Abstract
Expectations about an upcoming emotional event have the power to shape one’s subsequent affective response for better or worse. Here, we used mediation analyses to examine the relationship between brain activity when anticipating the need to cognitively reappraise aversive images, amygdala responses to those images and subsequent success in diminishing negative affect. We found that anticipatory activity in right rostrolateral prefrontal cortex was associated with greater subsequent left amygdala responses to aversive images and decreased regulation success. In contrast, anticipatory ventral anterior insula activity was associated with reduced amygdala responses and greater reappraisal success. In both cases, left amygdala responses mediated the relationship between anticipatory activity and reappraisal success. These results suggest that anticipation facilitates successful reappraisal via reduced anticipatory prefrontal ‘cognitive’ elaboration and better integration of affective information in paralimbic and subcortical systems.
Keywords: anticipation, fMRI, mediation, reappraisal
INTRODUCTION
Every day, we are faced with all manner of challenges to our emotional equilibrium. Although many of these aversive events are unexpected and catch us unprepared, in some cases we can anticipate that something unpleasant comes this way. As an example, imagine that you are going to visit a family member in the hospital who is gravely ill. In thinking about the upcoming visit, you might anticipate that seeing your loved one in distress will be upsetting and predict that you will need to regulate your emotional response when you see him or her. How do your expectations impact your reaction to the expected unpleasant event when it actually transpires? And what neural systems mediate these effects? Despite the relevance of these questions to our understanding of the neural systems that maintain emotional health and well-being, very little research links anticipatory brain processes with subjective and brain measures of emotion and emotion regulation.
Although little work has directly addressed these issues, previous studies have suggested two non-competing alternative hypotheses. Namely, anticipatory activity in key regions implicated in affective responsivity and cognitive control could be preparatory and positive or such activity could be maladaptive and negative.
One principal region of interest (ROI) is the prefrontal cortex (PFC), which could be involved in setting adaptive expectations that ultimately support subsequent reappraisal success. The PFC has been widely implicated in cognitive control, goal-directed behavior and high-level processes used to regulate responses to emotional events, with the predominant finding being that activity in a variety of prefrontal regions is associated with adaptive emotion regulation.
The majority of such studies have probed the involvement of PFC in the cognitive regulation of emotion via reappraisal, which involves cognitively changing the meaning of an affective stimulus in a way that alters its emotional impact (Gross, 1998; Ochsner and Gross, 2005, 2008). Numerous neuroimaging studies (e.g. Ochsner et al., 2002, 2004; Kalisch et al., 2005; Phan et al., 2005; van Reekum et al., 2007; Goldin et al., 2008; McRae et al., 2010;for a review, see Silvers et al., 2013) have shown that the down-regulation of negative emotion via reappraisal typically is associated with increased activation of control-related regions such as lateral and medial PFC, along with dorsal anterior cingulate cortex, and decreased activation of regions associated with triggering emotional responses, such as the amygdala, (Ochsner and Gross, 2008; Denny et al., 2009). A few studies have examined the use of reappraisal-like strategies to regulate affective responses elicited during anticipation of a stimulus, with results mostly parallel to those described above (Kalisch et al., 2005; Delgado et al., 2008a,b; Herwig et al., 2007b; Martin and Delgado, 2011). Critically, although these studies examined regulation of affective responses elicited during the anticipation of an upcoming event (like anticipatory anxiety or reward), none was designed to directly relate anticipatory PFC activity to success at reducing self-reported affective responses to the subsequently experienced event itself.
A second hypothesis, however, is that the PFC could be involved in setting maladaptive expectancies whereby PFC supports negative expectations that promote responses in affective appraisal-related regions like the amygdala. Behaviorally, anticipating negative events elicits self-report (Butler and Mathews, 1987; Savitsky et al., 2001) and psychophysiological (Grillon et al., 1991) markers of negative affect. Neurally, imaging studies have shown that anticipation of a clearly or potentially aversive event is associated with increased activation of both the amygdala (Herwig et al., 2007a,c; Kaffenberger et al., 2010) and, critically, the anterior and dorsomedial PFC and anterior cingulate cortex (Ueda et al., 2003; Mechias et al., 2010), with regions of rostral dorsal and pre-genual cingulate cortices (Wager et al., 2009) mediating the relationship between negative anticipation and peripheral physiological reactivity. Activity in these medial PFC regions has been associated more generally with the maintenance of beliefs that influence one’s emotions, including lowered expectations of drug effectiveness and reduced responses to a placebo analgesic (Wager et al., 2011), the top–down generation of negative emotion via cognitive appraisals (Ochsner et al., 2004, 2009b) and with the tendency to mind wander (Christoff et al., 2009; Mason et al., 2007), which has been shown to lead to general feelings of unhappiness (Killingsworth and Gilbert, 2010). These medial regions, along with other midline and temporal structures, also have been implicated in making judgments about mental states (like beliefs and emotions) more generally (Kober et al., 2008; Olsson and Ochsner, 2008; Denny et al., 2012), and are thought to be a key compoment of a ‘default’ network that is relatively more active during uninstructed periods (when one is wont to mind-wander) than during task performance (Gusnard et al., 2001; Raichle et al., 2001). Together, these data suggest that medial PFC (perhaps along with associated regions) support the tendency to spontaneously generate negative expectancies that could impair subsequent emotion regulation ability.
