Abstract
Using the event-related potential (ERP) approach, we tracked the time-course of white participants’ empathic reactions to white (own-race) and black (other-race) faces displayed in a painful condition (i.e. with a needle penetrating the skin) and in a nonpainful condition (i.e. with Q-tip touching the skin). In a 280–340 ms time-window, neural responses to the pain of own-race individuals under needle penetration conditions were amplified relative to neural responses to the pain of other-race individuals displayed under analogous conditions. This ERP reaction to pain, whose source was localized in the inferior frontal gyrus, correlated with the empathic concern ratings of the Interpersonal Reactivity Index questionnaire. In a 400–750 ms time-window, the difference between neural reactions to the pain of own-race individuals, localized in the middle frontal gyrus and other-race individuals, localized in the temporoparietal junction was reduced to nil. These findings support a functional, neural and temporal distinction between two sequential processing stages underlying empathy, namely, a race-biased stage of pain sharing/mirroring followed by a race-unbiased stage of cognitive evaluation of pain.
Keywords: race, empathy, event-related potentials, empathic concern, inferior frontal gyrus
INTRODUCTION
In the laboratories, a standard practice to study the effects race exerts at various levels of the human processing architecture is to monitor the behavioral and neural reflections trailing the onset of a face stimulus in designs in which the face belongs to a member of the same race as that of the participant or to a member of a different race. Over the last decade, a large body of neuroimaging studies adopting variants of this approach has established that own- and other-race faces are processed differently, likely at all stages of the identification process (Hart et al., 2000; Amodio et al., 2003; Cunningham et al., 2004; Wheeler and Fiske, 2005). Results from studies using event-related potentials (ERPs) are exemplar in this perspective. Race-driven modulations of face processing occur as early as the N1 component time-locked to face onset (Ito and Urland, 2003, 2005). Race, however, continues to modulate neural activity in cascade even during postsensory stages of processing, up to and including working memory maintenance of face stimuli, as reflected in race-dependent effects observed on subsequent P2, N2, P3 (Dickter and Bartholow, 2007) and sustained posterior contralateral negativity (Sessa et al., 2012; see also Luria et al., 2010; Lefebvre et al., 2011; Sessa et al. 2011) ERP components (see Ito and Bartholow, 2009, for a review).
A recent line of investigation has revealed that race can also bias the processing of elements other than those strictly necessary to build and maintain a visual representation of a face. One of these elements is the ability to empathize with other persons, which appear to be more natural when facing own-race than other-race members (Xu et al., 2009; Avenanti et al., 2010). This view seems to dovetail nicely with functional magnetic resonance imaging (fMRI) evidence suggesting that, in addition to the fusiform face area, which is assumed to be implicated in the generation of a visual representation of a face, race-driven differential activation following the exposure to a face can be found at the level of the amygdala, a crucial node in the neural circuitry underpinning the activation and control of emotional responses (Phelps et al., 2000; Golby et al., 2001; Golarai et al., 2004).
Empathy is a notoriously ambiguous term that has resisted a unitary definition for years, eliciting an active debate about the diverse components entailed in an empathic reaction. Current theorizing agrees on the proposal that a definition of empathy must encompass at least two aspects. One such aspect is neural resonance, which is envisaged as a mechanism enabling rapid sharing of other people’s internal states. A different aspect, usually referred to as cognitive empathy, is related to the construction of a mental representation of other people’ internal states (i.e. mentalizing) and self-regulation/control of internal emotional states. Interestingly, neuroimaging evidence suggests that these two components of empathy are subserved by distinct brain networks (Decety and Jackson, 2006; Decety and Lamm, 2006; Shamay-Tsoory, 2011). Neural resonance, in its various proposed declinations (e.g. experience sharing, affective empathy, emotional contagion and shared self-other representations; Zaki and Ochsner, 2012), has been framed as reflecting rapid bottom–up activation of subcortical/cortical circuitries supporting sensory-motor and affective resonance, with neural underpinnings in the mirror neuron system (i.e. intra-parietal lobule, inferior frontal gyrus (IFG) and dorsal premotor cortex) and the limbic system (i.e. amygdala, anterior cingulate cortex (ACC), anterior insula and ventral striatum; Keysers et al., 2010; Lamm and Singer, 2010; Rizzolatti and Sinigaglia, 2010). The cognitive component of empathy, on the other hand, has been shown to be influenced by higher-level, top–down, signals originating in prefrontal cortical circuitries (Decety and Jackson, 2006; Decety and Lamm, 2006; Zelazo et al., 2008), with neural underpinnings in the dorsomedial, dorsolateral and medial prefrontal cortices, middle frontal gyrus (MFG), temporoparietal junction (TPJ) and precuneus (Kanwisher, 2003; Amodio and Frith, 2006; Decety, 2011; Lamm et al., 2011).
In an attempt at forging a link between empathy and race, Xu et al. (2009) had Chinese and Caucasian participants watch short video clips featuring Chinese or Caucasian characters whose faces were stimulated with tools associated with the sensation of pain (e.g. a face penetrated by a needle) or with a more neutral sensation (e.g. a face touched by a Q-tip). The participants’ task was to categorize the video clips based on whether the characters were feeling pain or not, so-called pain decision task, disregarding their race. Blood oxygenation level–dependent (BOLD) responses recorded from the ACC, a region mediating the perception of self-experienced pain as well as vicarious pain, was increased when participants watched faces under painful stimulation relative to faces stimulated with the nonpainful tool. Notably, such BOLD response increase detected in ACC was of greater magnitude when the painful stimulation was applied to the faces of own-race characters compared with faces of other-race characters. Results and conclusions from this study were compatible with the view proposed in prior work (Singer et al., 2004) that, among the diverse structures composing the complex neural network subtended in the perception of pain, dubbed pain matrix, the selective involvement of the ACC is a direct reflection of an empathic reaction of emotional/affective nature (Decety and Jackson, 2006). More specifically, results by Xu et al. (2009) showed that this modulation depended on race.
An elegant demonstration that the interplay between empathy and race is however not confined to emotional/affective aspects of social interactions has been provided by Avenanti et al. (2005, 2010). Avenanti et al. used transcranial magnetic stimulation (TMS) to probe the excitability of sensory-motor corticospinal neurons in participants exposed to video clips of a hand stimulated painfully (i.e. needle penetration) or nonpainfully (i.e. Q-tip touch) in distinct skin regions. The experimental design included one condition in which the hand belonged to an own-race member and a different condition in which the hand belonged to an other-race member. The results were clear-cut in revealing a sizable reduction in excitability of the monitored neurons—which is typically observed in preparation of a self-experienced painful event—in participants watching the hand of own-race members penetrated by a needle, as though the painful stimulation was applied to their own hands. This reaction, termed sensory-motor contagion, was nil in participants watching the hand of other-race members. As further, complementary support, participants showing greater implicit pro-ingroup preference—as measured by the implicit association test (or IAT; Greenwald et al., 2003)—were less affected by sensory-motor contagion when observing the physical suffering of other-race individuals.
