Abstract
The devastating social, emotional and economic consequences of human aggression are laid bare nightly on newscasts around the world. Aggression is principally mediated by neural circuitry comprising multiple areas of the prefrontal cortex and limbic system, including the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), amygdala and hippocampus. A striking characteristic of these regions is their structural asymmetry about the midline (i.e. left vs right hemisphere). Variations in these asymmetries have been linked to clinical disorders characterized by aggression and the rate of aggressive behavior in psychiatric patients. Here, we show for the first time that structural asymmetries in prefrontal cortical areas are also linked to aggression in a normal population of early adolescents. Our findings indicate a relationship between parent reports of aggressive behavior in adolescents and structural asymmetries in the limbic and paralimbic ACC and OFC, and moreover, that this relationship varies by sex. Furthermore, while there was no relationship between aggression and structural asymmetries in the amygdala or hippocampus, hippocampal volumes did predict aggression in females. Taken together, the results suggest that structural asymmetries in the prefrontal cortex may influence human aggression, and that the anatomical basis of aggression varies substantially by sex.
Keywords: sex difference, brain asymmetry, aggression, anterior cingulate, orbitofrontal cortex
The pervasive nature of human aggression has made it the focus of nearly a century of intensive research, prompting a range of theories variously emphasizing its cognitive, behavioral, social, ethological and neurophysiological underpinnings (e.g. Dollard et al., 1939; Lorenz, 1958; Bandura, 1973; Davidson et al., 2000). Neurophysiological theories have typically attributed aggression to reduced thresholds for activating negative affect (e.g. anger, distress, agitation) combined with inappropriate responses to the negative consequences of behaving aggressively (Davidson et al., 2000). Consistent with this account, significant relationships have been found between aggression and the structure and function of neural systems involved in behavioral control, learning, emotion processing, emotion regulation and affective style (Grafman et al., 1996; Soderstrom et al., 2000; Dougherty et al., 2004; Raine, 2008; Motzkin et al., 2011; Juárez et al., 2013). This work has particularly emphasized the orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC) (Birbaumer et al., 2005), as well as the amygdala and hippocampus (Critchley et al., 2000; Raine et al., 2004; Bufkin and Luttrell, 2005).
Although there is general agreement that the structure and function of prefrontal and limbic regions are associated with aggression, the exact nature of the relationship remains elusive. In particular, it is unclear whether aggression-related differences are found bilaterally or limited to a single hemisphere. For example, increased bilateral OFC activity has been observed among populations characterized by aggression (e.g. Goyer et al., 1994; Juhasz et al., 2001; Coccaro et al., 2007), and individuals who report high levels of trait aggression show elevated bilateral ACC activation (Eisenberger et al., 2007). Aggression has also been linked to bilateral changes (Tonkonogy, 1991; Woermann et al., 2000) in amygdala volume and reduced bilateral hippocampal volumes in females with borderline personality disorder (Schmahla et al., 2003) and in male adolescents with conduct disorder (Huebner et al., 2008; Fairchild et al., 2011). On the other hand, a recent meta-analysis found only reduced right-hemisphere OFC and ACC volumes in violent, antisocial and psychopathic individuals (Yang and Raine, 2009), while other studies have shown increased hippocampal activity only in the right hemisphere in these individuals (e.g. Raine et al., 1998).
One potential source of variability in previous studies might be due to their focus on absolute size or activity in each hemisphere, rather than on relative differences between the hemispheres (i.e. asymmetries). Structural asymmetries are a prominent feature of many parts of the brain at the level of both gross anatomy and cellular structure (Toga and Thompson, 2003), and have been functionally linked to key systems such as language (Foundas et al., 1996) and motor control (Zilles et al., 1996). Thus, it is plausible to conjecture that a prominent human behavior such as aggression might also be linked to underlying structural asymmetries. Asymmetries are also of theoretical interest because they may be linked to early neurodevelopmental processes, rather than those that occur as the result of later environmental factors that are more likely to act similarly on both hemispheres (Bilder et al., 1999; Raine et al., 2004).
To date, studies that have linked aggression to brain morphology have focused on clinical samples of individuals who are likely to be aggressive, such as those with anti-social personality disorder, as there is no ‘aggressive disorder’ per se. Structural asymmetries have been shown in frontal and limbic areas in schizophrenia (Keshavan et al., 2002), anti-social personality disorder (Chesterman et al., 1994) and psychopathy (Raine et al., 2004). In addition, several studies have shown evidence for asymmetrical brain activity in relevant areas, such as the frontal lobes (Forbes et al., 2006), amygdalae and hippocampi (Raine et al., 1997). Structural asymmetries have not yet been examined in aggressive adolescents. However, it may be useful and more parsimonious to consider previous findings of volumetric differences in only one hemisphere that have been found in males with conduct disorder (e.g. lesser right but not left insula volumes, Fairchild et al., 2011; lesser left but not right amygdala volumes, Sterzer et al., 2007) as being due to hemispheric asymmetries.
Although previous research has focused on samples of clinical groups that are likely to be aggression, aggression also exists in the normal, non-clinical, population. Recently, Walhovd et al. (2012) found that brain morphology, including thinner left prefrontal and supramarginal areas, was related to conduct problems (including, but not limited to, aggression) in a normal sample of youth. Although this work is suggestive, it remains unclear whether structural asymmetries in frontal and limbic areas are linked to normal levels of human aggression in the absence of comorbid pathology. This question is a critical focus of the current study.
Our emphasis on understanding normal levels of aggression that occur in the community outside of a clinical context was a key motivator in our choice of an early adolescent sample. Early adolescence is a time when aggression begins to approach adult levels, and yet predates the development of many comorbid problems (e.g. incarceration, substance use, psychiatric conditions) that often accompany aggressive behavior. These problems frequently occur in adult samples conventionally used to study neural correlates of aggression, thus raising questions about whether comorbid symptoms or other life experiences might moderate observed brain–behavior relationships (Yang and Raine, 2009). By using an early adolescent sample, we aimed to minimize these ambiguities.