Further, previous work suggests that regions beyond PFC may be important as well in either facilitating adaptive or maladaptive reappraisals. In particular, evidence suggests that the insula may play a key role. In addition to PFC, insula also has been shown to be importantly involved in the anticipation and appraisal of emotional events, in addition to the integration of sensory and motor information (Augustine, 1996). Differences in anatomy (Mesulam and Mufson, 1982a,b; Mufson and Mesulam, 1982, 1984) and resting-state functional connectivity (Deen et al., 2011) suggest that the insula has separate posterior, ventral anterior and dorsal anterior subregions. Of interest here is the fact that the anterior insula has been associated with emotional and motivational states, interoceptive awareness of them and often is active along with adjacent ventrolateral preftontal regions during cognitive control tasks, including reappraisal of negative emotion (Critchley et al., 2004; Wager and Barrett, 2004; Nitschke et al., 2006; Craig, 2009). Wager and Barrett (2004) suggested that these diverse roles for the anterior insula can be understood in part by a ventral–dorsal distinction, such that ventral anterior insula is more strongly associated with emotional awareness (Carlson et al., 2011), and dorsal anterior insula is more strongly associated with updating goal states and top–down executive control (Wager et al., 2004; Nee et al., 2007). Ventral anterior insula activity has been reported during the anticipation of aversive events (Kalisch et al., 2006; Carlson et al., 2011), though these effects were not shown to be specifically attuned to negatively valenced anticipation states (Carlson et al., 2011). The fact that anterior insula has been implicated broadly in both affective integration and cognitive control, as well as the fact that people who are better at introspectively assessing their emotions tend to regulate their emotions more frequently (Barrett et al., 2001), suggests that it has the potential to promote reappraisal success via integrating information about the body, one’s current emotional state and current task goals.
To differentiate among these hypotheses, we employed a variant of a well-studied reappraisal task (Ochsner and Gross, 2008). This task presents participants with aversive images and asks them either to let themselves respond naturally (i.e. baseline ‘Look’ trials) or to reinterpret the meaning of the image in a way that lessens its unpleasant impact (i.e. ‘Reappraise’ trials). The present task modified the basic trial structure to insert an anticipatory gap between the presentation of the cue instructing participants that they would Look or Reappraise and the presentation of the upcoming aversive or neutral image.
Using this design, we adopted a two-step analysis procedure to address the two hypotheses described above concerning the way in which expectations of the need to reappraise influence subsequent neural and behavioral responses to affective events. We reasoned that the best way to determine how expectations influence ultimate reappraisal success was by first identifying a signature of successful reappraisal. To do this, our first step involved correlating reappraisal success (defined as the drop in self-reported negative affect on Reappraisal as opposed to Look trials) with activity in the amygdala, which is the affect-related region most commonly modulated by reappraisal of negative emotion (Ochsner and Gross, 2008). This identified a region of the left amygdala whose activity during reappraisal of an aversive image was negatively correlated with reappraisal success. In the second step, we used Mediation Effect Parametric Mapping (MEPM) to test for the hypothesized relationships among anticipatory neural activity, amygdala activity during reappraisal and self-reports of negative affect (Wager et al., 2008b; Atlas et al., 2010). Here, we aimed to determine how brain activity during anticipation of reappraisal (i.e. in the 6 s before the image to be reappraised was presented) is associated with subsequent reappraisal success, mediated by stimulus-related activity in the functionally defined area of the left amygdala described above. On one hand, if anticipating reappraisal enhances regulatory success, then we should find that anticipatory activation of reappraisal- or affect-related regions leads to larger drops in negative affect via down-regulation of subsequent amygdala responses to aversive images. On the other hand, if anticipating reappraisal diminishes regulatory success, then we should find that anticipatory activation of reappraisal- or affect-related regions leads to smaller drops in negative affect via a failure to down-regulate subsequent amygdala responses to aversive images.
METHODS
Participants
In total, 36 healthy participants (average age being 22.0 years; 13 female) provided informed consent in accordance with the human subjects regulations of Columbia University and were paid $20/h for their participation. All participants were right-handed and were screened with questionnaires to ensure good general health and functional magnetic resonance imaging (fMRI) scan eligibility. Six participants were excluded prior to analysis because they were not within movement, normalization or timing-accuracy tolerances. Thus, the present analyses were performed on data from 30 participants.
Materials
The basic stimuli, task design and procedures used in the current study have been detailed in a previous report focusing on activity solely during the presentation of aversive images (Wager et al., 2008b). In this previous report the anticipation period was not examined. The current study, while using the same dataset, focuses on the novel questions detailed above regarding the relationship between activity during the cue and anticipation periods and subsequent activity during the picture presentation period and reappraisal success. Forty-eight aversive images and 24 neutral images from the International Affective Picture System (IAPS; Lang et al., 1993) were presented. Stimulus details can be found in Supplementary Data.
Task design
Images were presented in one of three conditions. For the Look Neu and Look Neg conditions, participants were shown either neutral or aversive images and were asked to look at the image, understand its content and allow themselves to experience/feel any emotional response it might naturally elicit. In contrast, for the Reapp Neg condition, participants viewed aversive images and were asked to reinterpret their meaning so that they felt less negative in response to them [cf. previous published work from our laboratory (Ochsner and Gross, 2008)].
The assignment of negative images to conditions was randomized and counterbalanced across participants. Before presentation of each image, participants viewed a cue that signaled both the instruction type (Look or Reappraise) and the image type (aversive or neutral). Cues were white shapes—a circle, a square and a triangle (∼0.5° visual angle)—presented on a black background. The assignment of shape to condition was counterbalanced across participants.
