Abstract
Social interactions occur within a variety of different contexts––cooperative/competitive––and often involve members of our social network. Here, we investigated whether social network modulated the value placed on positive outcomes during a competitive context. Eighteen human participants played a simple card-guessing game with three different competitors: a close friend (in-network), a confederate (out-of-network) and a random number generator (non-social condition) while undergoing functional magnetic resonance imaging. Neuroimaging results at the time of outcome receipt demonstrated a significant main effect of competitor across multiple regions of medial prefrontal cortex, with Blood Oxygen Level Dependent (BOLD) responses strongest when competing against one’s friend compared with all other conditions. Striatal BOLD responses demonstrated a more general sensitivity to positive compared with negative monetary outcomes, which an exploratory analysis revealed to be stronger when interacting with social, compared with non-social, competitors. Interestingly, a Granger causality analysis indicated directed influences sent from an medial prefrontal cortex (mPFC) region, which shows social network differentiation of outcomes, and the ventral striatum bilaterally. Our results suggest that when competing against others of varying degrees of social network, mPFC differentially values these outcomes, perhaps treating in-network outcomes as more informative, leaving the striatum to more general value computations.
Keywords: effective connectivity, medial prefrontal cortex, social network, striatum, valuation
INTRODUCTION
Human behavior often occurs within varying social contexts that color our daily experiences and decisions. We often seek out social rewards, such as looking for acceptance from others (Somerville et al., 2006), which may be valued subjectively in putative neural reward circuitry (Izuma et al., 2008) akin to non-social rewards (Delgado et al., 2000; Knutson et al., 2001; O'Doherty et al., 2002; Knutson et al., 2003; Tricomi et al., 2006; Seymour et al., 2007). One interesting idea is that the value of social rewards and the influence of social context may in part be driven by a fundamental need to feel accepted or belong (Baumeister and Leary, 1995) and a desire to form meaningful relationships (van Winden et al., 2008), both of which can modulate behavior. The mere chance to receive social approval for our actions increases pro-social tendencies (e.g. charitable giving) and more strongly recruits reward circuitry than when there is no chance for approval (Izuma et al., 2010), and simply being in the presence of peers lends increased value to engaging in risk-taking behaviors (Steinberg and Monahan, 2007; Chein et al., 2011). Furthermore, both vicarious and shared positive experiences can receive differential value depending on whether they occur with others that are perceived as socially similar (vs dissimilar; Mobbs et al., 2009) or with someone from within (vs outside of) one’s social network (Fareri et al., 2012). Taken together, these findings lend credence to the notion that social context can influence neural signals involved in motivated behavior, in turn playing a significant role in our daily experiences.
An interesting question arises, however, when considering that social interactions can occur in contexts that are sometimes diametrically opposed––e.g. cooperative vs competitive. Within cooperative social contexts, humans often act against their own self-interest, forgoing maximal personal gains for lesser gains that carry greater social value in the long run; the motivation here is that concerns for social preferences––e.g. reciprocity, fairness and reputation––underlie social behavior (Berg et al., 1995; Fehr and Fischbacher, 2002; Fehr and Camerer, 2007). For example, achieving outcomes via mutual cooperation with another person elicits stronger BOLD responses in corticostriatal reward circuitry compared with when acting selfishly or when mutually cooperating with a computer (Rilling et al., 2002) as well as compared with when one’s cooperation goes unreciprocated (Rilling et al., 2004).
Competitive contexts, on the other hand, require keeping track of others’ behavior so as to be able to outperform a competitor. Such processes rely on cortical structures, particularly medial prefrontal cortex (mPFC), to monitor self and other performance (de Bruijn et al., 2009; Howard-Jones et al., 2010) and code for outcomes earned against another (Bault et al., 2011). A competitive social context has also been found to influence striatal BOLD signals, with responses to losses in an auction correlating with a tendency to overbid (Delgado et al., 2008). Taken together, these findings suggest diverging motives and mechanisms during cooperative and competitive contexts.
As many of our interactions occur with members of our social networks, it is critical to understand how social network might affect the value placed on earned outcomes within these differing social contexts. Sharing positive outcomes with a close, in-network partner more strongly recruits the striatum than sharing the same outcome with an out-of-network other (Fareri et al., 2012), suggesting a higher value attached to outcomes shared with close others. However, it is unclear how competing with an in-network other might affect outcome value; i.e. will earning a positive outcome against an in-network other carry higher or lower value than against someone out-of-networks? To investigate this, we administered a simple card-guessing task (adapted from Fareri et al., 2012) in which we manipulated participants’ competitors and roles. Participants competed for separate pots of money against three different competitors: an in-network close friend, an out-of-network other (confederate) and a random number generator (non-social control). Participants alternated roles between making guesses in the game (player) and watching their competitors guess (observer). Importantly, outcomes could benefit one or the other party irrespective of who was responding; i.e. monetary gains could go to a participant if they made a correct guess or if their competitor made an incorrect guess. Based on previous work from our group (Fareri et al., 2012), we hypothesized that outcome valuation as reflected by corticostriatal BOLD responses would be modulated by social network in this competitive context, with enhanced responses observed in mPFC and the striatum at the time of outcome when competing against an in- vs out-of-network competitor.
MATERIALS AND METHODS
Participants
Twenty-four gender-matched participant pairs were recruited using posted advertisements from Rutgers-Newark and the surrounding area. Six participant pairs were excluded from final analysis. One participant withdrew after reporting claustrophobia. Three participant pairs failed to meet inclusionary criteria: excessive head motion (>3 mm in any plane) across multiple runs of the session and observed artifact in BOLD images. Two final pairs were excluded because of reported explicit plans to split the money earned in the task prior to coming in for the scanning session, thus compromising the creation of a competitive social context. Final analysis was conducted on behavioral and functional magnetic resonance imaging (fMRI) data from the remaining 18 MRI participants (mean = 20.4 years, s.d. = 2.15, 8 female participants). Behavioral analyses were additionally conducted on questionnaire data from the cohort of behavioral participants (mean = 21 years, s.d. = 3.36). All participant pairs provided informed consent prior to participation. This study was approved by the Institutional Review Boards of Rutgers University and the University of Medicine and Dentistry of New Jersey.
