Abstract
Reminders of mortality influence human social cognition, but whether and how reminders of mortality affect brain activity underlying social cognition remains unclear. To test whether increasing mortality salience modulates neural responses to others’ suffering, we scanned healthy adults who viewed video clips showing others in pain using functional magnetic resonance imaging. One group of participants were primed to increase mortality salience and another group were primed with negative affect in terms of fear/anxiety. We found that perceiving painful vs non-painful stimuli in the pre-priming session activated the midcingulate/dorsal medial prefrontal cortex (MCC/dMPFC), bilateral anterior insula/inferior frontal cortex, bilateral secondary somatosensory cortex and left middle temporal gyrus. However, MCC/dMPFC activity in response to perceived pain in others was significantly decreased in the post-priming session by the mortality salience priming, but was not influenced by the negative affect priming. Moreover, subjective fear of death induced by the priming procedures mediated the change in MCC/dMPFC activity across the priming procedures. Subjective fear of death also moderated the co-variation of MCC/dMPFC and left insular activity during perception of others in pain. Our findings indicate that reminders of mortality decrease neural responses to others’ suffering and this effect is mediated by the subjective fear of death.
Keywords: mortality, empathy, midcingulate, fMRI
INTRODUCTION
Awareness of one’s own death is a unique feature of human thought and its conflict with yearning to life constitutes a basic motivation of human life (Becker, 1973; Koole et al., 2006). There has been increasing evidence that death-related thoughts influence social cognition and behavior. For example, people reported more favorability toward and gave more money to a charity after being reminded of mortality relative to a control condition (Jonas et al., 2002). When mortality was salient, individuals’ opinions were more close to that of ingroup members but more likely to contrast away from that of outgroup members relative to when thinking about something that was not related to death (Renkema et al., 2008). The effects of death-related thoughts on social cognition and behavior have been interpreted well by the Terror Management Theory (Pyszczynski et al., 1999) that proposes that reminding people of mortality enforce the symbols of cultural worldviews and self-esteem that function to buffer the anxiety related to increased mortality. However, to date, there still lacks a neuroscience account of the effect of death-related thoughts on social cognition and behavior.
Recent neuroimaging research has shown increasing interests in the neural correlates of death-related thoughts. For example, using functional magnetic resonance imaging (fMRI), Han and colleagues examined brain activity involved in the processing of linguistic cues related to death (Han et al., 2010; Shi and Han, 2012). They found that, although both death-related and death-unrelated negative valence words increased activity in the posterior cingulate and lateral frontal cortex relative to neutral valence words, viewing death-related words vs death-unrelated negative valence words decreased the neural activity in the bilateral anterior insula (AI) and the anterior midcingulate (MCC). Quirin et al. (2012) found that reading sentences related to mortality activated the ventral anterior cingulate (vACC). A recent event-related potential study also found increased activity over the parietal-occipital area to death-related words compared with death-unrelated words (Klackl et al., 2013). Although these neuroimaging findings provide evidence for modulations of the neural activity in specific brain regions by death-related thoughts, it remains unknown whether and how death-related thoughts modulate human brain activity involved in other social cognitive and affective processes.
The current work investigated how reminders of mortality affect neural responses to perceived pain in others that have been associated with empathy (Singer et al., 2004; Jackson et al., 2005; Gu and Han, 2007; Han et al., 2009) and altruistic behavior (Hein et al., 2010; Ma et al. 2011; Rameson et al., 2012). The previous brain imaging studies have shown ample evidence that perceiving others in pain is associated with activations in a neural network consisting of the AI, MCC, the supplementary motor area (SMA), the sensorimotor cortex, etc. (Singer et al., 2004; Avenanti et al., 2005; Jackson et al., 2005; Bufalari et al., 2007; Gu and Han, 2007; Saarela et al., 2007; Cheng et al., 2007; Han et al., 2009; Xu et al., 2009; Gu et al., 2010). The different brain regions in this neural network have been associated with distinct subcomponents of empathy. For example, the sensorimotor activity to perceived pain in others is associated with sensorimotor resonance (Avenanti et al., 2005; Bufalari et al., 2007), whereas the MCC and AI may play different functional roles in the cognitive-evaluative and affective-perceptual forms of empathy (Fan et al., 2011).
Behavioral studies that investigated the relationship between death-related thoughts and prosocial attitudes/behavior yielded conflicting results. Jonas et al. (2002) found that reminders of mortality induced positive attitudes toward charitable organizations and suggested that increasing mortality salience may enhance empathy and facilitate prosocial attitudes and behavior. In contrast, Hirschberger et al. (2005) reported that mortality salience decreased sympathy for the disabled and suggested that reminders of mortality may decrease empathy. We hypothesized that reminders of mortality may weaken empathic neural responses to others’ pain for two reasons. First, it has been shown that death-related thoughts make individuals avoid self-focused states (Arndt et al., 1998) and cause them to flee from their own physical sensations (Goldenberg et al., 2006). These findings support a postulate that mortality salience may induce self-avoidance. Empathy requires shared representation of one’s own feelings and others’ feelings (Decety and Jackson, 2004; Batson, 2009). The tendency to avoid one’s own feelings when mortality salience is increased may weaken the shared representation of one’s own emotion and others’ emotion and thus decrease empathy for others’ pain. Second, although the procedure to induce mortality threats activated the vACC (Quirin et al., 2012), the MCC and AI that are engaged in empathy for others in pain (Singer et al., 2004; Jackson et al., 2005; Gu and Han, 2007; Saarela et al., 2007; Han et al., 2009; Cheng et al., 2007; Xu et al., 2009) showed decreased activity during either implicit or explicit processing of death-related vs death-unrelated words (Han et al., 2010; Shi and Han, 2012). Thus, both the cognitive model of empathy and the findings of neural substrates underlying empathy and death-related thoughts make us to suspect that reminders of death may decrease the neural responses to others’ suffering.
To test our hypothesis, we scanned healthy adults using fMRI while they viewed video clips of real-life situations in which a person receives painful or non-painful stimuli (i.e. others’ faces being punctured with a needle and expressing pain or being touched with a Q-tip and maintaining a neutral expression). These stimuli were adopted from our previous research (Han et al., 2009; Xu et al., 2009). Previous research has shown that perception of both painful facial expression and painful stimuli applied to faces activated the pain matrix, including the MCC and AI (Saarela et al., 2007; Han et al., 2009; Xu et al., 2009). In this study, one group of participants viewed the videos before and after a priming procedure that increased mortality salience (MS group). Another group of participants were primed with negative affect in terms of fear/anxiety (NA group), because these affects are more closely related to mortality than other types of negative affect such as anger. To compare fMRI results from MS and NA groups would help to clarify whether the effect of MS priming on the neural responses to others’ suffering was similar to that induced by general negative affect.
We first identified empathic neural responses by contrasting painful vs non-painful stimuli during the pre-priming sessions. We then examined whether empathic neural responses to perceived pain in others were weaker in the post-priming sessions in MS group compared with NA group. We also examined whether the MS and NA groups differed in changes in empathic neural responses to perceived pain across the pre- vs post-priming sessions. To further estimate whether the priming effect on empathic neural responses, if any, was associated with death-related feelings, we measured subjective feelings of fear of death (FOD) from the two subject groups and conducted to a mediation analysis to assess whether the priming effect on the neural activity to perceived pain in others was mediated by FOD. We also conducted a hierarchical regression analysis to examine whether FOD moderated the co-variation of the neural activities in different brain regions (e.g. MCC and AI) across the pre- and post-priming sessions. Finally, to examine whether the empathic neural responses to perceived pain in others can predict participants’ prosocial behavior, we asked our participants to decide the intensity of electric shocks that would be applied to others in a pseudo-experiment. We then examined whether participants who showed stronger empathic neural responses would decide to give weaker electric shocks to others.