Previous studies of reappraisal have not separated brain activity related to anticipation and instruction processing, stimulus viewing and picture rating, and a goal of our task design was to provide the ability to separately estimate the magnitude of brain activation related to each of these three phases of the image viewing and rating procedure. To accomplish this, a partial trial design was employed (Ollinger et al., 2001). Three variants of each task condition (Look Neu, Look Neg and Reapp Neg) were used, with an equal distribution of each type of trial: full (AntStim) trials, anticipation-only (AntOnly) trials and stimulus-only (StimOnly) trials. On full trials, a 2 s instruction cue was followed by a 4 s anticipatory interval during which a fixation cross was presented on the screen. An image was subsequently presented for 8 s, followed by a fixation cross for 4 or 7 s as a jittered interstimulus interval (ISI; uniform distribution of 4 and 7 s intervals). Following the ISI period, the words ‘How negative do you feel?’ appeared onscreen for 2.1 s, and participants rated their current level of negative affect on a five-point scale by pressing a button with one of five fingers on a button-response unit (0 = ‘not at all negative’, indicated by a thumb button press, up to 4 = ‘extremely negative’, indicated by a fifth-finger button press). Following the rating, a 4 or 7 s jittered inter-trial-interval concluded the trial. This trial structure is shown in Figure 1 for a full trial. The AntOnly trials were identical to the full trials, except that the picture presentation period was omitted. The StimOnly trials were identical to the full trials, except that the 4 s anticipation interval was omitted.
Fig. 1.
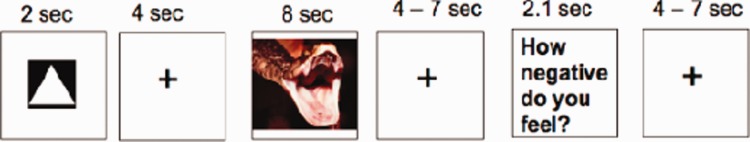
Trial structure for a full (AntStim) trial.
This design allowed us to construct orthogonal predictors for Cue-, Anticipation- and Image-related brain activity related to each trial type in the general linear model (GLM) that could provide efficient estimates of activation in each phase of the trial for each condition.
Procedure
A comprehensive pre-scanning training procedure was used to assure that participants understood the cue–task associations and the reappraisal strategy (see Wager et al., 2008b for details). During the task in the fMRI scanner, 108 total trials were presented (36 trials per condition). Within each condition, 12 trials were presented for each condition (Look Neu, Look Neg and Reapp Neg) × trial type (full trial, AntOnly, StimOnly) combination. Following scanning, participants completed a post-task questionnaire during which they confirmed that they reappraised as instructed.
Data acquisition and analysis
Behavioral
Behavioral data were analyzed using linear mixed models incorporating fixed effects estimates for trial type, condition and their interaction, and a random effect consisting of an intercept for each participant.
fMRI
Whole-brain fMRI data were acquired on a 1.5T GE Signa Twin Speed Excite HD scanner (GE Medical Systems). Acquisition details are given in Supplementary Data. Separate regressors in the GLM were specified for fMRI responses to the cue, anticipation, stimulus viewing and rating response periods.
Defining amygdala region-of-interest
In order to address our first question of interest concerning how reappraisal expectations modulate subsequent behavioral and neural responses to affective events, we first subjected values for the (Reapp Neg image viewing–Look Neg image viewing) contrast to second-level robust regression analysis (Wager et al., 2005) to localize regions correlated with reappraisal success, defined as each participant’s self-reported (Look Neg–Reapp Neg) average negative affect rating. This reappraisal success regressor excluded ratings made during AntOnly trials, as those ratings were made on trials in which no image was presented. Each participant’s global magnetic resonance (MR) signal during the picture presentation period was used as a covariate in this analysis, and the search threshold was P < 0.01 (one-tailed). Additional details are available in the Supplementary Data.
Mediation analysis
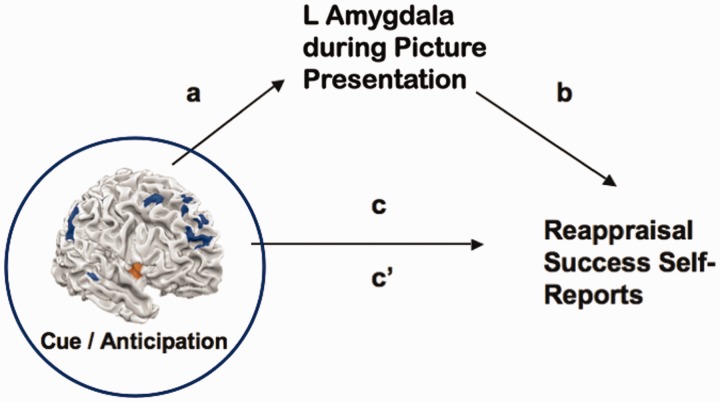
We then employed MEPM, which is based on a standard three-variable mediation model (Baron and Kenny, 1986) where a predictor (X) is related to an outcome (Y) via a mediator (M). MEPM analyses were performed using the (Reapp Neg–Look Neg] contrast values during the cue and anticipation period (the X or predictor variable; see Supplementary Data for details of the combination of these periods), the stimulus presentation period (the M or mediator variable) and behavioral reappraisal success scores (the Y or outcome variable). We performed a MEPM analysis in which the mediator values were pre-defined as beta weights from the amygdala seed region during the picture presentation period, and then the whole brain was searched for predictor (X) regions at cue/anticipation whose activity showed a relationship with reappraisal success (Y) that was mediated by the activity of the seed amygdala region (M) (Figure 2). In this analysis, by-participant average beta weights for global cue/anticipation activity and global stimulus presentation period activity were each entered as covariates to reduce regression confounds due to spurious covariance of task activity and global signal.
Fig. 2.
Mediation path diagram showing the predictor search variable (Reapp Neg–Look Neg brain activity during cue/anticipation), a priori mediator variable (amygdala activation during the picture presentation period) and outcome variable (reappraisal success self-reports). a and b are indirect paths, c is the total relationship, and c′ is the direct path (controlling for the mediator).
Family-wise error (FWE) thresholds for the mediation results were determined using AlphaSim (Ward, 2000). Significant clusters (FWE-corrected, P < 0.05) were thresholded at P < 0.05, two-tailed, uncorrected, with an extent of at least 50 voxels, based on the unresliced voxel size. For display purposes using NeuroElf software (neuroelf.net), mediation t-score images were then resliced to isometric voxels (2 × 2 × 2 mm), and FWE multiple comparison correction thresholds were again determined using AlphaSim. Clusters were thresholded at P < 0.05, two-tailed, uncorrected, with an extent of at least 333 voxels, resulting in a whole-brain corrected FWE rate of P < 0.05, two-tailed.
