Abstract
Behavioral inhibition (BI), a temperament characterized by vigilance to novelty, sensitivity to approach–withdrawal cues and social reticence in childhood, is associated with risk for anxiety in adolescence. Independent studies link reward hyper-responsivity to BI, adolescent anxiety and dopamine gene variants. This exploratory study extends these observations by examining the impact of DRD4 genotype and reward hyper-responsivity on the BI–anxiety link. Adolescents (N = 78) completed a monetary incentive delay task in the fMRI environment. Participants were characterized based on a continuous score of BI and the 7-repeat allele (7R+) of the DRD4 functional polymorphism. Parent-report and self-report measures of anxiety were also collected. Across the entire sample, striatal activation increased systematically with increases in the magnitude of anticipated monetary gains and losses. DRD4 status moderated the relation between BI and activation in the caudate nucleus. Childhood BI was associated with parent report of adolescent anxiety among 7R+ participants with elevated levels of striatal response to incentive cues. DRD4 genotype influenced the relations among neural response to incentives, early childhood BI and anxiety. The findings help refine our understanding of the role reward-related brain systems play in the emergence of anxiety in temperamentally at-risk individuals, building a foundation for future larger scale studies.
Keywords: fMRI, caudate, reward, genetics, temperament
INTRODUCTION
Behavioral inhibition (BI), a temperament identified in early childhood, is marked by an early-emerging and persistent tendency to display fear to novelty (Fox et al., 2005), social reticence (Garcia Coll et al., 1984; Coplan et al., 1994; Fox et al., 2005) and sensitivity to approach–avoidance cues (Fox et al., 2008). Children with stable, high levels of BI exhibit increased risk as adolescents for anxiety disorders (Schwartz et al., 1999; Prior et al., 2000; Kagan et al., 2001; Chronis-Tuscano et al., 2009), relative to non-behaviorally inhibited children. Similarities between BI and anxiety (Pérez-Edgar and Fox, 2005) are also evident at the neural level (Schwartz et al., 2003; Schwartz and Rauch, 2004; McClure et al., 2007; Pérez-Edgar et al., 2007), suggestive of common neural mechanisms underlying the phenotypic link between BI and anxiety.
Individuals with a history of BI also display unique patterns of striatal response to anticipated gains and losses (Guyer et al., 2006; Bar-Haim et al., 2009; Helfinstein et al., 2011, 2012). In a monetary incentive delay (MID) task, adolescents with a history of BI showed striatal hyper-reactivity to incentive cues relative to non-BI peers (Guyer et al., 2006). In a related task, this hyper-reactivity was specifically evident when incentive delivery was contingent on performance (Bar-Haim et al., 2009). In parallel, adolescents with social anxiety disorder (SAD) showed a similar pattern of hyper-reactivity to incentives, relative to adolescents with generalized anxiety disorder (GAD) and healthy adolescents (Guyer et al., 2012), suggesting that comparable alterations in striatal response to incentives may be at play for both BI and SAD. Although BI imparts an elevated risk for SAD (Chronis-Tuscano et al., 2009), most BI children do not manifest this disorder in adolescence (Degnan and Fox, 2007; Fox and Pine, 2012). Instead, there is often a broad pattern of elevated anxiety symptoms or internalizing difficulties across this generally healthy population (Williams et al., 2009). Thus, it is important to delineate factors that modulate the BI–anxiety link in order to eventually identify individuals more vulnerable to developing an anxiety disorder. Differential developmental trajectories are likely shaped by multiple mechanisms operating across a wide spectrum from genes to environment. In order to lay the foundation for future large-scale research and refine the targets of subsequent studies, the current exploratory work examines a genetic marker previously linked to both temperament and reward reactivity that may help better understand the relation between early BI and later anxiety.
Dopamine neurotransmission plays a fundamental role in reward functioning (Luciana et al., 2012) and can be indexed by dopamine-related genes. To date, the exon III polymorphism of the dopamine receptor D4 subtype (DRD4) has received the greatest empirical support, in terms of a specific gene associated with temperament-linked individual differences (Schmidt et al., 2007, 2009). In addition, DRD4 gene variants are directly linked to individual variations in reward sensitivity (Marco-Pallares et al., 2009; Camara et al., 2010). Specifically, the 7-repeat, long-allele variant of DRD4 (7R+) predicts weaker transmission of intracellular dopamine signals (Robbins and Everitt, 1999), and 7R+ carriers have been shown in previous work to display increased reward-related ventral striatum reactivity (Forbes et al., 2009) and risk for substance use in adolescence (Ellis et al., 2011). Tying these areas of research together, we also found that adolescents with a history of BI show higher levels of substance use (Williams et al., 2010), a risk pattern associated with striatal hyper-reactivity to incentives during the MID (Lahat et al., 2012).
Adolescence is a unique developmental window marked by peak incidence of psychopathology, particularly anxiety and substance use (Steinberg et al., 2006; Paus et al., 2008). Neurodevelopmental models (Ernst and Fudge, 2009; Somerville and Casey, 2010) suggest that this observed developmental pattern may be due to acute limbic and striatal sensitivity to salient stimuli, coupled with relatively immature regulatory mechanisms centered in the prefrontal cortex. This model suggests that the adolescent period may be particularly sensitive to differential gene effects on reward processing. Thus, this study, via its focus on adolescents, is well positioned to examine the contribution of dopamine gene effects and the neural correlates of reward sensitivity to previously observed patterns of socioemotional functioning in individuals with a history of BI.
Taken together, the literature suggests that the DRD4 gene might modulate previously seen links between BI and striatal hyper-reactivity. Striatal hyper-reactivity to incentives, in turn, has been documented in socially anxious adolescents (Guyer et al., 2012), further implicating this response pattern in the BI–anxiety association. Thus, the current exploratory study examines the inter-relations among striatal reactivity to incentives, BI and DRD4 variants. It is also examines the potential impact of striatal response and gene variants on anxiety levels in an at-risk sample.
We predict that (i) BI will be associated with striatal hyper-reactivity to incentive cues; (ii) this effect will be most pronounced in adolescents with the 7R+ allele and (iii) DRD4 status and striatal response to incentives will moderate the BI–anxiety relation.
METHODS
Participants
We recruited 78 adolescents (mean age = 16.33, s.d. = 2.83) from a larger longitudinal study of BI who had available DRD4 genotype and fMRI data (Tables 1 and 2). Data from subsets of the current participants have been published in previous studies involving the MID task (Guyer et al., 2006; Lahat et al., 2012).
Table 1.