METHODS
Participants
About 36 Chinese university students were recruited to participate in the study as paid volunteers. Half of the participants were randomly assigned to MS group (mean = 21.2 years, s.d. = 1.8 years, 9 males) and the other half to NA group (mean = 21.4 years, s.d. = 1.6 years, 9 males). All participants were right-handed, had normal or corrected-to-normal vision and reported no abnormal neurological history. Informed consent was obtained from all participants before scanning. This study was approved by a local ethics committee.
Stimuli and procedure
Before the fMRI scanning procedure, individuals’ trait-level death anxiety was assessed using Templer’s Death Anxiety Scale (Templer, 1970) and trait-level empathic ability was measured using the Interpersonal Reactivity Index (Davis, 1996).
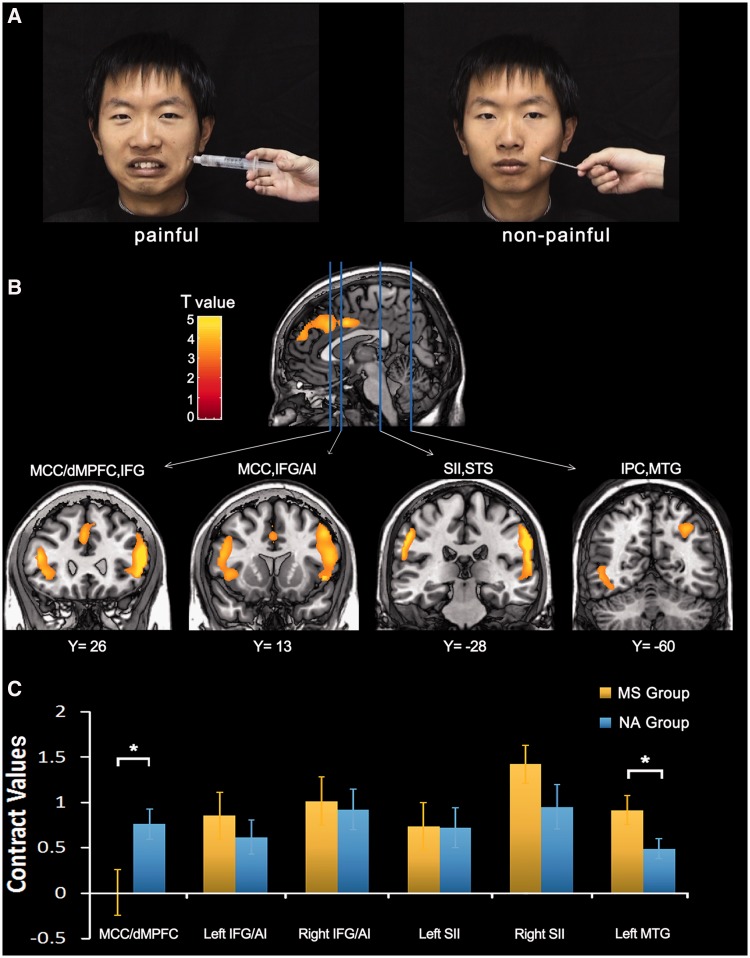
Stimuli used during fMRI scanning consisted of 24 video clips showing six Chinese faces (three males and three females) that received painful (needle penetration) stimulation and showed pain expressions or received non-painful (Q-tip touch) stimulation and showed neutral expressions (Figure 1A). The painful and non-painful stimulations were applied to the left or right cheeks. Each clip lasted for 3 s and subtended a visual angle of 21° × 17° (width × height) at a viewing distance of 80 cm. Participants indicated whether or not the model was feeling pain by a button press using the right index or middle finger after each video clip.
Fig. 1.
Illustration of stimuli and neural activity to perceived pain in others. (A) Illustration of the stimuli used in our study. Video clips showed painful faces receiving needle penetration or neutral faces with Q-tip touch. (B) Increased neural responses to painful vs non-painful stimuli during the pre-priming sessions. These were identified in the whole brain analysis in the MCC/dMPFC, AI/IFG, SII, STS, MTG and inferior parietal cortex (IPC). (C) Contrast values to painful vs non-painful stimuli during the post-priming sessions in MS and NA groups. NA group showed significantly greater MCC/dMPFC activity compared with MS group, whereas activation in the left MTG showed a reverse pattern.
There were three functional scans of 204 s in the pre- and post-priming sessions, respectively. During each scan, participants viewed eight video clips with painful stimulation and eight with non-painful stimulation. The video clips were presented in a random order for each participant. The interstimulus interval between two successive clips lasted 9 s during which participants fixated at a central cross. The last video clip in each scan was followed by a fixation of 12 s. After the three functional scans in the pre-priming session, each participant was given 28 statements and had to judge whether he/she agreed with each statement that lasted for 7 s. The materials for MS and NA priming (see Supplementary Materials) were created similar to those in the previous studies (e.g. Greenberg et al., 1990). All statements used for MS priming were related to death (e.g. ‘I won’t feel terrible even if I would die lonely’, ‘My body would rot after death’). All statements used for NA priming were not related to death but referred to negative emotions such as fear (e.g. ‘I am not frightened about life at all’) and anxiety (‘The coming exam makes me uneasy’). After the priming task, each participant was asked to perform 40 calculations in 5 min, which served as a delay between MS/NA induction and the critical dependent measures. Participants had to judge whether each calculation would give an odd or even number by a button press. Each calculation lasted for 7 s and two consecutive calculations were intervened with 0.5 s. The three functional scans in the post-priming session were the same as those in the pre-priming sessions except that the order of the video clips was changed.
After the fMRI scanning procedure, participants were shown half of the video clips again outside the scanner and were instructed to rate the intensity of pain experienced by the model (How painful do you think the model feels?) and the unpleasantness of viewing the clips (How unpleasant do you feel when observing the video clip?). To assess their feelings of closeness to death and FOD, participants were asked to rate their feelings about the priming task (e.g. ‘How close do you feel to death after reading all the sentences and making your judgments?’, ‘How fearful do you feel about death after reading all the sentences and making your judgments?’). A Likert-type scale was used for all ratings where 0 indicated no effect and 10 indicated maximal effect (e.g. ‘extremely painful’, ‘extremely unpleasant’, ‘extremely close’, ‘extremely fearful’).
Finally, all participants were invited to take part in another pseudo-experiment a week after the scanning procedure. They were informed that a new experiment would recruit a group of participants who would receive electric shocks during scanning. The default intensity was 2.1 mA that would produce moderately painful feeling. 0.9 mA would produce sensory feelings, whereas 3.3 mA would produce intolerant painful feeling. These values were chosen based on pain threshold measurements in our laboratory. Participants were asked to decide the intensity of electric shocks between 0.9 and 3.3 mA to apply to an individual participant to get ideal experimental effects. The intensity of electric shocks was used as an index of participants’ altruistic behavior.
fMRI imaging data acquisition
Scanning was performed at Peking University First Hospital, on a GE 3-T scanner using a standard head coil. About 32 transverse slices of functional images covering the whole brain were acquired using a gradient-echo echo-planar pulse sequence [64 × 64 × 32 matrix with a spatial resolution of 3.75 × 3.75 × 4 mm, repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, field of view= 24 × 24 cm, flip angle = 90°]. Anatomical images were obtained using a 3D FSPGR T1 sequence (256 × 256 × 128 matrix with a spatial resolution of 0.938 × 0.938 × 1.4 mm, TR = 7.4 ms, inversion time = 450 ms, TE = 3.0 ms, flip angle = 20°).
fMRI data analysis
The functional image data were analyzed by using the general linear model for event-related designs in SPM2 (the Wellcome Trust Centre for Neuroimaging, London, UK). In order to compensate for delays associated with acquisition time differences between slices during the sequential imaging, the functional data were first time-corrected. Then the functional images were realigned to the first scan to correct for head motion between scans. The anatomical image was co-registered with the mean functional image that was produced during the process of realignment. All images were then spatially normalized to the Montreal Neurological Institute (MNI) template and resampled to obtain images with a voxel size of 2 × 2 × 2 mm. Functional images were smoothed using a Gaussian filter with the full-width/half-maximum parameter set to 8 mm. The event-related neural activity was modeled using a canonical hemodynamic response function.