We then determined whether there were any other mediators of the relationship between anticipatory PFC and insula activity and reappraisal success that might have been overlooked by our ROI-based method that focused on the amygdala. To do this, we performed three additional mediation analyses using the same analysis procedure as above, with the exception that the predictor variable was fixed to be anticipatory activity [separately specifying the BA 10 RLPFC (rostrolateral PFC) region and the left and right insula regions described in the results], and a whole-brain search was performed for regions that showed stimulus period activity that mediated a relationship between anticipatory activity and reappraisal success.
RESULTS
Behavioral
Figure 3 shows average negative affect ratings for each condition for AntStim and StimOnly trials and separately for anticipation only (AntOnly) trials. Negative affect reports did not differ between AntStim and StimOnly trials [F(1,145) = 0.715, n.s.], nor was there a significant interaction between trial type and condition for AntStim and StimOnly trials [F(2,145) = 1.733, n.s.], so data are shown collapsed across those two trial types. However, there was a main effect of condition [F(2,145) = 428.36, P < 0.001]; negative affect ratings for both negative image viewing conditions (Reapp Neg and Look Neg) were significantly greater than those for the Look Neu condition [t(29) = 12.59, P < 0.001 and t(29) = 22.62, P < 0.001, respectively]. Critically, among AntStim and StimOnly trials, reappraising negative images significantly modulated participants’ self-reported negative affect relative to responding naturally to negative images [t(29) = 8.10, P < 0.001]. Means for all trial types (AntStim, StimOnly and AntOnly) are shown in Supplementary Figure S1.
Fig. 3.
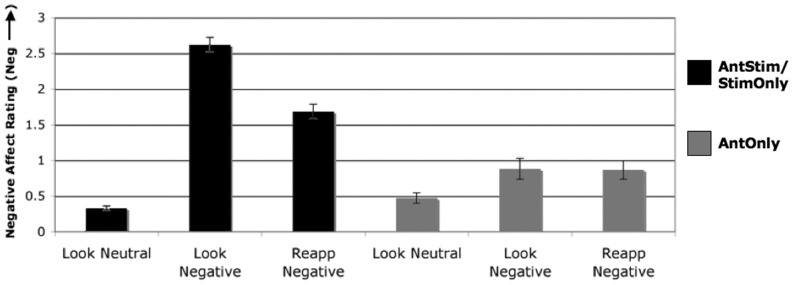
Negative affect ratings. Black bars represent negative affect ratings from trials containing a picture presentation (i.e. full AntStim trials and StimOnly trials) and gray bars represent negative affect ratings from anticipation only (AntOnly) trials.
For AntOnly trials, there was a main effect of condition [F(2,58) = 10.14, P < 0.001], with Look Neu ratings significantly lower than Look Neg [t(29) = 3.29, P < 0.003] and Reapp Neg [t(29) = 4.78, P < 0.001] ratings, but no significant difference between Look Neg and Reapp Neg ratings [t(29) = 0.15, n.s.].
Amygdala ROI
In order to address our primary question regarding potential amygdala-mediated relationships involving anticipatory brain activation that are correlated with reappraisal success, we first carried out a robust regression analysis to determine which voxels in the amygdala showed activation during the stimulus presentation period that was significantly correlated with reappraisal success. Three voxels in the left amygdala were significantly negatively correlated with reappraisal success at the search threshold (P < 0.01, one-tailed). These voxels in the left amygdala [Montreal Neurological Institute (MNI): (−21, −3, −23), (−14, −7, −23) and (−17, −7, −23)] comprised a volume of 160 mm3 and represented our amygdala seed ROI for the subsequent mediation analysis.
Mediation analysis
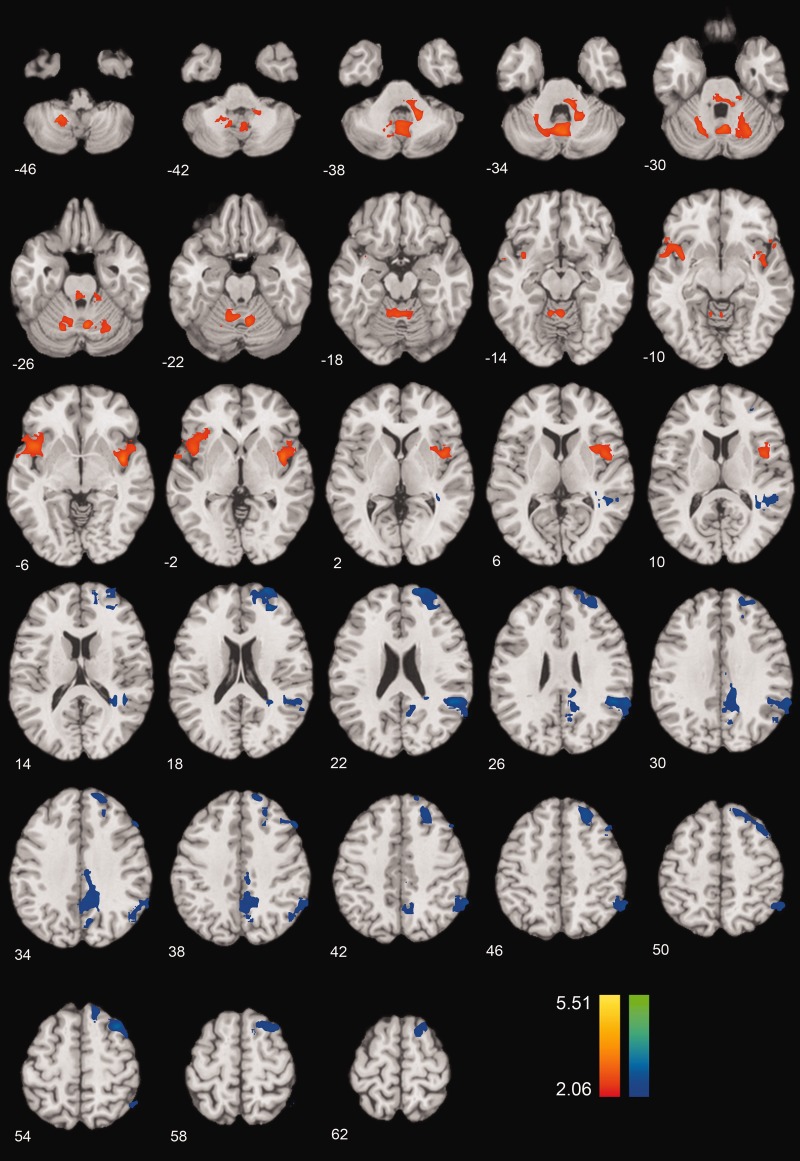