Our choice of an adolescent sample was also motivated by the fact that little is known about how aggression is linked to neuroanatomy in this age group. Adolescence is a particularly critical period in the social, emotional and neural development that shapes adult behavior. Many studies have suggested that there is a rapid increase in the frequency of physical aggression at this age (Farrell et al., 2005; Karriker-Jaffe et al., 2008). This has been suggested to parallel the onset of puberty and to reflect an increased desire to establish dominance in social groups, which is thought to be the main goal of overtly aggressive behavior such as hitting, kicking and taunting (e.g. Pepler and Craig, 2005). More worrisome, the increase in physical aggression in early adolescence is thought to set the stage, in some individuals, for more serious violence in late adolescence (Loeber and Hay, 1997; Tolan et al., 2000), and thus open the door to comorbid problems such as incarceration. Viewed from this perspective, it is clear that understanding neural correlates of aggression in this early adolescence may provide important clues about the foundations of overt aggression later in life.
A second issue of key importance to us was the impact of sex differences on structural correlates of aggression. Unlike past studies, which have often used exclusively single-sex samples, our participants comprised approximately equal numbers of males and females. This allowed us to directly compare structural correlates of aggression across sex using identical imaging techniques and behavioral measures. Such comparisons are crucial in light of abundant evidence that aggression differs significantly between the sexes. For example, it is well documented that males show greater overt displays of aggression, such as hitting, than females across the lifespan (Archer, 2004; Vazsonyi and Keiley, 2007). In addition, there is evidence for sex differences in the developmental trajectories of aggression (Bjorkqvist et al., 1992), in anatomical correlates of defiant behavior and irritable temperament (Boes et al., 2008), and in associations between aggression and other social, emotional and behavioral problems (Crick and Grotpeter, 1995). Hess and Hagen (2006) found that faced with an attack on their character, adolescent females preferentially retaliated with relational aggression, while males chose both overt and relational aggression (see also Bjorkqvist et al., 1992; Salmivalli et al., 2000; Archer, 2004 for evidence that females begin to prefer relational aggression during early adolescence). Finally, neurophysiological evidence suggests there is a relationship between the duration of adolescent conflicts with parents and ACC asymmetry in boys, but not girls (Whittle et al., 2008). In light of this evidence, this study investigated whether differences in aggression might be reflected in variations in volume or structural asymmetry across sex.
MATERIALS AND METHODS
Participants
We performed magnetic resonance imaging (MRI) scans on a sample of 153 early adolescent individuals (72 female) selected from a larger sample of 2479 grade six students in Melbourne, Australia. Participants were recruited as part of an ongoing research program investigating the relationship between adolescent temperament, brain development and risk for psychopathology. Participants were chosen to reflect the full range of resiliency and risk for aggression on the basis of an in-school temperament screening consisting of the Early Adolescent Temperament Questionnaire (Ellis and Rothbart, 2001). To this end, equal numbers of adolescents were selected across the following ranges of scores on two higher order factors of the EATQ-R (negative affectivity and effortful control): 0–1 s.d. above and below the mean, 1–2 s.d. above and below the mean, 2–2.5 s.d. above and below the mean and >2.5 s.d. above and below the mean. Of 425 selected adolescents, 153 agreed to participate in the MRI component of the study. No differences between participants who agreed to the MRI and those who were selected but declined were observed on temperament (negative affectivity, t[412] = 0.58, P = 0.56; effortful control, t[412] = 0.32, P = 0.75 or sex, χ2[1] = 0.54, P = 0.46). Handedness was established using the Edinburgh Handedness Inventory (Oldfield, 1971). No participants evidenced current or past case level axis I depressive, substance use or eating disorder, established using The Schedule for Affective Disorder and Schizophrenia for School-Aged Children: Epidemiologic Version (Orvaschel, 1995). Demographic information can be found in Table 1.
Table 1.
Demographic information
| Males | Females | Total sample | |
|---|---|---|---|
| Age (years) | 12.66 (0.47) | 12.58 (0.43) | 12.62 (0.45) |
| 11.36–13.59 | 11.40–13.69 | 11.36–13.69 | |
| Handedness, proportion right (%) | 87.50 | 92.30 | 90.83 |
| Neighborhood socioeconomic status | 14.44 (5.17) | 15.29 (4.16) | 14.83 (4.74) |
| 2–20 | 2–20 | 2–20 | |
| Ethnicity (%) | |||
| Australian | 92.62 | 91.42 | 92.06 |
| Mixed-Australian | 3.69 | 2.86 | 3.31 |
| European | 2.46 | 1.43 | 1.99 |
| Chinese | 1.23 | 1.43 | 1.32 |
| Indonesian | 0 | 1.43 | 0.66 |
| Indian | 0 | 1.43 | 0.66 |
Numbers in brackets represent 1 s.e.m.
Measurement of aggression
As part of a larger battery of measures, a parent (80% mothers) completed the overt aggression scale from the Social Behavior Scale (Crick, 1996), which is a four-item measure assessing physical aggression (e.g. hitting, kicking). Items are rated on a five-point scale ranging from 1 = ‘almost never’ to 5 = ‘almost always’. We chose this scale because it provides a brief measure that is free of non-aggressive items often found in other scales, such as over confidence. It also has a wealth of evidence attesting to its reliability (e.g. Chronbach’s alpha of 0.94 in a normative sample of school children; Crick, 1996) and validity (e.g. correlates with other measures of behavior problems more strongly than mood problems; e.g. Crick, 1996) across a variety of ages, cultures and clinical and normative samples (e.g. Tomada and Schneider, 1997; Prinstein et al., 2001; Little et al., 2003). As in previous studies, we found that participants were, on an average, occasionally aggressive, with males (mean = 132; s.d. = 0.52) significantly more aggressive than females [(mean = 1.26; s.d. = 0.30), t(1, 124) = 2.24, P < 0.03 and d = 0.34].
