhibit low levels of spontaneous spike-wave discharges (SWDs) (Frankel et al., 2005). Nevertheless, Scn8amed/+ mice have significantly more SWDs in a 24-h period than the WT littermates as we have previously reported (Papale et al., 2009), and the average duration of the SWDs exhibited by the mutants is longer than that of the WT littermates (Supplementary Fig. 1B–C). Consequently, Scn8amed/+ mice spend a significantly longer time engaged in SWD activity over a 24-h period than the WT littermates (Supplementary Fig. 1D).
Acute stress increases SWD frequency in Scn8amed/+ mice
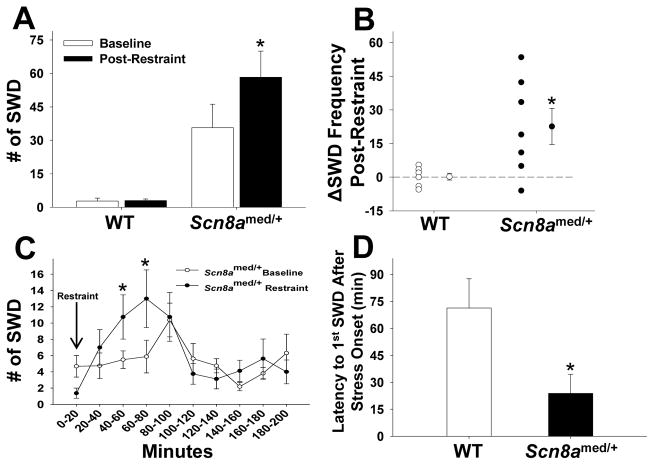
Scn8amed/+ mutants and WT littermates were restrained for 20 min while simultaneous EEG recordings were obtained. A statistically significant increase in SWD activity in the 2-h period immediately following the acute stress exposure was seen only in the mutants (Fig. 1A). A closer look at the absolute change in SWD activity for each animal (ΔSWD Frequency = SWDpost-restraint − SWDbaseline) over this 2-h period shows that the Scn8amed/+ mice had a significantly larger change than the WT littermates (Fig. 1B). To better characterize the temporal effect of stress on SWD activity in the mutants, the 3-h period following the restraint stress was subdivided into 20-min bins. Following an initial suppression of SWD activity while the animals were in the restraint tubes (0–20 min), there was a significant increase in SWD activity between 40 and 80 min after the onset of the stressor (Fig. 1C). Given the appearance of an initial suppression of SWD activity, we determined the average latency to the first absence seizure following stress exposure and found Scn8amed/+ mice have a significantly shorter latency, 24 min, to the first SWD event compared to WT littermates (Fig. 1D).
Figure 1. SWD activity in response to a 20-minute acute restraint stress.
(A) Scn8amed/+ mice have significantly more SWDs in the 2-hour period following restraint stress compared to the same 2-hour period at baseline, while WT mice show no increase in number of SWDs following restraint stress (genotype main effect: F(1,12)=17.969, p<0.01; stress main effect: F(1,12)=7.7.36, p<0.05; genotype x stress interaction effect: F(1,12)=7.448, p<0.05). *p<0.05 vs. Baseline within genotype, Tukey post hoc test. (B) Scn8amed/+ mice have a significant change in SWD frequency after restraint stress compared to WT mice: t(12)=−2.729, *p<0.05. (C) SWD activity in Scn8amed/+ mice in 20-minute intervals beginning with the restraint stress (min 0–20) and ending 3 hours after the end of the stressor. Following an initial suppression of SWD activity while in the restraint tube, Scn8amed/+ mice show a significant increase in SWDs 40 to 80 minutes post-restraint (time main effect: F(9,63)=3.818, p<0.001; time x stress interaction effect: F(9,63)=2.619, p<0.05). *p<0.05 vs. Baseline, Tukey post hoc test. (D) After the onset of a stressor, Scn8amed/+ mice show a shorter latency to the first post-stress SWD than WT mice: t(11)=6.321, *p<0.001. Error bars represent SEM. n=6–8 per group.
Acute stress affects SWD rhythm in Scn8amed/+ mice for up to 60 hours after the stress exposure
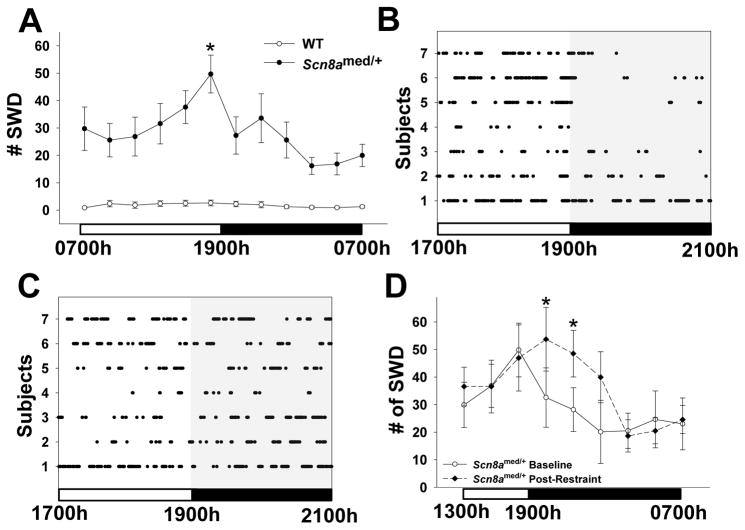
Upon analyzing SWD frequency during 24 h of continuous baseline recordings, we observed that the Scn8amed/+ mice, but not WT littermates, have a 24-h diurnal rhythm of absence seizures, with a significant peak of SWD activity between 1700h–1900h, coinciding with the end of the light period (Fig. 2A). As illustrated in a dot plot showing individual SWDs experienced by each of the 7 mice from 1700h to 2100h, this peak in SWD activity was followed by a sharp decrease in baseline SWD activity at 1900h (Fig. 2B). Interestingly, the decrease in SWD activity at 1900h was not seen the evening of the same day that a 20-min acute restraint stress was administered during the morning (0800h–1000h) (Fig. 2C). To examine this finding in more detail, post-restraint SWD frequency in Scn8amed/+ mice was analyzed in 2-h intervals from 1300h to 0700h. Analysis showed the mice that experienced restraint stress displayed an altered SWD pattern, with the peak of seizure activity extending beyond 1700h–1900h, resulting in increased SWD activity from 1900h–2300h (Fig. 2D). As the morning restraint was performed between 0800h–1000h, these changes in SWD activity occurred almost 12 h following stress exposure. We examined a separate group of animals to determine how long these changes persisted. We found that the evening SWD rhythm was still disrupted 36 h post-restraint, and did not return to the normal baseline pattern until the third evening, 60 h post-restraint (data not shown).