Using the amygdala ROI as a mediator, we found that a broad area of right RLPFC (BA 10) showed anticipatory activity prior to reappraisal that was negatively correlated with reappraisal success and significantly mediated by amygdala activity during the picture presentation period (Figures 2 and 4; Table 1). Increased anticipatory RLPFC activity was positively correlated with amygdala activation during the stimulus presentation period, and stimulus-related amygdala activity was negatively correlated with reappraisal success. In addition, a more dorsal medial PFC region (BA 8) also showed this negative mediated relationship, as did the posterior cingulate/precuneus, superior temporal gyrus/temporoparietal junction and pre- and post-central gyrus (Figure 4; Table 1).
Fig. 4.
Brain activity during cue/anticipation that satisfies the mediated relationship depicted in Figure 2. Orange-to-yellow regions show a positive mediated relationship, such that increases are associated with greater reappraisal success, mediated by amygdala activity during picture presentation. Blue-to-green regions show a negative mediated relationship, such that more activity in these regions at cue/anticipation is associated with less reappraisal success, mediated by amygdala activity during picture presentation. Thresholded at P < 0.05 unc, k = 333 voxels, FWE, P < 0.05, two-tailed.
Table 1.
Brain activity during cue/anticipation that shows a significant amygdala-mediated relationship with reappraisal success (a*b mediation path)
| Mediation path | x | y | z | k |
a*b |
a |
b |
c' |
c |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eff | t | P | Eff | t | P | Eff | t | P | Eff | t | P | Eff | t | P | |||||
| Negative-mediated relationship | |||||||||||||||||||
| RH Sup frontal gyrus (BA 10) | 24 | 62 | 18 | 94 | −1.22 | −2.35 | 0.03 | 2.46 | 2.79 | 0.01 | −0.48 | −2.64 | 0.01 | −0.25 | −0.22 | 0.83 | −1.48 | −1.27 | 0.22 |
| RH Sup Frontal Gyrus (BA 8) | 34 | 21 | 54 | 96 | −1.48 | −2.34 | 0.03 | 2.40 | 2.47 | 0.02 | −0.61 | −3.11 | 0.00 | 1.04 | 0.92 | 0.37 | −0.44 | −0.40 | 0.69 |
| RH Sup temporal gyrus (BA 40) | 48 | −48 | 23 | 174 | −1.84 | −2.33 | 0.03 | 2.99 | 2.58 | 0.02 | −0.62 | −2.96 | 0.01 | 1.37 | 1.02 | 0.32 | −0.47 | −0.40 | 0.69 |
| LH precentral gyrus (BA 4) | −21 | −21 | 54 | 74 | −2.82 | −2.27 | 0.03 | 5.78 | 2.62 | 0.01 | −0.49 | −2.54 | 0.02 | −0.64 | −0.12 | 0.91 | −3.47 | −1.44 | 0.16 |
| RH post cingulate gyrus (BA 31) | 17 | −41 | 32 | 105 | −1.06 | −2.25 | 0.03 | 2.20 | 2.59 | 0.02 | −0.48 | −2.67 | 0.01 | −0.31 | −0.22 | 0.83 | −1.37 | −1.26 | 0.22 |
| RH postcentral gyrus (BA 3) | 31 | −34 | 50 | 50 | −1.58 | −2.25 | 0.03 | 3.10 | 2.79 | 0.01 | −0.51 | −2.47 | 0.02 | 0.03 | 0.00 | 1.00 | −1.55 | −1.49 | 0.15 |
| Positive-mediated relationship | |||||||||||||||||||
| RH Insula (BA 13) | 41 | 0 | −5 | 71 | 1.83 | 2.37 | 0.03 | −2.78 | −2.78 | 0.01 | −0.66 | −2.82 | 0.01 | −1.79 | −1.09 | 0.28 | 0.04 | 0.04 | 0.97 |
| LH insula (BA 13) | −48 | 10 | −5 | 59 | 1.40 | 2.35 | 0.03 | −2.36 | −2.78 | 0.01 | −0.58 | −2.79 | 0.01 | −0.80 | −0.74 | 0.47 | 0.60 | 0.73 | 0.47 |
| RH anterior lobe cerebellum | 3 | −69 | −36 | 126 | 1.18 | 2.36 | 0.03 | −2.18 | −2.63 | 0.01 | −0.54 | −2.76 | 0.01 | −0.48 | −0.51 | 0.62 | 0.70 | 0.72 | 0.48 |
| RH anterior lobe cerebellum | 14 | −48 | −41 | 56 | 1.39 | 2.31 | 0.03 | −2.34 | −2.57 | 0.02 | −0.60 | −2.76 | 0.01 | −0.95 | −0.86 | 0.40 | 0.44 | 0.43 | 0.67 |
Regions are whole-brain FWE-corrected at P < 0.05, two-tailed, with thresholds of P < 0.05 and k ≥ 50 voxels. Coordinates are in MNI space. For each cluster, path coefficients, t-scores and significance levels are shown for each mediation path.