Neighborhood socioeconomic status
Postcode data for participants’ residences were used to provide an estimate of neighborhood disadvantage using the Socioeconomic Indexes for Areas (SEIFA) scale of Advantage/Disadvantage, a composite of 31 variables pertaining to neighborhood disadvantage including income, unemployment, occupation and education. The Australian Bureau of Statistics reports that the SEIFA index has good validity based on analyses using the Australian Census (Trewin, 2001).
Image acquisition
MRI scans were performed on a 3-T scanner at the Brain Research Institute, Austin and Repatriation Medical Centre, Melbourne, Australia, using a gradient echo volumetric acquisition sequence (repetition time = 36 ms; echo time = 9 ms; flip angle = 35°; field of view = 20 cm2; pixel matrix = 410 × 410) to obtain 124 T1-weighted contiguous 1.5 mm thick slices (voxel dimensions = 0.4883 × 0.4883 × 1.5 mm).
Image pre-processing
Images were transferred to a SGI/Linux workstation for morphometric analysis. Image pre-processing was carried out using tools from the FMRIB software library (http://www.fmrib.ox.ac.uk/fsl/). Each three-dimensional scan was stripped of all non-brain tissue (Smith, 2002) and aligned to the MNI 152 average template (six-parameter rigid body transform with trilinear interpolation) using FLIRT (Jenkinson and Smith, 2001). This registration served to align each image axially along the anterior commissure-posterior commissure (AC-PC) plane and sagittally along the interhemispheric fissure without any deformation. Images were resampled to 1 mm3.
Morphometric analysis
Regions of interest (ROIs) were defined and quantified based on previous techniques developed and published in the Melbourne Neuropsychiatry Centre (see below). All ROIs were traced using the software package ANALYZE (Mayo Clinic, Rochester, USA; http://www.mayo.edu/bir/). Brain tissue was segmented into grey matter, white matter and cerebrospinal fluid using an automated algorithm, as implemented in FAST (Zhang et al., 2001). An estimate of whole brain volume (WBV) was obtained by summing grey and white matter pixel counts (i.e. WBV included cerebral grey and white matter, the cerebellum, and brainstem, but not the ventricles, cisterns or cerebrospinal fluid). ACC and OFC estimates were based on grey matter pixel counts contained within the defined ROIs. Amygdala and hippocampus estimates were based on total voxels within the defined ROI.
Amygdala and hippocampus
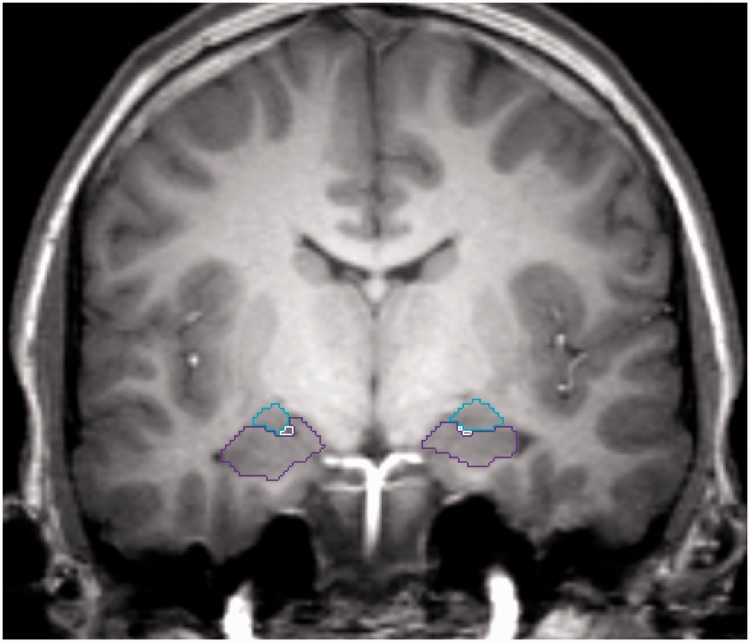
The guidelines for tracing the amygdala and hippocampus were adapted from those described by Velakoulis et al. (1999, 2006). Differences in method, which were adopted in efforts to maximize reliability for the current data set, relate to marking the anterior boundary of the amygdala and the boundary between the amygdala and hippocampus. The anterior boundary of the amygdala was identified as the section posterior to the most posterior of either the point where the optic chiasm joins, or the point where the lateral sulcus closes to form the endorhinal sulcus. Watson et al.’s (1992) protocol was used to separate the amygdala from the hippocampus (Figure 1).
Fig. 1.
Example of manual delineation of bilateral amygdalae (light blue) and hippocampi (purple) on a coronal MR image.
Anterior cingulate cortex
The boundaries of the ACC have been described in detail by Fornito et al. (2006). This protocol demarcates limbic and paralimbic portions of the ACC (ACCL and ACCP, respectively) regions by taking into account individual differences in morphology of the cingulate (CS), paracingulate (PCS) and superior rostral sulci. Briefly, the anterior ACCL contained all grey matter in the gyrus bound by the callosal sulcus and the CS. The ACCP contained all grey matter in the gyrus bound by the CS and PCS, except in cases where the PCS was absent, for which the ACCP contained only the grey matter on the upper bank of the CS. A PCS was considered present if there was a clearly identifiable sulcus running dorsal and parallel to the CS for more than or equal to three consecutive sagittal slices that was at least 20 mm in length.
Orbitofrontal cortex
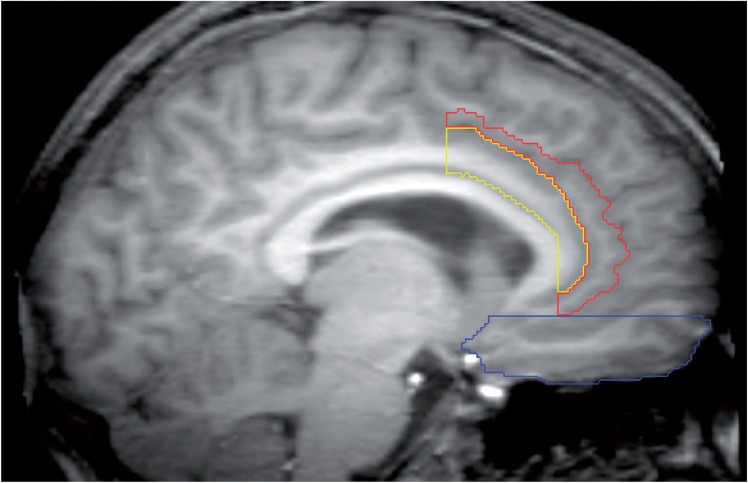
The boundaries of the OFC were based on a previously published method (Riffkin et al., 2005). A line through the AC-PC was used to define the superior boundary of the OFC. The posterior boundary was marked by a coronal plane passing through the most posterior aspect of the olfactory sulcus in each hemisphere. All images were manually edited, using the pencil and edit tools in the ANALYZE software, to eliminate subcortical tissue and artifacts related to the eye sockets and nasal bones (Figure 2).