Figure 2. Long-lasting SWD response to acute stress.
(A) Scn8amed/+ mice show a 24-hour rhythm in SWD frequency when analyzed in 2-hour intervals, with the peak in activity occurring between 1700h–1900h (genotype main effect: F(1,16)=19.866, p<0.001; time main effect: F(11,176)=4.990, p<0.001; genotype x time interaction effect: F(11,176)=4.054, p<0.001). *p<0.05 vs. other time points except 1500h–1700h within genotype, Tukey post hoc test. (B, C) Dots represent individual SWDs by Scn8amed/+ mice from 1700h until 2100h during both a baseline period (B) and the evening following a morning exposure to acute restraint stress (C). The sharp drop-off of SWD activity at 1900h disappears following the morning restraint stress. (D) The morning restraint stress alters the evening rhythm of SWD activity in Scn8amed/+ mice by shifting and broadening the peak from 1700h–1900h to 1900h–2300h (time main effect: F(8,48)=4.241, p<0.001: time x stress interaction effect: F(8,48)=2.524, p<0.05). *p<0.05 vs. Baseline, Tukey post hoc test. Error bars represent SEM. n=7–8 per group.
Acute stress reduces latencies to and increases severity of picrotoxin-induced and kainic acid-induced seizures in Scn8amed/+ mice
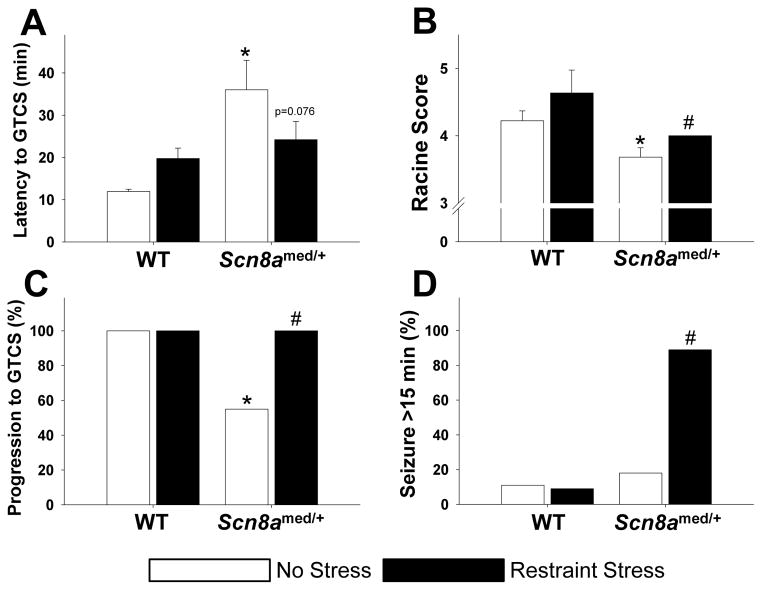
To determine the effect of acute stress on seizure thresholds, we first subjected Scn8amed/+ mice and WT littermates to a 20-min acute restraint stress immediately before injection with picrotoxin (5 mg/kg, IP). We had previously established that, under normal conditions, Scn8amed/+ mice have elevated thresholds to generalized tonic-clonic seizures (GTCSs) induced by flurothyl and reduced seizure severity in response to kainic acid (Martin et al., 2007). Consistent with this finding, under unstressed conditions the mutants exhibited increased latency to picrotoxin-induced GTCS (Fig. 3A, white bars) and reduced seizure severity compared to WT littermates (Fig. 3B, white bars). Furthermore, consistent with our previous findings, significantly fewer mutants in the unstressed group progressed to a GTCS when compared to WT littermates (Fig. 3C, white bars).
Figure 3. Effect of stress on picrotoxin-induced seizures.
(A) Under normal conditions, Scn8amed/+ mice have increased picrotoxin-induced seizure thresholds compared to WT littermates (genotype main effect: F(1,36)=9.761, p<0.01). However, while restraint stress increases thresholds in WT mice, it decreases thresholds in Scn8amed/+ mice (genotype x stress interaction effect: F(1,36)=4.594, p<0.05). *p<0.05 vs. WT littermates within condition, Tukey post hoc test. (B) Exposure to restraint stress increases seizure severity as measured by the Racine score, but the increase is only significant in Scn8amed/+ mice: Mann-Whitney, U=21, *p<0.05 vs. WT within condition; Mann-Whitney, U=27, #p<0.05 vs. No Stress within genotype. (C) Under normal conditions, fewer Scn8amed/+ mice progress to a GTCS than WT mice, but restraint stress significantly increases the percentage of Scn8amed/+ mice progressing to a GTCS: Fisher Exact, *p<0.05 vs. WT within condition; Fisher Exact, #p<0.05 vs. No Stress within genotype. (D) Acute restraint stress results in an increase in the percent of Scn8amed/+ mice experiencing seizures lasting longer than 15 minutes: Fisher’s Exact, #p<0.01 vs. No Stress within genotype. Error bars represent SEM. n=9–11 per group.