Means (s.d.) of demographic and temperament measures for study participants
| Study Measures | All subjects | 7R+ | 7R− | BI |
|---|---|---|---|---|
| Age | 16.33 (2.84) | 15.75+ (2.69) | 16.84+ (2.80) | 0.173 |
| Gender | 38/40 | 16/16 | 21/25 | 0.169 |
| IQ | 114.20 (10.68) | 116.30 (9.95) | 112.73 (11.14) | 0.072 |
| SCARED parent | 9.38 (9.61) | 9.72 (9.84) | 9.25 (9.62) | 0.243* |
| SCARED child | 14.55 (10.93) | 14.48 (11.39) | 14.69 (10.84) | 0.086 |
| BI composite | −0.04 (0.70) | −0.02 (0.66) | −0.04 (0.73) | — |
| N | 78 | 32 | 46 | — |
Data are presented for the full sample in the first column, followed separately for DRD4 genotype (7R+, 7R−) in the next two columns. The final column presents the correlation between BI score and the row variable. Gender = male/female. +P < 0.10, *P < 0.05.
Table 2.
Correlations between the central measures and outcomes incorporated in this study
| BI | DRD4 | SCARED parent | SCARED child | High incentive | Medium incentive | Low incentive | |
|---|---|---|---|---|---|---|---|
| BI | — | ||||||
| DRD4 | 0.02 | — | |||||
| SCARED parent | 0.24* | 0.01 | — | ||||
| SCARED child | 0.09 | −0.01 | 0.59** | — | |||
| High incentive | −0.01 | −0.02 | −0.00 | −0.03 | — | ||
| Medium incentive | 0.03 | −0.02 | 0.11 | 0.07 | 0.77** | — | |
| Low incentive | 0.02 | −0.17 | 0.05 | 0.14 | 0.58** | 0.72** | — |
*P < 0.05. **P < 0.01.
Initially, 433 four-month-old infants were screened for reactivity to novel sensory stimuli; 153 infants were retained based on their scores (Fox et al., 2001). Individual differences in BI were assessed at 14 and 24 months and individual differences in social reticence were observed at 4 and 7 years of age. Laboratory assessments at 14 and 24 months presented children with novel and unfamiliar objects and people (Fox et al., 2001). At 4 and 7 years, children’s reticent behavior with unfamiliar peers was measured using Rubin’s Play Observation Scale (Rubin, 1989). Maternal ratings of their child’s social fear were collected at 14 and 24 months with the Toddler Behavior Assessment Questionnaire (Goldsmith, 1996). Mothers rated their child’s shyness at ages 4 and 7 years with the Colorado Child Temperament Inventory (Rowe and Plomin, 1977).
Individual behavioral and maternal report scores across the four testing waves were taken from the entire cohort (regardless of participation in this study). Past research has shown that high, stable levels of BI are most closely linked to elevated levels of anxiety (Chronis-Tuscano et al., 2009). Thus, we standardized and then averaged the individual Z-scores to create a composite measure of BI (mean = −0.008, s.d. = 0.641, range = −1.33 to 2.82). To capitalize on our large sample and examine the broad spectrum of BI, we used the continuous scores in our analyses below.
The participants included in this study were representative of the larger cohort with respect to mean BI scores overall (−0.04 for participants vs 0.02 for the cohort, P = 0.53).
Participants and their parents provided written informed assent/consent and were compensated for their participation.
DRD4 genotyping
Genomic DNA was prepared from saliva samples collected in the laboratory using Oragene•DNA kits (DNA Genotek, Ottawa, Ontario, Canada). DRD4 48-bp VNTR Polymorphism in exon 3 was amplified from 20 ng genomic DNA using the primer sequences: forward 5′-(GACCGCGACTACGTGGTCTACTC)-3′ and reverse 5′-(CTCTTGCAGCTTCGCCGCCAG)-3′ (Monuteaux et al., 2008).
Due to the high GC content in the VNTR region, amplification was performed using GC-rich PCR system (Roche Applied Science, 68298 Mannheim, Germany). PCR conditions were 98°C (3 min), 40 cycles of 98°C (30 s), 60.5°C (30 s), 68°C (45 s) and a final elongation, 68°C (10 min). The forward primer was labeled with the fluorescent dye 6-FAM, amplicons were visualized with GeneScan-1200 LIZ Size Standard (Applied Biosystems, Foster City, CA, USA) and analyzed on an ABI 3730 capillary sequencer. Allele sizes (allele 2, 404 bp; allele 3, 452 bp; allele 4, 500 bp; allele 5, 548 bp; allele 6, 596 bp; allele 7, 642 bp; allele 8, 692 bp; allele 9, 740 bp; allele 10, 785 bp and allele 11, 836 bp) were determined using GeneMapper v4.0 (Applied Biosystems).
Genotyping accuracy was determined empirically by duplicate genotyping of 25% of the samples selected randomly. The error rate was less than 0.005, and the completion rate was greater than 0.95. Genotype distributions for the full cohort, including adolescents not retained for the final analyses presented, were 2R/7R (N = 8, 6.0%), 3R/7R (N = 1, 0.7%), 4R/7R (N = 42, 31.3%), 7R/7R (N = 7, 4.7%), 7R/8R (N = 1, 0.7%), 2R/2R (N = 1, 0.7%), 2R/4R (N = 15, 11.2%), 3R/4R (N = 7, 5.2%), 4R/4R (N = 52, 38.8%) and 4R/5R (N = 1, 0.7%). The distribution was in line with the Hardy–Weinberg equilibrium (χ2 = 1.65, P = 0.20).
For the final sample, participants were classified as 7-repeat carriers (7R+; N = 32; 25 heterozygous and 7 homozygous) or non-carriers (7R−; N = 46). The DRD4 genotype groups did not differ on age, IQ, BI or sex (Ps > 0.10; Table 1). All participants were Caucasian.
Anxiety
BI is associated with increased risk for anxiety disorders (Chronis-Tuscano et al., 2009) and our previous work has linked SAD with an enhanced caudate and putamen response to incentives during the MID task (Guyer et al., 2012). Thus, we examined participants’ anxiety based on parent-report and self-report of anxiety on the Screen for Child Anxiety Related Emotional Disorders-Revised (SCARED-R) (Muris et al., 1999) concurrent with their neuroimaging scan. This study focused on a broad-band anxiety scale because of the low incidence of identified diagnoses, including social anxiety, in the current sample (see below). In addition, we wished to avoid the increased risk of generating Type I errors by running multiple analyses on the five instrument-specific subscales.
Analyses were run separately for the parent and child report scores. Although correlated, r = 0.59, P < 0.001, prior work has noted discrepancies between informants’ reports (Edelbrock et al., 1986; Salbach-Andrae et al., 2009), particularly in adolescence. Anxiety scores were not associated with age, IQ or sex (Ps > 0.11).