Experimental paradigm
This study took part over the course of 2 days. Recruited MRI participants were asked to bring a same gender close friend to the experimental session (not a romantic partner or family member). After providing informed consent on Day 1, participants and their friends separately completed the Inclusion of Other in Self Scale (IOS; Aron et al., 1992). This served as a manipulation check by which to assess the degree of closeness within the in-network relationship, because social network was a factor of interest here. The IOS consists of a series of sets of circles varying in their degree of overlap, with increased overlap indicating increased closeness. Participant pairs were separately instructed to choose the set that best characterized their relationship. Prior to the end of the Day 1 session, a facial photograph was taken of the same gender friend and programmed as a stimulus into the Day 2 task.
The experimental session took place on Day 2 (typical delay between sessions was 1–2 days) at the University Heights Advanced Imaging Center (Newark, NJ, USA). MRI participants were told that they were going to be playing a simple card-guessing game in which they would be competing for monetary outcomes. We manipulated two factors of interest: MRI participants’ competitors (1) and role (2) during the game. MRI participants played the game against three different competitors: their friend (in-network), a gender-matched confederate from the laboratory (out-of-network other) and a random number generator (RNG). MRI participants were told that their goal was to earn more money than each competitor. The confederate was portrayed as another participant in the study who had been trained separately on the task and met the MRI participant and their friend at the start of the experimental session on Day 2. Both the MRI participant and their friend rated the confederate on the IOS as a manipulation check. The confederate’s true identity was not revealed until the end of the task to limit suspicions of an unfair advantage in the task. The RNG served as a non-social control condition and was represented in the task by a photo of a matrix of random numbers (see Supplementary Materials for further discussion). MRI participants alternated roles during the task between making the guesses in the game (player) and watching their competitors make the guesses (observer).
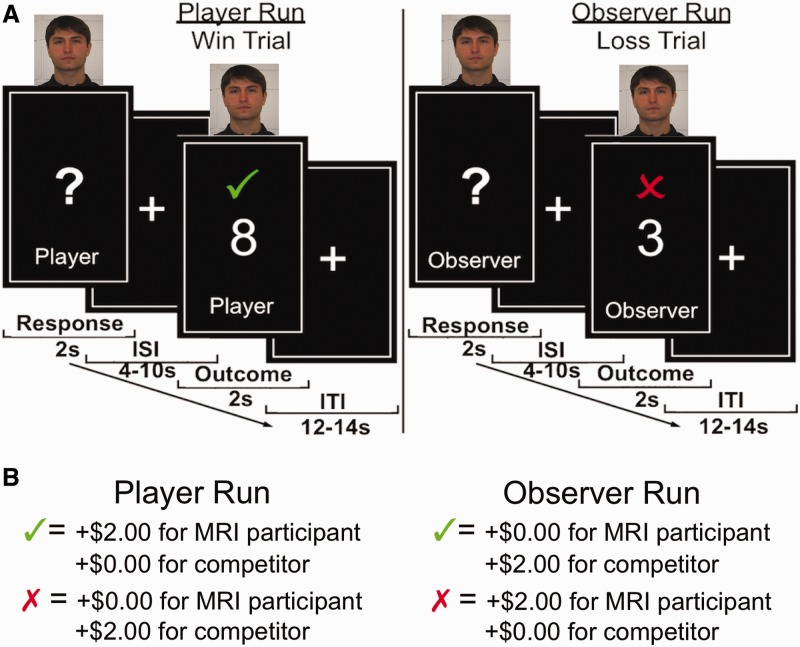
Participants’ task (adapted from Fareri et al., 2012), was simply to guess whether the value of a card was lower (1, 2, 3, 4) or higher (6, 7, 8, 9) than the number 5 (Figure 1 and Supplementary Materials for trial timeline). The task consisted of 96 trials in total, evenly distributed across four functional runs. Each run contained eight trials per partner condition, randomly presented. Participants’ roles alternated across runs––two player runs and two observer runs, with 48 trials total per role condition––the order of which was counterbalanced across sessions. During player runs, MRI participants responded using buttons designated ‘high’ and ‘low’ on an MRI-compatible fiber optic response box (Current Designs, Inc.); the friend was seated in the control room and pressed a designated button on a computer keyboard to ‘release’ the MRI participants’ responses (i.e. allow them to be counted) on trials in which they were competing. During observer runs, MRI participants made a button press to release their competitors’ responses. This served as both a motor control as well as to keep MRI participants and their friends engaged in the task at all times. Unknown to MRI participants, confederate and computer responses were pre-programmed into the task.
Fig. 1.
Task structure. MRI participants played a simple card-guessing task in which they competed for monetary outcomes against one of three competitors on each trial––a random number generator, a gender matched confederate or a close, same gender friend (adapted from Fareri et al., 2012). (A) A picture at the top of the screen indicated MRI participants’ competitor on each trial. MRI participants’ roles alternated between making guesses (player runs) and observing their competitors make the guesses (observer runs). Responses were made during a 2-s response period, which was followed by jittered inter-stimulus interval (4–10 s) and an ensuing outcome phase (2 s). (B) Correct guesses indicated by a green check mark resulted in +$2.00 for the respondent and $0.00 for the other party on a given trial. Incorrect guesses indicated by a red ‘X’ resulted in $0.00 for the respondent and +$2.00 for the other party. Trials were separated by a jittered inter-trial interval (12–14 s).
All trials had $2.00 at stake. Correct guesses resulted in +$2.00 for the respondent and $0.00 for the other party; incorrect guesses resulted in the opposite distribution. Thus, the MRI participant could experience positive (+$2.00) and negative outcomes ($0.00) regardless of whether they (player runs) or their competitors (observer runs) were making the guesses. No monetary losses were incurred in this task unless a trial was missed. If either the MRI participant or their competitor did not respond within the requisite amount of time (Figure 1 and Supplementary Materials), the ‘#’ symbol would appear; participants were told this indicated a monetary loss of $1.00 for both parties involved. This was intended to encourage responding and to protect against participants potentially not responding in order to prevent a competitor from earning money. Importantly, all outcomes were pre-determined (50% positive, 50% negative) and randomly presented to ensure equivalent experiences across all participants.
We assessed MRI participants’ motivation to beat each competitor prior to the task as a subjective baseline measure of competitiveness. Post-session ratings were acquired to assess participants’ experience during the task (e.g. how excited/disappointed they were to win/lose against each competitor). Ratings were made on 7-point Likert scales (1 = not at all, 7 = a lot).