Fig. 2.
Example of manual delineation of the OFC (blue), ACCL (yellow) and ACCP (red) on a mid-sagittal MR image.
Data analysis
We computed brain volumes for the ACCL, ACCP, OFC, hippocampus and amygdala (corrected for WBV) and then for each region created: (i) total volumes by adding left and right hemisphere volumes and (ii) asymmetry indexes by subtracting right hemisphere from left hemisphere volumes. Total volumes and asymmetry indexes were used to predict adolescent aggression using hierarchical linear regression models. Demographic information (age and neighborhood socioeconomic status) and sex were entered in the first block of the regression. The total volume or asymmetry index was entered in the second block and the interaction between sex and total volume or sex and asymmetry index was computed and entered in the third block. For significant main or interactive effects, follow-up correlations between brain region volume and aggression were performed separately by hemisphere (and/or sex) to determine the basis of the sex difference or hemispheric imbalance.
RESULTS
As shown in Table 2, asymmetries in all frontal regions (ACCL, ACCP, OFC) predicted adolescent aggression, whereas total volumes did not. Reduced (left–right) OFC asymmetry predicted aggression for both sexes, and for this region aggression was associated with greater right OFC volume, r = 0.16, P < 0.05 (and no association with left OFC volume, P > 0.19). However, in the ACCL and ACCP, follow-up tests showed that asymmetries significantly predicted aggression only for male adolescents. For the ACCL, aggression was linked to increased (left–right) asymmetry, which was driven by an association between aggression and greater left volumes (r = 0.27, P < 0.03); for the ACCP, aggression was linked to reduced (left–right) asymmetry, which was driven by associations between aggression and greater right (r = 0.27, P < 0.03) and smaller left (r = −0.24, P < 0.05) volumes. In both cases, these relationships were absent in females (all Ps > 0.64).
Table 2.
Summary of hierarchical regressions predicting adolescent aggression with adolescent ‘frontal’ brain volume/asymmetry measures
| Predictors and dependent variables | β | t (P) | ΔF (P) (for the final model) | ΔR2 (R2) |
|---|---|---|---|---|
| ACCL total | 0.16 | 1.45 (0.15) | 1.15 (0.34) | 0.004 (0.030) |
| ACCL total × sex | −0.12 | −1.07 (0.29) | 0.005 (0.037) | |
| ACCL asymmetry | 0.25 | 2.24 (0.02)* | 1.84 (0.10) | 0.005 (0.031) |
| ACCL asymmetry × sex | −0.23 | −2.06 (0.04)* | 0.027 (0.058) | |
| ACCP total | −0.003 | 0.031 (0.97) | 0.77 (0.61) | −0.003 (0.024) |
| ACCP total × sex | −0.019 | −0.17 (0.86) | −0.009 (0.024) | |
| ACCP asymmetry | −0.41 | −3.78 (0.001)*** | 3.63 (0.004)** | 0.039 (0.064) |
| ACCP asymmetry × sex | 0.30 | 2.76 (0.007)** | 0.079 (0.109) | |
| OFC total | 0.056 | 0.52 (0.60) | 0.81 (0.54) | 0.001 (0.027) |
| OFC total × sex | 0.001 | 0.007 (0.99) | −0.006 (0.027) | |
| OFC asymmetry | −0.22 | −1.94 (0.05)* | 1.49 (0.20) | 0.007 (0.033) |
| OFC asymmetry × sex | 0.18 | 1.53 (0.13) | 0.016 (0.048) |
Statistically significant results highlighted in bold (*P < 0.05, **P < 0.01, ***P < 0.001). Hierarchical linear regression models used with standardized variables. Neighborhood economic status, sex and age entered at first step as covariates. Brain volume entered in second step, brain volume by sex interaction entered at third step. Brain volumes used have all been corrected by whole brain volume. Beta weight reported is the standardized coefficient.
As shown in Table 3, neither hippocampal nor amygdala asymmetries significantly predicted adolescent aggressive behavior. However, total hippocampal volume-predicted adolescent aggression. There was a significant hippocampus × sex interaction, and follow-up tests showed that the link between total hippocampal volume and aggression was significant for females (r = 0.40, P < 0.002) but not males (P > 0.54). Further correlational tests for females indicated that both the left (r = 0.46, P < 0.001) and right (r = 0.37, P < 0.004) hippocampal volume significantly predicted aggression. Notably, amygdala volume did not predict aggression in either sex.
Table 3.
Summary of hierarchical regressions predicting adolescent aggression with adolescent ‘limbic system’ brain volume/asymmetry measures
| Predictors and dependent variables | β | t (P) | ΔF (P) (for the final model) | ΔR2 (R2) |
|---|---|---|---|---|
| Hippocampus total | 0.28 | 2.20 (0.03)* | 1.89 (0.10) | 0.004 (0.028) |
| Hippocampus total × sex | −0.27 | −2.19 (0.03)* | 0.025 (0.057) | |
| Hippocampus asymmetry | 0.17 | 1.52 (0.13) | 1.21 (0.31) | 0.009 (0.035) |
| Hippocampus asymmetry × sex | −0.08 | −0.77 (0.44) | 0.007 (0.039) | |
| Amygdala total | −0.01 | −0.09 (0.93) | 1.43 (0.22) | 0.007 (0.033) |
| Amygdala total × sex | 0.16 | −1.44 (0.15) | 0.014 (0.047) | |
| Amygdala asymmetry | −0.07 | −0.61 (0.54) | 0.84 (0.52) | −0.003 (0.024) |
| Amygdala asymmetry × sex | 0.08 | 0.78 (0.44) | −0.005 (0.028) |
Statistically significant results highlighted in bold (*P < 0.05). Hierarchical linear regression models used with standardized variables. Neighborhood economic status, sex and age entered at first step as covariates. Brain volume entered in second step, Brain volume by sex interaction entered at third step. Brain volumes used have all been corrected by whole brain volume. Beta weight reported is the standardized coefficient.