However, acute restraint stress affected GTCS latencies differently in Scn8amed/+ mice than in WT littermates (Fig. 3A). While the latency in WT animals increased, the latency in Scn8amed/+ mice decreased, resulting in a significant stress-genotype interaction (F(1,36)=4.594, p<0.05). Although the magnitude of the change was not statistically significant in either genotype, there was a trend towards decreased latency in the Scn8amed/+ mice (Tukey, p=0.076). Furthermore, acute stress significantly increased the severity of the seizures in the Scn8amed/+ mice, while the increase in the WT animals was not significant (Fig. 3B), and stress increased the number of Scn8amed/+ mice that progressed to the GTCS stage (Fig. 3C). Stress also increased the percentage of Scn8amed/+ mice having seizures lasting longer than 15 min, whereas acute stress did not affect seizure duration in WT mice (Fig. 3D).
To measure the effect of stress on thresholds to seizures induced by a different mechanism, mice were subjected to 20-min acute restraint stress immediately prior to injection with kainic acid (30 mg/kg, IP). We were able to replicate our previous findings of increased latency to a GTCS in unstressed Scn8amed/+ mice (Supplementary Fig. 2C, white bars) and decreased seizure severity in unstressed mutant mice (Supplementary Fig. 2D, white bars) compared to WT littermates following kainic acid administration (Martin et al., 2007). We were also able to show that the unstressed mutants have an increased latency to Stage 3 seizure activity (unilateral forelimb clonus) compared to unstressed WT littermates (Supplementary Fig. 2B, white bars).
Following acute stress, there was a significant effect of stress on both Stage 2 (automatisms) and Stage 3 (unilateral forelimb clonus) seizure latencies. Stress significantly reduced the latency to Stage 2 in Scn8amed/+ mice (Supplementary Fig. 2A) and showed a trend for reducing the latency to Stage 3 in the mutants (Supplementary Fig. 2B). However, acute stress affected neither the latency to Stage 6 (GTCS) nor the Racine score for seizure severity (Supplementary Figs. 2C and 2D). Overall, these data show that acute restraint stress worsens seizure outcome by decreasing seizure latency and increasing seizure severity in Scn8amed/+ mice following administration of a chemiconvulsant, although stress had more of an effect on picrotoxin-induced seizures than on kainic acid-induced seizures. In both cases, stress affected mutants differently than WT littermates.
Scn8amed/+ Mice have normal HPA axis activity
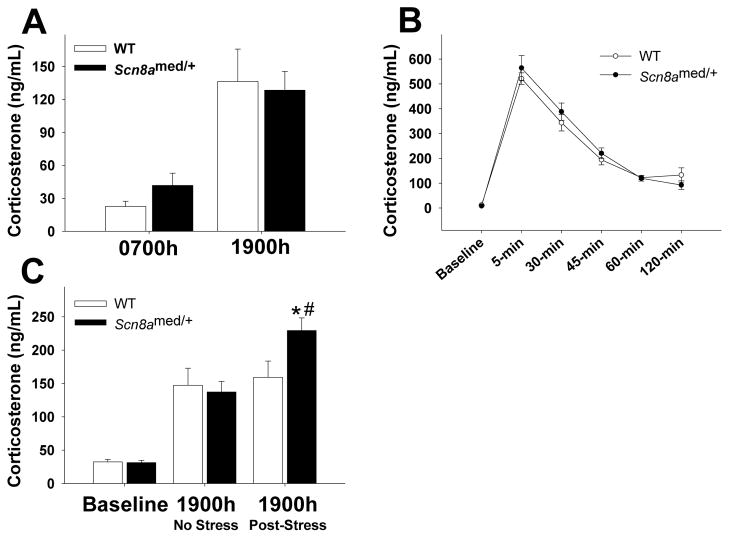
We examined the diurnal HPA axis activity by measuring corticosterone (CORT) levels at the beginning of the light (0700h) and dark (1900h) phases. Both mutants and WT littermates have an intact diurnal variation in CORT (Fig. 4A). We tested the HPA axis response to an acute stressor by measuring CORT responses at baseline and at 5 different time points following an acute 20-min restraint stress. Stress exposure in both genotypes resulted in an immediate surge in CORT levels, followed by a gradual decline over the next 2 h (Fig. 4B). Thus, Scn8amed/+ mice display a normal HPA axis response to an acute stressor, as well as normal negative feedback regulation to terminate the stress response.
Figure 4. HPA axis function.
(A) Snc8amed/+ mice have normal diurnal fluctuations in plasma corticosterone levels, with similar corticosterone levels as WT mice during both the nadir (0700h) and peak (1900h) of the circadian HPA axis rhythm (time main effect: F(1,16)=34.011, p<0.001). (B) Plasma corticosterone levels before (baseline) and at 5, 30, 45, 60, and 120 min following a 20-minute restraint stress. There were no differences in the HPA axis response between Scn8amed/+ mice and WT mice (time main effect: F(5,130)=100.337, p<0.001). (C) Although there are no basal differences in plasma corticosterone levels prior to the stressor (baseline) between genotypes (t(20)=0.216, p=0.831), morning exposure to acute restraint stress increases corticosterone levels at 1900h in Scn8amed/+ mice, but not in WT littermates (stress main effect: F(1,20)=14.505, p<0.01; stress x genotype interaction effect: F(1,20)=8.605, p<0.01). *p<0.05 vs. WT within Post-Stress condition and #p<0.05 vs. No Stress condition within genotype, Tukey post hoc test. Error bars represent SEM. n=9–12 per group.
Acute stress affects HPA axis circadian activity in Scn8amed/+ mice for up to 12 hours after the stress exposure
To determine whether acute restraint stress could have a long-term effect on HPA axis circadian activity, we first sampled blood from a group of animals at 1900h to establish the normal CORT baseline levels. A week later, we took a basal blood draw immediately before subjecting each mouse to a 20-min acute restraint stress that occurred between 0800h–0900h, followed by a final blood draw at 1900h of the same day. Consistent with the data presented in Fig. 4B, we found no differences in basal CORT levels between Scn8amed/+ mice and their WT littermates (Fig. 4C). However, following the restraint stress, the 1900h CORT levels in the Scn8amed/+ mice were significantly higher than both the baseline 1900h levels in Scn8amed/+ mice and the post-stress 1900h levels in the WT littermates (Fig. 4C).