Behavioral analysis
We conducted Pearson’s correlations between MRI participants’ and friends’ responses on the IOS to probe whether they held similar views of their relationship. MRI participants’ ratings of friend and confederate on the IOS were tested with paired sample t-tests; the same was done for friends’ responses. Pre- and post-session ratings were examined with separate one-way repeated measures analyses of variance (ANOVAs). A Greenhouse–Geiser correction was applied for violations of sphericity. Where appropriate, post hoc comparisons were conducted and corrected for multiple comparisons using the sequential Bonferonni method (Holm, 1979; Rice, 1989).
fMRI acquisition and analysis
Images were acquired using a 3T Siemens Allegra head-only scanner. Anatomical images were collected with a T1-weighted MPRAGE sequence (256 × 256 matrix; FOV = 256 mm: 176 1 mm sagittal slices). Functional images were acquired using a single-shot gradient echo EPI sequence (TR = 2000 ms, TE = 25 ms, FOV = 192, flip angle = 80°, bandwidth = 2604 Hz/Px, echo spacing = 44) and comprised 35 contiguous oblique-axial slices (3 × 3 × 3 mm voxels) parallel to the anterior commissure–posterior commissure line. Pre-processing and analysis of neuroimaging data were performed using BrainVoyager QX (v2.2, Brain Innovation). Pre-processing consisted of 3D motion correction (six parameters) slice scan time correction (cubic spline interpolation), 3D Gaussian spatial smoothing (4-mm Full width at half maximum (FWHM)), voxelwise linear detrending and high-pass filtering of frequencies (three cycles per time course). Individual anatomical and functional datasets were warped to standard Talairach stereotaxic space (Talairach and Tournoux, 1988). Individual whole-brain masks were created and additively combined to create a group mask excluding the skull.
We constructed a single random effects General Linear Model (GLM) using role, competitor and outcome as factors. We modeled the response and outcome phases of the task with separate regressors as a function of competitor and role conditions in order to capture variance unique to each phase. Thus, we included a total of 18 regressors of interest in our model. Six regressors were included modeling the response phase (2 s in duration; two levels of role and three levels of competitor); 12 regressors were included modeling the outcome phase (2 s in duration; two levels of role, three levels of competitor and two levels of outcome). One missed trial regressor and six motion parameters served as regressors of no interest. Regressors of interest and missed trial regressors were convolved with a 2-gamma hemodynamic response function. All regressors were z-transformed at the single participant level. Statistical parametric maps (SPMs) were initially set to an uncorrected height threshold of P < 0.001, unless otherwise noted, and were subsequently corrected for multiple comparisons with a corrected threshold of P < 0.05 at the group level, using the Cluster Level Statistical Threshold Estimator plugin in BrainVoyager. This correction method runs a series of Monte Carlo simulations across the whole brain to determine the probability that observed significant clusters of activation are not false positives in a given SPM (Forman et al., 1995; Goebel et al., 2006; see also Lieberman and Cunningham, 2009). A cluster threshold of three contiguous voxels (equivalent to 81 mm3) as determined by the plugin was applied, unless otherwise noted. We conducted three main types of analyses:
Whole-brain analyses.
We conducted a 2 (role) × 3 (competitor) × 2 (outcome valence) whole-brain repeated measures ANOVA to investigate BOLD responses during the outcome phase of the task. We additionally conducted a 2 (role) × 3 (competitor) whole-brain repeated measures ANOVA in order to probe BOLD responses during the response phase (see Supplementary Materials for results). Mean parameter estimates were extracted from functional clusters to characterize resulting significant effects based on an average across all voxels in a region of interest centered around the peak voxel. Where appropriate, post hoc comparisons were conducted and corrected using the sequential Bonferonni method (Holm, 1979; Rice, 1989). We probed modulation of outcome-related BOLD responses by social closeness (Fareri et al., 2012) with whole-brain correlations between self-reported closeness (IOS) with their friends and outcome-related BOLD responses collapsed across all competitor types (e.g. ∑ positive > ∑ negative outcomes).
Second-order contrast.
We also conducted a second-order contrast to explore differences in outcome value signals (e.g. positive greater than negative) as a function of whether a competitor was a social or non-social entity. We performed separate contrasts of positive vs negative outcomes for social (friend + confederate) and non-social (RNG) trials at the single participant level. We then conducted a subtraction of social–non-social outcome maps for each participant and combined single-subject subtractions to form a group map that was subjected to a t-test against zero. This exploratory analysis was set at a more lenient threshold of P < 0.005, whole-brain corrected at the cluster level to five contiguous voxels (135 mm3) of brain tissue as determined by the Cluster Level Statistical Estimator.
Granger causality analysis.
As previous investigations have demonstrated connectivity within corticostriatal circuitry during competitive and strategic social interactions (e.g. Hampton et al., 2008; Bault et al., 2011), we conducted a Granger causality analysis in BrainVoyager. Granger causality assesses interactions between a seed region of interest and all other areas of the brain by assuming a linear dependence between two time series x and y when using vector autoregression (Geweke, 1982). Linear dependence Fx,y between two time series can be quantified as a summation of the extent to which: past values of one time series x can better predict values of a second time series y (Fx→y) than past values of y and vice versa (Fy→x) as well as the undirected instantaneous influence that may occur between time series x and y (Fx*y) (Goebel et al., 2003; Roebroeck et al., 2005). Granger causality thus tests for both effective (directed) and functional (instantaneous) connectivity between a seed region and all other areas of the brain (Goebel et al., 2003; Roebroeck et al., 2005, 2011). We computed separate functional and effective connectivity maps demonstrating interactions between this seed region and all other voxels in the brain across the entire timecourse of each functional run (290 TRs) for each participant. As we were primarily interested in directed influences to and from this seed region, we focused on effective connectivity results. Connectivity maps were computed for each participant and were combined to form a group map which was subjected to a t-test against zero (Dickerson et al., 2010). Group comparison maps were thresholded at P < 0.005 and corrected using a cluster threshold of six contiguous voxels (equivalent to 162 mm3 of contiguous brain tissue) as determined by the Cluster Level Statistical Estimator.
RESULTS
Behavioral results
A simple Pearson’s correlation between MRI participants’ and their friends’ responses on the IOS revealed a significant correlation [r(16) = 0.68, P = 0.002], suggesting similar perceptions of the friendship. Supporting these results, both MRI participants [t(17) = 12.83, P < 0.001] and their friends [t(17) = 12.70, P < 0.001] reported feeling closer to each other than to the confederate, suggesting an effective in- vs out-of-network manipulation.