DISCUSSION
These findings indicate that aggression in a non-clinical sample is strongly linked to structural asymmetries in the OFC and ACC that previously have been shown to play a role in impulse control, emotional regulation and activation of negative affect (Critchley et al., 2000). Thus, as in multiple domains where structural asymmetries are associated with lateralization of neural function (Powell et al., 2006), it would seem that functional and neurochemical asymmetries thought to play a role in aggressive behavior have structural correlates. These new findings further buttress arguments for the critical role of frontal areas in the regulation of normal human aggressive impulses (Critchley et al., 2000), and refute suggestions that comorbid symptoms and life experiences are the chief determinants of frontal and limbic differences observed between aggressive patient populations and normal-matched controls (Yang and Raine, 2009).
Our results fit well with an emergent pattern uniquely linking ACC asymmetries with male aggression and disorders characterized by aggression. For example, asymmetries like those shown here have also been found in males with schizophrenia (Yücel et al., 2001, 2002). Similarly, as noted earlier, previous work by our group with this sample has shown a strong relationship between the duration of adolescent conflicts with parents and ACC asymmetry uniquely in boys (Whittle et al., 2008). On the other hand, the implication of the link between OFC asymmetries in boys and aggression is less clear. For example, the increased size of the right OFC relative to the left is consistent with findings from patients with a history of borderline personality disorder, but opposite to that reported in teenagers with first presentation of the disorder (Chanen et al., 2008). This is somewhat surprising given that one might expect first-presentation sufferers to be more similar to our own sample of adolescents without a psychiatric condition than long-term sufferers. It would seem, then, that further work is thus needed to understand how OFC asymmetries are reflected in aggressive behavior.
Unlike frontal areas, asymmetries in the hippocampus and amygdala were unrelated to aggression. Instead, only increased hippocampal volumes predicted aggression, and this relationship was obtained only in females. Interestingly, this finding is the opposite of that shown in previous clinical literature, which linked borderline personality disorder with reduced hippocampal volumes in female patients (Schmahla et al., 2003). It also fails to replicate previous evidence for the presence of hippocampal asymmetries in jailed male psychopaths (Raine et al., 2004). Tentatively, we suggest that differences between our results and earlier work may reflect our use of an early adolescent non-clinical population, and that previous evidence for hippocampal asymmetries in criminal psychopaths reflected a link with comorbid symptoms rather than aggression per se.
At first glance, our failure to find a link between amygdala volumes and aggression also seems surprising. The amygdala has conventionally been linked to individual differences in processing of negative emotions and emotional dysregulation (Minzenberg et al., 2007) that can lead to aggressive behavior. In addition, past research has demonstrated reduced amygdala volume in patients with borderline personality disorder relative to normal controls (Driessen et al., 2000; Schmahla et al., 2003; van Elst et al., 2003; Nunes et al., 2009). On the other hand, we have shown elsewhere that amygdala volumes were larger in normal adolescents who showed increased durations of aggressive episodes during conflicting interactions with their mothers (Whittle et al., 2008). In sum, then, the relationship between volumetric changes in the amygdala and aggression remains unclear. It may be that such a link is found only in patient populations such as those with borderline personality disorder, and thus is related to the non-aggressive behaviors that are specific to such clinical populations. Such differences would not be observable in our community-based sample of young adolescents. Alternatively, it could be that structural changes are related to other types of aggression (e.g. relational), but not to overt aggression. This is clearly an issue that requires further investigation.
A key issue that remains to be addressed concerns how sex differences observed here are linked to aggressive behavior. For males, our findings suggest that increased overt aggression is linked to a reversal of the normal pattern of ACC asymmetries characterized by leftward asymmetry of the ACCP and rightward asymmetry of the ACCL (Yücel et al., 2001). Generally speaking, greater lateralization of brain function seems to confer advantages to tasks suited to more localized (i.e. intra-hemispheric) brain function (Toga and Thompson, 2003) via decreased interhemispheric information exchange and/or from interhemispheric inhibition (Luders et al., 2003). Thus, the observed reversal in asymmetries may reflect a disruption to the patterns of inter- or intra-hemispheric neural function that are normally recruited to promote non-aggressive behavior. Another possibility is suggested by studies that have linked reduced leftward asymmetry in the ACCP and poorer cognitive performance (Fornito et al., 2008). This finding opens up the possibility that abnormal development of the ACC in males may bias them toward increased overt aggression in place of more sophisticated social strategies that would likely depend heavily on ACC functioning (Carter et al., 2000; Sanders et al., 2002).
As noted earlier, the relationship between increased hippocampal volume and aggression in our female sample was the opposite of that seen in earlier studies with both male psychopaths (Laakso et al., 2001) and females with borderline personality disorder (Nunes et al., 2009). Nevertheless, in formulating a hypothesis to explain the impact of increased hippocampal volume on female aggression, it is useful to consider the putative roles of the hippocampus in aggression suggested in these earlier reports. In particular, Raine et al. (2004) have linked changes in hippocampal structure to disruption of a frontal–hippocampal circuit that is critical for emotional regulation, fear learning and an appreciation of cues that may lead to avoidance of punishment. If we assume that structural changes can also produce enhanced functioning in some circumstances, then it is possible that an enlarged hippocampus might be indicative of increased efficiency in this circuit. In turn, this might imply that females with an enlarged hippocampus use aggression more often because they are better able to avoid the punishments that are usually administered in response to this behavior.
