Abstract
Behavioral research has demonstrated an advantage for females compared with males in social information processing. However, little is known about sex-related differences in brain activation during understanding of self and others. In the current functional magnetic resonance imaging study, this was assessed in late adolescents (aged 18–19) and young adults (aged 23–25) when making appraisals of self and other as well as reflected self-appraisals. Across all groups and for all appraisal conditions, activation was observed in the medial prefrontal cortex, medial posterior parietal cortex, left and right dorsolateral prefrontal cortex and left posterior parietal cortex. Males activated the medial posterior parietal cortex and bilateral temporoparietal junction more than females. The precuneus showed stronger activation in males compared with females specifically during appraisals of others. No differences between late adolescents and young adults were found. These results indicate that sex differences exist in the neural bases of social understanding.
Keywords: brain, males, other, self, age
INTRODUCTION
Important research areas in social cognitive neuroscience include understanding others, understanding oneself and the interaction between self and social understanding (Lieberman, 2007). These processes have been investigated using diverse tasks, such as making appraisals about traits (Johnson et al., 2002), the attribution of mental states or ‘mentalizing’ (Frith and Frith, 2003) and reflecting on emotions (Ruby and Decety, 2004). Sex differences have been demonstrated on a range of social phenomena and across age. For example, Bosacki (2000) showed that girls 10–13 years old perform better on self-understanding and perspective taking than boys of the same age. Female adolescents have been found to be faster than male adolescents in mentalizing about emotions and actions of self and others (Keulers et al., 2010). In addition, empathy scores are on average higher for female adults compared with male adults (Baron-Cohen and Wheelwright, 2004). The presence of sex differences seems to depend on the type of assessment, as males and females have similar physiological responses to others’ emotional state (Eisenberg and Lennon, 1983). It has been suggested that observed differences in empathic accuracy reflect a higher motivation, and not necessarily a higher ability, in females (Ickes et al., 2000; Klein and Hodges, 2001). Overall, it appears that females show an advantage over males on self-report measures and behavioral tasks aimed at social information processing. However, little is known about sex differences in the neural bases of understanding self and others.
Previous brain imaging research investigating differences between males and females has mainly focused on emotions (Kret and De Gelder, 2012). For example, sex-specific activation patterns have been observed in response to threat (McClure et al., 2004; Kret et al., 2011) and fairness (Singer et al., 2006). Much less attention has been paid to sex differences in more cognitive aspects of social processing, related to mentalizing and self- or other-appraisals. The current functional magnetic resonance imaging (fMRI) study aims to fill this gap by comparing males and females during appraisals of attributes and preferences.
Brain regions involved in understanding self and others include the cortical midline structures, medial prefrontal cortex and medial posterior parietal cortex (Uddin et al., 2007). These areas also play a role in ‘reflected self-appraisals’, judging what someone else thinks about you (Ochsner et al., 2005; Pfeifer et al., 2009). The medial prefrontal cortex, encompassing the anterior cingulate, is preferentially engaged in understanding oneself (Kelley et al., 2002; D'Argembeau et al., 2005; Ochsner et al., 2005; Heatherton et al., 2006; Qin et al., 2012). The medial posterior parietal cortex, particularly the posterior cingulate and precuneus, is more engaged during understanding of others compared with self (Ruby and Decety, 2004; Pfeifer et al., 2007; Lombardo et al., 2010; Qin et al., 2012; Veroude et al., 2012). An additional region, the temporoparietal junction, seems to be especially important for mentalizing about someone else’s state of mind (Saxe and Kanwisher, 2003; Van Overwalle, 2009; Veroude et al., 2012).
To our knowledge, only one study focused on neural differences between males and females during mentalizing. Krach et al. (2009) used a ‘Prisoner’s dilemma’ in which participants played against a human or a computer partner. To earn points in this game, players can choose to defect or cooperate and therefore have to take the perspective of the other player into account. Results indicated that activation in the medial prefrontal cortex when playing against a human partner was larger for males compared with females. Some fMRI studies on understanding self and others include only females (Blakemore et al., 2007) or males (Sebastian et al., 2012), therefore excluding possible confounding differences between the groups. However, it is important to further investigate sex differences in brain activation. This will provide more insight into the underlying mechanisms of social processing, independent of possible sex differences at the behavioral level.