Diagnostic status
Experienced clinicians assessed participants using the Schedule for Affective Disorders and Schizophrenia for School Aged Children–Present and Lifetime Version (Kaufman et al., 1997) or the Structured Clinical Interview for DSM Disorders (Spitzer et al., 1992), depending on participant age. Twenty-one participants met criteria for one or more current or past Axis I diagnoses [five SAD, three GAD, seven attention deficit hyperactivity disorder, two tic disorder, two adjustment disorder, one post-traumatic stress disorder, two specific phobia, one major mood disorder and two enuresis]. Of these, eight were diagnosed with an anxiety disorder (SAD, GAD or both).
Participants with an anxiety diagnosis (N = 8), participants with another diagnosis (N = 13) and healthy participants (N = 57), were equivalent on all measures (Ps > 0.09), except for BI and parent-report SCARED scores. Individuals with current/past anxiety diagnoses had higher BI (0.21 vs −0.47 vs −0.12), F(2,73) = 3.52, P = 0.03, f = 0.31 and higher parent-report SCARED (18.88 vs 6.29 vs 6.53), F(2,73) = 7.36, P = 0.002, f = 0.45, scores vs participants with a non-anxiety diagnosis or no diagnosis, respectively. A similar, albeit non-significant, pattern emerged for self-report SCARED scores (21.25 vs 11.93 vs 11.57), F(2,73) = 2.75, P = 0.07, f = 0.27. Excluding individuals with current/past diagnoses did not change the pattern of findings presented below; they were therefore retained in the final analyses.
MID task
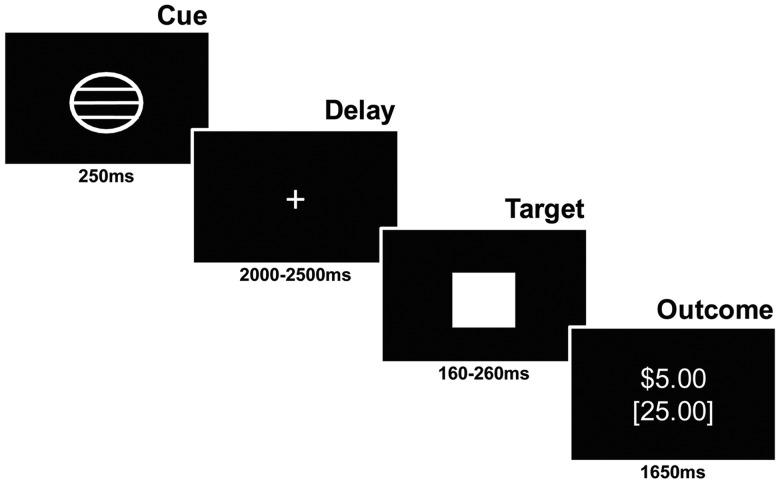
The task consisted of two runs of 72 contiguous 6 s trials involving presentation of a cue (250 ms), followed by a variable delay (2000–2500 ms) displaying a crosshair point, then a response target (160–260 ms), and finally outcome information (1650 ms) (Figure 1). Three cue types established trial condition: circle cues (n = 64) indicated potential monetary gain; square cues (n = 64) indicated potential loss and triangle cues (n = 16) indicated no incentive (neutral). Magnitude of the incentive value was conveyed via lines within the circle or rectangle: single line for small incentive ($0.20; n = 32), two lines for medium incentive ($1; n = 32) or three lines for high incentive ($5; n = 32). Correct responses made during target presentation yielded the anticipated gain or avoidance of potential loss. Participants were notified of their current and cumulative dollar amount. Trial type order was randomized. Participants were told that they would receive a percentage of the dollar amount won.
Fig. 1.
Schematic of the MID task employed in this study. The figure depicts a trial with a large potential reward ($5), which the participant was able to attain through a rapid button press response at the onset of the target.
Participants completed a practice MID task, providing an estimate of their reaction time (RT). Task difficulty was standardized to an ∼66% success rate by adjusting target duration (five difficulty levels) to minimize potential confounds from large performance differences across participants. After the task, participants rated each of the cues (large, medium and small potential gains and equivalent potential losses) on a scale from −5 (dislike very much) to +5 (like very much).
Behavioral data analysis
Dependent variables included mean difficulty level, accuracy, RT and post-scan ratings. Repeated-measures analyses of covariance (ANCOVAs) employed DRD4 group and continuous BI score, with valence and incentive magnitude as within-subjects factors. To parallel the imaging analyses, which used $0 trials as a baseline, RTs to $0 cues were also included in the models as a covariate.
fMRI acquisition
Scanning occurred in a Signa 3 tesla scanner (General Electric, Waukesha, WI, USA) for the first 64 participants. For the final 14 participants, scanning occurred in a GE 3 tesla Excite HDX scanner. Both groups used the same GE head coil. The two scanning groups did not differ on major demographic or research factors (Ps > 0.11), other than age (P < 0.01). To assess for any potential impacts of scanner, we first carried out our analyses (below) with scanner type as a between-subjects factor and again as a covariate. We found no significant effect of scanner on blood oxygen level-dependent (BOLD) signal (Ps > 0.12). We then replicated the reported analyses with only the first 64 participants. One critical finding for this study was a three-way BI-by-DRD4-by-incentive interaction (below), which remained significant (P = 0.03) in the participants studied on the initial scanner. The equivalent analyses in the remaining 14 participants could not be completed due to sample size.
A Cedrus Lumina response box (Psychological Software Tools, San Pedro, CA, USA) recorded behavioral data. Task stimuli were projected onto a screen at the foot of the scanner bed and viewed with mirrors mounted on the head coil. Foam padding constrained head movement.
Each brain volume consisted of 30 interleaved slices, 4-mm thick, acquired in the sagittal plane using a T2*-weighted echo-planar sequence with a repetition time (TR) of 2500 ms, echo time (TE) of 23 ms and flip angle of 90°. Voxel dimension was 3.75 × 3.75 × 4.0 mm. Matrix size was 64 × 64, and field of view (FOV) was 24 cm. To allow for signal stabilization, four acquisitions were obtained before task onset. A high-resolution structural image was also acquired for each subject using a T1-weighted standardized magnetization prepared spoiled gradient recalled echo sequence: 124 1-mm slices, 8100 ms TR, 32 ms TE, 15° flip angle, 256 × 256 matrix and 24 cm FOV.
fMRI data analysis
Analysis of functional and neural images (AFNI) software was used for the analyses (Cox, 1996). As with the behavioral data, error trials were removed from processing. Standard preprocessing of echo-planar data included slice time correction, motion correction and spatial smoothing with a 6-mm full-width half-maximum smoothing kernel. Signal deviations >2.5 s.d. from the mean were removed using an AFNI despiking algorithm applied on a voxel-wise basis and a band-pass filtering algorithm to remove cyclical fluctuations in signal (either >0.01 or <0.15 Hz) not temporally indicative of a hemodynamic response. We then normalized BOLD signal intensity to percentage signal change using each subject’s voxel-wise time series mean as a baseline.