Another question to be considered is the potential origins of individual differences in brain structure and asymmetry. Distally, the structural changes seen here may reflect genetically linked neurodevelopmental changes. Consistent with this proposition, recent research suggests that lateralization and aggression are intimately linked across diverse vertebrate species (Rogers, 2002). Moreover, several genes have been directly implicated in aggression associated with antisocial personality disorder (Meyer-Lindenberg et al., 2006; Raine, 2008). However, the genetic origins of the asymmetries obtained here cannot be definitively demonstrated because they are based on differences in regional volumes, which may be influenced by a variety of environmental factors (Weinberger and McClure, 2002). In light of this caveat, it may be useful for future work to search for morphological differences between the left and right ACC that can be confidently attributed to pre- or peri-natal neurodevelopmental influences (Armstrong et al., 1995).
While the present work has provided a window into the structural correlates of overt aggression in the brains of young adolescents, the results must be considered in light of some limitations. One such limitation is the correlational nature of our results, which renders us unable to determine causal relationships between structure and aggression. Another limitation of our work is that we focused exclusively on overt aggression. Thus, we cannot speak to the issue of how structural characteristics observed here might be related to other forms of aggression, such as relational aggression, which also increase in frequency in this age group (Karriker-Jaffe et al., 2008). This is clearly an important area for future investigation. It might also be argued that our choice of young adolescent participants limits our ability to generalize our findings to other age groups, particularly because of the significant neuroanatomical changes that occur in early adolescence (Giedd et al., 1999; Lenroot and Giedd, 2006). While this may be true, we think that the increasing prevalence of physical aggression in this age group, along with the evidence that this forms the behavioral foundation for more serious violence in later years, makes understanding the anatomical underpinnings of young adolescent aggression particularly crucial to delineating the development aggression into adulthood. Finally, it must be noted that this study represents a snapshot of adolescent brain structure. Longitudinal data are of great importance, and we are currently following up this sample in order to begin to examine developmental associations.
In conclusion, we have shown that structural asymmetries in the ACC and OFC can be linked to overt aggressive behavior in young adolescents, and that these associations vary with sex. This opens up many new research questions aimed at discovering the functional significance of these structural differences for both normal and abnormal levels of human aggression. Such work will likely be closely linked with establishing the source of asymmetries at genetic, environmental and cellular levels and the implications of these asymmetries for diverse displays of human aggression. This future research will be particularly important to develop a comprehensive view of the origins of aggression, incorporating its origins from a complex network of cognitive, behavioral, social, ethological and neurophysiological factors. The present findings here provide one piece of evidence about the biological origins of this important human behavior. It remains to be shown how structural brain asymmetries are intertwined with other factors in yielding aggression.
Acknowledgments
Neuroimaging analysis was facilitated by the Neuropsychiatry Imaging Laboratory, managed by Ms Bridget Soulsby, at the Melbourne Neuropsychiatry Centre and supported by Neurosciences Victoria. The authors would like to thank the Brain Research Institute for support in acquiring the neuroimaging data. This research was supported by grants from the Colonial Foundation; the National Health and Medical Research Council (NHMRC; Australia; program grant 350241); the Australian Research Council (discovery grants DP0878136 and DP120102313) and a Queensland University of Technology Women in Research Award. S.W. was supported by an NHMRC Career Development Fellowship (ID: 1007716). M.Y. was supported by an NHMRC Clinical Career Development Award (509345).
REFERENCES
- Archer J. Sex differences in aggression in real-world settings: a meta-analytic review. Review of General Psychology. 2004;8:291–322. [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cerebral Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Bandura A. Aggression: A Social Learning Analysis. Englewood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- Bilder RM, Wu H, Bogerts B, et al. Cerebral volume asymmetries in schizophrenia and mood disorders: a quantitative magnetic resonance imaging study. International Journal of Psychophysiology. 1999;34:197–205. doi: 10.1016/s0167-8760(99)00077-x. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Archives of General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist L, Osterman K, Kaukiainen A. The development of direct and indirect aggressive strategies in males and females. In: Bjorkqvist K, Niemelae P, editors. Of Mice and Women: Aspects of Female Aggression. San Diego: Academic Press; 1992. pp. 51–64. [Google Scholar]
- Boes AD, Tranel D, Anderson SW, Nopoulos P. Right anterior cingulate: a neuroanatomical correlate of aggression and defiance in boys. Behavioral Neuroscience. 2008;122:677–84. doi: 10.1037/0735-7044.122.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufkin JL, Luttrell VR. Neuroimaging studies of aggressive and violent behavior: current findings and implications for criminology and criminal justice. Trauma, Violence, & Abuse. 2005;6:176–91. doi: 10.1177/1524838005275089. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1944–8. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanen AM, Velakoulis D, Carison K, et al. Orbitofrontal, amygdala and hippocampal volumes in teenagers with first-presentation borderline personality disorder. Psychiatry Research. 2008;163:116–25. doi: 10.1016/j.pscychresns.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Chesterman LP, Taylor PJ, Cox T, Hill M. Multiple measures of cerebral state in dangerous mentally disordered inpatients. Criminal Behaviour and Mental Health. 1994;4:228–39. [Google Scholar]
- Coccaro EF, McCloskey M, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 2007;62:168–78. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Crick NR. The role of overt aggression, relational aggression, and prosocial behavior in children’s future social adjustment. Child Development. 1996;67:2317–27. [PubMed] [Google Scholar]