Fixed effect analyses were first performed to estimate effects at each voxel and to compare regionally specific effects in individual participants using linear contrasts. To define pain-specific neural activations, the contrast of painful vs non-painful stimuli was calculated. Random effect analyses were then conducted across all participants based on statistical parameter maps from each individual participant to allow population inference. Significant activations in the pre-priming sessions were defined in the whole-brain analysis using a threshold of P < 0.05 [false discovery rate (FDR) corrected for multiple comparisons]. Region of interests (ROIs) were defined as spheres with a radius of 5 mm centered at the peak voxel of these activations for assessment of the priming effect that compared MS and NA groups during the post-priming sessions. To assess the difference in the neural activity related to empathy between pre- and post-priming sessions and determine the ROIs independent of the present data, we defined ROIs based on previous studies that used similar stimuli to induce empathy for pain [MNI coordinates: MCC: x/y/z = 4/40/38 (Han et al., 2009); right AI: x/y/z = 40/20/−10; left AI: x/y/z = −36/16/−2 (Cheng et al., 2007)]. As models in video clips showed eye/mouth movement when receiving painful stimulation but did not show eye/mouth movement when receiving non-painful stimulation, we also examined the priming effect on the neural activity in the superior temporal sulcus (STS) that is involved in the processing of eye/mouth movements [Talairach coordinates: right STS: x/y/z = 50/−49/3; left STS: x/y/z = −47/−50/2 (Puce et al., 1998)]. The ROIs were defined as spheres with radii of 5 mm centered at the peak voxel of activated clusters using MarsBar toolbox in SPM2 (Brett et al., 2002). Talairach coordinates were converted to MNI using a non-linear transformation (http://imaging.mrccbu.cam.ac.uk/imaging/MniTalairach).
Mediation analysis
The mediation analysis (Baron and Kenny, 1986) was conducted to test whether FOD induced by the priming procedure mediated the difference in the brain activations between MS and NA groups. To do this, three regression models were constructed. First, we regressed a dependent variable [changes of MCC/dMPFC (dorsal medial prefrontal cortex) activity to painful vs non-painful stimuli across the priming procedure] on an independent variable (Group: MS vs NA) and demonstrated that the independent variable was correlated with the dependent variable. Second, we regressed the mediator (FOD) on the independent variable (Group) and demonstrated that the independent variable was correlated with the mediator. Finally, we regressed the dependent variable (changes of MCC/dMPFC activity to painful vs non-painful stimuli across the priming procedure) on both the mediator (FOD) and the independent variable (Group) to test whether the mediator affected the dependent variable and whether the effect of the independent variable on the dependent variable was significantly reduced when the mediator was included in the regression model. The Sobel test (Sobel, 1982) was conducted to further confirm the significance of the mediator.
Moderator analysis
To examine whether MS priming influenced inter-regional interactions between the two key brain regions involved in empathy (i.e. MCC/dMPFC and AI), using the MarsBaR (http://marsbar.sourceforge.net/), we extracted the time course of blood-oxygen-level-dependent (BOLD) signals in pre- and post-priming sessions from the MCC/dMPFC and AI that showed increased activity to perceived pain in others in the previous work (Cheng et al., 2007; Han et al., 2009). The time course of BOLD signals consisted of β-values at each sampling point in a scan. We then calculated the correlation of BOLD signals in the two brain regions and defined the MCC/dMPFC–AI co-variation using the correlation coefficient in each participant. Hierarchical regression analyses were then conducted to examine whether FOD induced by the priming procedure affected the relationship between MCC/dMPFC–AI co-variation in the pre-priming sessions (independent variable, IV) and MCC/dMPFC–AI co-variation in the post-priming sessions (dependent variable). The IV (the MCC/dMPFC–AI co-variation in pre-priming sessions) and the moderator (FOD) were normalized before the hierarchical regression analysis. The interactions between the MCC/dMPFC–AI co-variation in pre-priming sessions and FOD were calculated by multiplying the normalized variables together (Aiken and West, 1991). Normalized MCC/dMPFC–AI co-variation in the pre-priming sessions, FOD and their interactions were then sequentially entered into the hierarchical regression. The moderator effect was indicated by a significant interaction of the MCC/dMPFC–AI co-variation in the pre-priming sessions and FOD on the MCC/dMPFC–AI co-variation in the post-priming sessions. As there was a significant moderator effect of FOD on the MCC/dMPFC–left-AI co-variation in pre- and post-priming sessions, we divided participants into low and high FOD groups. The mean FOD across all participants was 2.65 ± 2.35. The top 40% participants constituted the high FOD group (14 individuals, mean ± s.d. = 5.11 ± 1.71) and the bottom 40% participants constituted the low FOD group (15 individuals, mean ± s.d. = 0.57 ± 0.50). Post hoc regression analyses were then conducted for the high and low FOD groups, respectively.
RESULTS
Behavioral results
MS and NA groups identified painful and non-painful stimuli during scanning with similar accuracy (95.2 vs 95.0%, t = 0.246, P = 0.5; Table 1 for response accuracy in each condition). A repeated measures analysis of variance (ANOVA) with a within-subjects variable (Pain: painful vs non-painful stimuli) and a between-subjects variable (Group: MS vs NA) showed that rating scores of pain intensity and self-unpleasantness were higher for painful than non-painful stimuli [pain intensity: 7.97 vs 0.24; self-unpleasantness: 6.88 vs 0.69; F(1,34) = 1173.06 and 227.45, P-values < 0.001], but did not differ between MS and NA groups [F(1,34) = 0.03 and 0.23, P-values > 0.5; Table 2 for rating scores in each condition). Two sample t-tests confirmed higher rating scores of closeness to death [4.22 ± 2.40 vs 1.58 ± 2.10, t(34) = 3.52, P = 0.001] and of FOD [3.53 ± 2.42 vs 1.78 ± 1.97, t(34) = 2.38, P < 0.05] in MS group than in NA group. There was no significant difference between MS and NA groups in rating scores on subscales of the Interpersonal Reactivity Index [empathic concern: 20.8 ± 2.53 vs 20.3 ± 3.94, t(34) = 0.45, P = 0.65; perspective-taking: 17.7 ± 2.97 vs 16.5 ± 3.75, t(34) = 1.09, P = 0.29; fantasy: 19.6 ± 4.84 vs 19.0 ± 4.28, t(34) = 0.40, P = 0.69 and personal distress scale: 17.4 ± 3.54 vs 16.8 ± 4.05, t(34) = 0.53, P = 0.60]. There was no significant difference in rating scores of the Death Anxiety Scale between MS and NA groups [6.88 ± 2.78 vs 6.40 ± 2.11, t(34) = 0.73, P = 0.31].
Table 1.
Response accuracy for pain judgment tasks in each condition (mean ± s.d.)
| Pre-priming groups |
Post-priming groups |
|||
|---|---|---|---|---|
| MS | NA | MS | NA | |
| Painful | 0.94 ± 0.05 | 0.92 ± 0.09 | 0.93 ± 0.09 | 0.91 ± 0.13 |
| Non-painful | 0.97 ± 0.04 | 0.98 ± 0.03 | 0.97 ± 0.04 | 0.99 ± 0.02 |
Table 2.