Although both studies reported in the foregoing section appear to provide unequivocal evidence for the role of race as modulatory factor of the neural resonance component of empathy, we note that, though providing fundamental information about where the potential neural loci of the interplay between racial bias and empathy may be localized in the brain, these studies are virtually tacit relative to when such interplay takes place. The interpretation of results of Xu et al. (2009) hinged on variations in BOLD signal, whose time-scale is suboptimal to capture effects arising from subtle factorial interactions (Logothetis, 2008; Scarpa et al., 2010; Cutini et al., 2011, 2012, 2014). Second, in studies by both Xu et al. (2009) and Avenanti et al. (2010), participants were exposed to face/hand stimuli conveying racial and empathy-eliciting information for a relatively long interval prior to recording their joint effects on stimuli processing. The use of long stimuli exposure makes it plausible that the affective/emotional and sensory-motor reactions described in those studies were in fact both mediated by higher level processes linked to the cognitive aspects of empathy, including attention, emotion regulation and cognitive evaluation of others’ pain. In this vein, the long stimuli exposure provided participants with the opportunity to create a cognitive representation of others’ suffering which may have in turn mediated—in a top–down fashion, as hinted by Decety and Jackson (2004, 2006; see also Decety, 2011)—their emotional/affective and sensory-motor reactivity to the stimuli used by Xu et al. (2009) and Avenanti et al. (2010).
Mapping out the temporal locus of the interplay between race and empathy-related subprocesses, which is the primary scope of the present investigation, may capitalize on ERP studies describing ERP responses to pain of different temporal and functional origins. Fan and Han (2008), for instance, presented participants with a varying number of hands (i.e. one vs. two) that were displayed in a painful or nonpainful condition. Participants had to carry out a pain decision task (disregarding hands’ number) in one condition and a counting task (disregarding pain) in a different condition. ERP responses to painful vs. nonpainful stimulation diverged from 140 ms until about 660 ms after stimulus, including the P2, N2 and N3 components of ERP. This ERP reaction to pain, however, exhibited a different sensitivity to the task manipulation, remaining constant across task conditions in a 140–360 ms time range and decreasing substantially from 380 ms onward (i.e. in a time range typically associated with the P3 component) in the counting task. The dependency on task-set of the P3 reaction to pain was taken as a clear indication of its cognitive origin (see also Donchin, 1981; Donchin and Coles, 1988; Verleger, 1988, 1997; Sessa et al., 2007; Li and Han, 2010), whereas the earlier, pre-P3, portion of the reaction to pain was hypothesized to reflect a prompt reaction to others’ pain analogous to neural resonance (Decety et al. 2010).
Having therefore a temporal hallmark of when, during the flow of processing triggered by stimuli associated with pain, the neural resonance and the cognitive components of a reaction to others’ pain become manifest in ERP is valuable in our perspective, as it allows us to make specific predictions concerning an hypothetical ERP scenario coming about by implementing in a single design an orthogonal variation of race and painful stimulation conditions. More specifically, white participants in the present ERP study were presented with own- (i.e. white) and other-race (i.e. black) faces in a painful condition (i.e. penetrated by a needle) or in a nonpainful condition (i.e. touched by a Q-tip) under the requirement to perform a pain decision task. Extant evidence of modulations of ERP responses to faces as a function of race occurring as early as the N1 component would lead to hypothesize that, if an interaction between race and pain stimulation conditions were evident in ERPs, it might surface relatively early in time, within a pre-P3 onset time window, thereby suggesting that neural resonance is reduced when facing other-race individuals in pain. An alternative scenario would be more congruent with our interpretation of the results by Xu et al. (2008) and Avenanti’s et al. (2010), namely, that of a cognitive involvement in the reduction of brain responses to the pain of other-race individuals reflected in interactive effects largely confined to the P3 time range.
Note, however, there is no a priori theoretical ground to hypothesize that these two empirical outcomes would be mutually exclusive. In this vein, to better qualify the meaning of each ERP component modulated by pain and/or race, participants in this study were also administered, at the end of the pain decision task, a standard-race IAT and the Italian version of the Interpersonal Reactivity Index (IRI; Albiero et al., 2006; original versions by Davis, 1980, 1983), a self-report questionnaire that measures affective and cognitive components of empathy. These measures were collected to assess parametrically whether implicit racial bias, as assessed by the IAT, influenced ERP reactions to pain, and whether affective and cognitive components of empathy, as assessed by the IRI, correlated with earlier (i.e. pre-P3) and later (P3 and following) ERP reactions to pain.
METHOD
Participants
Informed consent was obtained from 12 white students at the University of Padova [8 females; mean age = 26.4 years, standard deviation (s.d.) = 8.6; 1 left-handed] volunteered to participate in the present experiment.
Stimuli and procedure
ERP recording session
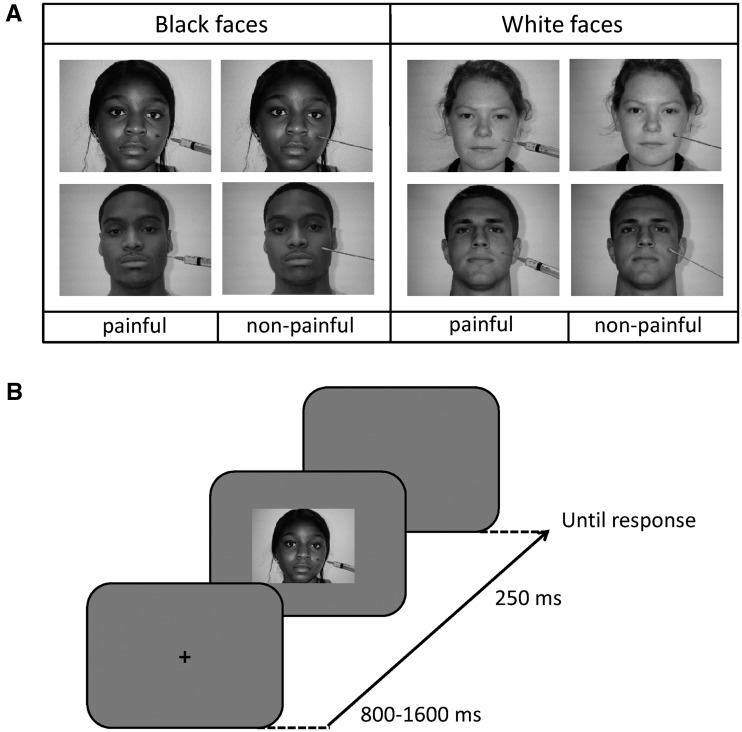
The stimuli were colored digital photographs of 20 white (i.e. own-race; 10 males and 10 females) and 20 black (i.e. other-race; 10 males and 10 females) faces with a neutral expression selected from the database of Minear and Park (2004). Each photograph was scaled to fit in a rectangular portion of the computer screen subtending 3.7° × 3.1° (width × height) of visual angle at a viewing distance of approximately 70 cm. Each face was manipulated digitally so as to be displayed in two different conditions. In the painful stimulation condition, the face was displayed with a needle of a syringe penetrating the cheek. In the nonpainful stimulation condition, the face was displayed with a Q-tip touching the cheek. Figure 1A shows a sample of the stimuli (in grayscale), one for each experimental condition.
Fig. 1.
(A) Examples of stimuli, one for each experimental condition (stimulation condition: painful vs. non-painful; race: white/own-race faces vs. black/other-race faces). (B) Schematic illustration of the procedure.
Each trial began with a fixation cross at the center of the computer screen that remained visible for a randomly variable duration (800–1600 ms, jittered in 100 ms steps). The fixation cross was then replaced by a centrally presented face, which was displayed for a fixed duration of 250 ms, and followed by a blank interval that lasted until response detection (discussed later). Figure 1B illustrates the temporal structure of one trial.
Participants were required to decide whether each face was painfully or not painfully stimulated by pressing one of two appropriately labeled keys of the computer keyboard (‘F’ or ‘J’, counterbalanced across participants) as quickly and accurately as possible. The experimental list of stimuli was organized in 12 blocks of 80 trials, which were preceded by a brief session of practice with stimuli that were not included in the set of experimental stimuli. In each block, all possible combinations of face’s sex, race and stimulation condition were equally represented and randomly ordered at run-time for each participant.