Scn8amed/+ mice show increased anxiety-like behavior
The first behavioral difference we observed was that Scn8amed/+ mice struggled significantly less while in the restraint tubes than WT littermates (Fig. 5A). This result prompted us to further examine the behavior of Scn8amed/+ mice in other behavioral tasks. We exposed mice to predator odor using the synthetic fox feces compound TMT dissolved in ethanol. Analysis of freezing showed that, while both genotypes exhibited increased freezing in the presence of the predator odor, the Scn8amed/+ mice froze significantly more than the WT littermates (Fig. 5B). There were no differences in freezing behavior between genotypes in the absence of the predator odor (Fig. 5B). Examination of rearing, a characteristic exploratory behavior in rodents that should decrease in situations of perceived danger, revealed that both mutants and WT mice reared significantly less in the presence of the odor as expected (Fig. 5C). Interestingly, in the no-odor (vehicle) condition, the Scn8amed/+ mice reared significantly less than the WT littermates, suggesting reduced exploratory behavior in the mutants (Fig. 5C).
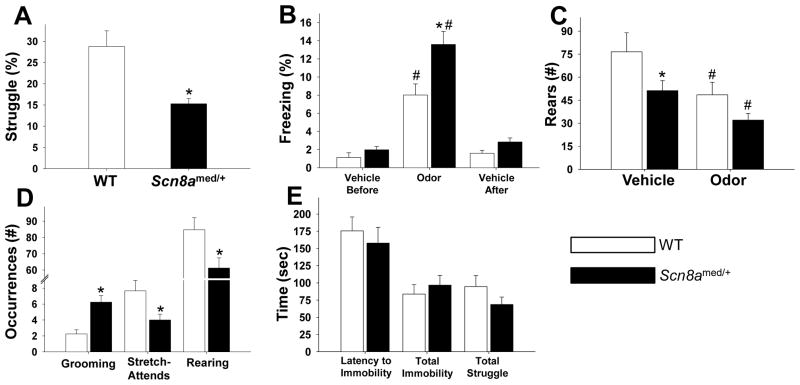
Figure 5. Behavior in stressful situations.
(A) Scn8amed/+ mice struggled less in the restraint tubes during the 20-minute period of restraint stress compared to WT littermates: t(12)=3.504, *p<0.01. (B) In response to predator odor exposure, both Scn8amed/+ mice and WT mice showed increased freezing, but the Scn8amed/+ mice froze significantly more than their WT littermates (genotype main effect: F(1,24)=10.447, p<0.01; odor exposure main effect: F(2,48)=88.992, p<0.001; genotype x odor exposure interaction effect: F(2,48)=5.729, p<0.01). *p<0.05 vs. WT within condition and #p<0.05 vs. Vehicle (before and after) within each genotype, Tukey post hoc test. (C) Under control conditions, Scn8amed/+ mice rear less than WT mice, and exposure to predator odor significantly reduces rearing in both genotypes (odor exposure main effect: F(1,24)=21.454, p<0.001); trend for genotype main effect: F(1,24)=3.803, p=0.063). *p<0.05 vs. WT within condition and #p<0.05 vs. Vehicle within genotype, Tukey post hoc test. (D) In response to novelty exposure, Scn8amed/+ mice show increased grooming and reduced exploratory behaviors (stretch-attends and rearing) compared to their WT littermates (grooming: t(22)=−4.119, *p<0.001; stretch-attends: t(22)=2.454, *p<0.05; rearing: t(22)=2.432, *p<0.05). (E) There were no differences between Scn8amed/+ mice and WT mice in forced swim test measures. Error bars represent SEM. n=7–13 per group.
To further characterize exploratory behavior, animals were next placed into a novel environment and observed for grooming and exploratory behaviors. Increased grooming has been linked to increased levels of anxiety, as grooming is a displacement response in stress-inducing situations (Espejo, 1997). Scn8amed/+ mice were observed to engage in significantly more grooming episodes than the WT littermates (Fig. 5D). Scn8amed/+ mice also exhibit fewer stretch-attends, a risk-assessment behavior used by a rodent in exploration of a novel environment (Choleris et al., 2001), than WT littermates (Fig. 5D). Scn8amed/+ mice also reared significantly less than WT littermates, in agreement with observations during the vehicle condition of the predator odor experiment (Fig. 5D). Taken together, the reduced struggling, increased freezing, increased grooming, and decreased exploratory activities indicate a higher level of anxiety in the Scn8amed/+ mice.
We also measured depressive-like behavior using the Porsolt forced swim test and found no significant differences between Scn8amed/+ mice and WT littermates in any of the measures tested (Fig. 5E), indicating that Scn8amed/+ mice do not exhibit depressive-like behaviors as measured by this test.
Discussion
The present study provides important new information linking a specific monogenic mutation to diurnal seizure activity and demonstrating the effects of an acute stressor on the diurnal rhythm of seizures. Specifically, the principal findings of the present study are: (1) Acute stress can have both short- and long-term effects on spontaneous absence seizure frequency, (2) Acute stress reduces seizure thresholds and increases the severity of chemically induced seizures in Scn8amed/+ mice, but not in WT littermates, (3) Acute stress affects HPA axis diurnal rhythm in Scn8amed/+ mice, but not in WT littermates, and (4) The Scn8amed/+ mutation is sufficient to increase anxiety-like behaviors in response to mildly stressful situations. In contrast to much of the previous research on the relationship between stress and epilepsy that mostly used wild-type animal models with normal brain and stress system development (Sawyer and Escayg, 2010), the current study demonstrates that an inherited sodium channel mutation can alter the seizure response to stress. Furthermore, because sodium channels play a major role in the regulation of neuronal excitability, and altered neuronal excitability is believed to underlie a number of neurological and neuropsychiatric disorders, findings from this study may have broader implications for understanding the impact of stress on disease processes.