Assessing MRI participants’ pre-task ratings of competitiveness (e.g. ‘How much do you want to beat this competitor in the game?’) with a one-way repeated measures ANOVA revealed a marginally significant main effect [F(1.335, 22.694) = 3.045, P = 0.084]. Participants were marginally more motivated to compete against the RNG (mean = 6.39, s.d. = 0.98) compared with their friend [mean = 5.55, s.d. = 1.54; t(17) = 1.97, P = 0.065]; this effect was weaker between confederate (mean = 6.11, s.d. = 0.96) and friend [t(17) = 1.49, P = 0.15]. These results suggest that competing against an in-network other may have differentially affected participants’ motivation in the task as expected, though they did not quite reach significance. Probing post-session ratings of excitement and disappointment for winning/losing revealed no significant effects.
Neuroimaging results
Whole-brain analyses.
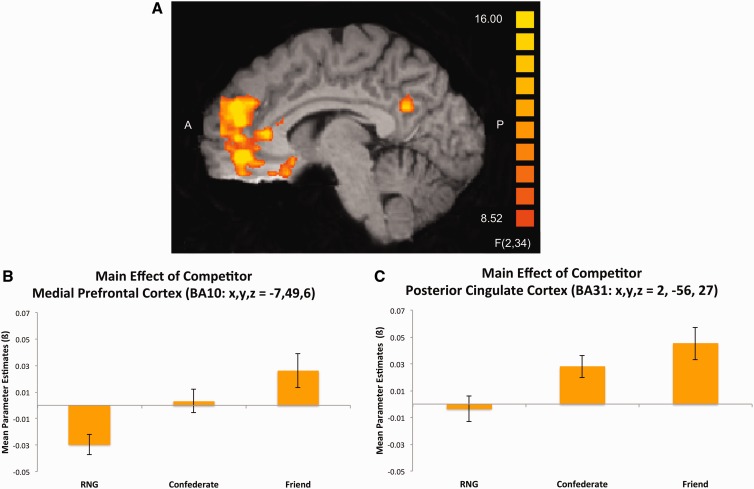
Our primary interest in this study was whether level of social network with a competitor would modulate corticostriatal outcome value signals. A 2 (role) × 3 (competitor) × 2 (outcome) whole-brain repeated measures ANOVA revealed a main effect of competitor (Table 1) that was highly robust across many mPFC regions (Figure 2A). Importantly, a cluster emerged in a dorsal part of BA10 in mPFC (x, y, z = −7, 49, 6), encompassing voxels previously implicated as being sensitive to social gains from risky choices compared to social losses and non-social outcomes (Bault et al., 2011). When collapsing across outcome valence (Figure 2B), BOLD responses here were more positive when competing against one’s friend as compared with the random number generator [t(17) = 6.45, P = 0.000006], or confederate [t(17) = 2.42, P = 0.027]. This region also showed a more positive BOLD response when competing against the confederate than when competing against the RNG [t(17) = 3.74, P = 0.002]. Other areas of mPFC––dorsomedial PFC (BA9), ventromedial PFC (BA10) and a cluster bordering orbitofrontal cortex (BA11)––all showed this same general pattern. A cluster in posterior cingulate cortex bordering the cuneus (BA31) showed similar effects (Figure 2C): BOLD responses when competing against the confederate [t(17) = 5.19, P = 0.000074] and friend [t(17) = 4.34, P = 0.0004] were more positive than when competing against the RNG. A marginally significant trend emerged when comparing activation in this region during friend vs confederate trials [t(17) = 1.92, P = 0.07].
Table 1.
Outcome phase 2 × 3 × 2 ANOVA: main effect of competitor
| Region of activation | Brodmann area | Direction | Laterality | Talairach coordinates |
No. of voxels (mm3) | F-statistic | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Cerebellum | * | R | 44 | −53 | −21 | 464 | 14.45 | |
| Inferior/middle frontal gyrus | BA11/47 | * | R | 29 | 31 | −15 | 278 | 19.20 |
| Medial frontal gyrus/OFC | BA11 | * | L | −4 | 31 | −15 | 222 | 14.67 |
| Subgenual anterior cingulate | BA25 | * | L | −7 | 19 | −15 | 152 | 13.82 |
| Inferior frontal gyrus | BA47 | * | L | −37 | 19 | −15 | 239 | 14.24 |
| Medial frontal gyrus | BA10 | * | L | −7 | 46 | −6 | 2110 | 28.50 |
| Medial frontal gyrus | BA10 | * | L | −7 | 49 | 6 | 2016 | 19.66 |
| Cingulate gyrus/corpus callosum | BA24 | ** | L | −4 | 25 | 15 | 94 | 11.56 |
| Medial frontal gyrus | BA9 | * | L | −1 | 46 | 24 | 2554 | 27.50 |
| Inferior parietal lobule/angular gyrus | BA39 | *** | L | −49 | −62 | 24 | 710 | 15.30 |
| Posterior cingulate | BA31 | * | R | 2 | −56 | 27 | 1810 | 20.16 |
* = friend > confederate > RNG; ** = friend > RNG, friend > confederate; *** friend > RNG, confederate > RNG.
Fig. 2.
Main effect of competitor (outcome phase). (A) A 2 (role) × 3 (competitor) × 2 (outcome) whole-brain repeated measures ANOVA revealed a significant main effect of competitor in a number of regions. (B) Parameter estimates extracted from a cluster in BA10 (x, y, z = −7, 49, 6) demonstrate this effect to be driven by enhanced value signals to outcomes on friend trials compared with confederate or RNG trials. (C) Similar results emerged in a cluster of posterior cingulate cortex bordering the cuneus (BA31). Activation maps were set to an initial uncorrected height threshold of P < 0.001 and subsequently, whole-brain corrected at the cluster level to a threshold of P < 0.05.