IAT
The stimuli were colored digital photographs of 10 white male and 10 black male faces that were different than the faces used for the ERPs recording session. The attributes were 10 positive words (e.g. peace, love and paradise) and 10 negative words (e.g. disgust, vomit and earthquake). A standard race-IAT was administered to each participant after the pain decision task. A description of the IAT procedure is illustrated in Table 1.
Table 1.
Schematic description of the IAT procedure
| Block | Type of judgment | Instructions | Number of trials |
|---|---|---|---|
| 1. | Race discrimination | Press ‘D’ to categorize pictures as whites and ‘K’ to categorize pictures as blacks | 20 |
| 2. | Attribute discrimination | Press ‘D’ to categorize positive words and ‘K’ to categorize negative words | 20 |
| 3. | Prejudice-consistent combination | Press ‘D’ to categorize pictures of whites and positive words and ‘K’ to categorize pictures of blacks and negative words | 40 |
| 4. | Race discrimination reversed | Press ‘D’ to categorize pictures as blacks and ‘K’ to categorize pictures as whites | 20 |
| 5. | Prejudice-inconsistent combination | Press ‘D’ to categorize pictures of blacks and positive words and ‘K’ to categorize pictures of whites and negative words | 40 |
IAT consisted of five classification tasks administered in a sequential order on the computer screen. Block 1, 2 and 4 were learning blocks, whereas blocks 3 and 5 were critical to compute IAT scores. The order of the critical blocks was counterbalanced across participants. In each block, participants were required to classify stimuli by pressing one of two different keys on the computer keyboard (‘D’ and ‘K’). Instructions and key assignments were displayed on the computer screen before each block. Stimuli were presented at the center of the screen and remained visible until response.
IRI
The Italian version of the IRI is a self-report questionnaire composed of 28 items measuring affective and cognitive components of empathy. The questionnaire is organized in four 7-item subscales, two affective subscales and two cognitive subscales. The affective component is assessed by the empathic concern (EC) and personal distress (PD) subscales. The EC subscale taps the feelings of warmth, compassion and concern for others. The PD subscale assesses the feelings of anxiety in situations of social relations. The cognitive component is assessed by the perspective-taking (PT) and fantasy subscales (FS). The PT subscale measures the tendency to spontaneously adopt the psychological point of view of others. The FS subscale measures the tendency to imaginatively transpose oneself into fictional situations (e.g. like movies or book characters). Both the PT and FS subscales have been shown to be positively correlated with other validated measures of cognitive empathy, such as the Hogan (1969) empathy scale.
EEG acquisition and analysis
EEG activity was recorded from 64 electrodes distributed over the scalp according to an extension of the international 10/20 system referenced to the left earlobe. The EEG was re-referenced offline to the average of the left and right earlobes. Trials contaminated by eye blinks, large horizontal eye movements or incorrect responses in the pain decision task were discarded from analysis.
Mean N1, P2 and N2 component amplitudes were measured at frontocentral electrode sites in 80–100 ms, 120–150 ms and 200–240 ms time-windows locked to the onset of the face stimuli, respectively. The mean P3 amplitude was measured in a 400–750 ms time-window at Pz, P3 and P4 electrode sites. An additional 280–340 ms window, spanning the trough between N2 and N3 peaks, was selected based on visual localization of factor effects maximum values. Statistical analyses were conducted on individual amplitude estimates of activity recorded at each of frontal (AF3/AF4, AF7/AF8, Fz, FCz, F1/F2, F3/F4, F5/F6, F7/F8, FC1/FC2, FC3/FC4, FC5/FC6 and FT7/FT8), central (Cz, C1/C2, C3/C4 and C5/C6) and parietal (Pz and P3/P4) electrode sites. In all multi-factorial analyses, a Greenhouse–Geisser correction was used where appropriate.
The standardized Low Resolution Brain Electromagnetic Tomography (sLORETA) (Pascual-Marqui, 2002) was used for brain localization of the potential sources of ERP reactions to pain. sLORETA analyses were conducted following the creation of a boundary element method model, with about 5000 nodes from MRI data, the selection of a temporal window in which ERP responses differentiated between painful and nonpainful stimulations, and a location-wise inverse weighting from the minimum norm least square analysis with estimated variances.
RESULTS
Pain decision task
Reaction times (RTs) exceeding each individual mean RT in a given condition ±2.5 s.d. and RTs associated with incorrect responses were excluded from the RT analysis. Individual mean proportions of correct responses and RTs were submitted to separate repeated measure analyses of variance (ANOVA), both considering stimulation condition (painful vs. nonpainful) and race (white faces vs. black faces) as within-subjects factors. Neither ANOVA showed significant factor effects (max F = 2.2; min P = 0.16).
IRI
Scores were computed by summing 1–7 scores to each item of the four subscales. Interindividual mean rating scores were 27.1 (s.d. = 4.03) for the PT subscale, 25.0 (s.d. = 4.94) for the FS subscale, 26.8 (s.d. = 2.96) for the EC subscale and 21.4 (s.d. = 5.70) for the PD subscale.
IAT
Score calculation followed the improved algorithm proposed by Greenwald et al. (2003). D positive scores were taken to indicate a preference towards white people. Mean D scores were significantly different from zero (mean D ± standard error: 0.37 ± 0.17; t(11) = 2.2, P < 0.05), reflecting a successful detection of a positive bias toward own-race members.
ERP: N1, P2 and N2
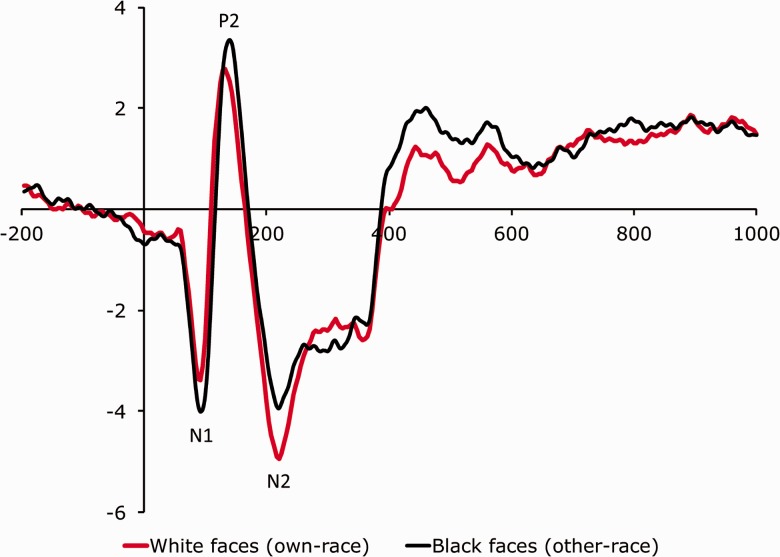
Figure 2 shows mean amplitudes of the subset of face-locked ERP components recorded at electrode site Fz that previous studies have indicated as sensitive to race, namely, N1, P2 and N2. We submitted to ANOVA individual amplitude values of each component considering stimulation (painful vs. nonpainful), race (white faces vs. black faces) and electrode site as within-subjects factors. The ANOVAs carried out on N1, P2 and N2 mean amplitude values revealed main effects of race over all electrodes of the frontal area (all Ps < 0.05). As expected, black (other-race) faces elicited N1 and P2 of greater amplitude than white (own-race) faces, and white faces elicited an N2 of greater amplitude than black faces. The ANOVAs detected no other main effect or interaction (all Ps > 0.05).
Fig. 2.
ERPs time-locked to the presentation of the faces recorded at electrode site Fz in response to white/own-race and black/other-race faces collapsed across stimulation conditions (painful vs. nonpainful).