Preprocessed time series data for each individual were analyzed by multiple regression (Neter et al., 1996). In line with previous work with the task (Guyer et al., 2006, 2012), the regression model included both cues and feedback, although only the cue period was analyzed for this study. Regressors modeled effects attributable to residual motion (using the motion correction factors in the x, y and z planes and in the yaw, pitch and roll dimensions), as well as baseline and linear trends for each of the two runs. Regressors of interest included cues signaling trial type (e.g. large, medium and small potential gains and equivalent potential losses) and were convolved with a gamma variate function that modeled a prototypical hemodynamic response (Cohen, 1997). Idealized signal time courses were estimated based on onset time of different event types during the task.
The first analysis examined the effects of BI and DRD4 on BOLD signal during the MID task. The second set of analyses treated anxiety ratings on the SCARED (separately for parent and child scores) as a dependent measure to examine the effects of childhood BI score, DRD4 group and adolescent BOLD activation.
BI and DRD4 modulation of striatal activity
Contrasts of BOLD activation were created for each subject for cues signaling magnitude of (i) large, (ii) medium and (iii) small vs no incentive. Our main analysis predicting levels of BOLD activity targeted the interaction of BI score with DRD4 group on striatal activation in response to incentive anticipation. Based on our a priori hypothesis focused on striatal activation, and our previous findings with this task (Guyer et al., 2006, 2012), we used a region of interest (ROI) approach to assess bilateral caudate (body and head), putamen and nucleus accumbens activation. Previous BI and anxiety studies (Guyer et al., 2006, 2012; Bar-Haim et al., 2009; Helfinstein et al., 2011) have found varying patterns of results across ROIs. Therefore, we examined each ROI separately.
ROIs were defined by anatomical boundaries provided by AFNI after spatial normalization (Talairach and Tournoux, 1988), again in line with previous work with this task (Guyer et al., 2006, 2012). Specifically, we extracted values from the ROIs to reflect the main effects of task for our contrasts of interest (large, medium and small vs no incentive). Thus, three contrast values were generated per subject for each ROI, thereby minimizing Type I errors associated with multi-voxel testing.
Individual contrast values were extracted from the ROIs and entered into group-level repeated-measures ANCOVA performed in SPSS (SPSS 19.0, Chicago, IL, USA). The initial model included DRD4 group (7R+, 7R−) and BI score as the main predictors. Incentive magnitude (small, medium and large), valence (gain and loss) and laterality (right and left) served as within-subjects factors. To minimize Type 1 errors, the Greenhouse–Geisser (G–G) procedure was applied when appropriate. The degrees of freedom indicated in the text are those before the G–G correction. However, epsilon (∈) is noted when less than 1.0.
The initial analyses revealed only the expected linear main effect of incentive level in the nucleus accumbens, F(1,73) = 7.38, P = 0.008, d = 0.64, ∈ = 0.84. Similarly, for the putamen, there were linear main effects of incentive level, F(1,73) = 22.84, P < 0.001, d = 1.12, ∈ = 0.93, and valence, F(1,73) = 5.54, P = 0.02, d = 0.55, with greater activation for gain cues than loss cues. As neither was associated with either BI or DRD4 genotype, they were not included in the full analyses.
The central findings for the caudate are presented below. Our focus on the caudate was also confirmed by a whole-brain analysis (threshold of P = 0.001 with a 10-voxel-size minimum) which found significant activation in this region (peak t = −4.18).
Predicting anxiety levels with BI, DRD4 and caudate activation
To test hypothesized DRD4 genotype and caudate activation modulation of the BI–anxiety link, we regressed SCARED scores in adolescence on early-childhood BI score (continuous), DRD4 (7R+, 7R−) and adolescent BOLD activation at each incentive level (high, medium and low) in the caudate. Continuous predictive measures were mean centered before use in the regressions. The regressions were run separately for the parent-report and self-report SCARED scores.
Predictors were initially entered into a hierarchical linear regression in six sequential blocks: (i) scanner, age and task difficulty level; (ii) BI, DRD4, high, medium and low; (iii) BI-by-DRD4; (iv) BI-by-high, BI-by-medium and BI-by-low; (v) DRD4-by-high, DRD4-by-medium and DRD4-by-low and (vi) BI-by-DRD4-by-high, BI-by-DRD4-by-medium and BI-by-DRD4-by-low. None of the predictors in Step 1 contributed significantly to the model, as such they were removed from the subsequent analysis presented here. Given the large number of predictors, we then completed a backward elimination procedure, removing predictors failing to reach probability cut-offs, in order to create a more parsimonious model for comparison. As a final confirmatory analysis, we repeated the model using 5000 bootstrapping samples.
RESULTS
Behavioral data
Post-scan ratings of likeability
Behavioral data acquired after scanning demonstrated the expected patterns of performance, confirming that adolescents understood cue manipulations. Both incentive values and valence influenced the post-scan ratings, F(2,146) = 64.94, P < 0.001, f = 0.94, which increased linearly from a large potential loss to a large potential win.
There was a significant BI by valence interaction, F(1,73) = 5.76, P = 0.02, f = 0.28, such that BI was positively associated with ratings of loss cues (r = 0.32, P = 0.007) and negatively associated with ratings of gain cues (r = −0.30, P = 0.012).
Task performance scores
BI was positively associated with difficulty level, F(1,70) = 5.58, P = 0.02, f = 0.28. As such, we initially included difficulty level as a nuisance covariate for all analyses. There were no significant effects involving this covariate for DRD4, P = 0.24.
Performance did not differ across BI scores or DRD4 groups on RTs (Ps > 0.10) or accuracy (Ps > 0.09). Thus, with the individualized task parameters, the groups attained comparable levels of success for both the gain and loss trials.
fMRI data
BI and DRD4 modulation of caudate activity
The initial hypothesis was tested in a multi-factorial omnibus ANCOVA. Laterality and valence showed no significant effects. In addition, age, sex and difficulty level had no significant impact when included as covariates. We removed non-significant factors and the presented analysis is restricted to the three main factors of interest: incentive, BI and DRD4.
As expected, caudate activity varied by incentive value, indicating a linear increase in caudate activation with increased monetary value (0.050 vs 0.099 vs 0.126), F(1,73) = 27.60, P < 0.001, d = 1.23, ∈ = 0.85. We also found a three-way incentive-by-BI-by-DRD4 interaction, F(1,73) = 7.26, P = 0.009, d = 0.63. The finding is illustrated in Figure 2 using the GroupAna procedure within AFNI (Cox, 1996).
Fig. 2.