Interestingly, a brief, 20-minute restraint stress in the morning resulted in a transient increase in SWD activity, yet also had long-lasting effects on the circadian rhythm of SWD activity. At the transition from the light to the dark period on the same day as the stressor, we found that Scn8amed/+ mice had an extended period of elevated SWD activity lasting well into the evening, an altered rhythm that did not resume its normal pattern until 60 hours following the stress exposure. In humans, daily stressful events and perceived stress levels are reported to be strongly associated with seizures over both the short-term and up to 24 hours later (Temkin and Davis, 1984), consistent with our findings. Wistar Albino Glaxo rats from Rijswijk (WAG/Rij), another animal model of absence epilepsy, also demonstrate a baseline 24-hour rhythm in SWD occurrence, with a maximal peak of seizure activity at the end of the light phase (Smyk et al., 2011), consistent with our results. However, the current study is the first to describe the long-term effects of acute stress on SWD activity in an animal model of absence epilepsy. Discovery of this long-term effect now gives us the opportunity to investigate how stress works to prime the epileptic brain for seizure activity long after the stressor has passed.
Interestingly, the diurnal SWD activity in our mice matches the diurnal HPA axis activity: we saw a peak in SWD activity from 1700h–1900h corresponding with increased CORT levels at 1900h. Furthermore, the time course of HPA axis activity after an acute stressor follows a similar pattern as the SWD activity, with both responses increasing soon after stress exposure and returning to baseline about two hours later. While an argument could be made that the increased seizure activity is contributing to an increase in HPA axis activity, our results are more consistent with the converse relationship of increased HPA axis activity leading to increased SWD activity. The elevation in CORT levels following the acute stress precedes the increase in seizure activity by 20 minutes or more, suggesting that the increase in HPA axis activity precedes the short-term increase in SWD activity. In a study with WAG/Rij rats, direct injections of CORT increased SWD activity 15–30 minutes after injection (Schridde and van Luijtelaar, 2004), further evidence that it may be the increase in CORT that is driving the increase in SWD activity. The immediate anti-convulsant response to stress is likely meditated by the rapid noradrenergic stress response which precedes the longer-lasting genomic actions of the HPA axis response (Giorgi et al., 2004). CORT is also known to alter sodium currents by changing voltage-dependent activation and inactivation and recovery from inactivation in hippocampal neurons (Werkman et al., 1997), as well as voltage-dependent sodium conductances (Joels, 1997), providing a possible mechanistic explanation for how HPA axis activity may induce seizure activity. However, further investigation into the relationship between seizure activity and HPA axis activity in the Scn8amed/+ mutants is needed to establish whether the link between the immediate increase in HPA axis activity and the short-term increase in seizure activity is directly causative, or whether these changes may result from a common upstream mediator.
Our study also provides evidence of a link between increased HPA axis activity and long-lasting alterations in SWD activity. Following a morning exposure to an acute stressor, we found that 1900h CORT levels in the mutants were elevated above baseline, and the normal evening rhythm of SWD activity was altered. There is evidence that seizure activity can have long-term effects on HPA axis activity, possibly resulting in a positive feedback loop that could raise the chances of spontaneous seizures in the future. In seizure-sensitive gerbils, corticotropin-releasing factor (CRF) immunoreactivity following a seizure does not return to pre-seizure levels until 24 hours later (An et al., 2003), and pilocarpine-induced status epilepticus (SE) can increase CRF gene expression significantly for up to a day after the SE (Wu et al., 2012). In both cases, CRF activity is altered, and CRF is consistently found to be pro-convulsant (Sawyer and Escayg, 2010). Considering that only the Scn8amed/+ mutants, and not the wild-type littermates, show a long-lasting change in HPA axis activity after an acute stressor, altered sodium channel function may well mediate such long-lasting HPA axis-seizure feedback loops.
Given the proposed link between hyperemotionality and epilepsy and the possible involvement of the HPA axis in both phenomena, we also assessed the behavioral response of Scn8amed/+ mice to mildly stressful situations. We found that Scn8amed/+ mice show higher levels of anxiety-like behavior, characterized by decreased struggling during restraint stress, increasing freezing, increased grooming, and decreased exploratory behavior, but they did not show any measure of depressive-like behavior in the forced swim test. These behavioral changes are unlikely to be due to elevated SWD activity in response to the stressful nature of the task, as we have also shown that SWD activity is suppressed for an average of 24 minutes following the onset of a stressor, and all of the behavioral tasks were completed in 15 minutes or less. Furthermore, the mutants exhibited an increase in grooming in response to novelty and no difference in struggling during the forced swim test, behaviors that would be inconsistent with SWD-induced behavioral arrest. Our behavioral findings are consistent with other reported findings, and increased anxiety-like behavior in animal models of epilepsy appears to be highly reproducible (Heinrichs, 2010). For example, the WAG/Rij rats show increased agitation, decreased exploration, increased grooming, and hyperlocomotion in response to novelty stress (Midzyanovskaya et al., 2005). Intriguingly, anxiety levels in WAG/Rij rats are positively correlated with the propensity for SWD activity, with rats having the greatest number of SWDs showing the highest anxiety levels (Midzyanovskaya et al., 2005). Both WAG/Rij rats and the Genetic Absence Epilepsy Rats from Strasbourg (GAERS) also show depressive-like behaviors (Jones et al., 2008; Sarkisova and van Luijtelaar, 2011), whereas the Scn8amed/+ mutants did not differ from wild-type littermates in depressive measures tested. Linkage studies in the GAERS and WAG/Rij rats show that the genes linked with SWD activity in these two models are on different chromosomes, suggesting different genetic causes for SWD activity and perhaps even the behavioral abnormalities seen in these models (Gauguier et al., 2004; Rudolf et al., 2004). The Scn8amed/+ model, on the other hand, allows us to speculate that a VGSC mutation might preferentially affect systems involved in the anxiety response. Evidence provided by Mirza et al. (2005) shows that VGSCs can indeed mediate the anxiety response, while McKinney et al. (2008) saw increases in anxiety-like behaviors in the Scn8atg mutant, and we previously found minor changes in anxiety-like behavior in the Scn8amed-jo mutant (Papale et al., 2010). Increased levels of anxiety are also seen in mutants with deficits in another VGSC gene, Scn1a (Han et al., 2012; Ito et al., 2012). The evidence from these experiments and the results from the current study suggest that Scn8a and GSCs in general do play a role in anxiety-like behaviors.