Rating scores of pain intensity and self-unpleasantness in each condition (Mean±s.d.)
| MS group |
NA group |
|||
|---|---|---|---|---|
| Pain intensity | Self-unpleasantness | Pain intensity | Self-unpleasantness | |
| Painful | 7.80 ± 1.33 | 6.70 ± 2.55 | 8.13 ± 1.37 | 7.05 ± 1.55 |
| Non-painful | 0.29 ± 0.41 | 0.71 ± 1.24 | 0.22 ± 0.47 | 0.66 ± 1.33 |
Seventy-five percent of the participants reduced the intensity and 16.7% raised the intensity of electric shock to be applied to an individual participant in the pseudo-experiment. 8.3% of the participants did not change the intensity of electric shock applied to others. The intensity of assigned electric shock was from 0.9 to 3.3 mA (mean = 1.5 mA, s.d. = 0.72).
Neuroimaging results
Neural responses to perceived others in pain.
We first conducted whole-brain analyses of the fMRI data in the pre-priming sessions across all participants to identify empathic neural responses to others’ suffering. The contrast of painful vs non-painful stimuli revealed significant activations in the MCC and the dMPFC, the bilateral AI and the inferior frontal gyrus (IFG), the bilateral parietal operculum/secondary somatosensory cortex (SII), the left middle temporal gyrus (MTG) and the superior temporal sulcus (STS) (Figure 1B and Table 3).
Table 3.
Brain activations to painful vs non-painful stimuli in the pre-priming sessions
| MNI coordinates |
|||||
|---|---|---|---|---|---|
| Brain region | k | t-value | X | y | z |
| Right MCC/dMPFC | 686 | 4.15 | 8 | 46 | 36 |
| Left MCC | 153 | 5.12 | −2 | 8 | 38 |
| Left AI/IFG | 825 | 5.32 | −46 | 32 | 14 |
| 4.24 | −42 | 18 | 0 | ||
| Right AI/IFG | 1680 | 6.48 | 54 | 26 | 18 |
| 5.57 | 50 | 30 | 0 | ||
| Left parietal operculum/SII | 482 | 6.77 | −58 | −34 | 36 |
| Right parietal operculum/SII | 1530 | 7.7 | 62 | −24 | 32 |
| Right STS | 5.02 | 60 | −42 | 8 | |
| Right IPC | 4.47 | 32 | −62 | 44 | |
| Left MTG | 139 | 3.95 | −50 | −60 | −2 |
To test the association between empathic neural responses and altruistic behavior in our participants, we first calculated parameter estimates of signal intensity in the pre-priming sessions from the ROIs that showed greater activity in responses to painful vs non-painful stimuli at 8/46/36 (MCC/dMPFC); −46/32/14 (left AI/IFG); 54/26/18 (right AI/IFG); −58/−34/36 (left parietal operculum/SII); 62/−24/32 (right parietal operculum/SII) and −50/−60/−2 (left MTG/STS). We then conducted regression analyses to assess whether the empathic neural responses can predict participants’ prosocial behavior. The regression analyses showed that MCC/dMPFC and bilateral AI/IFG activities were significantly correlated with participants’ subsequent altruistic behavior in the pseudo-experiment. Participants who showed stronger MCC/dMPFC and AI/IFG activities applied electric shocks with less intensity to others (MCC/dMPFC: r = −0.61, P < 0.001; left AI/IFG: r = −0.33, P < 0.05; right AI/IFG: r = −0.47, P < 0.005; Supplementary Figures S1–S3). Thus, these analyses validate the idea of an association between the empathic neural responses and prosocial behavior.
Group difference in empathic neural responses in the post-priming sessions.
To compare neural responses with perceived pain in others between MS and NA groups after priming, we extracted parameter estimates of signal intensity from the fMRI data in the post-priming sessions in the brain regions that were significantly activated in the pre-priming sessions. ANOVAs of MCC/dMPFC activity with a within-subjects variable (Pain: painful vs non-painful stimuli) and a between-subjects variable (Group: MS vs NA) showed a significant main effect of Pain [F(1,34) = 6.40, P < 0.05] and a significant interaction of Pain × Group [F(1,34) = 6.25, P < 0.05] suggesting that MCC/dMPFC activity in response to painful vs non-painful stimuli in the post-priming sessions was significantly weaker in MS group compared with NA group. Simple main effect analyses confirmed that viewing painful vs non-painful stimuli activated the MCC/dMPFC in NA group [t(17) = 4.59, P < 0.001] but not in MS group [t(17) = 0.02, P = 0.99; Figure 1C]. ANOVAs of the bilateral AI/IFG activity and the bilateral parietal operculum/SII activity showed significant main effects of Pain [F(1,34) = 6.20–54.16, P-values < 0.05). However, the interaction of Pain × Group was not significant (P-values > 0.1) suggesting comparable neural responses in these brain regions to perceived pain in others in the MS and NA groups in the post-priming sessions. The ANOVA of the left MTG/STS activity also showed a significant main effect of Pain [F(1,34) = 51.66, P < 0.001] and a significant interaction of Pain × Group [F(1,34) = 4.76, P < 0.05] due to greater MTG/STS activity to perceived pain in others in MS group than in NA group. The simple main effect analyses confirmed that viewing painful vs non-painful stimuli activated the left MTG/STS in both MS [t(17) = 5.73, P < 0.001] and NA groups [t(17) = 4.34, P < 0.001].
Whole-brain analyses of the fMRI data in the post-priming sessions revealed that for both MS and NA groups, the contrast of painful vs non-painful stimuli revealed increased activations in the bilateral parietal operculum/SII, the bilateral inferior frontal cortex, the right inferior parietal cortex and right STS. Moreover, brain activations that characterized NA group were observed in the SMA, MCC/MPFC, the bilateral AI and the right thalamus. Brain activations that characterized MS group were found in the bilateral MTG/STS and the right fusiform gyrus (Supplementary Figure S4).
Changed empathic neural responses across the priming procedures.
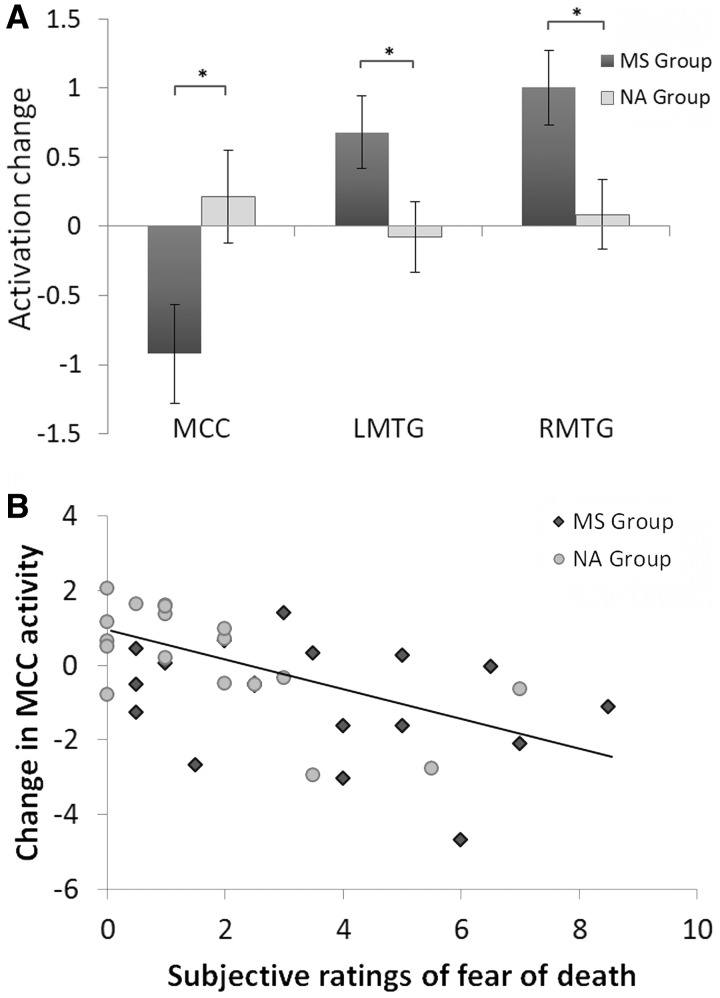
Next, we assessed the group difference in variation of MCC/dMPFC and MTG/STS activities in response to perceived pain in others across the priming procedures. As fMRI data in both pre- and post-priming sessions were included from this analysis, we defined the ROIs in the MCC/dMPFC and MTG/STS independently based on the results of previous studies (Puce et al., 1998; Han et al., 2009). The parameter estimates of signal intensity were extracted from both the pre- and post-priming sessions in the ROIs. ANOVAs were then performed with Pain (painful vs non-painful stimuli) and Priming (pre- vs post-priming sessions) as within-subjects independent variables and Group (MS vs NA) as a between-subjects variable. The ANOVA of MCC/dMPFC activity showed a significant three-way interaction of Pain × Priming × Group [F(1,34) = 5.73, P < 0.05; Figure 2A] suggesting different priming effects on MCC/dMPFC activity to perceived pain in others in the MS and NA groups. Separate analyses showed a significant interaction of Pain × Priming in MS group [F(1,17) = 6.68, P < 0.05] but not in NA group [F(1,17) = 0.57, P > 0.4] suggesting that MS priming significantly reduced MCC/dMPFC activity to perceived pain in others in the post- vs pre-priming sessions, whereas NA priming did not induce significant changes in MCC/dMPFC activity to perceived pain in others between the post- and pre-priming sessions.