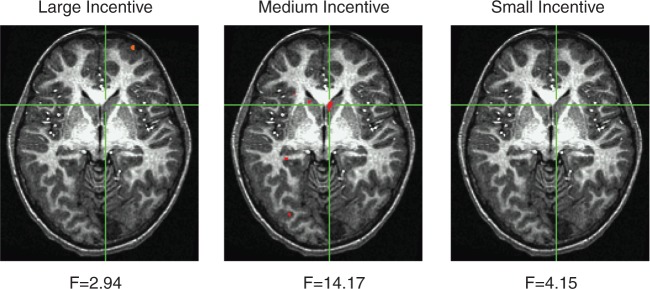
Changes in BOLD signal for the BI by DRD4 (7R+, 7R−) interaction using the GroupAna procedure in AFNI. Activation patterns are presented separately for the small, medium and large incentive cues. A significant interaction pattern was evident for the medium cues (peak differences were found at Talairach coordinates −5, −16, 0, P < 0.001).
To interpret the three-way interaction, we decomposed the initial ANCOVA and repeated the analysis separately for the DRD4 groups. For the 7R+ group, we found a significant BI-by-incentive interaction, F(1,29) = 4.10, P = 0.05, d = 0.75. There was little relation between BI and caudate activity for the high (r = 0.01, P = 0.95) and low (r = 0.03, P = 0.86) incentive cues. The significant interaction was likely driven by the BI–activation relation for medium incentives, although the individual correlation was non-significant, (r = 0.28, P = 0.13). In contrast, the 7R− group showed no significant interaction, F(1,44) = 2.41, P = 0.13, d = 0.47.
Unlike our initial BI study (Guyer et al., 2006), the main effect of BI on striatal activation was not significant in the initial omnibus ANCOVA, P = 0.80 (see also Table 2). This may be due to the fact that the original study employed an extreme-groups approach, while here we relied on a larger, broader sample of the BI distribution. The inclusion of participants with a wide range of scores on BI may have diluted direct temperament-related differences, thus suggesting the need to closely examine potential moderators.
Predicting anxiety levels with BI, DRD4 and caudate activation
We first regressed parent report SCARED scores on childhood BI scores, DRD4 genotype group and adolescent BOLD activity at each incentive level (Table 3). The full model accounted for 35.5% of the total variance, F(15,74) = 2.16, P = 0.018. Although there were multiple significant contributors, the highest order predictors were the BI-by-DRD4-by-low interaction, the BI-by-DRD4-by-medium interaction and the BI-by-high interaction. Given the large number of predictors relative to sample size in the initial regression, we then repeated the analysis using a backward removal process. The three higher order predictors were retained in the final model. Finally, we completed a confirmatory analysis with 5000 bootstrapping samples, again confirming three core predictors, as each was found to be significant and the 95% confidence intervals did not include zero. We then examined the individual contributions of these three predictors to the model.
Table 3.
Predicting SCARED scores using childhood BI, DRD4 group and concurrent BOLD activation levels
| Predictor | β | t | P |
|---|---|---|---|
| BI | 0.151 | 0.980 | 0.331 |
| DRD4 | −0.228 | −1.728 | 0.089 |
| BI-by-DRD4 | −0.327 | −1.532 | 0.131 |
| High incentive | −0.250 | −0.992 | 0.325 |
| Medium incentive | 0.833 | 2.591 | 0.012 |
| Low incentive | 0.622 | 2.326 | 0.023 |
| BI-by-high incentive | 0.319 | 1.971 | 0.050 |
| BI-by-medium incentive | 0.173 | 0.699 | 0.487 |
| BI-by-low incentive | 0.048 | 0.241 | 0.810 |
| DRD4-by-high incentive | −0.227 | −0.936 | 0.353 |
| DRD4-by-medium incentive | 0.030 | 0.104 | 0.917 |
| DRD4-by-low incentive | 0.026 | 0.109 | 0.914 |
| BI-by-DRD4-by-high incentive | 0.133 | 0.790 | 0.432 |
| BI-by-DRD4-by-medium incentive | 0.528 | 1.976 | 0.050 |
| BI-by-DRD4-by-low incentive | 0.642 | 2.693 | 0.009 |
| F(15,74) = 2.17, P = 0.02 | |||
The table presents the predictors from the initial hierarchical regression model. The equivalent model for self-report SCARED scores was not significant. Standardized β are presented. Significant predictors are in bold (p < 0.05).
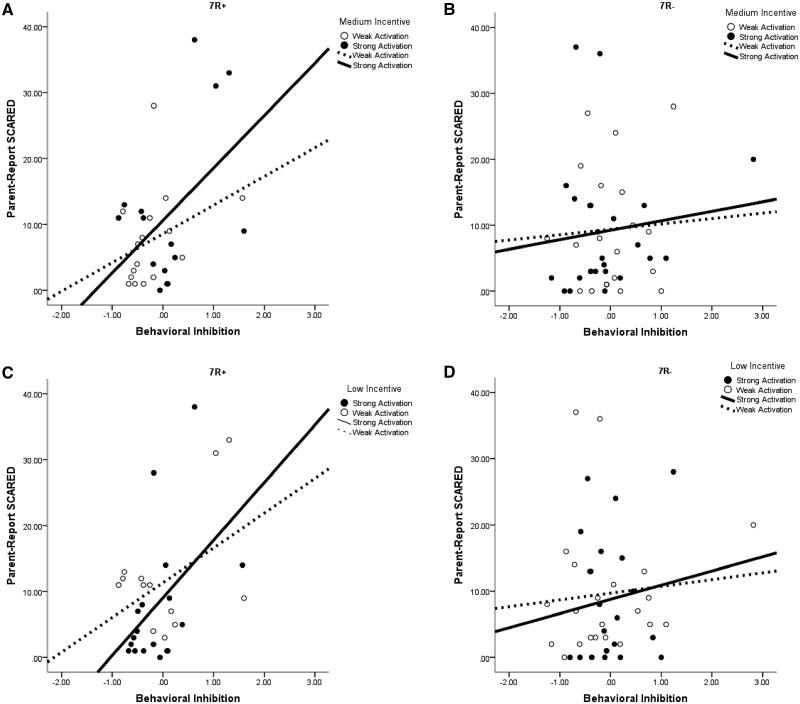
The BI-by-DRD4-by-low interaction accounted for 8.3% of the variance. The zero-order correlations between BI and SCARED scores were then calculated separately by DRD4 group and activation level (median split creating strong-BOLD and weak-BOLD groups). For adolescents with 7R+ and strong-BOLD activation, BI predicted anxiety, r = 0.59, P = 0.04 (Figure 3). In contrast, the relation was non-significant for adolescents with 7R+ and weak-BOLD activation, r = 0.46, P = 0.08. For 7R−, neither BOLD group showed a significant relation, rs < 0.07, Ps > 0.75.