This analysis also identified several regions showing a significant positively mediated relationship, including bilateral insula (Figure 4; Table 1). Left ventral anterior insula and right ventral mid-insula activity was positively correlated with reappraisal success, mediated by amygdala stimulus-related activity. Anticipatory insula activity in these ROIs was negatively correlated with stimulus-related amygdala activity. Two separate clusters in the anterior lobe of the cerebellum also exhibited this pattern.
Critically, to confirm and extend the ROI-based analyses, we fixed anticipatory activity (RLPFC, left and right insula, in three separate analyses) as the predictor and searched the whole brain for mediators of the anticipatory activity–reappraisal success relationship. For RLPFC, this analysis found that left amygdala and parahippocampal gyrus activity adjacent to our prior left amygdala seed represented the only significant negative mediator [Supplementary Figure S2; cluster comprising 554 voxels, thresholded at P < 0.05, k = 333 voxels, peak at (−24, −10, −34)]. The only other mediator that was significant at this threshold was a region in dorsal parietal cortex [peak at (−36, −54, 54)], which showed activity that was negatively related to anticipatory RLPFC activity and negatively related to reappraisal success. No regions in the two mediation analyses incorporating anticipatory insula activity exceeded whole-brain FWE-correction thresholds.
DISCUSSION
The present results serve as the first investigation of mediated relationships among anticipatory brain activity and subsequent self-reported emotion regulation success. In this study, we sought to identify regions of the brain that show anticipatory activity prior to the instruction to reappraise that predict changes in amygdala activity during reappraisal itself, which in turn predict changes in self-reported reappraisal success or failure. We found that anticipatory RLPFC activity, which is not typically observed in reappraisal studies (Buhle et al., 2011), predicted ‘increased’ amygdala activity during the picture presentation period, which in turn predicted reappraisal failure. In addition, we found that anticipatory activity in left ventral anterior and right ventral mid-insula predicted ‘decreased’ stimulus-related amygdala activity and in turn greater reappraisal success. These results were confirmed by a whole-brain search for mediators of the anticipatory activity–success relationship, which found that a region spanning the left amygdala and parahippocampal gyrus was the only region mediating a negative relationship between anticipatory RLPFC activity and reappraisal success, and no additional mediators were present for the relationship between anticipatory insula activity and reappraisal success. Thus, in addition to our a priori focus on interrogating amygdala activity as a mediator, we have provided empirical evidence of the significant role played by an extensive region of left amygdala in mediating the relationship between anticipatory brain activity and reappraisal success.
Notably, the network of brain regions that were related to reappraisal failure and success during the anticipation period did not bear great similarity to the network of regions recruited during emotion regulation implementation itself, including ventrolateral PFC (Ochsner and Gross, 2008; Buhle et al., 2011) but instead were similar to regions implicated in the default state and emotion more generally.
Implications for neural mechanisms of expectancy × regulatory strategy interactions
Anticipatory activity associated with less regulatory success
In thinking about the meaning of anticipatory activations that predicted less reappraisal success, it is important to emphasize that participants were not explicitly instructed to perform any regulation during the anticipation interval, in contrast to previous work (Kalisch et al., 2005; Herwig et al., 2007b; Delgado et al., 2008a,b; Martin and Delgado, 2011). Rather, they were simply told to get ready for the upcoming picture and be ready to employ the cued strategy during the picture presentation.
In this context, it is interesting that we observed activity in RLPFC and other areas that have been associated with executive control and mentalizing. RLPFC has been previously associated with emotional awareness and the self-generation of information—including intentions for future actions (Christoff and Gabrieli, 2000; Burgess et al., 2003; Ray et al., 2005; Gilbert et al., 2006; Ochsner et al., 2009a; Costa et al., 2011). This suggests that the anticipatory RLPFC activity observed here may reflect self-generation of negative expectancies on the part of the participant (Sawamoto et al., 2000) in advance of the need to regulate that ultimately exert influence over their reappraisal success. Indeed, a very similar region of RLPFC has shown positive correlations with state negative affect when viewing aversive pictures (Nitschke et al., 2006).
Additional regions that were negatively associated with reappraisal success have been associated with a network for mentalizing—i.e. thinking about one’s own or another’s mental state—including dorsomedial PFC, posterior cingulate/precuneus and superior temporal gyrus/temporoparietal junction activity (Gilbert et al., 2006; Northoff et al., 2006; van der Meer et al., 2010; Qin and Northoff, 2011; Van Overwalle, 2009, 2011; Denny et al., 2012). In combination with RLPFC activity, this suggests that participants may have been imagining how they might feel when the picture is presented, and were elaborating on it, which served to create a negative expectation that was ultimately confirmed and led to poorer regulation outcomes (Wilson et al., 1989; Klaaren et al., 1994).
This interpretation raises the question of whether participants were feeling negative during the anticipation interval as a result of developing the proposed negative expectation. Given that we did not observe significant differences in self-reported negative affect scores for AntOnly Reappraise and Look Neg trials, however, the idea that participants felt especially ‘negative’ during reappraisal anticipation in particular is not supported in the present work. That said, the lack of behavioral evidence for anticipatory negative affect may be due to the fact that multiple brain regions exhibited anticipatory activity that was both positively and negatively associated with reappraisal success via amygdala activity. Thus, it is possible that there are both benefits and costs of reappraisal anticipation and this may have obscured a behavioral main effect on self-reported emotion.