ERP: N2–N3
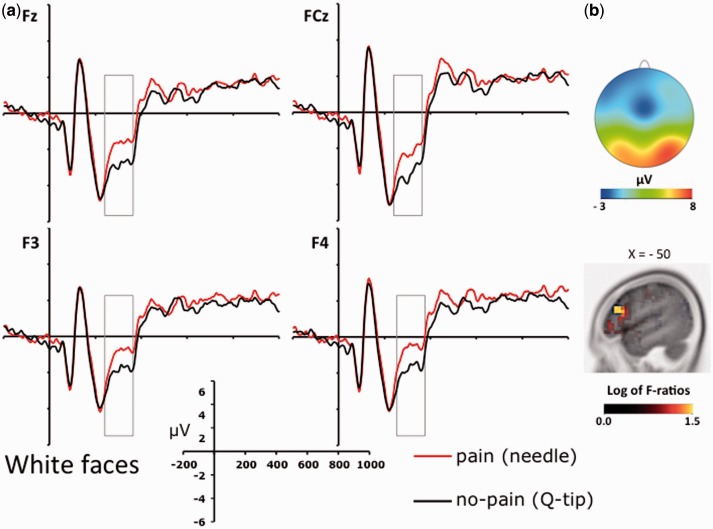
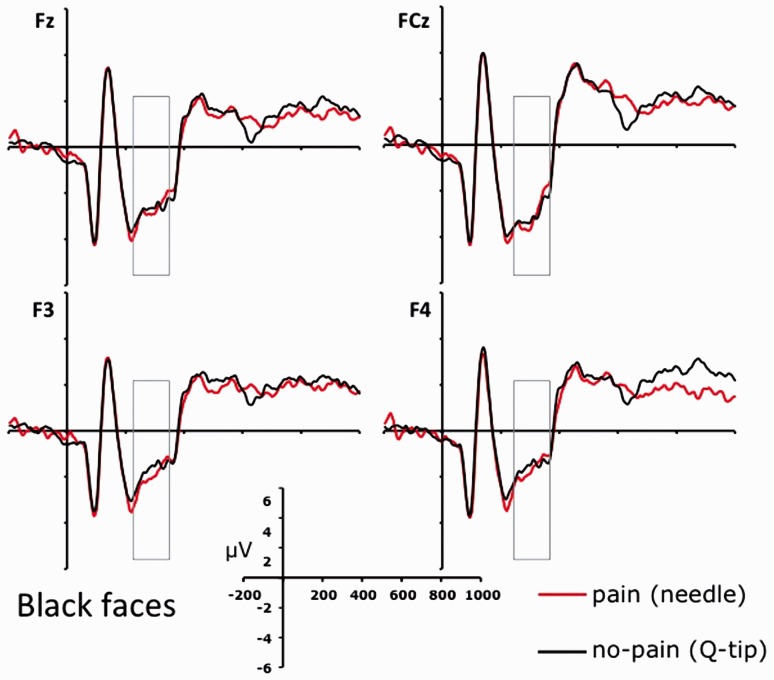
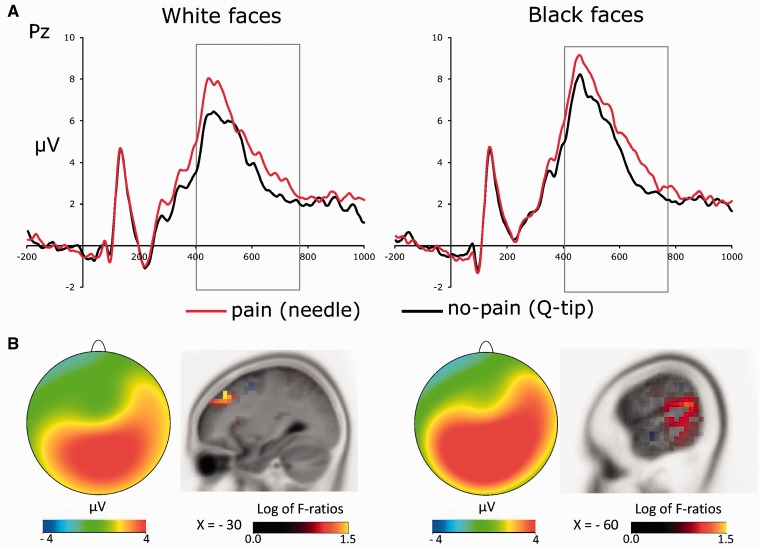
Figures 3A and 4 show face-locked ERPs recorded from a selection of frontal electrode sites, separately for white and black faces. A visual inspection of Figures 3A and 4 suggests that participants processed differently the faces in painful and nonpainful conditions, exhibiting a positive shift in response to painful stimulation only when applied to white (own-race) faces relative to black (other-race) faces. This observation was substantiated by an ANOVA carried out on individual ERP mean amplitude values recorded in a 280–340 ms time-window considering recording side (left vs. right hemisphere electrodes) as additional factor, which showed a significant interaction between stimulation condition and race on all the frontal and central electrode sites (min F = 5.4; max P = 0.013, min 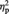 = 0.516). This pattern was bilaterally distributed, for recording site did not produce significant effects either as main factor or in interactions with race and stimulation conditions (all Fs < 1; min P > 0.45). Planned comparison indicated that painful stimuli elicited a positive shift relative to nonpainful stimuli in the N2–N3 time window only when applied to white (own-race) faces (min t = 2.39; max P = 0.036). This shift was absent when black (other-race) faces were painfully stimulated (all Ps < 0.23). These findings suggest that, in a temporal window of 280–340 ms, participants were in a state of experience sharing elicited by the presentation of suffering own-race members, but not of suffering other-race members. A scalp topographic map of N2–N3 activity elicited by white (own-race) faces in the painful stimulation condition is depicted in Figure 3B (upper panels).
= 0.516). This pattern was bilaterally distributed, for recording site did not produce significant effects either as main factor or in interactions with race and stimulation conditions (all Fs < 1; min P > 0.45). Planned comparison indicated that painful stimuli elicited a positive shift relative to nonpainful stimuli in the N2–N3 time window only when applied to white (own-race) faces (min t = 2.39; max P = 0.036). This shift was absent when black (other-race) faces were painfully stimulated (all Ps < 0.23). These findings suggest that, in a temporal window of 280–340 ms, participants were in a state of experience sharing elicited by the presentation of suffering own-race members, but not of suffering other-race members. A scalp topographic map of N2–N3 activity elicited by white (own-race) faces in the painful stimulation condition is depicted in Figure 3B (upper panels).
Fig. 3.
(A) ERPs recorded at a selection of frontal electrode sites (i.e. Fz, FCz, F3 and F4) relative to the two stimulation conditions (painful vs. nonpainful) for white/own-race faces. (B) Voltage topography of the N2–N3 activity recorded in the painful condition (upper panels) and source estimation of the N2–N3 activity in the painful vs. nonpainful conditions for white/own-race faces (lower panels).
Fig. 4.
ERPs recorded at a selection of frontal electrode sites (i.e. Fz, FCz, F3 and F4) relative to the two stimulation conditions (painful vs. nonpainful) for black/other-race faces.
The results of sLORETA analysis revealed that the neural activity in the N2–N3 time window that differentiated between painful and nonpainful stimulations applied to white faces was mainly localized in the left IFG (Brodman area, BA, 45; peak Montreal Neurological Institute (MNI) coordinates: −50, 25, 20, Figure 3B, bottom), a core region of the mirror neuron system. This finding provides further support to the view that the early ERP reaction to pain was a likely reflection of neural resonance elicited by own-race faces in a painful condition.