Fig. 3.
The figures depict the BI by DRD4 by incentive interaction evident in the regression analyses predicting parent report of adolescent anxiety for medium incentives in the 7R+ (A) and 7R− (B) groups, as well as the low incentive trials for 7R+ (C) and 7R− (D).
The BI-by-DRD4-by-medium interaction accounted for 4.9% of the variance. Again, the zero-order correlation between BI and SCARED scores was significant, r = 0.60, P = 0.02, for adolescents with 7R+and strong-BOLD activation. The relation was non-significant for the 7R+/weak BOLD-activation group, r = 0.33, P = 0.24. Again, neither 7R− activation group was significant, rs < 0.08, Ps > 0.75.
Finally, the two-way BI-by-high interaction accounted for 4.5% of the variance. The zero-order correlation between BI and SCARED scores was significant, r = 0.47, P < 0.01, for adolescents with strong-BOLD activation. In contrast, for participants with relatively weak activation, the correlation was not significant, r = 0.02, P = 0.92.
The second regression used self-report SCARED scores as the outcome measure. In the end, the model did not reach significance, F(15,74) = 1.01, P = 0.46. To compare with the parent report regression, we examined the three interactions that emerged in that analysis. Together, they accounted for only 7.4% of the total variance, F(3,74) = 1.81, P = 0.15.
In summary, this analysis revealed that BOLD response to high levels of reward modulated the relation between early BI and anxiety in adolescence, independently of DRD4 allelic status. However, for low and medium magnitude incentives, the relation between BI and anxiety emerged only for individuals with both a large striatal response and the 7R+ DRD4 allele.
DISCUSSION
The current exploratory study examined the relations between early childhood BI and neural sensitivity to anticipated reward in adolescence. Based on the central role of dopamine in reward processing (Luciana et al., 2012) and its previous association with temperament-linked behaviors (Schmidt et al., 2007, 2009; Ellis et al., 2011), we examined whether the BI–reward processing association was modulated by variations in DRD4 allelic status. Finally, given the phenotypic link between BI and anxiety (Pérez-Edgar and Fox, 2005; Fox and Pine, 2012) and recent neuroimaging work showing parallel patterns of neural functioning during the MID task for behaviorally inhibited and anxious adolescents (Guyer et al., 2006, 2012), we examined whether the BI–anxiety link was modulated by variations in DRD4 and the neural response to reward.
This study examines the moderating effects of allelic status on neural activation associated with both temperament and reward processing. It also examines the relation between neural activation and anxiety symptomatology within a sample selected for early temperamental variations. Thus, these data examine the functional significance of the reward-related variations seen across previous studies of temperament (Guyer et al., 2006; Bar-Haim et al., 2009; Helfinstein et al., 2011). These preliminary results will allow future larger scale studies to more effectively target individuals most vulnerable to anxiety and suggest mechanisms that can modulate the long-term consequences of early temperament.
No relations were observed between BI and striatal activation when relying on continuous scores in our full sample. The participants in our study were characterized based on BI and social reticence scores derived from multiple laboratory and questionnaire-based observations over a 7-year period. This continuous score and our large sample reflected a broad spectrum of BI, suggesting that our previous finding directly linking BI to heightened striatal activation is not distributed linearly across the population. Debate within the literature has centered on whether temperament is best characterized as gradual variation across a broad spectrum or discrete shifts in functioning across qualitatively distinct subgroups (Rothbart, 2004; Kagan et al., 2007). The current data suggest that while extreme groups that reflect the prototypic markers of a trait may show significant differences in an outcome of interest (Guyer et al., 2006), additional moderators may need to be incorporated in order for similar patterns to emerge across a wider, more heterogeneous, spectrum.
In this study, DRD4 modulated the relation between BI and caudate activation. The current data support this previous work (Zink et al., 2004) suggesting that striatal activity tracks stimulus salience rather than the absolute value of a cue. Adolescents with a history of BI were previously found to exhibit elevated striatal response to reward only when the outcome was contingent on performance (Bar-Haim et al., 2009) and thus more salient, in line with data noting increased levels of performance monitoring in BI (McDermott et al., 2009; Henderson, 2010). This hypothesis could be tested in future work with larger samples, investigating the unique conditions during which individuals with a history of BI invest stimuli with salience, and whether salience is indeed the main parameter responsible for distinctive striatal responses in BI.
Our focus was on the caudate nucleus, as no significant findings emerged for the nucleus accumbens or putamen. Recent work suggests striatal sub-region dissociations in response to different types of reward, particularly in adolescence (Ernst and Fudge, 2009). The caudate nucleus plays a central role in instrumental responding (Yin et al., 2008) and goal-based processes (Yin and Knowlton, 2006). Children with, or at risk for, anxiety may be particularly sensitive to reward- or loss-related environmental cues and feedback (Fox and Helfinstein, 2013). The caudate, as well as the broader dopaminergic system, is integral to learning the reward or punishment values of an environmental cue. This general sensitivity to salient incentive cues may contribute to the sensitivity behaviorally inhibited children show to social feedback. That is, for the temperamentally vulnerable child, this sensitivity may mean that only a relatively few negative social encounters may be needed before strong avoidance patterns become entrenched and lead to the manifestation of anxiety (Helfinstein et al., 2012). Indeed, recent work found an atypical caudate response to social feedback in adolescents with a history of BI (Guyer et al., in press).
In line with the larger literature, early childhood BI was associated with increased levels of parent-reported anxiety. At high levels of incentive, there was a direct effect of striatal activation on anxiety for adolescents with a history of BI, relative to their non-inhibited peers. In contrast, for medium and low incentives, the pattern was only evident when early temperamental inhibition was coupled with the 7R+ variant of DRD4. This suggests that these moderate markers of gain and loss may be more open to subjective assessments of salience, as opposed to the clearly significant high-incentive cue. To the best of our knowledge, this is the first study to demonstrate the role of caudate functioning, manifest during adolescence, in the emergence of anxiety within a prospectively followed, non-clinical sample selected for a temperamental trait in infancy and early childhood.
We found no relations between early BI and adolescent anxiety for participants lacking the genetic or neural marker of reward sensitivity. Typically, early risk can be mitigated by protective factors that are either environmental (e.g. sensitive parenting) or biological (e.g. more efficient self-regulation mechanisms), such that most behaviorally inhibited children do not go on to show extremely high levels of social anxiety in adolescence. However, individuals who also harbor additional mechanisms of risk for anxiety, such as genetic risk factors, may be ‘resistant’, to these protective mechanisms. A similar argument has been made within the attention literature, suggesting that early BI is associated with later social withdrawal only if also coupled with attention biases to threat (Pérez-Edgar et al., 2010a, 2011). Our current data suggest that sensitivity to incentive cues, manifest in the caudate response and coupled with markers of genetic risk, may also act to link early risk to the later emergence of maladaptation, as in attention (Pérez-Edgar et al., 2010b).