Anticipatory activity associated with greater regulatory success
In the present study, not all anticipatory activation was maladaptive, however. We observed a substantial area of left ventral anterior and right ventral mid-insula activation that predicted diminished amygdala activation during picture presentation and in turn ultimate reappraisal success. This result is consistent with previous work showing that the ventral anterior insula is importantly involved in integration of affective information, including meta-awareness of bodily states and awareness of emotional and motivational states more generally (Wager and Barrett, 2004) and that greater emotional awareness may yield better regulatory outcomes (Barrett et al., 2001). Here, it is possible that greater insula activity reflects greater internal and emotional awareness (Critchley et al., 2004; Zaki et al., 2012), which in turn helps participants to be ready to clearly identify their subsequent emotional responses to presented photos, which in turn helps them to more easily pick effective reappraisals.
In addition to insula, anticipatory cerebellar activity promoted reappraisal success via stimulus period amygdala deactivation. This is consistent with other works implicating similar deep cerebellar foci in arousal and affective valuation (Wager et al., 2008a, 2011; Stoodley and Schmahmann, 2009).
Finally, the findings of the current study are further illuminated when viewed in the context of previous studies (Kalisch et al., 2005; Erk et al., 2006; Herwig et al., 2007b; Delgado et al., 2008a,b; Martin and Delgado, 2011) that suggest the regulation of emotions elicited by anticipation (previous studies) may be different than anticipating the need to regulate the emotions elicited by a future event (present study). Studies of the former tend to show that activation of posterior regions of medial prefrontal cortex (MPFC) and/or dorsolateral PFC associated with performance monitoring and cognitive control (Botvinick et al., 2001; Amodio and Frith, 2006) accompanies successful regulation. These regions may have been used to effectively implement a regulatory strategy to quell anticipatory emotions. In contrast, our study of the latter found that anticipatory activation in anterior MPFC and RLPFC regions associated with judgments about beliefs and emotions (Kober et al., 2008; Olsson and Ochsner, 2008) and the self-generation of relational thoughts (Christoff et al., 2001) predicted subsequent regulatory failure. These regions may have been used to generate maladaptive forecasts about what emotions one might later feel in response to a stimulus and ineffective or unhelpful ideas about how one might ultimately reappraise it.
Future directions
Future research may target at least two questions not addressed here. First, in our study the nature of the expectancy was open-ended in the sense that participants did not know the precise characteristics of the forthcoming stimulus and our reappraisal strategy required a stimulus-specific reinterpretation. Thus, we may have set participants up for some degree of failure insofar as their expectations couldn’t help but be incorrect and potentially unhelpful. Although this in many ways mirrors real-life events where our advance knowledge of an upcoming negative event is more general than specific, future work may unpack whether expectations about alternative types of stimuli and/or reappraisal strategies may prove more adaptive. For example, for situations where one isn’t sure of the particulars of upcoming events, psychological distancing (Mischel and Baker, 1975; Ochsner and Gross, 2008) may be effective. Distancing involves viewing a stimulus in a detached, objective and impartial manner. Such a strategy may invoke more of a task ‘mindset’ that is not stimulus-specific and relatively adaptive, even during anticipation.
Also, it would be very interesting to know whether individuals that vary within the normal or abnormal range of emotional responding and regulatory ability would show more or less RLPFC or insula activity during reappraisal anticipation. Among healthy individuals, future work may examine individual differences that may lead to greater or less RLPFC activity, including whether adaptive response patterns are more prevalent over time in aging. Regarding clinical implications, in one of the few neuroimaging studies to investigate the anticipation of emotional stimuli in a clinical population, Abler et al. (2007) reported elevated dorsolateral PFC (BA 9) activation in depressed patients for anticipation of negative vs positive stimuli in the absence of explicit instructions to subsequently regulate during stimulus presentation (Abler et al., 2007), which is consistent with the results of the current study. It would be similarly interesting to know whether patients with different forms of psychopathology involving emotion dysregulation would show greater anticipatory RLPFC activity in our paradigm, coupled with diminished ability to subsequently down-regulate amygdala responses to aversive stimuli.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We would like to thank Matthew Davidson, Brent Hughes and Melissa Brandon for assistance with data collection.
This work was supported by NIH Grant MH076137 (to K.N.O.) and MH076136 (to T.D.W.).