- Crick NR, Grotpeter JK. Relational aggression, gender, and social-psychological adjustment. Child Development. 1995;66:710–22. doi: 10.1111/j.1467-8624.1995.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Simmons A, Daly EM, et al. Prefrontal and medial temporal correlates of repetitive violence to self and others. Biological Psychiatry. 2000;15:928–34. doi: 10.1016/s0006-3223(00)00231-6. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Dollard J, Doob L, Miller N, Mowrer O, Sears R. Frustration and Aggression. New Haven, CT: Yale University Press; 1939. [Google Scholar]
- Dougherty DD, Rauch SL, Deckersbach T, et al. Ventromedial prefrontal cortex and amygdala dysfunction during an anger induction positron emission tomography study in patients with major depressive disorder with anger attacks. Archives of General Psychiatry. 2004;61:795–804. doi: 10.1001/archpsyc.61.8.795. [DOI] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of General Psychiatry. 2000;57:1115–22. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Way BM, Taylor SE, Welch WT, Lieberman MD. Understanding genetic risk for aggression: clues from the brain’s response to social exclusion. Biological Psychiatry. 2007;35:1601–12. doi: 10.1016/j.biopsych.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Ellis LK, Rothbart MK. Minneapolis, MN: 2001. Revision of the Early Adolescent Temperament Questionnaire. Poster presented at the Biennial Meeting of the Society for Research in Child Development. [Google Scholar]
- Fairchild G, Passamonti L, Hurford G, et al. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. American Journal of Psychiatry. 2011;168:624–33. doi: 10.1176/appi.ajp.2010.10081184. [DOI] [PubMed] [Google Scholar]
- Farrell AD, Sullivan TN, Esposito LE, Meyer AL, Valois RF. A latent growth curve analysis of the structure of aggression, drug use, and delinquent behaviours and their interrelations over time in urban and rural adolescents. Journal of Research on Adolescence. 2005;15:179–204. [Google Scholar]
- Forbes EE, Shaw DS, Fox NA, Cohn JF, Silk JS, Kovacs M. Maternal depression, child frontal asymmetry, and child affective behavior as factors in child behavior problems. Journal of Child Psychology and Psychiatry. 2006;47:79–87. doi: 10.1111/j.1469-7610.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- Fornito A, Whittle S, Wood SJ, Velakoulis D, Pantelis C, Yücel M. The influence of sulcal variability on morphometry of the human anterior cingulate and paracingulate cortex. Neuroimage. 2006;33:843–54. doi: 10.1016/j.neuroimage.2006.06.061. [DOI] [PubMed] [Google Scholar]
- Fornito A, Wood SJ, Whittle S, et al. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: associations with cortical thickness, surface area, volume, and sulcal depth. Human Brain Mapping. 2008;29:222–36. doi: 10.1002/hbm.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Gilmore RL, Fennell EB, Heilman KM. Pars triangularis asymmetry and language dominance. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:719–22. doi: 10.1073/pnas.93.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goyer PF, Andreason PJ, Semple WE, et al. Positron emission tomography and personality disorders. Neuropsychopharmacology. 1994;10:21–8. doi: 10.1038/npp.1994.3. [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology. 1996;46:1231–8. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Hess NH, Hagen EH. Sex differences in informational aggression: psychological evidence from young adults. Evolution and Human Behavior. 2006;27:231–45. [Google Scholar]
- Huebner T, Voet TD, Marx I, et al. Morphometic brain abnormalities in boys with conduct disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:540–7. doi: 10.1097/CHI.0b013e3181676545. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Juárez M, Kiehl KA, Calhoun VD. Intrinsic limbic and paralimbic networks are associated with criminal psychopathy. Human Brain Mapping. 2013;34(8):1921–30. doi: 10.1002/hbm.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz C, Behen ME, Muzik O, Chugani DC, Chugani HT. Bilateral medial prefrontal and temporal neocortical hypometabolism in children with epilepsy and aggression. Epilepsia. 2001;42:991–1001. doi: 10.1046/j.1528-1157.2001.042008991.x. [DOI] [PubMed] [Google Scholar]
- Karriker-Jaffe KJ, Foshee VA, Ennett ST, Suchindran C. The development of aggression during adolescence: sex differences in trajectories of physical and social aggression among youth in rural areas. Journal of Abnormal Child Psychology. 2008;36:1227–36. doi: 10.1007/s10802-008-9245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Dick E, Mankowski I, et al. Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophrenia Research. 2002;58:173–83. doi: 10.1016/s0920-9964(01)00404-2. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Koivisto E, et al. Psychopathy and the posterior hippocampus. Behavioral Brain Research. 2001;118:187–93. doi: 10.1016/s0166-4328(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Little T, Henrich C, Jones S, Hawley P. Dientangling the “whys” and the “whats” of aggressive behaviour. International Journal of Behavioral Development. 2003;27:122–33. [Google Scholar]
- Loeber R, Hay DF. Key issues in the development of aggression and violence from childhood to early adulthood. Annual Review of Psychology. 1997;48:371–410. doi: 10.1146/annurev.psych.48.1.371. [DOI] [PubMed] [Google Scholar]
- Lorenz KZ. The evolution of behavior. Scientific American. 1958;199:67–78. doi: 10.1038/scientificamerican1258-67. [DOI] [PubMed] [Google Scholar]
- Luders E, Rex DE, Narr KL, et al. Relationships between sulcal asymmetries and corpus callosum size: gender and handedness effects. Cerebral Cortex. 2003;23:1084–93. doi: 10.1093/cercor/13.10.1084. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6269–74. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Front-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Research. 2007;155:231–43. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced prefrontal connectivity in psychopathy. Journal of Neuroscience. 2011;31:17348–57. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes PM, Wenzel A, Borges KT, Porto CR, Caminha RM, de Oliveira IR. Volumes of the hippocampus and amygdala in patients with borderline personality disorder: a meta-analysis. Journal of Personality Disorders. 2009;23:333–45. doi: 10.1521/pedi.2009.23.4.333. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Lewinsohn PM, Seeley JR. Continuity of psychopathology in a community sample of adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:1525–35. doi: 10.1097/00004583-199511000-00020. [DOI] [PubMed] [Google Scholar]