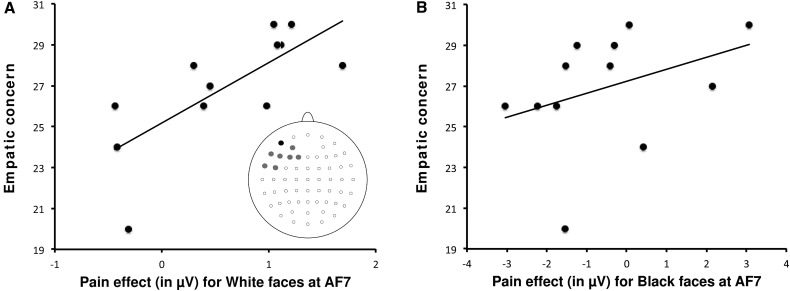
We also correlated individual pain reactions detected in the N2–N3 temporal window for each electrode site over frontal and central areas with the ratings collected with the IRI subscales. Pain reactions in the present time-window were isolated by subtracting ERPs elicited in the nonpainful stimulation condition from ERPs elicited in the painful condition, separately for white (own-race) faces and black (other-race) faces. Pain reactions recorded from a subset of frontal electrodes positioned on the left hemisphere (i.e. AF7, AF3, F7, F5, F3, F1, FT7 and FC5) correlated with the EC ratings (measuring the affective component of empathy) but only when white faces were presented. Spearman rs ranged from 0.50 at electrode site F7 to 0.73 at electrode site AF7 (all Ps < 0.05). Figure 5A shows the scatter plot of individual EC ratings and pain effects recorded at electrode site AF7, and a graphical indication of the additional electrode sites where EC ratings and pain effects were significantly correlated. Pain reactions in this temporal window, however, did not correlate with IAT D score (all Ps > 0.05). Figure 5B shows the scatterplot of individual EC ratings and pain reactions recorded at the electrode site AF7 for black faces. At all frontal electrode sites, the correlations between EC ratings and pain reactions elicited by black faces were not significant (rs ranged from 0.33 to 0.45 all Ps > 0.05).
Fig. 5.
(A) Scatterplot of the correlation between individual EC ratings and the pain effect observed at the electrode site AF7 in the time range of the N2–N3 components for white faces. Pain effect was computed by subtracting waveforms elicited in the nonpainful stimulation condition from the waveforms elicited in the painful condition. A schematic illustration of the EEG montage shows the electrode sites on the scalp for which correlations were significant (AF7 in black color, AF3, F7, F5, F3, F1, FT7 and FC5 in gray color). (B) Scatterplot of the correlation between individual EC ratings and the pain effect observed at the electrode site AF7 in the time range of the N2–N3 components for black/other-race faces.
ERP: P3
An ANOVA on individual P3 amplitude values recorded at Pz electrode site revealed a main effect of race. P3 amplitude was greater for black (other-race) faces (4.4 µV) than white (own-race) faces [3.3 µV; F(1, 11) = 11.38, P < 0.01, 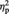 = 0.532). The main effect of stimulation condition was also significant [F(1, 11) = 7.05, P < 0.05,
= 0.532). The main effect of stimulation condition was also significant [F(1, 11) = 7.05, P < 0.05, 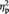 = 0.414). Notably, the interaction between these two variables did not reach significance (F < 1), suggesting no role of race in modulating a neural reflection of the cognitive component of empathy. P3 pain reactions were again isolated by subtracting amplitude values in the nonpainful stimulation condition from amplitude values in the painful condition, separately for white (own-race) faces and black (other-race) faces. P3 pain reactions amounted to 0.80 and 0.94 µV for white and black faces, respectively. An analogous pattern was observed when separate ANOVAs were carried out on P3 mean amplitude values recorded at P3 and P4 electrode sites. Both ANOVAs indicated main effects of race [P3: F(1, 11) = 7.45, P < 0.05,
= 0.414). Notably, the interaction between these two variables did not reach significance (F < 1), suggesting no role of race in modulating a neural reflection of the cognitive component of empathy. P3 pain reactions were again isolated by subtracting amplitude values in the nonpainful stimulation condition from amplitude values in the painful condition, separately for white (own-race) faces and black (other-race) faces. P3 pain reactions amounted to 0.80 and 0.94 µV for white and black faces, respectively. An analogous pattern was observed when separate ANOVAs were carried out on P3 mean amplitude values recorded at P3 and P4 electrode sites. Both ANOVAs indicated main effects of race [P3: F(1, 11) = 7.45, P < 0.05, 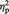 = 0.404; P4: F(1, 11) = 6.27, P < 0.05,
= 0.404; P4: F(1, 11) = 6.27, P < 0.05, 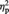 = 0.363] and marginally significant main effects of stimulation condition [P3: F(1, 11) = 4.09, P = 0.068,
= 0.363] and marginally significant main effects of stimulation condition [P3: F(1, 11) = 4.09, P = 0.068, 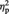 = 0.271; P4: F(1, 11) = 3.58, P = 0.085,
= 0.271; P4: F(1, 11) = 3.58, P = 0.085, 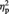 = 0.246]. No interaction between race and stimulation condition was observed [F < 1, P > 0.7). Face-locked P3 components recorded at Pz in painful and nonpainful stimulation conditions are shown in Figure 6A for white (own-race) faces black (other-race) faces.
= 0.246]. No interaction between race and stimulation condition was observed [F < 1, P > 0.7). Face-locked P3 components recorded at Pz in painful and nonpainful stimulation conditions are shown in Figure 6A for white (own-race) faces black (other-race) faces.
Fig. 6.
(A) P3 ERP component recorded at the electrode site Pz relative to the two stimulation conditions (painful vs. nonpainful) for white/own-race faces and for black/other-race faces. (B) Voltage topographies and source estimation of P3 activity as a function of race and stimulation conditions (painful vs. nonpainful).
Figure 6B shows scalp topographic maps of P3 activity elicited in the painful stimulation condition for white faces and black faces and the source estimations of the P3 that differentiated between painful and nonpainful stimulations applied to white and black faces. The results of the sLORETA analysis suggested that reactions to the pain of white own-race individuals and black other-race individuals in the P3 time window had potential sources in different brain regions underpinning the mentalizing system. The left MFG (BA 9; peak MNI coordinates: −30, 35, 40) was the likely source of the reaction to the pain of own-race individuals. The left TPJ (BA 40; peak MNI coordinates: −60, −50, 20; BA 22; peak MNI coordinates: −63, −40, 20) was the likely source of the reaction to the pain of other-race individuals. Since both these brain regions are involved in the attribution of mental states to others (Frith and Frith, 1999; Saxe and Kanwisher, 2003; Amodio and Frith, 2006; Moriguchi et al., 2006; Spiers and Maguire, 2006), these results suggest that participants devolved the same amount of cognitive resources while mentalizing others’ pain.
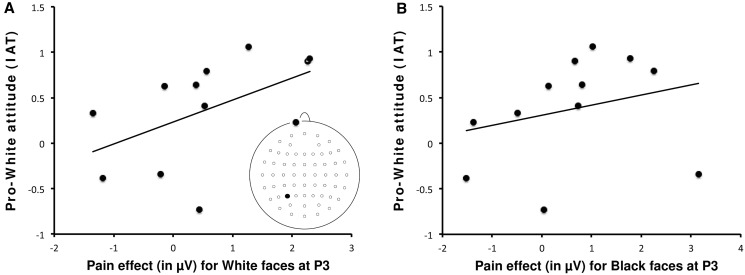
P3 reactions to pain did not correlate with any of the IRI subscales, including the PT and the FS subscales. Differential P3 pain reactions between white and black faces recorded at Pz, P3 and P4 electrode sites did not correlate with IAT D scores (P > 0.38). As the different potential sources of the P3 reactions to the pain of own-race and other-race individuals could suggest that these reactions are differently sensitive to the implicit pro-ingroup preference, we decided to compute correlations between P3 pain reactions and IAT D scores for white and black faces, separately. Interestingly, P3 pain reactions recorded at P3 electrode site correlated with IAT D scores for white (own-race) faces (r = 0.512, P < 0.05), but not for black faces (P > 0.10; Figure 7A and B), likely reflecting a link between a pro-white (i.e. pro-ingroup) attitude and the magnitude of the P3 reaction to pain in the left hemisphere. No correlations were found between P3 pain reactions recorded at Pz and P4 electrode sites and IAT D scores (P = 0.153 and P = 0.499, respectively).