In contrast to the stress-induced changes discussed above, baseline HPA axis activity in the mutants is not altered; however, this finding is consistent with literature showing that humans with generalized anxiety disorders, when analyzed separately from those with comorbid depression or post-traumatic stress disorder, often show no changes in baseline HPA axis activity (Vreeburg et al., 2010; Staufenbiel et al., 2012). Considering that an anxiety disorder is characterized by a mental and physiological overreaction to a stressful situation (Hoehn-Saric and McLeod, 2000), it is likely that at baseline in a non-stressed state, individuals appear physiologically normal. We found the CORT response to an acute stressor was the same in the Scn8amed/+ mutants as in WT littermates, suggesting a normal initial response to a stressor, but the mutants showed altered CORT levels up to 12 hours after the stressor. Thus, abnormalities in the HPA axis function of Scn8amed/+ mice may not become apparent until after the system is challenged by a stressful situation.
In contrast to the spontaneous SWD activity, Scn8a-deficient mice show elevated thresholds to induced seizures (Martin et al., 2007; Blumenfeld et al., 2009), a finding also confirmed by the present study. Interestingly, we found that acute stress affects chemically induced seizures in Scn8amed/+ mice differently than in WT littermates. Specifically, acute stress increased thresholds to picrotoxin-induced seizures in WT littermates, but decreased thresholds in Scn8amed/+ mice. Acute stress also increased seizure severity, the number of animals progressing to a GTCS, and the number of animals experiencing seizures lasting longer than 15 minutes in the Scn8amed/+ mutants, but not the WT littermates. Given that seizure activity, anxiety, and the stress response all involve limbic structures, we propose that the hyperemotionality seen in the Scn8amed/+ mice could be working in a feed-forward manner to influence the seizure response to a stressor. During the 20 minutes that the mice are in the restraint tubes prior to seizure induction, they are not only experiencing a stress response in terms of increased corticosterone levels, but they are also experiencing an anxiety response. The behavioral response to the stressor precedes the altered seizure response, suggesting that the alterations in anxiety-like behaviors may be driving the alterations in the seizure response to stress and elevated CORT, which then lead to long-lasting changes in HPA axis activity that in turn influence future seizure activity.
The results of this study support a role for Scn8a in anxiety-like behaviors. In addition, this study suggests a connection between altered anxiety-like behaviors, prolonged changes in HPA axis circadian activity, and spontaneous seizure activity in response to a stressor. Furthermore, previous work examining the role of stress in multifactorial models of epilepsy, such as the epilepsy (EL) mouse and the WAG/Rij rat, have shown that stress or fear can trigger spontaneous seizure activity (Seyfried et al., 1999; Tolmacheva et al., 2012). While these are validated models of genetic epilepsy, it is difficult to ascribe the results of those experiments to a single mechanism or gene. In contrast, we have shown that acute stress worsens seizure outcome and alters behavior in Scn8amed/+ mice. The differences between mutants and WT animals suggest that Scn8a, by altering neuronal excitability, plays a role in the stress response, highlighting the need for further research into the interplay between stress, anxiety, and seizures in genetically predisposed animal models, as well as future research into the role of VGSCs in the stress response. A better understanding of the impact of Scn8a on the stress response may give us insight into the mechanisms of stress-evoked disorders and point the way to new therapeutic targets for the treatment of epilepsy.
Supplementary Material
Acknowledgments
Research in this publication was supported by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH) under award numbers R01NS07221 (AE) and F31NS073237 (NTS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Support was also provided by an ARCS Fellowship (NTS). We would like to thank Cheryl Strauss for editorial assistance.