Fig. 2.
Changes of neural activity to perceived pain in others across the priming procedures. (A) The activation change across the priming procedures was defined as the contrast values to painful vs non-painful stimuli in the post-priming sessions minus those in the pre-priming sessions. MS group showed significantly decreased MCC/dMPFC activity, but increased MTG/STS activity in the post-priming than pre-priming sessions. (B) The association between changed MCC/dMPFC activity across the priming procedures and subjective feelings of fear of death induced by the priming procedure.
The ANOVA of MTG/STS activity also showed significant three-way Pain × Priming × Group interactions [left MTG/STS: F(1,34) = 4.29, P < 0.05; right MTG/STS: F(1,34) = 6.21, P < 0.05]. Separate analyses confirmed that, for NA group, there was a significant main effect of Pain [left MTG/STS: F(1,17) = 19.02, P < 0.001; right MTG/STS: F(1,17) = 15.42, P = 0.001], but this effect did not significantly differ between the pre- and post-priming sessions [left MTG/STS: F(1,17) = 0.10, P > 0.7; right MTG/STS: F(1,17) = 0.11, P > 0.7]. In contrast, the ANOVA of MTG/STS activity in MS group showed a significant interaction of Pain × Priming [left MTG/STS: F(1,17) = 6.70, P < 0.05; right MTG/STS: F(1,17) = 14.00, P < 0.01] suggesting that MTG/STS activity related to the perception of others’ pain increased significantly in the post-priming sessions when compared with the pre-priming sessions in the MS group.
FOD and the priming effect on empathic neural responses.
To assess whether variations of neural responses to perceived pain in others across the priming procedures were associated with subjective feelings of FOD induced by the priming procedures, we conducted a correlation analysis of changes in neural activity in response to perceived pain in others across the priming procedure (i.e. differential activity to painful vs non-painful stimuli in the post-priming sessions minus that in the pre-priming sessions) and subjective ratings of FOD. Subjective ratings of FOD were negatively correlated with changes of MCC/dMPFC activity (r = 0.55, P = 0.001; Figure 2B). Greater FOD was associated with greater decrease in MCC/dMPFC activity in response to perceived pain in others across the priming procedures. A similar analysis failed to show a significant correlation between changes of MCC/dMPFC activity across the priming procedure and subjective ratings of death anxiety (r = −0.07, P > 0.7).
Next, we examined whether the group difference in changes of MCC/dMPFC activity across the priming procedures was mediated by FOD. The mediation analysis showed that Group (MS vs NA) was a significant predictor of both MCC/dMPFC activity changes across the post- and pre-priming sessions (r = 0.37, P < 0.05; Figure 3) and FOD (r = 0.38, P < 0.05). FOD also predicted MCC/dMPFC activity changes (r = 0.47, P < 0.01). Moreover, the effect of Group on MCC/dMPFC activity changes was significantly reduced when FOD was included in the regression model (r = 0.19, P > 0.2), suggesting that the priming effect on MCC/dMPFC activity changes was mediated by FOD. We also conducted the Sobel test to further confirm that FOD was a significant mediator variable of the relationship between priming and MCC/dMPFC activity (z = 2.07, P < 0.05).
Fig. 3.
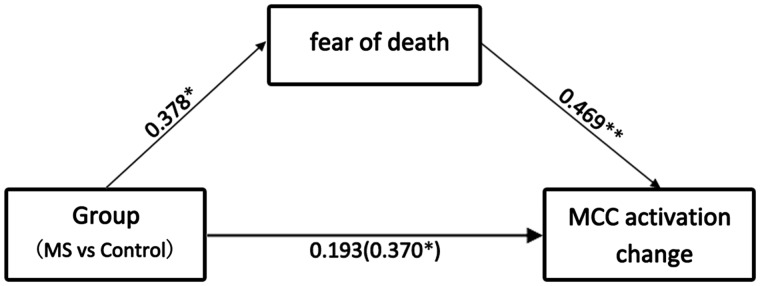
Illustration of the mediation effect. The effect of Group on MCC/dMPFC activity changes was significantly reduced when ratings of fear of death induced by the priming procedure was included in the regression model.
Moderation of MCC/dMPFC-AI/IF co-variation by MS and FOD.
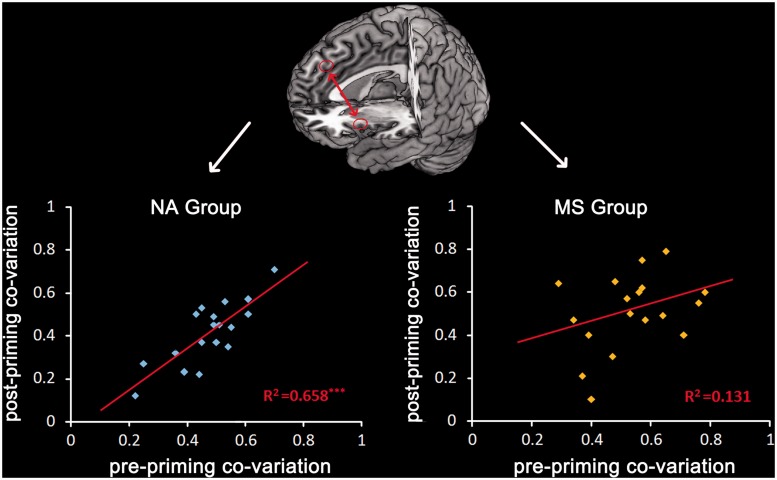
To assess whether the priming procedure influenced the co-variation of activities in different brain regions involved in empathy for pain, we first obtained BOLD signals from independently defined ROIs in the MCC/dMPFC, AI/IFG and MTG/STS in the pre-priming sessions. We then calculated the correlation between activities in any two of these brain regions and only found significant correlation between MCC/dMPFC and left AI/IFG activities in all participants (P-values < 0.05). We then examined whether MS and NA priming influenced the co-variation of neural responses in these two brain regions. We conducted regression analyses and found that co-variation of MCC/dMPFC–left AI/IFG activity in the pre-priming sessions predicted the co-variation of MCC/dMPFC–left AI/IFG activity in the post-priming sessions in NA group (R2 = 0.66, P < 0.001), but not in MS group (R2 = 0.13, P > 0.1; Figure 4). Next, we conducted hierarchical regression analyses to further examine whether the behavioral measurement of the priming effect on death-related thoughts (i.e. rating scores of FOD) moderated the co-variation of MCC/dMPFC–left AI/IFG activity across the pre- and post-priming sessions. The model regressed the moderator (normalized FOD), IV (normalized MCC/dMPFC–left AI/IF co-variation in the pre-priming sessions) and their interaction. This analysis showed that the interaction of FOD and MCC/dMPFC–left AI/IFG co-variation in the pre-priming sessions was predictive of MCC/dMPFC–left AI/IF co-variation in the post-priming sessions (Table 4). This confirmed that FOD significantly moderated the co-variation of MCC/dMPFC–left AI/IFG activity across the pre- and post-priming sessions. A separate analysis revealed a positive correlation between MCC/dMPFC–left AI/IFG co-variations in the pre-priming and post-priming sessions in low FOD individuals (β = 0.71, P < 0.01) but not in high FOD individuals (β = 0.06, P > 0.8). Similar hierarchical regression analyses were conducted to test whether FOD moderated the co-variation of MCC/dMPFC–right AI/IF and MCC/dMPFC–MTG/STS activities across the pre- and post-priming sessions but failed to find significant moderator effects.