The current fMRI study targets the neural bases of self- and other-appraisals as well as reflected self-appraisals in males and females aged 18–19 and 23–25. Overall, we expected cortical midline activation and potential involvement of the temporoparietal junction, in line with prior results (Saxe and Kanwisher, 2003; Ochsner et al., 2005; Uddin et al., 2007). Based on the finding that females are generally better than males in social understanding (Baron-Cohen, 2002), sex differences were predicted mainly during appraisals of others. We expected to find the largest effect in the medial posterior parietal cortex, as this area is particularly important for other-appraisals (Qin et al., 2012).
A similar paradigm has previously revealed differences between children and adults as well as adolescents and adults (Pfeifer et al., 2007, 2009). We hypothesized that the neural correlates of social appraisals might continue to change after age 18, as self-development takes place not only in early and middle adolescence but also during late adolescence and beyond (Steinberg and Morris, 2001). Although most researchers consider participants of 18 years and older to be adults, the transition from late adolescence to young adulthood is prolonged in modern western society (Casey et al., 2010). Social experiences, for example, in the educational environment, play a vital role during this stage (Crone and Dahl, 2012). Arnett (2000) pointed out that the age range between 18 and 25 years could be considered a separate life phase, named emerging adulthood, characterized by identity exploration. In addition, structural maturation of the brain is not complete before the early 20s (Giedd, 2004; Gogtay et al., 2004). To examine age differences during this transitional period, we compare 18- to 19-year olds with 23- to 25-year olds. Participants aged 18–19 were first-year and second-year students in Medical College, whereas the participants aged 23–25 were medical students in year four, five or six. The first group will be referred to as late adolescents and the latter group as young adults, in line with the notion that within this age range, important developmental changes occur. Thus, we explored age-related activation differences during understanding of self and others, in addition to sex differences.
METHODS
Participants
A total of 73 healthy right-handed volunteers were included in this study. Participants consisted of 20 female late adolescents (range = 18.39–19.98 years, mean = 19.14, s.d. = 0.43), 17 male late adolescents (range = 18.36–19.91 years, mean = 18.92, s.d. = 0.53), 18 female young adults (range = 23.24–24.95 years, mean = 24.07, s.d. = 0.46) and 18 male young adults (range = 23.05–25.95 years, mean = 24.03, s.d. = 0.89). They were recruited from Medical College at VU University Amsterdam and the University of Amsterdam.
Mean estimation of verbal IQ was 110.2 (s.d. = 6.68) on the Peabody Picture Vocabulary Test-III-NL (Schlichting, 2005). There was no significant difference between scores of the four groups: female late adolescents, male late adolescents, female young adults and male young adults (F = 1.21, P = 0.31). The groups also did not differ on two measures of executive functioning, the Concept Shifting Test (Van der Elst et al., 2006a) and the Letter Digit Substitution Test (Van der Elst et al., 2006b).
All volunteers had normal or corrected-to-normal vision and no history of neurological or psychiatric disorders. Written informed consent was obtained prior to the study and participants received monetary compensation. This study was approved by the Medical Ethics Committee of VU University Medical Center Amsterdam.
Procedure
The volunteers completed a behavioral session of 1.5 h and an fMRI session of 1 h. During the behavioral session, the fMRI tasks were practiced. In addition, neuropsychological tests were performed, and questionnaires were filled out. During the fMRI session, participants performed an Appraisal task. A Stroop task and a Go/NoGo paradigm were also administered and will be described elsewhere.
When practicing the Appraisal task, participants were asked to think about a same-sex friend from Medical College. Afterwards they gave a rating on a 10-point scale for the quality of the relationship with this person. The mean rating was 7.6 (s.d. = 1.11), and no significant difference between the four groups was found in a one-way analysis of variance (ANOVA; F = 0.86, P = 0.47). During the fMRI session, participants were instructed to think about the same friend as in the behavioral session.