Footnotes
Conflict of Interest
The authors report no financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An SJ, Park SK, Hwang IK, Kim HS, Seo MO, Suh JG, Oh YS, Bae JC, Won MH, Kang TC. Altered corticotropin-releasing factor (CRF) receptor immunoreactivity in the gerbil hippocampal complex following spontaneous seizure. Neurochem Int. 2003;43:39–45. doi: 10.1016/s0197-0186(02)00195-x. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Lampert A, Klein JP, Mission J, Chen MC, Rivera M, Dib-Hajj S, Brennan AR, Hains BC, Waxman SG. Role of hippocampal sodium channel Nav1.6 in kindling epileptogenesis. Epilepsia. 2009;50:44–55. doi: 10.1111/j.1528-1167.2008.01710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnjak J, Vukovic-Bobic M, Mejaski-Bosnjak V. Effect of war on the occurrence of epileptic seizures in children. Epilepsy Behav. 2002;3:502–509. doi: 10.1016/s1525-5050(02)00602-9. [DOI] [PubMed] [Google Scholar]
- Burgess DL, Kohrman DC, Galt J, Plummer NW, Jones JM, Spear B, Meisler MH. Mutation of a new sodium channel gene, Scn8a, in the mouse mutant ‘motor endplate disease’. Nat Genet. 1995;10:461–465. doi: 10.1038/ng0895-461. [DOI] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Espejo EF. Effects of weekly or daily exposure to the elevated plus-maze in male mice. Behav Brain Res. 1997;87:233–238. doi: 10.1016/s0166-4328(97)02286-9. [DOI] [PubMed] [Google Scholar]
- Feldman RG, Paul NL. Identity of emotional triggers in epilepsy. J Nerv Ment Dis. 1976;162:345–353. doi: 10.1097/00005053-197605000-00005. [DOI] [PubMed] [Google Scholar]
- Frankel WN, Beyer B, Maxwell CR, Pretel S, Letts VA, Siegel SJ. Development of a new genetic model for absence epilepsy: spike-wave seizures in C3H/He and backcross mice. J Neurosci. 2005;25:3452–3458. doi: 10.1523/JNEUROSCI.0231-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frucht MM, Quigg M, Schwaner C, Fountain NB. Distribution of seizure precipitants among epilepsy syndromes. Epilepsia. 2000;41:1534–1539. doi: 10.1111/j.1499-1654.2000.001534.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Morales I, de la Pena Mayor P, Kanner AM. Psychiatric comorbidities in epilepsy: identification and treatment. Neurologist. 2008;14:S15–25. doi: 10.1097/01.nrl.0000340788.07672.51. [DOI] [PubMed] [Google Scholar]
- Gauguier D, van Luijtelaar G, Bihoreau MT, Wilder SP, Godfrey RF, Vossen J, Coenen A, Cox RD. Chromosomal mapping of genetic loci controlling absence epilepsy phenotypes in the WAG/Rij rat. Epilepsia. 2004;45:908–915. doi: 10.1111/j.0013-9580.2004.13104.x. [DOI] [PubMed] [Google Scholar]
- Giorgi FS, Pizzanelli C, Biagioni F, Murri L, Fornai F. The role of norepinephrine in epilepsy: from the bench to the bedside. Neurosci Biobehav Rev. 2004;28:507–524. doi: 10.1016/j.neubiorev.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO, Catterall WA. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut SR, Hall CB, Masur J, Lipton RB. Seizure occurrence: precipitants and prediction. Neurology. 2007;69:1905–1910. doi: 10.1212/01.wnl.0000278112.48285.84. [DOI] [PubMed] [Google Scholar]
- Haut SR, Vouyiouklis M, Shinnar S. Stress and epilepsy: a patient perception survey. Epilepsy Behav. 2003;4:511–514. doi: 10.1016/s1525-5050(03)00182-3. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC. Neurobehavioral consequences of stressor exposure in rodent models of epilepsy. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:808–815. doi: 10.1016/j.pnpbp.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, McLeod DR. Anxiety and arousal: physiological changes and their perception. J Affect Disord. 2000;61:217–224. doi: 10.1016/s0165-0327(00)00339-6. [DOI] [PubMed] [Google Scholar]
- Ito S, Ogiwara I, Yamada K, Miyamoto H, Hensch TK, Osawa M, Yamakawa K. Mouse with Na(v)1.1 haploinsufficiency, a model for Dravet syndrome, exhibits lowered sociability and learning impairment. Neurobiol Dis. 2012;49C:29–40. doi: 10.1016/j.nbd.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Joels M. Steroid hormones and excitability in the mammalian brain. Front Neuroendocrinol. 1997;18:2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Salzberg MR, Kumar G, Couper A, Morris MJ, O’Brien TJ. Elevated anxiety and depressive-like behavior in a rat model of genetic generalized epilepsy suggesting common causation. Exp Neurol. 2008;209:254–260. doi: 10.1016/j.expneurol.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Krzemien DM, Schaller KL, Levinson SR, Caldwell JH. Immunolocalization of sodium channel isoform NaCh6 in the nervous system. J Comp Neurol. 2000;420:70–83. [PubMed] [Google Scholar]
- Martin MS, Tang B, Papale LA, Yu FH, Catterall WA, Escayg A. The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy. Hum Mol Genet. 2007;16:2892–2899. doi: 10.1093/hmg/ddm248. [DOI] [PubMed] [Google Scholar]
- McKinney BC, Chow CY, Meisler MH, Murphy GG. Exaggerated emotional behavior in mice heterozygous null for the sodium channel Scn8a (Nav1.6) Genes Brain Behav. 2008;7:629–638. doi: 10.1111/j.1601-183X.2008.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzyanovskaya IS, Shatskova AB, Sarkisova KY, van Luijtelaar G, Tuomisto L, Kuznetsova GD. Convulsive and nonconvulsive epilepsy in rats: effects on behavioral response to novelty stress. Epilepsy Behav. 2005;6:543–551. doi: 10.1016/j.yebeh.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Bright JL, Stanhope KJ, Wyatt A, Harrington NR. Lamotrigine has an anxiolytic-like profile in the rat conditioned emotional response test of anxiety: a potential role for sodium channels? Psychopharmacology (Berl) 2005;180:159–168. doi: 10.1007/s00213-005-2146-1. [DOI] [PubMed] [Google Scholar]
- Nakken KO, Solaas MH, Kjeldsen MJ, Friis ML, Pellock JM, Corey LA. Which seizure-precipitating factors do patients with epilepsy most frequently report? Epilepsy Behav. 2005;6:85–89. doi: 10.1016/j.yebeh.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Papale LA, Beyer B, Jones JM, Sharkey LM, Tufik S, Epstein M, Letts VA, Meisler MH, Frankel WN, Escayg A. Heterozygous mutations of the voltage-gated sodium channel SCN8A are associated with spike-wave discharges and absence epilepsy in mice. Hum Mol Genet. 2009;18:1633–1641. doi: 10.1093/hmg/ddp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papale LA, Paul KN, Sawyer NT, Manns JR, Tufik S, Escayg A. Dysfunction of the Scn8a voltage-gated sodium channel alters sleep architecture, reduces diurnal corticosterone levels, and enhances spatial memory. J Biol Chem. 2010;285:16553–16561. doi: 10.1074/jbc.M109.090084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Raman IM, Sprunger LK, Meisler MH, Bean BP. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron. 1997;19:881–891. doi: 10.1016/s0896-6273(00)80969-1. [DOI] [PubMed] [Google Scholar]