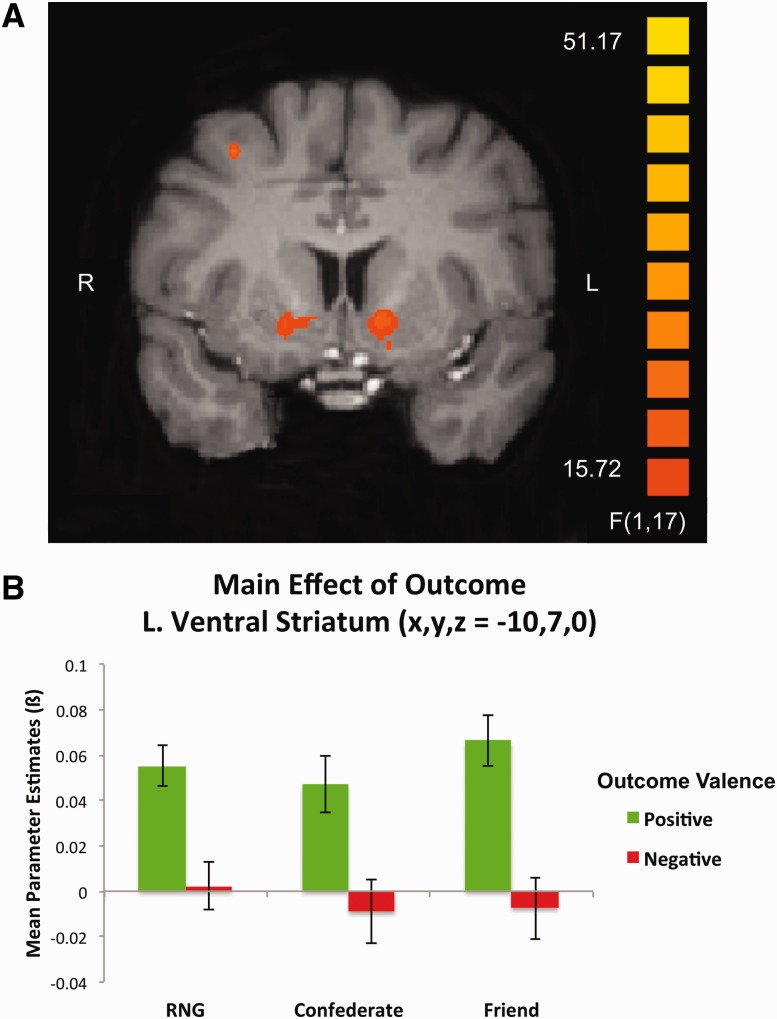
Interestingly, we observed no modulation of BOLD activation in the striatum as a function of social network in this task. Rather, a main effect of outcome was observed in multiple striatal subregions, including bilateral ventral caudate nucleus and bilateral putamen (Table 2). Striatal BOLD responses were significantly greater for positive compared with negative outcomes, irrespective of competitor (Figure 3A and B). No regions emerged showing a stronger response for negative outcomes (Supplementary Materials for additional ANOVA results).
Table 2.
Outcome phase 2 × 3 × 2 ANOVA: main effect of outcome
| Region of activation | Brodmann area | Laterality | Talairach coordinates |
No. of voxels (mm3) | F-statistic | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Cerebellum | R | 20 | −77 | −33 | 214 | 24.72 | |
| Cerebellum | R | 35 | −77 | −30 | 690 | 50.29 | |
| Middle frontal gyrus | BA11 | L | −22 | 37 | −15 | 262 | 35.65 |
| Putamen | L | −19 | 1 | −9 | 179 | 22.25 | |
| Medial frontal/cingulate gyrus | BA10/32 | L | −19 | 40 | −6 | 253 | 31.97 |
| Middle frontal gyrus | BA10/47 | L | −43 | 46 | −3 | 405 | 40.69 |
| Putamen | R | 17 | 4 | 0 | 187 | 27.48 | |
| Caudate nucleus | R | 8 | 10 | 0 | 155 | 19.58 | |
| Caudate nucleus/ventral striatum | L | −10 | 7 | 0 | 428 | 23.87 | |
| Middle frontal gyrus | BA10 | R | 23 | 58 | 3 | 81 | 27.62 |
| Caudate nucleus | L | −13 | 16 | 6 | 112 | 23.12 | |
| Inferior frontal gyrus | BA45/46 | R | 35 | 31 | 9 | 142 | 32.37 |
| Medial occipital gyrus | BA18 | R | 20 | −89 | 12 | 99 | 22.17 |
| Cingulate gyrus | BA31 | L | −16 | −44 | 27 | 103 | 21.84 |
| Cingulate gyrus | BA33 | L | −4 | −32 | 30 | 399 | 25.64 |
| Superior parietal lobule | BA7 | R | 35 | −65 | 48 | 249 | 27.79 |
| Middle frontal gyrus | BA8 | R | 29 | 10 | 48 | 408 | 29.95 |
| Middle frontal/superior frontal gyrus | BA8 | L | −28 | 19 | 51 | 82 | 24.83 |
Fig. 3.
Main effect of outcome. (A) A significant main effect of outcome emerged during a 2 (role) × 3 (competitor) × 2 (outcome) whole-brain repeated measures ANOVA in bilateral ventral striatum. (B) BOLD responses in the left ventral striatum (x, y, z, = −10, 7, 0) demonstrated enhanced value signals to positive compared with negative outcomes across all competitor conditions. Activation map was set to an initial uncorrected height threshold of P < 0.001 and subsequently, whole-brain corrected at the cluster level to a threshold of P < 0.05.
Given previous findings suggesting social closeness with an in-network other as a modulator of shared reward value (Fareri et al., 2012), we probed a potential role for this factor here. We explored whether any regions demonstrating increased BOLD responses to positive vs negative outcomes (collapsed across competitors) were further modulated by social closeness with an in-network competitor. Whole-brain correlations between a contrast of positive greater than negative outcomes and IOS ratings of one’s friend revealed no significant activation, suggesting that social closeness was not playing a significant role during outcome valuation within this competitive social context.
Second-order Contrast.
Previous results suggest differential striatal responses during social compared with non-social conditions during outcome receipt (Rilling et al., 2002; Delgado et al., 2008). We explored whether striatal BOLD responses might similarly demonstrate a more general social vs non-social distinction in the current paradigm. We conducted an exploratory analysis using a second-order contrast of positive greater than negative outcomes for social–non-social competitors. Results from this exploratory analysis (Supplementary Materials and Supplementary Figure S1 for additional discussion) revealed increased BOLD responses in a number of striatal subregions (Table 3), including bilateral putamen, when experiencing positive compared with negative outcomes against a social compared with non-social competitor.
Table 3.
Second-order contrast: social–non-social, win > loss
| Region of activation | Brodmann area | Laterality | Talairach coordinates |
No. of voxels (mm3) | t-statistic | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Inferior frontal gyrus | BA47 | R | 26 | 22 | −21 | 182 | 5.51 |
| Putamen/globus pallidus | L | −19 | 1 | −9 | 418 | 4.62 | |
| Inferior frontal gyrus | BA45 | L | −43 | 25 | 0 | 463 | 6.28 |
| Putamen | L | −25 | 7 | 3 | 203 | 4.28 | |
| Putamen | R | 17 | 13 | 6 | 233 | 3.92 | |
| Putamen | L | −28 | −14 | 6 | 264 | 4.77 | |
| Middle frontal gyrus | BA10 | L | −40 | 52 | 12 | 193 | 4.69 |
| Middle temporal gyrus/inferior parietal lobule | BA19 | L | −52 | −62 | 18 | 154 | 4.01 |
Granger causality analysis.