Fig. 7.
(A) Scatterplot of the correlation between individual IAT scores and the pain effect observed at the electrode site P3 in the time range of the P3 components for white/own-race faces. Pain effect was computed by subtracting waveforms elicited in the nonpainful stimulation condition from the waveforms elicited in the painful condition. (B) Scatterplot of the correlation between individual IAT scores and the pain effect observed at the electrode site P3 in the time range of the P3 components for black/other-race faces.
DISCUSSION
Neural reactions to the pain of own-race individuals recorded in a 280–340 ms time range, spanning the N2 and N3 ERP components, were magnified relative to neural reactions to the pain of other-race individuals. Neural reactions of comparable magnitude toward the pain of own-race and other-race individuals were observed in a 400–750 ms time-window, a time range typically associated with the P3 ERP component. In line with previous studies using similar experimental paradigms (Fan and Han, 2008; Xu et al., 2009; Avenanti et al., 2010; Decety et al., 2010; Li and Han, 2010), we interpret these neural reactions to pain as reflecting the engagement of distinct empathy-related subprocesses.
As others (Fan and Han, 2008; Han et al., 2008), we argue that pre-P3 ERP reaction to pain can be ascribed to an automatic component of empathy and, in this vein, the correlation between such ERP modulations and the EC ratings of the IRI can be taken as evidence compatible with this interpretation. The set of correlations was particularly evident at left-frontal electrode sites, nicely dovetailing with recent fMRI work reporting analogous correlations between EC ratings and BOLD responses recorded from the left insula and ACC (Singer et al., 2004). The likely source of this ERP response was found in the left IFG, a key region of the mirror neuron system, thus dovetailing with prior similar evidence (Shamay-Tsoory et al., 2004, 2009; Chakrabarti et al., 2006; Minio-Paluello et al., 2006; Schulte-Ruther et al., 2007; Jabbi and Keysers, 2008; Frühholz and Grandjean, 2012).
In the P3 time range, the racial bias on neural reactions to pain was reduced to nil. The source of P3 responses to pain was localized in the MFG and in the TPJ for own-race faces and other-race faces, respectively, in line with the cognitive functional characterization of this activity. The MFG is held to be involved in mentalizing (Ohnishi et al., 2004; Moriguchi et al., 2006; Spiers and Maguire, 2006; David et al., 2008; Lombardo et al., 2011). The TPJ is crucial in detecting goals from observed behavior, in self/others distinctions (Frith and Frith, 2001) and in representing beliefs of other individuals (Saxe and Wexler, 2005). To note, Cheon et al. (2011) reported an opposite response pattern compared with that observed in this study when monitoring a more anterior portion of the TPJ and this suggests that TPJ likely subserves distinct functional roles in empathic reactions (Mars et al., 2012).
One may obviously wonder why an apparently similar P3 ERP reaction to the pain of own-race and other-race individuals should be driven by different brain regions. In their meta-analysis, Lamm et al. (2011) pointed out the pervasive bilateral activation of AI and medial/ACC whenever an empathic response is elicited. Additional brain structures are however engaged based on the stimuli used to elicit an empathic reaction. More specifically, while realistic empathy-eliciting stimuli (e.g. body parts in painful situations) engage selectively IFG and MFG (among other regions), abstract visual symbols (e.g. colors displayed on a screen indicating a painful stimulation) engage preferentially TPJ (among other regions). Lamm et al. (2011) proposed that these two different circuitries concur to the empathic state throughout two different pathways. The first, including IFG and MFG, allows action understanding that in turn triggers inferences about potential (affective) consequences of observed actions. The second pathway, including TPJ, subserves mentalizing and autobiographical memory, allowing the understanding of others’ mental states on the basis of inferences based on one’s own past experience. These considerations suggest that P3 reactions to the pain of own-race and other-race individuals are qualitatively different, and further research is needed to disentangle between the different contribution of MFG and TPJ in representing others’ pain.
We do not have at present a definite account for the lack of a correlation between the P3 reaction to pain and the PT and FS ratings of the IRI measuring cognitive aspects of empathy, which would have provided a natural complement to the correlation between the EC ratings and early ERP reaction to pain observed in this study and discussed in a foregoing section. A plausible justification relies on evidence suggesting that the ability of taking the other individuals’ perspectives and mentalizing are two partially different aspects of cognitive empathy as indicated, for instance, by the lack of correlations between PT subscale scores and tasks involving mentalizing (Shamay-Tsoory et al., 2009).
In this study, we found a correlation between P3 reactions to pain of white own-race faces and IAT D scores that was confined to the left hemisphere. This finding bears a close conceptual analogy with results by Avenanti et al. (2010), who showed reduced sensory-motor contagion following the exposure to other-race individuals’ pain in participants exhibiting a higher degree of implicit pro-ingroup preference. Conversely, an analogous correlation was absent when we considered pre-P3 ERP reactions to pain. This finding suggests that, in the P3 time-window, participants’ implicit pro-white preference interacted with the process of evaluation of the painful condition, such that the evaluation tended to be greater for participants who showed a particularly high implicit pro-white preference. However, the interactive effect did not spread throughout the scalp, and the P3 recorded at the other electrode sites (Pz and P4) did not correlate with the IAT D scores, suggesting that the overall evaluation of painful stimuli was largely independent on the implicit pro-white preference.