- Pepler DJ, Craig WM. Aggressive girls on troubled trajectories: a developmental perspective. In: Pepler DJ, editor. Development and Treatment of Girlhood Aggression. Mahwah, NJ: Erlbaum; 2005. pp. 3–28. [Google Scholar]
- Powell HWR, Parker GJM, Alexander DC, et al. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32:388–99. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Boergers J, Vernberg EM. Overt and relational aggression in adolescents: psychological adjustment of aggressors and victims. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;30:479–91. doi: 10.1207/S15374424JCCP3004_05. [DOI] [PubMed] [Google Scholar]
- Raine A. From genes to brain to antisocial behaviour. Current Directions in Psychological Science. 2008;17:323–8. [Google Scholar]
- Raine A, Buchsbaum M, LaCasse L. Brain abnormalities in murderers indicated by positron emission tomography. Biological Psychiatry. 1997;42:495–508. doi: 10.1016/S0006-3223(96)00362-9. [DOI] [PubMed] [Google Scholar]
- Raine A, Ishikawa SS, Arce E, et al. Hippocampal structural asymmetry in unsuccessful psychopaths. Biological Psychiatry. 2004;55:185–91. doi: 10.1016/s0006-3223(03)00727-3. [DOI] [PubMed] [Google Scholar]
- Raine A, Meloy JR, Bihrle S, Stoddard J, LaCasse L, Buchsbaum MS. Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behavioral Sciences & the Law. 1998;16:319–32. doi: 10.1002/(sici)1099-0798(199822)16:3<319::aid-bsl311>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Riffkin J, Yücel M, Maruff P, et al. A manual and automated MRI study of anterior cingulate and orbito-frontal cortices, and caudate nucleus in obsessive-compulsive disorder: comparison with healthy controls and patients with schizophrenia. Psychiatry Research. 2005;138:99–113. doi: 10.1016/j.pscychresns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Rogers LJ. Lateralization in vertebrates: its early evolution, general pattern, and development. Advances in the Study of Behavior. 2002;31:107–61. [Google Scholar]
- Salmivalli C, Kaukiainen A, Lagerspetz K. Aggression and sociometric status among peers: do gender and type of aggression matter? Scandinavian Journal of Psychology. 2000;41:17–24. doi: 10.1111/1467-9450.00166. [DOI] [PubMed] [Google Scholar]
- Sanders GS, Gallup GG, Heinsen H, Hof PR, Schmitz C. Cognitive deficits, schizophrenia, and the anterior cingulate cortex. Trends in Cognitive Sciences. 2002;6:190–2. doi: 10.1016/s1364-6613(02)01892-2. [DOI] [PubMed] [Google Scholar]
- Schmahla CG, Vermettenb E, Elzingac BM, Bremnerde JD. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Research. 2003;122:193–8. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom H, Tullberg M, Wikkelso C, Ekholm S, Forsman A. Reduced regional cerebral blood flow in non-psychotic violent offenders. Psychiatry Research. 2000;98:29–41. doi: 10.1016/s0925-4927(99)00049-9. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37:335–42. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nature Reviews Neuroscience. 2003;4:37–8. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Tolan PH, Gorman-Smith D, Loeber R. Developmental timing of onsets of disruptive behaviors and later delinquency of inner-city youth. Journal of Child and Family Studies. 2000;9:203–30. [Google Scholar]
- Tomada G, Schneider BH. Relational aggression, gender, and peer acceptance: invariance across culture, stability over time, and concordance among informants. Developmental Psychobiology. 1997;33:601–9. doi: 10.1037//0012-1649.33.4.601. [DOI] [PubMed] [Google Scholar]
- Tonkonogy JM. Violence and temporal lobe lesion: Head CT and MRI data. Journal of Neuropsychiatry and Clinical Neurosciences. 1991;3:189–96. doi: 10.1176/jnp.3.2.189. [DOI] [PubMed] [Google Scholar]
- Trewin D. Census of Population and Housing: Socio-Economic Indexes for area’s (SEIFA) Canberra: Australian Bureau of Statistics; 2001. [Google Scholar]
- van Elst LT, Hesslinger B, Thiel T, et al. Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biological Psychiatry. 2003;54:163–71. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- Vazsonyi AT, Keiley MK. Normative developmental trajectories of aggressive behaviors in African American, American Indian, Asian American, Caucasian, and Hispanic children and early adolescents. Journal of Abnormal Child Psychology. 2007;35:1047–62. doi: 10.1007/s10802-007-9154-z. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Pantelis C, McGorry PD, et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Archives of General Psychiatry. 1999;56:133–41. doi: 10.1001/archpsyc.56.2.133. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MT, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Archives of General Psychiatry. 2006;63:139–49. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Tames CK, Østby Y, Due-Tønnessen P, Fjell AM. Normal variation in behavioural adjustment relates to regional differences in cortical thickness in children. European Child & Adolescent Psychiatry. 2012;21:133–40. doi: 10.1007/s00787-012-0241-5. [DOI] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic-resonance-imaging. Neurology. 1992;42:1743–50. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, McClure RK. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry: what is happening in the schizophrenic brain? Archives of General Psychiatry. 2002;59:533–58. doi: 10.1001/archpsyc.59.6.553. [DOI] [PubMed] [Google Scholar]
- Whittle S, Yap MBH, Yücel M, et al. Prefrontal and amygdala volumes are related to adolescents’ affective behaviors during parent-adolescent interactions. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3652–7. doi: 10.1073/pnas.0709815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woermann FG, van Elst LT, Koepp MJ, et al. Reduction of frontal neocortical grey matter associated with affective aggression in patients with temporal lobe epilepsy: an objective voxel by voxel analysis of automatically segmented MRI. Journal of Neurology, Neurosurgery, and Psychiatry. 2000;68:162–9. doi: 10.1136/jnnp.68.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Research. 2009;174:81–8. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Stuart GW, Maruff P, et al. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cerebral Cortex. 2001;11:17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]
- Yücel M, Stuart GW, Maruff P, et al. Paracingulate morphologic differences in males with established schizophrenia: a magnetic resonance imaging morphometric study. Biological Psychiatry. 2002;52:15–23. doi: 10.1016/s0006-3223(02)01312-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zilles K, Dabringhaus A, Geyer S, et al. Structural asymmetries in the human forebrain and the forebrain of non-human primates and rats. Neuroscience and Biobehavioral Reviews. 1996;20:593–605. doi: 10.1016/0149-7634(95)00072-0. [DOI] [PubMed] [Google Scholar]