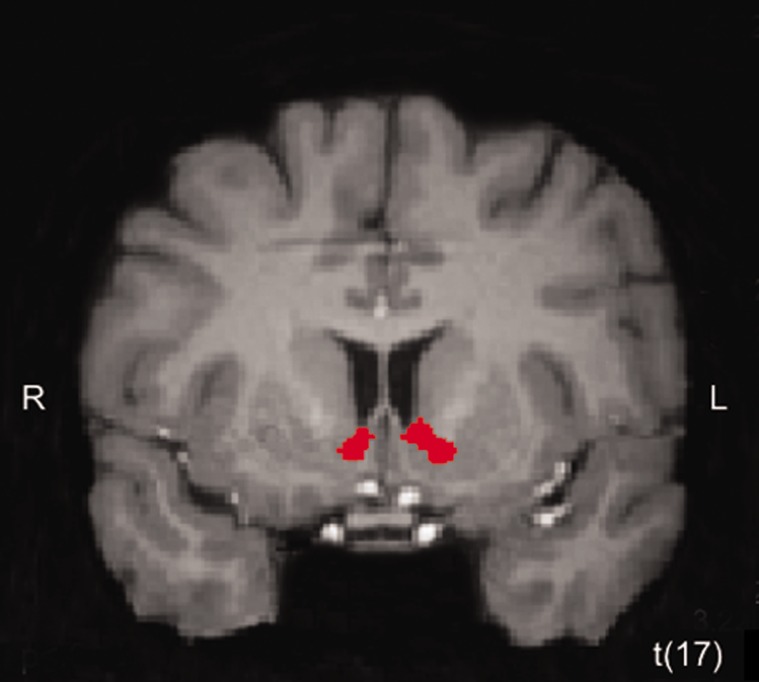
Based on a main effect of competitor emerging in mPFC but not the striatum and evidence showing connectivity between these two areas during strategic and competitive interactions (e.g. Hampton et al., 2008; Bault et al., 2011), we examined interactions between mPFC and the rest of the brain with a Granger causality analysis. We chose a cluster in BA10 (x, y, z = −7, 49, 6) as the seed region for this analysis, given our results showing its sensitivity to social network. This cluster also contains the peak voxel reported in a recent study by Bault and colleagues (2011) as demonstrating increased BOLD responses when experiencing positive outcomes gained in comparison to against another person. Figure 4 depicts effective connectivity results; clusters in red are targets of the mPFC seed region. As can be seen, directed influences are sent to bilateral ventral striatum (Table 4 for complete list of regions identified in this analysis), which demonstrated a main effect of outcome in our whole-brain ANOVA.
Fig. 4.
Effective connectivity results. (A) Granger causality analysis using a seed region in mPFC that demonstrated a main effect of competitor (x, y, z = −7, 49, 6) revealed directed influences sent from this region to bilateral ventral striatum (right: x, y, z = 11, 13, −3; left: x, y, z = −7, 7, 0). The clusters depicted in red denote directed influences received from the mPFC seed. Activation map was set to an initial uncorrected height threshold of P < 0.005 and subsequently, whole-brain corrected at the cluster level to a threshold of P < 0.05.
Table 4.
Granger causality analysis: Effective connectivity.
| Region of activation | Brodmann area | Laterality | Talairach coordinates |
No. of voxels (mm3) | t-statistic | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Medial frontal gyrus | BA11 | L | −4 | 40 | −12 | 228 | 4.93 |
| Medial temporal lobe | BA35 | L | −22 | −20 | −12 | 367 | 4.49 |
| Ventral striatum | R | 11 | 13 | −3 | 431 | 6.62 | |
| Ventral striatum | L | −7 | 7 | 0 | 428 | 4.30 | |
| PCC/corpus callosum | BA29 | R | 2 | −41 | 6 | 1441 | 4.77 |
| Medial frontal gyrus/cingulate gyrus | BA10/32 | L | −4 | 49 | 6 | 5616 | 4.69 |
| Thalamus | L | −4 | −14 | 12 | 364 | 4.54 | |
| Superior frontal gyrus | BA10 | L | −22 | 49 | 24 | 176 | 4.35 |
| Cingulate gyrus | BA32 | L | −1 | 28 | 27 | 172 | 4.98 |
| Precuneus/PCC | BA31 | L | −4 | −44 | 36 | 2234 | 5.43 |
| Medial frontal gyrus | BA8 | L | −7 | 49 | 42 | 216 | 4.02 |
Regions receiving directed influence from mPFC seed region (x, y, z = −7, 49, 6).
DISCUSSION
We investigated whether outcomes experienced during a competitive social context would carry differential value as a function of social network. Our results demonstrate that competing against an in-network other elicits enhanced outcome value signals in corticostriatal circuitry. BOLD responses across a wide range of mPFC showed sensitivity to social network, with activation strongest when evaluating outcomes experienced against an in-network competitor. In the ventral striatum, BOLD responses were characterized by a main effect of outcome—stronger for positive compared with negative outcomes—and a more general social vs non-social distinction, with increased activation observed for positive vs negative outcomes on social compared with non-social trials. A Granger causality analysis further revealed corticostriatal interactions: an mPFC cluster showing social network sensitivity sent directed influences to bilateral ventral striatum. Together, our findings suggest that during a competitive social context involving competitors of differing levels of social network, mPFC differentiates outcome value as a function of competitor, leaving the ventral striatum to process outcome value in a more coarse or general sense.