A comment is in order on a particularly recent study by Sheng and Han (2012) that bears an apparent close resemblance to the present design. These authors presented participants with suffering and neutral facial expressions of Asian (own-race) and Caucasian (other-race) individuals, while monitoring ERP responses during a race judgment task and a pain judgment task. When faces belonged to own-race members in both tasks, ERP responses to suffering facial expressions were characterized by a more positive deflection in the P2–N2 range relative to neutral facial expressions. The potential source of this ERP reaction to pain in the P2 time window was the ACC. Interestingly, this ERP reaction to pain was nil for faces belonging to other-race members in the race judgment task, and fully reinstated however in the pain decision task. Furthermore, P2 amplitude values in subtracted face-locked ERPs (i.e. ERPs elicited by suffering facial expressions minus ERPs elicited by neutral facial expressions) correlated positively with EC ratings of the IRI. The P3 component was generally influenced by race, with greater values for other-race than own-race faces, although no sign of empathic reaction was observed in this time range. At first sight, thus, estimates by Sheng and Han (2012) of the time-course of the interplay between race and pain reactions are slightly different from the estimates proposed in the present context, posing the question of which are the possible sources of such discrepancy. In our view, a possible explanation can be traced back to the type of stimuli used in the two studies, which were substantially different. To elicit the reaction to pain, Sheng and Han (2012) manipulated the facial expression (suffering vs. neutral) of members of distinct races, whereas we arbitrarily chose faces of members of distinct races with neutral facial expressions in combination with tools sharing a strong conceptual association with distinct feelings (pain vs. no pain). Our choice was motivated by the need to provide the most stringent control for low-level—sensory-driven—factors potentially influencing ERP estimates. Using physically identical faces with a neutral facial expression and tools which did not differ macroscopically (being both fusiform, equally oriented and globally of similar color) was compelling in this perspective. The physical difference between differing facial expressions is more evident. Besides, we avoided manipulating facial expressions to not induce ‘spurious’ automatic reactions in participants like mimicry (Hatfield et al., 2009), which cannot be taken, in and of itself, as symptomatic of an empathic state in the observers, although it clearly concurs to a thorough manifestation of empathy (Decety and Jackson, 2006). Differing facial expressions may also be related to possible variations in the degree of difficulty in decoding others’ feelings from faces belonging to distinct races (Anthony et al., 1992; Elfenbein and Ambidi, 2002). To note, ERP studies investigating the perception of others’ pain have reported different estimates of the time at which ERPs start to differentiate between painful and nonpainful conditions, ranging from 90 ms (Decety et al., 2010) to 230 ms (Li and Han, 2010). These different estimates are likely related to differences in the type of stimuli and task requirements that mediate the relative contribution of neural resonance and cognitive components of empathy (Zaki and Ochsner, 2012; see also Lamm et al., 2011). An experimental manipulation implemented in the design of Fan and Han (2008) is illustrative in this regard. As described in the Introduction, these authors used as stimuli images of hands in painful or neutral situations. The images were either pictures or cartoons, and, interestingly, the time at which ERPs started to differentiate between painful and neutral situations was postponed by 80 ms for cartoons relative to pictures. Fan and Han (2008) interpreted their findings as indicating that a lack of contextual reality of stimuli reduced the affective empathic responses, delaying the starting time of ERP reactions to pain. Therefore, one plausible way to reconcile the study by Sheng and Han (2012) and this study on the interplay between race and empathy is to ascribe the slightly dissimilar temporal maps to the use of suffering facial expressions in the absence of a context indicating the source of pain in the study by Sheng and Han (2012) that may have enhanced the involvement of neural resonance and reduced the contribution of cognitive empathy vis-a-vis the use of neutral facial expressions in this study in the context of a painful stimulation, that may have triggered neural resonance to a lesser extent, whereupon mentalizing was instead necessary to infer others’ painful states on the basis of the stimulation condition, as proposed by Zaki and Ochsner (2012).
In conclusion, our findings provide insights into the nature of cross-racial empathy. The present results complement and extend previous fMRI (Xu et al., 2009) and TMS (Avenanti et al., 2010) work by mapping out the time-course of the temporally asynchronous engagement of an early neural resonance component of empathy, which amplifies responses to the pain of own-race members, and of a cognitive component likely related to mentalizing which magnitude appears not to be influenced by their race, although the underlying different potential source estimates suggest that this later reaction qualitatively differs between own-race and other-race conditions.
Conflict of Interest
None declared.
REFERENCES
- Albiero P, Ingoglia S, Lo Coco A. Contributo all’adattamento italiano dell’Interpersonal Reactivity Index [A contribution to the Italian validation of the Interpersonal Reactivity Index] Testing Psicometria Metodologia. 2006;13:107–25. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Harmon-Jones E, Devine PG. Individual differences in the activation and control of affective race bias as assessed by startle eyeblink responses and self-report. Journal of Personality and Social Psychology. 2003;84:738–53. doi: 10.1037/0022-3514.84.4.738. [DOI] [PubMed] [Google Scholar]
- Anthony T, Cooper C, Mullen B. Cross-racial facial identification: a social cognitive integration. Personality and Social Psychology Bulletin. 1992;18:296–301. [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nature Neuroscience. 2005;8:955–60. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Sirigu A, Aglioti SM. Racial bias reduces empathic sensory-motor resonance with other-race pain. Current Biology. 2010;20:1018–22. doi: 10.1016/j.cub.2010.03.071. [DOI] [PubMed] [Google Scholar]
- Chakrabarti B, Bullmore E, Baron-Cohen S. Empathizing with basic emotions: common and discrete neural substrates. Social Neuroscience. 2006;1:364–84. doi: 10.1080/17470910601041317. [DOI] [PubMed] [Google Scholar]
- Cheon BK, Im D, Harada T, et al. Cultural influences on neural basis of intergroup empathy. NeuroImage. 2011;57:642–50. doi: 10.1016/j.neuroimage.2011.04.031. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Nezlek JB, Banaji MR. Implicit and explicit ethnocentrism: revisiting the ideologies of prejudice. Personality and Social Psychology Bulletin. 2004;30:1332–46. doi: 10.1177/0146167204264654. [DOI] [PubMed] [Google Scholar]
- Cutini S, Basso Moro S, Bisconti S. Functional near infrared optical imaging in cognitive neuroscience: an introductory review. Journal of Near Infrared Spectroscopy. 2012a;20:75–92. [Google Scholar]
- Cutini S, Scarpa F, Scatturin P, Dell’Acqua R, Zorzi M. Number-space interactions in the human parietal cortex: enlightening the SNARC effect with functional near-infrared spectroscopy. Cerebral Cortex. 2014;24(2):444–51. doi: 10.1093/cercor/bhs321. [DOI] [PubMed] [Google Scholar]
- Cutini S, Scarpa F, Scatturin P, et al. A hemodynamic correlate of lateralized visual short-term memories. Neuropsychologia. 2011;49:1611–21. doi: 10.1016/j.neuropsychologia.2010.12.009. [DOI] [PubMed] [Google Scholar]
- David N, Aumann C, Santos NS, et al. Differential involvement of the posterior temporal cortex in mentalizing but not perspective taking. Social Cognitive and Affective Neuroscience. 2008;3:279–89. doi: 10.1093/scan/nsn023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology. 1980;10:85. [Google Scholar]
- Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44:113–26. [Google Scholar]
- Decety J. The neuroevolution of empathy. Annals of the New York Academy of Sciences. 2011;1231:35–45. doi: 10.1111/j.1749-6632.2011.06027.x. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson P. A social-neuroscience perspective on empathy. Current Directions in Psychological Science. 2006;15:54–8. [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. Human empathy through the lens of social neuroscience. Scientific World Journal. 2006;6:1146–63. doi: 10.1100/tsw.2006.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Yang C, Cheng Y. Physicians down-regulate their pain empathy response: an event-related brain potential study. NeuroImage. 2010;50:1676–82. doi: 10.1016/j.neuroimage.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Dickter CL, Bartholow BD. Event-related brain potential evidence of ingroup and outgroup attention biases. Social Cognitive and Affective Neuroscience. 2007;2:189–98. doi: 10.1093/scan/nsm012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E. Presidential address, 1980. Surprise!…Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–74. [Google Scholar]