REFERENCES
- Abler B, Erk S, Herwig U, Walter H. Anticipation of aversive stimuli activates extended amygdala in unipolar depression. Journal of Psychiatric Research. 2007;41(6):511–22. doi: 10.1016/j.jpsychires.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. Journal of Neuroscience. 2010;30(39):12964–77. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Brain Research Review. 1996;22(3):229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personal Social Psychology. 1986;51(6):1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Gross J, Christensen TC, Benvenuto M. Knowing what you're feeling and knowing what to do about it: Mapping the relation between emotion differentiation and emotion regulation. Cognition and Emotion. 2001;15(6):713–24. [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Buhle J, Wager TD, Lopez R, Onyemekwu C, Silvers JA, Ochsner KN. Poster presented at 2011 Annual Meeting of Society for Neuroscience. Washington, D.C: 2011. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Scott SK, Frith CD. The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia. 2003;41(8):906–18. doi: 10.1016/s0028-3932(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Butler G, Mathews A. Anticipatory anxiety and risk perception. Cognitive Therapy and Research. 1987;11(5):551–65. [Google Scholar]
- Carlson JM, Greenberg T, Rubin D, Mujica-Parodi LR. Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Social Cognitive Affective Neuroscience. 2011;6(1):74–81. doi: 10.1093/scan/nsq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JD. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28(2):168–86. [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences USA. 2009;106(21):8719–24. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, et al. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14(5):1136–49. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Costa A, Oliveri M, Barban F, et al. Keeping memory for intentions: A cTBS investigation of the frontopolar cortex. Cerebral Cortex. 2011;21(12):2696–703. doi: 10.1093/cercor/bhr052. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex. 2011;21(7):1498–506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nature Neuroscience. 2008a;11(8):880–1. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008b;59(5):829–38. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24(8):1742–52. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Silvers JA, Ochsner KN. How we heal what we don’t want to feel: The functional neural architecture of emotion regulation. In: Kring AM, Sloan DM, editors. Emotion Regulation and Psychopathology: A Transdiagnostic Approach to Etiology and Treatment. New York: Guilford Press; 2009. pp. 59–87. [Google Scholar]
- Erk S, Abler B, Walter H. Cognitive modulation of emotion anticipation. European Journal of Neuroscience. 2006;24(4):1227–36. doi: 10.1111/j.1460-9568.2006.04976.x. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. Journal of Cognitive Neuroscience. 2006;18(6):932–48. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28(5):588–95. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2(3):271–99. [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98(7):4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U, Abler B, Walter H, Erk S. Expecting unpleasant stimuli–an fMRI study. Psychiatry Research. 2007a;154(1):1–12. doi: 10.1016/j.pscychresns.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Herwig U, Baumgartner T, Kaffenberger T, et al. Modulation of anticipatory emotion and perception processing by cognitive control. Neuroimage. 2007b;37(2):652–62. doi: 10.1016/j.neuroimage.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Herwig U, Kaffenberger T, Baumgartner T, Jancke L. Neural correlates of a ‘pessimistic' attitude when anticipating events of unknown emotional valence. Neuroimage. 2007c;34(2):848–58. doi: 10.1016/j.neuroimage.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Kaffenberger T, Bruhl AB, Baumgartner T, Jancke L, Herwig U. Negative bias of processing ambiguously cued emotional stimuli. Neuroreport. 2010;21(9):601–5. doi: 10.1097/WNR.0b013e328337ff18. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, et al. Anxiety reduction through detachment: subjective, physiological, and neural effects. Journal of Cognitive Neuroscience. 2005;17(6):874–83. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Herrmann K, Dolan RJ. Neural correlates of self-distraction from anxiety and a process model of cognitive emotion regulation. Journal of Cognitive Neuroscience. 2006;18(8):1266–76. doi: 10.1162/jocn.2006.18.8.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330(6006):932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- Klaaren KJ, Hodges SD, Wilson TD. The role of affective expectations in subjective experience and decision-making. Social Cognition. 1994;12(2):77–101. [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Martin LN, Delgado MR. The influence of emotion regulation on decision-making under risk. Journal of Cognitive Neuroscience. 2011;23(9):2569–81. doi: 10.1162/jocn.2011.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22(2):248–62. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage. 2010;49(2):1760–8. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. Journal of Comparative Neurology. 1982a;212(1):1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. Journal of Comparative Neurology. 1982b;212(1):38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Mischel W, Baker N. Cognitive appraisals and transformations in delay behavior. Journal of Personality and Social Psychology. 1975;31(2):254–61. [Google Scholar]
- Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. Journal of Comparative Neurology. 1982;212(1):23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. Journal of Comparative Neurology. 1984;227(1):109–20. doi: 10.1002/cne.902270112. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cognitive Affective and Behavioral Neuroscience. 2007;7(1):1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29(1):106–16. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17(2):153–8. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Hughes B, Robertson ER, Cooper JC, Gabrieli JD. Neural systems supporting the control of affective and cognitive conflicts. Journal of Cognitive Neuroscience. 2009a;21(9):1842–55. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, et al. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychological Science. 2009b;20(11):1322–31. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13(1):218–29. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends in Cognitive Sciences. 2008;12(2):65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57(3):210–19. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57(3):1221–33. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JD, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cognitive Affective and Behavioral Neuroscience. 2005;5(2):156–68. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- Savitsky K, Epley N, Gilovich T. Do others judge us as harshly as we think? Overestimating the impact of our failures, shortcomings, and mishaps. Journal of Personality and Social Psychology. 2001;81(1):44–56. doi: 10.1037//0022-3514.81.1.44. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Okada T, et al. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20(19):7438–45. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Buhle JT, Ochsner KN. The neuroscience of emotion regulation: Basic mechanisms and their role in development, aging and psychopathology. In: Ochsner KN, Kosslyn SM, editors. The Handbook of Cognitive Neuroscience. Vol. I. New York, NY: Oxford University Press; 2013. [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Ueda K, Okamoto Y, Okada G, Yamashita H, Hori T, Yamawaki S. Brain activity during expectancy of emotional stimuli: an fMRI study. Neuroreport. 2003;14(1):51–5. doi: 10.1097/00001756-200301200-00010. [DOI] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience and Biobehavioral Reviews. 2010;34(6):935–46. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2009;30(3):829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. A dissociation between social mentalizing and general reasoning. Neuroimage. 2011;54(2):1589–99. doi: 10.1016/j.neuroimage.2010.09.043. [DOI] [PubMed] [Google Scholar]
- van Reekum CM, Johnstone T, Urry HL, et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007;36(3):1041–55. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. Journal of Neuroscience. 2011;31(2):439–52. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Barrett LF. From affect to control: Functional specialization of the insula in motivation and regulation. Online publication at PsycExtra. 2004 http://www.apa.org/pubs/databases/psycextra. [Google Scholar]
- Wager TD, Barrett LF, Bliss-Moreau E, et al. The neuroimaging of emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotions. 3rd edn. New York: Guilford Press; 2008a. [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008b;59(6):1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22(4):1679–93. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26(1):99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47(3):836–51. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for FMRI data. 2000. http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf (3 July 2011, date last accessed)
- Wilson TD, Lisle DJ, Kraft D, Wetzel CG. Preferences as expectation-driven inferences: effects of affective expectations on affective experience. Journal of Personality and Social Psychology. 1989;56(4):519–30. doi: 10.1037//0022-3514.56.4.519. [DOI] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage. 2012;62(1):493–9. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.