The striatum and mPFC are well-recognized components of a neural valuation system (for reviews see Daw and Doya, 2006; Delgado, 2007; Rangel et al., 2008; Haber and Knutson, 2010), which assigns value to expected and experienced outcomes (Delgado et al., 2000; Knutson et al., 2001, 2003; O'Doherty et al., 2002, 2004; Delgado et al., 2004; Galvan et al., 2005; Hare et al., 2008) to help guide decision-making (Kennerley et al., 2006; Rushworth, 2008; Rushworth and Behrens, 2008). Importantly, areas of mPFC also encode social information pertaining to self and others (for reviews, see Amodio and Frith, 2006; Wagner et al., 2012), responding to socially dominant others (Rudebeck et al., 2006) and close friends in comparison with similar others (Krienen et al., 2010). The present results merge these two literatures, showing that during a competitive social context, mPFC differentially assigns value to experienced positive outcomes as a function of whether one’s competitor was from within or outside of one’s social network. A possible explanation for this might be that participants attempted to use outcomes on in-network trials to inform behavior more so than outcomes with other competitors, thus lending heavier weight to them. Although we could not directly test this, given the random nature of outcome distribution and no opportunity for learning, this interpretation would be consistent with a role for mPFC in outcome monitoring and strategic thinking during competitive contexts in humans (Hampton et al., 2008; de Bruijn et al., 2009; Bault et al., 2011) as well as in rats and non-human primates (Hillman and Bilkey, 2012; Yoshida et al., 2012). Future investigations could more fruitfully explore effects of social network on outcome processing in competitive situations involving dynamic learning scenarios.
Our whole-brain analyses demonstrated a main effect of outcome in bilateral ventral striatum, with increased BOLD responses observed for positive vs negative outcomes, consistent with previous iterations of this paradigm (e.g. Delgado et al., 2000, 2003, 2004). This also supports recent investigations of competitive interactions in which the striatum generally comes online during outcome processing (de Bruijn et al., 2009; Hampton et al., 2008) sometimes coding for social compared to non-social outcomes (Bault et al., 2011), but more putative cortical and prefrontal cortical regions support behavioral updating as a function of more complex social information (Hampton et al., 2008). In conjunction with a second-order contrast showing striatal sensitivity to positive social outcomes in the striatum, but not social network, these findings implicate the striatum as performing a more general role in outcome valuation during a competitive social context.
We observed effective connectivity within corticostriatal circuitry as a result of a Granger causality analysis: bilateral ventral striatum was a target of directed influence from a cluster of mPFC demonstrated to value positive outcomes earned after risky choices in comparison with another person (Bault et al., 2011). It is possible that the directed influences sent from this cluster of mPFC, which demonstrated an in- vs out-of-network distinction in the present study, led to a more general representation of outcome value in the striatum as opposed to one that was specifically sensitive to social network in a more motivationally salient, cooperative context (Fareri et al., 2012). This may additionally help explain why participants did not differentially rate excitement for winning or losing against each competitor. We thus suggest that the striatum in part may have processed outcome value more coarsely here due to directed modulation from mPFC, which was coding a finer sensitivity to social network.
It is important to consider potential caveats regarding this connectivity analysis. Granger causality is an exploratory analysis requiring no specific predictions about directionality or an a priori specified network of neural regions involved (Roebroeck et al., 2005). This analysis searches for correlations and predictive relationships between the timecourse of activation in a specified seed region and the rest of the brain. One study (David et al., 2008) contends that Granger causality may not be optimal for fMRI data, because the temporal dynamics of the hemodynamic response may be heterogeneous across the brain. However, other evidence suggests that considering temporal dynamics of fMRI data, and particularly temporal precedence, is necessary when attempting to model or detect causal influences (Roebroeck et al., 2011; for further discussion also see Valdes-Sosa et al., 2011).
Social closeness did not modulate outcome valuation as a function of social network in the present study. Closeness ratings in the present cohort of participants may have lacked sufficient range or variability to serve as an adequate predictor variable. All potential values of the IOS scale were not represented as selected responses in this sample of participants. Perhaps, with a sample exhibiting more diversity in their IOS responses, an effect may have emerged. However, given previous evidence from our group (Fareri et al., 2012) as well as complementary evidence suggesting general social reward sensitivity may be related to other measures of interpersonal closeness (Vrticka et al., 2008; for review see Vrticka and Vuilleumier, 2012), this result is not necessarily surprising. Competing against a close, in-network other may in fact be orthogonal to the notion of a merged representation of a social relationship inherent in the construct of social closeness (Aron et al., 1992). Rather than sharing a positive experience with another and perhaps reaffirming a friendship with said shared reward (Fareri et al., 2012), positive outcomes in the present task necessarily came at the expense of an in-network friend, which would not be a mutually positive and reaffirming experience.
The striatum demonstrated a general sensitivity to positive compared with negative outcomes, which was greater when competing against a social entity. We did not observe any significant competitor effects in response to negative outcomes as previously observed (Delgado et al., 2008). This could have been in part due to the competitive social context here not being salient enough. Although positive outcomes for the MRI participant in this task resulted in monetary gains, negative outcomes only led to a gain for the competitor and no gain (or cost) for the MRI participant. Previous work in which striatal BOLD responses to social losses correlated with overbidding in an auction (Delgado et al., 2008) necessitated more meaningful decisions. It is plausible that because participants in the present investigation simply made guesses, with no true opportunity to maximize earnings, the social manipulation may not have been as motivationally salient as intended. Future work could probe the effects of social network in a competition through the creation of a more salient and meaningful competitive context, one in which learning an optimal behavioral strategy is necessary to beat in- vs out-of-network competitors. Such an alternative design might better parse contributions of mPFC and striatum when competing against in-/out-of-network others. This may also further delineate behavioral correlates of competitiveness. Although it is conceivable that competing against one’s friend may elicit a stronger competitive desire, it seems equally likely that participants might be less motivated to beat their friend and a more salient design may further elucidate these divergent predictions.
The value placed on experienced outcomes is subject to a great deal of influence across varying social contexts. A common and important modulator of experienced outcomes is with whom they occur––someone from within or outside our social network. Our findings demonstrate that when competing against in-network other, increased value signals emerge in mPFC upon outcome receipt, as compared with receiving the same outcome in competition with an out-of-network other or non-social entity. This supports an integrating role for the mPFC, combining social information with value signals in a competitive social context.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
FUNDING
This research was funded by the National Institute of Mental Health (R01 MH084081 to M.R.D.).
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors would like to thank Michael Niznikiewicz and Meredith Johnson for assistance with data collection and Dr Anthony Porcelli for helpful discussion. This research was supported by funding from the National Institute of Mental Health (grant R01MH084081 to M.R.D.).