- Elfenbein HA, Ambidi N. On the universality and cultural specificity of emotion recognition: a meta-analysis. Psychological Bulletin. 2002;128:203–35. doi: 10.1037/0033-2909.128.2.203. [DOI] [PubMed] [Google Scholar]
- Fan Y, Han S. Temporal dynamic of neural mechanisms involved in empathy for pain: an event-related brain potential study. Neuropsychologia. 2008;46:160–73. doi: 10.1016/j.neuropsychologia.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds: a biological basis. Science. 1999;286:1692–95. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith C. The biological basis of social interaction. Current Directions in Psychological Science. 2001;10:151–5. [Google Scholar]
- Fruühholz S, Grandjean D. Towards a fronto-temporal neural network for the decoding of angry vocal expressions. NeuroImage. 2012;62:1658–66. doi: 10.1016/j.neuroimage.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Golarai G, Ghahremani DG, Eberhardt JL, Grill-Spector K, Gabrieli GDE. Representation of parts and canonical face configuration in the amygdala, superior temporal sulcus (STS) and the fusiform “face area” (FFA) Journal of Vision. 2004;4:a131. [Google Scholar]
- Golby A, Gabrieli J, Chiao J, Eberhardt J. Differential responses in the fusiform region to same-race and other-race faces. Nature Neuroscience. 2001;4:845–50. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and using the implicit association test. I. An improved scoring algorithm. Journal of Personality and Social Psychology. 2003;85:197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Han S, Fan Y, Mao L. Gender difference in empathy for pain: an electrophysiological investigation. Brain Research. 2008;1196:85–93. doi: 10.1016/j.brainres.2007.12.062. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, McInerney SC, Fischer H, Rauch SL. Differential response in the human amygdala to racial outgroup vs. ingroup face stimuli. NeuroReport. 2000;11:2351–55. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- Hatfield E, Rapson RL, Le YC. Emotional contagion and empathy. In: Decety J, Ickes W, editors. The Social Neuroscience of Empathy. Cambridge, MA: MIT Press; 2009. pp. 19–30. [Google Scholar]
- Hogan R. Development of an empathy scale. Journal of Consulting and Clinical Psychology. 1969;33:307–16. doi: 10.1037/h0027580. [DOI] [PubMed] [Google Scholar]
- Ito TA, Bartholow BD. The neural correlates of race. Trends in Cognitive Sciences. 2009;13:524–31. doi: 10.1016/j.tics.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito TA, Urland GR. Race and gender on the brain: electrocortical measures of attention to race and gender of multiply categorizable individuals. Journal of Personality and Social Psychology. 2003;85:616–26. doi: 10.1037/0022-3514.85.4.616. [DOI] [PubMed] [Google Scholar]
- Ito TA, Urland GR. The influence of processing objectives on the perception of faces: an ERP study of race and gender perception. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:21–36. doi: 10.3758/cabn.5.1.21. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Keysers C. Inferior frontal gyrus activity triggers anterior insula response to emotional facial expressions. Emotion. 2008;8:775–80. doi: 10.1037/a0014194. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nature Reviews Neuroscience. 2010;11:417–28. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Structure and Function. 2010;214:579–91. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Lefebvre C, Dell’Acqua R, Roelfsema P, Jolicœur P. Surfing the attentional waves during visual curve tracing: evidence from the sustained posterior contralateral negativity. Psychophysiology. 2011;48:1509–15. doi: 10.1111/j.1469-8986.2011.01228.x. [DOI] [PubMed] [Google Scholar]
- Li W, Han S. Perspective taking modulates event-related potentials to perceived pain. Neuroscience Letters. 2010;469:328–32. doi: 10.1016/j.neulet.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–78. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Baron-Cohen S. Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. NeuroImage. 2011;56:1832–8. doi: 10.1016/j.neuroimage.2011.02.067. [DOI] [PubMed] [Google Scholar]
- Luria R, Sessa P, Gotler A, Jolicœur P, Dell’Acqua R. Visual short-term memory capacity for simple and complex objects. Journal of Cognitive Neuroscience. 2010;22:496–512. doi: 10.1162/jocn.2009.21214. [DOI] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Schüffelgen U, Jbabdi S, Toni I, Rushworth MF. Connectivity-based subdivisions of the human right “temporoparietal junction area”: evidence for different areas participating in different cortical networks. Cerebral Cortex. 2012;22:1894−903. doi: 10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behavior Research Methods, Instruments, & Computers. 2004;36:630–3. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Minio-Paluello I, Avenanti A, Aglioti SM. Left hemisphere dominance in reading the sensory qualities of others’ pain? Social Neuroscience. 2006;1:320–33. doi: 10.1080/17470910601035954. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Lane RD, et al. Impaired self-awareness and theory of mind: an fMRI study of mentalizing in alexithymia. NeuroImage. 2006;32:1472–82. doi: 10.1016/j.neuroimage.2006.04.186. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Moriguchi Y, Matsuda H, et al. The neural network for the mirror system and mentalizing in normally developed children: an fMRI study. NeuroReport. 2004;15:1483–7. doi: 10.1097/01.wnr.0000127464.17770.1f. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods and Findings in Experimental and Clinical Pharmacology. 2002;24(Suppl. D):5–12. [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Cunningham WA, et al. Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience. 2000;12:729–38. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nature Reviews Neuroscience. 2010;11:264–74. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: The role of the temporo-parietal junction in theory of mind. NeuroImage. 2003;19:1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–9. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Scarpa F, Cutini S, Scatturin P, Dell’Acqua R, Sparacino G. Bayesian filtering of human brain hemodynamic activity elicited by visual short-term maintenance recorded through functional near-infrared spectroscopy (fNIRS) Optics Express. 2010;18:26550–68. doi: 10.1364/OE.18.026550. [DOI] [PubMed] [Google Scholar]
- Schulte-Ruther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. Journal of Cognitive Neuroscience. 2007;19:1354–72. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- Sessa P, Luria R, Gotler A, Jolicœur P, Dell’Acqua R. Inter-hemispheric ERP asymmetries over inferior parietal cortex reveal differential visual working memory maintenance for fearful versus neutral facial identities. Psychophysiology. 2011;48:187–97. doi: 10.1111/j.1469-8986.2010.01046.x. [DOI] [PubMed] [Google Scholar]
- Sessa P, Luria R, Verleger R, Dell’Acqua R. P3 latency shifts in the attentional blink: further evidence for second target processing postponement. Brain Research. 2007;1137:131–9. doi: 10.1016/j.brainres.2006.12.066. [DOI] [PubMed] [Google Scholar]
- Sessa P, Tomelleri S, Luria R, Castelli L, Reynolds M, Dell’Acqua R. Look out for strangers! Sustained neural activity during visual working memory maintenance of other-race faces is modulated by implicit racial prejudice. Social Cognitive and Affective Neuroscience. 2012;7:314–21. doi: 10.1093/scan/nsr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist. 2011;17:18–24. doi: 10.1177/1073858410379268. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Goldsher D, Berger BD, Aharon-Peretz J. Impairment in cognitive and affective empathy in patients with brain lesions: anatomical and cognitive correlates. Journal of Clinical and Experimental Neuropsychology. 2004;26:1113–27. doi: 10.1080/13803390490515531. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–27. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Sheng F, Han S. Manipulations of cognitive strategies and intergroup relationships reduce the racial bias in empathic neural responses. NeuroImage. 2012;61:786–97. doi: 10.1016/j.neuroimage.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. Spontaneous mentalizing during an interactive real world task: an fMRI study. Neuropsychologia. 2006;44:1674–82. doi: 10.1016/j.neuropsychologia.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Verleger R. Event-related potentials and cognition: a critique of the context updating hypothesis and an alternative interpretation of the P3. Behavioral and Brain Sciences. 1988;11:343–427. [Google Scholar]
- Verleger R. On the utility of P3 latency as a measure of mental chronometry. Psychophysiology. 1997;34:131–56. doi: 10.1111/j.1469-8986.1997.tb02125.x. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Fiske ST. Controlling racial prejudice and stereotyping: social cognitive goals affect amygdala and stereotype activation. Psychological Science. 2005;16:56–63. doi: 10.1111/j.0956-7976.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Zuo X, Wang X, Han S. Do you feel my pain? Racial group membership modulates empathic neural responses. Journal of Neuroscience. 2009;29:8525–9. doi: 10.1523/JNEUROSCI.2418-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Ochsner KN. The neuroscience of empathy: progress, pitfalls, and promise. Nature Neuroscience. 2012;15:675–80. doi: 10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Carlson SM, Kesek A. The development of executive function in childhood. In: Nelson C, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. 2nd edn. Cambridge, MA: MIT Press; 2008. pp. 553–74. [Google Scholar]