Fig. 4.
Illustration of the moderator effect. The co-variation of MCC/dmPFC–left AI/IFG activity in the pre-priming sessions predicted the co-variation of MCC/dmPFC–left AI/IFG activity in the post-priming sessions in NA group but not in MS group.
Table 4.
Hierarchical regression analysis of co-variation of MCC/dMPFC and left AI/IFG activities in the pre-priming and post-priming sessions
| Step1β | Step2β | |
|---|---|---|
| Pre-priming MCC/dMPFC-left AI/IF co-variation | 0.53** | 0.44** |
| FOD | 0.26 | 0.33* |
| Pre-priming MCC/dMPFC-left AI/IF co-variation × FOD | −0.30* | |
| R2 change | 0.40 | 0.08 |
| F change | 11.01*** | 5.04* |
| R2 | 0.40 | 0.48 |
| Adjusted R2 | 0.36 | 0.43 |
| Over all F | 11.01*** | 9.92*** |
| Df | 33 | 32 |
*P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
Our fMRI results first showed that viewing others in pain significantly activated the neural circuit consisting of the MCC/dMPFC, the AI/IFG, the parietal operculum/SII and the MTG/STS. These results are consistent with the previous findings (Singer et al., 2004; Jackson et al., 2005; Gu and Han, 2007; Saarela et al., 2007; Han et al., 2009; Xu et al., 2009; Corradi-Dell’Acqua et al., 2011). In addition, we showed that the activation in the MCC/dMPFC and the bilateral AI/IFG observed in our study were able to predict participants’ decision of the intensity of painful stimulation applied to others, suggesting an association between MCC/dMPFC and AI/IFG activities and altruistic behavior in our participants. More importantly, we found that MCC/dMPFC activity in response to viewing others’ pain in the post-priming sessions was significantly decreased in MS group than in NA group. MCC/dMPFC activity was also significantly reduced in the post- vs pre-priming sessions in MS group but not in NA group.
These effects were not due to group differences in trait-level empathy or trait-level death anxiety that was matched between the two subject groups. Furthermore, the effect of MS priming cannot be explained as merely a result of negative affect such as fear or anxiety, as priming NA did not modulate MCC/dMPFC responses to perceived pain in others. It is also unlikely that the variation of MCC/dMPFC activity across MS priming simply reflected the decreased activity in the same brain region induced by MS priming because the MS priming procedure and the post-priming session were separated by a 5-min calculation task. Our neuroimaging findings support the hypothesis that reminders of mortality decrease empathic neural responses to perceived pain in others. In addition, we showed that the modulation of MCC/dMPFC activity was mediated by subjective FOD induced by the priming procedure. Furthermore, the co-variation of MCC/dMPFC and left AI/IFG activity in the pre-priming sessions predicted that in the post-priming sessions in NA group but not in MS group. This indicates that the priming procedures moderated the co-variation of MCC/dMPFC and left AI/IFG activities associated with perceived pain in others.
A recent meta-analysis study (Lamm et al., 2010) identified the core network involved in empathy for pain. This network consists of brain regions similar to those observed in our work. The fact that empathy-related activity in the brain regions overlapped with that evoked by first-hand pain experience suggests shared neural mechanisms involved in empathy for others’ pain and in one’s own pain experience. However, activity in the different nodes in this network has distinct functional significance for empathy. The somatosensory cortex was usually activated during perception of others’ pain in studies using picture-based paradigms (Lamm and Singer, 2010) and this may reflect a simple form of empathy based on somatic resonance (Avenanti et al., 2005). Another meta-analysis suggests that the right AI is more likely to be activated in affective–perceptual forms of empathy, whereas activity in the MCC is more often observed in cognitive–evaluative forms of empathy and the left AI was involved in both forms of empathy (Fan et al., 2011). Thus, the AI may play a key role in a more complex form of empathy (e.g. both affective and cognitive components) that deals with shared emotion. Interestingly, our results suggest that reminders of mortality do not influence the neural substrates underlying the sensory and affective dimensions of empathic neural responses.
Why did reminders of mortality weaken MCC/dMPFC response to perceived pain in others? Besides the potential functional role of the MCC in cognitive–evaluative forms of empathy (Fan et al., 2011), the MCC is consistently activated by elicitation of negative affect, pain and cognitive control and that there is an overlap in regions of the MCC that are activated by these seemingly unrelated emotional and cognitive processes (Shackman et al., 2011). It was suggested that the primary role of the MCC is to use information about punishment to control aversively motivated actions in response to uncertain threatening environments. Similarly, the dMPFC has been suggested to play a key role in intentional inhibitory control over one’s own actions (Brass and Haggard, 2007; Filevich et al., 2012) and expressive suppression of emotions (Kühn et al., 2011). Neural responses to others’ suffering enable humans to evaluate and to respond to threatening and aversive stimuli, so as to adapt to environmental conditions with affective and nociceptive importance (Decety, 2011). Indeed, pain-responsive MCC neurons are activated by anticipation of and instrumental escape from physical threats such as pain (Hutchison et al., 1999; Iwata et al., 2005). Perception of others’ suffering also signals uncertain threats in environments and this also requires aversively motivated actions such as escaping or helping. We suggest that MCC/dMPFC activity in response to perceived pain in others may play a key role in the regulation of aversively motivated actions associated with uncertain threats signaled by others’ suffering. However, reminders of mortality make salient the worst situation that human individuals have to undergo and this to a certain degree reduces the uncertainty of the effects of environmental risks and in turn, weakens the demand to control aversively motivated actions in response to suffering others. Consistent with this proposal, we found evidence that the effect of mortality salience on MCC/dMPFC activity was mediated by subjective FOD resulting from the priming procedures. It is possible that the priming procedures first induced changes in death-related affect, which then further caused modulation of MCC/dMPFC activity in response to others’ suffering. Such influences were limited to specific components of empathy related to evaluation and regulation, rather than the entire network activated by perceived pain.