REFERENCES
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Aron A, Aron EN, Smollan D. Inclusion of the Other in the Self Scale and the structure of interpersonal closeness. Journal of Personality and Social Psychology. 1992;63:596–612. [Google Scholar]
- Bault N, Joffily M, Rustichini A, Coricelli G. Medial prefrontal cortex and striatum mediate the influence of social comparison on the decision process. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16044–9. doi: 10.1073/pnas.1100892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- Berg J, Dickhaut J, McCabe K. Trust, reciprocity, and social history. Games and Economic Behavior. 1995;10:122–42. [Google Scholar]
- Chein J, Albert D, O'Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Developmental Science. 2011;14:F1–10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David O, Guillemain I, Saillet S, et al. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS Biology. 2008;6:2683–97. doi: 10.1371/journal.pbio.0060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Doya K. The computational neurobiology of learning and reward. Current Opinion in Neurobiology. 2006;16:199–204. doi: 10.1016/j.conb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- de Bruijn ER, de Lange FP, von Cramon DY, Ullsperger M. When errors are rewarding. The Journal of Neuroscience. 2009;29:12183–6. doi: 10.1523/JNEUROSCI.1751-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Annals of the New York Academy of Sciences. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cognitive, Affective & Behavioral Neuroscience. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84:3072–7. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Schotter A, Ozbay EY, Phelps EA. Understanding overbidding: using the neural circuitry of reward to design economic auctions. Science. 2008;321:1849–52. doi: 10.1126/science.1158860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Stenger VA, Fiez JA. Motivation-dependent responses in the human caudate nucleus. Cerebral Cortex. 2004;14:1022–30. doi: 10.1093/cercor/bhh062. [DOI] [PubMed] [Google Scholar]
- Dickerson KC, Li J, Delgado MR. Parallel contributions of distinct human memory systems during probabilistic learning. NeuroImage. 2010;55:266–76. doi: 10.1016/j.neuroimage.2010.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Niznikiewicz MA, Lee VK, Delgado MR. Social network modulation of reward-related signals. The Journal of Neuroscience. 2012;32:9045–52. doi: 10.1523/JNEUROSCI.0610-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr E, Camerer CF. Social neuroeconomics: the neural circuitry of social preferences. Trends in Cognitive Sciences. 2007;11:419–27. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Fehr E, Fischbacher U. Why social preferences matter: The impact of non-selfish motices on competition, cooperation and incentives. The Economic Journal. 2002;112:C1–33. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ. The role of ventral frontostriatal circuitry in reward-based learning in humans. The Journal of Neuroscience. 2005;25:8650–6. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geweke J. Measurement of linear dependence and feedback between multiple time series. Journal of the American Statistical Association. 1982;77:304–13. [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with BrainVoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel R, Roebroeck A, Kim DS, Formisano E. Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magnetic Resonance Imaging. 2003;21:1251–61. doi: 10.1016/j.mri.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, Bossaerts P, O'Doherty JP. Neural correlates of mentalizing-related computations during strategic interactions in humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6741–6. doi: 10.1073/pnas.0711099105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. The Journal of Neuroscience. 2008;28:5623–30. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman KL, Bilkey DK. Neural encoding of competitive effort in the anterior cingulate cortex. Nature Neuroscience. 2012;15:1290–7. doi: 10.1038/nn.3187. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Howard-Jones PA, Bogacz R, Yoo JH, Leonards U, Demetriou S. The neural mechanisms of learning from competitors. NeuroImage. 2010;53:790–9. doi: 10.1016/j.neuroimage.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–94. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of the incentive for social approval in the ventral striatum during charitable donation. Journal of Cognitive Neuroscience. 2010;22:621–31. doi: 10.1162/jocn.2009.21228. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nature Neuroscience. 2006;9:940–7. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage. 2003;18:263–72. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Tu PC, Buckner RL. Clan mentality: evidence that the medial prefrontal cortex responds to close others. The Journal of Neuroscience. 2010;30:13906–15. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Meyer M, et al. A key role for similarity in vicarious reward. Science. 2009;324:900. doi: 10.1126/science.1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–4. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–26. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nature Reviews Neuroscience. 2008;9:545–56. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;6:223–5. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, Cohen JD. Opposing BOLD responses to reciprocated and unreciprocated altruism in putative reward pathways. Neuroreport. 2004;15:2539–43. doi: 10.1097/00001756-200411150-00022. [DOI] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. NeuroImage. 2005;25:230–42. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, Goebel R. The identification of interacting networks in the brain using fMRI: Model selection, causality and deconvolution. NeuroImage. 2011;58:296–302. doi: 10.1016/j.neuroimage.2009.09.036. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Buckley MJ, Walton ME, Rushworth MF. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313:1310–2. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- Rushworth MF. Intention, choice, and the medial frontal cortex. Annals of the New York Academy of Sciences. 2008;1124:181–207. doi: 10.1196/annals.1440.014. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience. 2008;11:389–97. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Seymour B, Daw N, Dayan P, Singer T, Dolan R. Differential encoding of losses and gains in the human striatum. The Journal of Neuroscience. 2007;27:4826–31. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9:1007–8. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Monahan KC. Age differences in resistance to peer influence. Developmental Psychology. 2007;43:1531–43. doi: 10.1037/0012-1649.43.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: An approach to medical cerebral imaging. New York: Theime Medical Publisher; 1988. [Google Scholar]
- Tricomi E, Delgado MR, McCandliss BD, McClelland JL, Fiez JA. Performance feedback drives caudate activation in a phonological learning task. Journal of Cognitive Neuroscience. 2006;18:1029–43. doi: 10.1162/jocn.2006.18.6.1029. [DOI] [PubMed] [Google Scholar]
- Valdes-Sosa PA, Roebroeck A, Daunizeau J, Friston K. Effective connectivity: influence, causality and biophysical modeling. NeuroImage. 2011;58:339–61. doi: 10.1016/j.neuroimage.2011.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Winden F, Stallen M, Ridderinkhof R. On the nature, modeling, andn neural bases of social ties. In: Houser D, Mccabe K, editors. Neuroeconomics. Bingley, UK: Emerald Group; 2008. [PubMed] [Google Scholar]
- Vrticka P, Andersson F, Grandjean D, Sander D, Vuilleumier P. Individual attachment style modulates human amygdala and striatum activation during social appraisal. PloS One. 2008;3:e2868. doi: 10.1371/journal.pone.0002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrticka P, Vuilleumier P. Neuroscience of human social interactions and adult attachment style. Frontiers in Human Neuroscience. 2012;6:212. doi: 10.3389/fnhum.2012.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Haxby JV, Heatherton TF. The representation of self and person knowledge in the medial prefrontal cortex. Wiley Interdisciplinary Reviews. Cognitive Science. 2012;3:451–70. doi: 10.1002/wcs.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Saito N, Iriki A, Isoda M. Social error monitoring in macaque frontal cortex. Nature Neuroscience. 2012;15:1307–12. doi: 10.1038/nn.3180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.