The current findings should be viewed in light of study limitations. The use of an adolescent sample allowed us to focus on a developmental window marked by the peak incidence of both reward sensitivity and psychopathology. However, while the sample size is fairly large for a neuroimaging study, the sub-groups were not large enough to allow for further exploration of additional moderators, such as sex, age or pubertal status. Although sex- and age-related differences in dopamine transmission may influence striatal function (Andersen et al., 1997), the direction of these differences is not well understood (Andersen et al., 2002). In addition, since reward processes were only assessed in adolescence, we cannot comment on any changes in the relation between the core measures over time. A longitudinal study incorporating multiple measures of each predictor over time would be able to address this issue.
DRD4 only indirectly reflects levels of dopaminergic activity. Our use of a healthy adolescent sample precluded more invasive measures or manipulations of dopamine levels. However, there is recent evidence that supports our presumptive link between DRD4 allelic status and dopamine variation. Past work has found modulations in striatal activation to reward with dopaminergic medication (Riba et al., 2008) and BOLD signal changes in the striatum have been correlated with dopamine release (Knutson and Gibbs, 2007). We were also unable to address variations across multiple candidate genes that may impact both temperament and reward processing (Smoller et al., 2001).
Finally, the relatively small sample did not allow us to explicitly incorporate psychiatric status into the analyses. Post-hoc analyses found that removal of affected adolescents did not alter the reported findings. A direct comparison of BI and clinically anxious adolescents would allow researchers to assess the extent to which at-risk and affected adolescents parallel each other in striatum- (Guyer et al., 2006, 2012) and amygdala-linked (McClure et al., 2007; Pérez-Edgar et al., 2007) processing. A larger sample would have also allowed us to further explore the differing pattern of results for the parent report and self-report measures of anxiety. Recent work has attempted to determine whether informant discrepancies are simply measurement error or meaningful indicators of differences in intra-personal vs inter-personal assessments (De Los Reyes, 2011).
In summary, this study expands our understanding of the scope of neural functions associated with BI (Fox et al., 2008) and their relations, in turn, to psychological outcomes. In doing so, the data document a common gene variant interacting with temperament measured in the first years of life to modulate neural functioning across childhood into adolescence. In addition, we examine the functional impact of these relations on the emergence of anxiety in an at-risk sample.
Funding
Funding was provided by the Intramural Research Program of National Insitutes of Health (NIH), as well as grants from NIH (MH073569, MH094633) and NARSAD Foundation (Blowitz-Ridgeway Young Investigator Award to K.P.E.); the NIH (MH074454, R37HD17899) and NARSAD Foundation (Distinguished Investigator Award to N.A.F.) and the NIH (MH080076 to A.E.G.).
Conflict of Interest
None declared.
Acknowledgments
The authors thank the research team at the University of Maryland and NIMH for their assistance in the longitudinal data collection. They would especially like to thank the parents of the children who participated and continue to participate in our studies.
REFERENCES
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–8. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AP, Kerenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27:683–91. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, et al. Neural correlates of reward processing in adolescents with a history inhibited temperament. Psychological Science. 2009;20:1009–18. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara E, Kramer UM, Cunillera T, et al. The effects of COMT (Val108/158Met) and DRD4 (SNP-521) dopamine genotypes on brain activations related to valence and magnitude of rewards. Cerebral Cortex. 2010;20:1985–96. doi: 10.1093/cercor/bhp263. [DOI] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan K, Pine D, et al. Stable behavioral inhibition during infancy and early childhood predicts the development of anxiety disorders in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:928–35. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Coplan RJ, Rubin KH, Fox NA, Calkins SD, Stewart SL. Being alone, playing alone, and acting alone: distinguishing among reticence and passive and active solitude in young children. Child Development. 1994;65:129–37. [PubMed] [Google Scholar]
- Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- De Los Reyes A. More than measurement error: discovering meaning behind informant discrepancies in clinical assesments of children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2011;40:1–9. doi: 10.1080/15374416.2011.533405. [DOI] [PubMed] [Google Scholar]
- Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: multiple levels of a resilience process. Development and Psychopathology. 2007;19:729–46. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- Edelbrock C, Costello A, Dulcan M, Conover N. Parent–child agreement on child psychiatric symptoms assessed via structured interview. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1986;27:181–90. [PubMed] [Google Scholar]
- Ellis JA, Olsson CA, Moore E, Greenwood P, Van den Ven MOM, Patton GC. A role of the DRD4 Exon III VNTR in modifying the association between nicotine dependence and neuroticism. Nicotine & Tobacco Research. 2011;13:64–9. doi: 10.1093/ntr/ntq210. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge J. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews. 2009;33:367–82. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity [Article] Molecular Psychiatry. 2009;14(1):60–70. doi: 10.1038/sj.mp.4002086. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Helfinstein SM. The contribution of temperament to the study of social cognition: learning whether the glass is half empty or half full. In: Banaji MR, Gelman S, editors. The Development of Social Cognition. Cambridge, MA: Harvard University Press; 2013. [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–62. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Pérez-Edgar K, White L. The biology of temperament: an integrative approach. In: Nelson C, Luciana M, editors. The Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press; 2008. pp. 839–54. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Pine DS. Temperament and the emergence of anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:125–8. doi: 10.1016/j.jaac.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Coll C, Kagan J, Reznick JS. Behavioral inhibition in young children. Child Development. 1984;55:1005–19. [Google Scholar]
- Goldsmith HH. Studying temperament via construction of the toddler behavior assessment questionnaire. Child Development. 1996;67:218–35. [PubMed] [Google Scholar]
- Guyer AE, Benson B, Choate VR, et al. Behaviorally inhibited temperament in early childhood is associated with attenuated response in ventral striatum to positive social feedback in adolescence. Development and Psychopathology. in press [Google Scholar]
- Guyer AE, Choate VR, Detloff A, et al. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. American Journal of Psychiatry. 2012;169:205–12. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26(24):6399–405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein SM, Benson B, Pérez-Edgar K, et al. Striatal responses to negative monetary outcomes differ between behaviorally inhibited and non-inhibited adolescents. Neuropsychologia. 2011;49:479–85. doi: 10.1016/j.neuropsychologia.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein SM, Fox NA, Pine DS. Approach-withdrawal and the role of the striatum in the temperament of behavioral inibition. Developmental Psychology. 2012;48:815–26. doi: 10.1037/a0026402. [DOI] [PubMed] [Google Scholar]