Most fMRI studies of empathy for pain have found increased activity in both the MCC/dMPFC and AI/IFG in response to perceived pain in others. Reciprocal anatomical connectivity exists between these two brain regions (Mesulam et al., 1982). Functional connectivity is also observed between the AI and MCC during the resting state (Taylor et al., 2009; Deen et al., 2011), which is, however, significantly reduced in patients with chronic pain (Malinen et al., 2010). These findings suggest that inter-regional interactions between the AI and MCC may contribute to affective processing related to pain in humans. Our finding that the activities in the MCC/dMPFC and AI/IFG co-varied during perception of others in pain indicates that both the increased activity in the pain matrix and the enhanced functional connectivity between different brain regions in the pain matrix contribute to the understanding and sharing of others’ painful feelings. More interestingly, the co-variation between MCC/dMPFC and left AI/IF activities across pre- and post-priming sessions was moderated by FOD. For those with low FOD, the co-variation between MCC/dMPFC and left AI/IF activities in the pre- and post-priming sessions was positively correlated, whereas individuals with high FOD failed to show such an association between the co-variation of MCC/dMPFC and left AI/IF activities in the pre- and post-priming sessions. This is consistent with the finding that the co-variation of MCC/dMPFC and left AI/IF activity in pre-priming sessions predicted that in post-priming sessions in NA group, but not in MS group, as MS priming induced higher FOD compared with NA priming. These findings have two implications. First, the functional connectivity between the MCC/dMPFC and left AI/IF related to empathy for pain varied across individuals but was consistent in the pre- and post-priming sessions in NA group. Second, MS priming seemed to break the consistency of MCC/dMPFC–left AI/IF connectivity related to empathy for others’ suffering and this effect seemed to be stronger in those who reported high FOD. Taken together, our results suggest that MS priming may not only modulate the magnitude of MCC/dMPFC activity in response to others’ suffering, MS priming-induced FOD may also affect the pattern of functional connectivity between brain regions in the neural network involved in empathy.
Empathy has been hypothesized to generate concern for others’ welfare (e.g. Batson, 1998; Batson et al., 2002) and as a consequence, to promote prosocial behavior (de Waal, 2008). Recent brain imaging studies have shown evidence for associations between empathy-related neural activity and altruistic behaviors (Hein et al., 2010; Ma et al., 2011; Rameson et al., 2012). However, empathy-related neural activity can be altered by a number of factors. For example, MCC and AI/IFG responses to others’ suffering decreases when an observer’s attention is directed away from cues of others’ suffering in stimuli (Gu and Han, 2007). MCC and AI activity during the observation of animated visual stimuli depicting needles being inserted into different body parts decreased significantly in physicians who practiced acupuncture compared with naive participants (Cheng et al., 2007). Racial group relationship has also been shown to modulate empathy-related neural activity, such that MCC activity in response to perceived pain in others was decreased for racial ingroup members compared with racial outgroup members (Xu et al., 2009; Sheng and Han, 2012). Such modulations of empathic neural responses may have two behavioral consequences. On the one hand, if empathic neural responses mediate spontaneously empathic emotions in response to people in need, decreased empathy-related neural activity may then make people less likely to help those who are suffering. On the other hand, altruistic actions in responses to others’ suffering requires inhibition of empathy-related responses in order to regulate one’s own feelings of unpleasantness induced by the perception of others’ suffering. This may then facilitate altruistic actions so that one can efficiently help others who are suffering without being interrupted by one’s own negative affective states elicited by empathy. Similarly, MS priming that decreased MCC/dMPFC activity and affected the co-variation of MCC/dMPFC–left AI/IFG activity related to others’ suffering may influence prosocial behaviors in two opposite directions. Thus, the effect of MS on altruistic behavior may depend on social context and social group relationship between an empathizer and the target. Future research should further investigate the relationship between prosocial behavior and the modulation of empathy-related activity by reminders of mortality.
Unexpectedly, we also found that compared with NA priming, MS priming enhanced MTG/STS activity in response to others’ suffering. The MTG/STS has been demonstrated to play a pivotal role in the processing of facial features. Dynamic facial expressions strongly activated the MTG/STS and the neighboring occipital cortex compared with static facial expressions (Sato et al., 2004; Schultz and Pilz, 2009). Perception of eye gaze also activates the STS (Hoffman and Haxby, 2000; Ethofer et al., 2011). Thus, MTG/STS is involved in perceptual analysis of changing facial features. In our experiment, the models receiving painful stimuli showed dynamic painful expression, whereas the models receiving non-painful stimuli showed little changes of facial features. Thus, the MTG/STS were also engaged during perception of painful stimuli in order to encode the models’ dynamic facial features. However, as a consequence of decreased MCC/dMPFC responses to others’ suffering that may reflect weakened affect and regulation processing during viewing others’ pain, participants might pay more attention to perceptual features of others’ faces and thus show greater activity in the brain regions involved in the processing of facial features. In support for this analysis, we found negative correlation between changed activations in the MCC/dMPFC and MTG/STS (RMTG/STS: r = −0.43, P < 0.01; LMTG/STS: r = −0.45, P < 0.01; Supplementary Figures S5 and S6). Individuals who showed greater decreases in MCC/dMPFC activity also exhibited greater increases in the MTG/STS activity across the priming procedure. This pattern of neural activity changes suggests that reminders of mortality do not inhibit brain activity in general. Instead, reminders of mortality may produce opposite effects on the neural activity engaged in the perceptual and affective processing of others’ faces with pain expression.
Finally, while our fMRI results showed evidence for modulations of empathic neural responses by MS/NA priming, behavioral measurements were not influenced by mortality salience. Rating scores of pain intensity and self-unpleasantness were slightly lower in MS than NA group, but this difference did not reach significance. It happens often in fMRI studies of empathy that brain activations significantly differentiate between two conditions, whereas behavioral rating scores do not (e.g. Xu et al., 2009; Sheng and Han, 2012). Such results indicate that, on the one hand, fMRI signals are more sensitive to perceived pain in others compared with subjective reports. On the other hand, subjective reports compared with brain activations may be more easily influenced by social context and social desire and thus may not co-vary with empathic neural responses.
In sum, we found evidence that reminders of mortality decreased MCC/dMPFC activity during perception of others’ suffering, whereas neural activity in the MTG/STS was increased by reminders of mortality. The modulation of MCC/dMPFC activity was mediated by subjective feelings of FOD induced by the priming procedure. Reminders of mortality also affected the co-variation of MCC/dMPFC and left AI/IFG activity during perception of others in pain. These brain imaging results provide evidence that reminders of mortality influence neural activity related to empathy for others’ suffering. Future research should investigate how reminders of mortality affect prosocial behavior by changing empathy-related brain activity.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank M. Varnum for commenting on the manuscript.
This work was supported by National Natural Science Foundation of China [Projects 91024032, 81161120539, 30910103901]; National Basic Research Program of China [973 Program 2010CB833903].