- Rudolf G, Bihoreau MT, Godfrey RF, Wilder SP, Cox RD, Lathrop M, Marescaux C, Gauguier D. Polygenic control of idiopathic generalized epilepsy phenotypes in the genetic absence rats from Strasbourg (GAERS) Epilepsia. 2004;45:301–308. doi: 10.1111/j.0013-9580.2004.50303.x. [DOI] [PubMed] [Google Scholar]
- Sarkisova K, van Luijtelaar G. The WAG/Rij strain: A genetic animal model of absence epilepsy with comorbidity of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011 doi: 10.1016/j.pnpbp.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Sawyer NT, Escayg A. Stress and Epilepsy: Multiple Models, Multiple Outcomes. J Clin Neurophysiol. 2010;27:445–452. doi: 10.1097/WNP.0b013e3181fe0573. [DOI] [PubMed] [Google Scholar]
- Schaller KL, Krzemien DM, Yarowsky PJ, Krueger BK, Caldwell JH. A novel, abundant sodium channel expressed in neurons and glia. J Neurosci. 1995;15:3231–3242. doi: 10.1523/JNEUROSCI.15-05-03231.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schridde U, van Luijtelaar G. Corticosterone increases spike-wave discharges in a dose- and time-dependent manner in WAG/Rij rats. Pharmacol Biochem Behav. 2004;78:369–375. doi: 10.1016/j.pbb.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Seyfried TN, Todorova MT, Poderycki MJ. Experimental models of multifactorial epilepsies: the EL mouse and mice susceptible to audiogenic seizures. Adv Neurol. 1999;79:279–290. [PubMed] [Google Scholar]
- Smyk MK, Coenen AM, Lewandowski MH, van Luijtelaar G. Endogenous rhythm of absence epilepsy: relationship with general motor activity and sleep-wake states. Epilepsy Res. 2011;93:120–127. doi: 10.1016/j.eplepsyres.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Spatt J, Langbauer G, Mamoli B. Subjective perception of seizure precipitants: results of a questionnaire study. Seizure. 1998;7:391–395. doi: 10.1016/s1059-1311(05)80008-1. [DOI] [PubMed] [Google Scholar]
- Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology. 2012;38:1220–1235. doi: 10.1016/j.psyneuen.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Swinkels WA, Engelsman M, Kasteleijn-Nolst Trenite DG, Baal MG, de Haan GJ, Oosting J. Influence of an evacuation in February 1995 in The Netherlands on the seizure frequency in patients with epilepsy: a controlled study. Epilepsia. 1998;39:1203–1207. doi: 10.1111/j.1528-1157.1998.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Temkin NR, Davis GR. Stress as a risk factor for seizures among adults with epilepsy. Epilepsia. 1984;25:450–456. doi: 10.1111/j.1528-1157.1984.tb03442.x. [DOI] [PubMed] [Google Scholar]
- Thapar A, Kerr M, Harold G. Stress, anxiety, depression, and epilepsy: investigating the relationship between psychological factors and seizures. Epilepsy Behav. 2009;14:134–140. doi: 10.1016/j.yebeh.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Tolmacheva EA, Oitzl MS, van Luijtelaar G. Stress, glucocorticoids and absences in a genetic epilepsy model. Horm Behav. 2012;61:706–710. doi: 10.1016/j.yhbeh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Trudeau MM, Dalton JC, Day JW, Ranum LP, Meisler MH. Heterozygosity for a protein truncation mutation of sodium channel SCN8A in a patient with cerebellar atrophy, ataxia, and mental retardation. J Med Genet. 2006;43:527–530. doi: 10.1136/jmg.2005.035667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, Matthews G. Impaired firing and cell-specific compensation in neurons lacking nav1.6 sodium channels. J Neurosci. 2006;26:7172–7180. doi: 10.1523/JNEUROSCI.1101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeramah KR, O’Brien JE, Meisler MH, Cheng X, Dib-Hajj SD, Waxman SG, Talwar D, Girirajan S, Eichler EE, Restifo LL, Erickson RP, Hammer MF. De Novo Pathogenic SCN8A Mutation Identified by Whole-Genome Sequencing of a Family Quartet Affected by Infantile Epileptic Encephalopathy and SUDEP. Am J Hum Genet. 2012;90:502–510. doi: 10.1016/j.ajhg.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velisek L. Models of Chemically-Induced Acute Seizures. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of Seizures and Epilepsy. Elsevier; Amsterdam: 2006. pp. 127–152. [Google Scholar]
- Veliskova J. Behavioral Characterization of Seizures in Rats. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of Seizures and Epilepsy. Elsevier; Amsterdam: 2006. pp. 601–611. [Google Scholar]
- Vreeburg SA, Zitman FG, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Hoogendijk WJ, Smit JH, Penninx BW. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosom Med. 2010;72:340–347. doi: 10.1097/PSY.0b013e3181d2f0c8. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang J, Li X, Ji J, Yang F, Wan C, Feng G, Wan P, He L, He G. SCN8A as a novel candidate gene associated with bipolar disorder in the Han Chinese population. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1902–1904. doi: 10.1016/j.pnpbp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Wasserman D, Geijer T, Rozanov V, Wasserman J. Suicide attempt and basic mechanisms in neural conduction: relationships to the SCN8A and VAMP4 genes. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:116–119. doi: 10.1002/ajmg.b.30128. [DOI] [PubMed] [Google Scholar]
- Werkman TR, Van der Linden S, Joels M. Corticosteroid effects on sodium and calcium currents in acutely dissociated rat CA1 hippocampal neurons. Neuroscience. 1997;78:663–672. doi: 10.1016/s0306-4522(96)00624-0. [DOI] [PubMed] [Google Scholar]
- Wu J, Ma DL, Ling EA, Tang FR. Corticotropin releasing factor (CRF) in the hippocampus of the mouse pilocarpine model of status epilepticus. Neurosci Lett. 2012;512:83–88. doi: 10.1016/j.neulet.2012.01.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.