- Henderson HA. Electrophysiological correlates of cognitive control and the regulation of shyness in children. Developmental Neuropsychology. 2010;35:177–93. doi: 10.1080/87565640903526538. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N, Kahn V, Towsley S. The preservation of two infant temperaments into adolescence. Monographs of the Society for Research in Child Development. 2007;72:1–75. doi: 10.1111/j.1540-5834.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N, McManis M, Woodward S. Temperamental contributions to the affect family of anxiety. The Psychiatric Clinics of North America. 2001;24(4):677–88. doi: 10.1016/s0193-953x(05)70257-4. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–88. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology. 2007;191:813–22. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Lahat A, Pérez-Edgar K, Degnan KA, et al. Early childhood temperament predicts substance use in young adults. Translational Psychiatry. 2012;2:e157. doi: 10.1038/tp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Wahlstrom D, Porter JN, Collins P. Dopaminergic modulation of incentive motivation in adolescence: age-related changes in signaling, individual diffdrences, and implications for the development of self-regulation. Developmental Psychology. 2012;48:844–61. doi: 10.1037/a0027432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco-Pallares J, Cucurell D, Cunillera T, et al. Genetic variability in the dopamine system (dopamine receptor D4, catechol-O-methyltransferase) modulates neurophysiological responses to gains and losses. Biological Psychiatry. 2009;66:154–61. doi: 10.1016/j.biopsych.2009.01.006. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64:109–116. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Pérez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox N. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–8. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monuteaux MC, Seidman LJ, Faraone SV, et al. A preliminary study of dopamine D4 receptor genotype and structural brain alterations in adults with ADHD. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2008;147B:1436–41. doi: 10.1002/ajmg.b.30870. [DOI] [PubMed] [Google Scholar]
- Muris P, Merckelbach H, Schmidt H, Mayer B. The revised version of the screen for child anxiety related emotional disorders (SCARED-R): factor structure in normal children. Personality and Individual Differences. 1999;26:99–112. [Google Scholar]
- Neter J, Kutner M, Machtsheim C, Wasserman W. Applied Linear Statistical Models. 4 edn. Chicago: Irwin; 1996. [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Review Neuroscience. 2008;9:947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Bar-Haim Y, McDermott JM, Chronis-Tuscano A, Pine DS, Fox NA. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010a;10:349–57. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Fox NA. Temperament and anxiety disorders. Child and Adolescent Psychiatric Clinics of North America. 2005;14:681–706. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, McDermott JM, Korelitz K, et al. Patterns of sustained attention in infancy shape the developmental trajectory of social behavior from toddlerhood through adolescence. Developmental Psychology. 2010b;46:1723–30. doi: 10.1037/a0021064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Reeb-Sutherland BC, McDermott JM, et al. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. Journal of Abnormal Child Psychology. 2011;39:885–95. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Roberson-Nay R, Hardin MG, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35:1538–46. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior M, Smart D, Sanson A, Oberklaid F. Does shy-inhibited temperament in childhood lead to anxiety problems in adolescence? Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(4):461–8. doi: 10.1097/00004583-200004000-00015. [DOI] [PubMed] [Google Scholar]
- Riba J, Kramer UM, Heldmann M, Richter S, Munte TF. Dopamine agonist increases risk taking but blunts reward-related brain activity. PLoS One. 2008;3:e2479. doi: 10.1371/journal.pone.0002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Motivation and reward. In: Zigmond MJ, editor. Fundamental Neuroscience. San Diego: Academic Press; 1999. pp. 1246–60. [Google Scholar]
- Rothbart MK. Commentary: differentiated measures of temperament and multiple pathways to childhood disorders. Journal of Clinical Child and Adolescent Psychology. 2004;33(1):82–7. doi: 10.1207/S15374424JCCP3301_8. [DOI] [PubMed] [Google Scholar]
- Rowe DC, Plomin R. Temperament in early childhood. Journal of Personality Assessment. 1977;41:150–6. doi: 10.1207/s15327752jpa4102_5. [DOI] [PubMed] [Google Scholar]
- Rubin KH. The Play Observation Scale (POS) Ontario, Canada: University of Waterloo; 1989. [Google Scholar]
- Salbach-Andrae H, Lenza K, Lehmkuhl U. Patterns of agreement among parent, teacher and youth ratings in a referred sample. European Psychiatry. 2009;24:345–51. doi: 10.1016/j.eurpsy.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox N, Hamer D. Evidence for a gene–gene interaction in predicting children’s behavior problems: association of serotonin transporter short and dopamine receptor D4 long genotypes with internalizing and externalizing behaviors in typically developing 7-year-olds. Development and Psychopathology. 2007;19:1105–16. doi: 10.1017/S0954579407000569. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Pérez-Edgar K, Hamer DH. Linking gene, brain, and behavior: DRD4, frontal asymmetry, and temperament. Psychological Science. 2009;20:831–7. doi: 10.1111/j.1467-9280.2009.02374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Rauch SL. Temperament and its implications for neuroimaging of anxiety disorders. CNS Spectrums. 2004;9(4):284–91. doi: 10.1017/s1092852900009226. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–15. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300:1952–3. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Rosenbaum JF, Biederman J, et al. Genetic association analysis of behavioral inhibition using candidate loci from mouse models. American Journal of Medical Genetics: Neuropsychiatric Genetics. 2001;105:226–35. doi: 10.1002/ajmg.1328. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinions in Neurobiology. 2010;20:236–41. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. The structured clinical interview for DSM-III-R (SCID) I: history, rationale, and description. Archives of General Psychiatry. 1992;49(8):624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Dahl R, Keating D, Kupfer DJ, Masten AS, Pine D. The study of developmental psychopathology in adolescence: integrating affective neuroscience with the study of context. In: Cicchetti D, Cohen DJ, editors. Psychophysiological Methods for the Study of Developmental Psychopathology. New York: Wiley; 2006. pp. 710–41. [Google Scholar]
- Talairach J, Tournoux P. Co-planar Sterotaxic Atlas of the Human Brain. Stuttgart, Germany: Thieme Verlag; 1988. [Google Scholar]
- Williams LR, Degnan KA, Pérez-Edgar K, et al. Impact of behavioral inhibition and parenting style on internalizing and externalizing problems from early childhood through adolescence. Journal of Abnormal Child Psychology. 2009;37:1063–75. doi: 10.1007/s10802-009-9331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LR, Fox NA, Lejuez CW, et al. Early temperament, propensity for risk-taking and adolescent substance-related problems: a prospective multi-method investigation. Addictive Behaviors. 2010;35:1148–51. doi: 10.1016/j.addbeh.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Review Neuroscience. 2006;7:464–76. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. European Journal of Neuroscience. 2008;28:1437–48. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–17. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]