REFERENCES
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Arndt J, Greenberg J, Simon L, Pyszczynski T, Solomon S. Terror management and self-awareness: Evidence that mortality salience provokes avoidance of the self-focused state. Personality and Social Psychological Bulletin. 1998;24:1216–27. [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nature Neuroscience. 2005;8:955–60. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Batson CD. Altruism and prosocial behavior. In: Gilbert DT, Fisk S, Gardner L, editors. The Handbook of Social Psychology. Boston: McGraw Hill; 1998. pp. 282–316. [Google Scholar]
- Batson CD. These things called empathy: Eight related but distinct phenomena. In: Decety J, Ickes W, editors. The Social Neuroscience of Empathy. Cambridge. MA: The MIT Press; 2009. pp. 3–15. [Google Scholar]
- Batson CD, Chang J, Orr R, Rowland J. Empathy, attitudes, and action: Can feeling for a member of a stigmatized group motivate one to help the group? Personality and Social Psychological Bulletin. 2002;28:1656–66. [Google Scholar]
- Becker E. The Denial of Death. New York: Free Press; 1973. [Google Scholar]
- Brass M, Haggard P. To do or not to do: The neural signature of self control. Journal of Neuroscience. 2007;27:9141–5. doi: 10.1523/JNEUROSCI.0924-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract] 2002 8th International Conference on Functional Mapping of the Human Brain Neuroimage. Sendai, Japan. [Google Scholar]
- Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cerebral Cortex. 2007;17:2553–61. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Lin CP, Liu HL, et al. Expertise modulates the perception of pain in others. Current Biology. 2007;17:1708–13. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Corradi-Dell'Acqua C, Hofstetter C, Vuilleumier P. Felt and seen pain evoke the same local patterns of cortical activity in insular and cingulate cortex. Journal of Neuroscience. 2011;31:17996–8006. doi: 10.1523/JNEUROSCI.2686-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH. Empathy: A Social Psychological Approach. Boulder: Westview Press; 1996. [Google Scholar]
- de Waal FB. Putting the altruism back into altruism: The evolution of empathy. Annual Review of Psychology. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- Decety J. The neuroevolution of empathy. Annals of the New York Academy of Sciences. 2011;1231:35–45. doi: 10.1111/j.1749-6632.2011.06027.x. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behavior and Cognitive Neuroscience Review. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex. 2011;21:1498–506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethofer T, Gschwind M, Vuilleumier P. Processing social aspects of human gaze: a combined fMRI-DTI study. Neuroimage. 2011;55:411–9. doi: 10.1016/j.neuroimage.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience and Biobehavior Review. 2011;35:903–11. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Filevich E, Kühn S, Haggard P. Intentional inhibition in human action: The power of ‘no’. Neuroscience and Biobehavior Review. 2012;36:1107–18. doi: 10.1016/j.neubiorev.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Goldenberg JL, Hart J, Pyszczynski T, Warnica GM, Landau M, Thomas L. Terror of the body: Death, neuroticism, and the flight from physical sensation. Personality and Social Psychological Bulletin. 2006;32:1264–77. doi: 10.1177/0146167206289505. [DOI] [PubMed] [Google Scholar]
- Greenberg J, Pyszczynski T, Solomon S, et al. Evidence for terror management theory. II: The effects of mortality salience on reactions to those who threaten or bolster the cultural worldview. Journal of Personality and Social Psychology. 1990;58:308–18. doi: 10.1037//0022-3514.57.4.681. [DOI] [PubMed] [Google Scholar]
- Gu X, Han S. Attention and reality constraints on the neutral processes of empathy for pain. Neuroimage. 2007;36:256–67. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Gu X, Liu X, Guise KG, Naidich TP, Hof PR, Fan J. Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. Journal of Neuroscience. 2010;30:3739–44. doi: 10.1523/JNEUROSCI.4844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Fan Y, Xu X, et al. Empathic neural responses to others’ pain are modulated by emotional contexts. Human Brain Mapping. 2009;30:3227–37. doi: 10.1002/hbm.20742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Qin J, Ma Y. Neurocognitive processes of linguistic cues related to death. Neuropsychologia. 2010;48:3436–42. doi: 10.1016/j.neuropsychologia.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Hein G, Silani G, Preuschoff K, Batson CD, Singer T. Neural responses to ingroup and outgroup members' suffering predict individual differences in costly helping. Neuron. 2010;68:149–60. doi: 10.1016/j.neuron.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Hirschberger G, Florian V, Mikulincer M. Fear and compassion: A terror management analysis of emotional reactions to physical disability. Rehabilitation Psychology. 2005;50:246–57. [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nature Neuroscience. 2000;3:80–4. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO. Pain-related neurons in the human cingulate cortex. Nature Neuroscience. 1999;2:403–5. doi: 10.1038/8065. [DOI] [PubMed] [Google Scholar]
- Iwata K, Kamo H, Ogawa A, et al. Anterior cingulate cortical neuronal activity during perception of noxious thermal stimuli in monkeys. Journal of Neurophysiology. 2005;94:1980–91. doi: 10.1152/jn.00190.2005. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–9. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jonas E, Schimel J, Greenberg J, Pyszczynski T. The Scrooge effect: Evidence that mortality salience increases prosocial attitudes and behavior. Personality and Social Psychological Bulletin. 2002;28:1342–53. [Google Scholar]
- Klackl J, Jonas E, Kronbichler M. Existential neuroscience: Neurophysiological correlates of proximal defenses against death-related thoughts. Social Cognitive and Affective Neuroscience. 2013;8(3):333–40. doi: 10.1093/scan/nss003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole L, Greenberg J, Pyszczynski T. Introduction science to the psychology of the soul. Current Direction in Psychological Science. 2006;15:121–216. [Google Scholar]
- Kühn S, Gallinat J, Brass M. “Keep calm and carry on”: structural correlates of expressive suppression of emotions. PLoS One. 2011;6:e16569. doi: 10.1371/journal.pone.0016569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2010;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Structure and Function. 2010;214:579–91. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wang C, Han S. Neural responses to perceived pain in others predict real-life monetary donations in different socioeconomic contexts. NeuroImage. 2011;57:1273–80. doi: 10.1016/j.neuroimage.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Malinen S, Vartiainen N, Hlushchuk Y, et al. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proceedings of the National Academy of Sciences USA. 2010;107:6493–7. doi: 10.1073/pnas.1001504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. Journal of Comparative Neurology. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. Journal of Neuroscience. 1998;18:2188–99. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyszczynski T, Greenberg J, Solomon S. A dual-process model of defense against conscious and unconscious death-related thoughts: An extension of terror management theory. Psychological Review. 1999;106:835–45. doi: 10.1037/0033-295x.106.4.835. [DOI] [PubMed] [Google Scholar]
- Quirin M, Loktyushin A, Arndt J, et al. Existential neuroscience: a functional magnetic resonance imaging investigation of neural responses to reminders of one's mortality. Social Cognitive and Affective Neuroscience. 2012;7:193–8. doi: 10.1093/scan/nsq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameson LT, Morelli SA, Lieberman MD. The neural correlates of empathy: experience, automaticity, and prosocial behavior. Journal of Cognitive Neuroscience. 2012;24:235–45. doi: 10.1162/jocn_a_00130. [DOI] [PubMed] [Google Scholar]
- Renkema LJ, Stapel DA, Van Yperen NW. Go with the flow: Conforming to others in the face of existential threat. European Journal of Social Psychology. 2008;38:747–56. [Google Scholar]
- Saarela MV, Hlushchuk Y, Williams AC, Schürmann M, Kalso E, Hari R. The compassionate brain: humans detect intensity of pain from another’s face. Cerebral Cortex. 2007;17:230–7. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Yoshikawa S, Naito E, Matsumura M. Enhanced neural activity in response to dynamic facial expressions of emotion: an fMRI study. Cognitive Brain Research. 2004;20:81–91. doi: 10.1016/j.cogbrainres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Schultz J, Pilz KS. Natural facial motion enhances cortical responses to faces. Experimental Brain Research. 2009;194:465–75. doi: 10.1007/s00221-009-1721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Review Neuroscience. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng F, Han S. Manipulations of cognitive strategies and intergroup relationships reduce the racial bias in empathic neural responses. NeuroImage. 2012;61:786–97. doi: 10.1016/j.neuroimage.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Shi Z, Han S. Transient and sustained neural responses to death-related linguistic cues. Social Cognitive and Affective Neuroscience. 2013;8(5):573–8. doi: 10.1093/scan/nss034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, editor. Sociological Methodology. San Francisco: Jossey-Bass; 1982. pp. 290–312. [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping. 2009;30:2731–45. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templer DI. The construction and validation of a death anxiety scale. Journal of General Psychology. 1970;82:165–77. doi: 10.1080/00221309.1970.9920634. [DOI] [PubMed] [Google Scholar]
- Xu X, Zuo X, Wang X, Han S. Do you feel my pain? Racial group membership modulates empathic neural responses. Journal of Neuroscience. 2009;29:8525–9. doi: 10.1523/JNEUROSCI.2418-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.