Appraisal paradigm
The fMRI Appraisal task consisted of 40 short descriptive phrases from the social domain (e.g. ‘I have many friends’) and the academic domain (e.g. ‘I like going to the library’). The phrases were translated from the study of Pfeifer et al. (2007) and adapted to the context of university. Stimuli were presented visually on a back-projection screen that could be seen through a tilted mirror attached to the scanner head coil.
The task included three Appraisal conditions and a Baseline condition. During the condition Self, participants had to indicate whether the phrases described themselves (e.g. ‘I often eat lunch by myself. True or False?’). During the condition Other, participants indicated whether the phrases described their friend from Medical College (e.g. ‘He/She reads very quickly. True or False?’). In the condition Reflective, participants judged what their friend would think about them (e.g. ‘I feel lonely at the university. True or False?’). In the Baseline condition, participants judged if the descriptions were positive or negative (e.g. ‘He/She always spells things wrong. Positive or Negative?’). True or false responses (and positive or negative responses for Baseline) were given by button press.
The task, programmed in E-Prime 2.0 (http://www.pstnet.com/eprime.cfm), was divided into two runs. One run contained 16 blocks with a length of 20 s. In each block, five sentences from a given domain (social or academic) were presented. The phrases stayed on the screen for 4 s during which the participants gave a response. If no response was given within this time frame, a missed response was recorded. Four consecutive blocks contained the same five phrases for the different conditions (Self, Other, Reflective and Baseline). This was followed by four blocks with five phrases of a different domain, another four blocks with five phrases of the first domain and ending with four blocks containing five phrases of the second domain. Thus, one run consisted of 20 unique sentences repeated for each condition and in total, 160 phrases were presented divided over two runs. The order of conditions and domain was counterbalanced across participants. For each participant, the order of conditions between blocks was fixed. Positive and negative phrases were intermixed. Blocks were preceded by an instruction screen shown for 6 s. Between blocks there was a rest period of 10 s. The total run length was 8 min.
Data acquisition
Images were acquired on a General Electric 3-T head-only MRI scanner. A T2*-weighted echo planar imaging sequence was used with the following parameters: time to repetition (TR) = 2000 ms, time to echo (TE) = 35 ms, flip angle (FA) = 80°, field of view (FOV) = 22 × 22 cm, number of slices = 35 and voxel size = 3.5 × 3.5 × 3 mm. A T1-weighted anatomical scan was acquired to aid with spatial normalization (TR = 7.876 ms, TE = 3.06 ms, FA = 12°, FOV = 22 × 22 cm, number of slices = 166 and voxel size = 1 × 1 × 1 mm).
Behavioral data analysis
Responses and reaction times were recorded. Due to technical malfunctions, behavioral data of four participants (one male late adolescent, three male young adults) were missing. The reported analyses were conducted on the remaining 69 participants. For each of the 69 participants, the total of missed responses did not exceed 10% (mean = 1.22%, s.d. = 1.25%). Accuracy was calculated for the Baseline condition to ensure participants were paying attention to the task. An ANOVA with number of errors as the dependent variable and fixed factors Sex (female, male) and Age (late adolescent, young adult) was conducted to test for differences between groups.
There were no correct answers for the Appraisal conditions, but differences between answers for Self and Other as well as Self and Reflective were assessed. This provides information on the extent to which participants judge the other as similar to oneself and in how far participants think the other judges them similar as they judge themselves. To investigate if there were differences between groups in how far appraisals of other and self were similar, an ANOVA was carried out with number of different answers between Self and Other as dependent variable and Sex and Age as fixed factors. In addition, an ANOVA for number of different answers between Self and Reflective was conducted to see if groups differed in the extent to which reflected self-appraisals and self-appraisals were similar.
Reaction times were approximately normally distributed. A repeated measures ANOVA was performed with within-subject factor Condition (Self, Other, Reflective and Baseline) and between-subject factors Sex and Age. For each ANOVA, a threshold of P < 0.05 was used. If appropriate, post hoc tests were carried out with a threshold of P < 0.05, Bonferroni corrected for multiple comparisons.
fMRI data analysis
fMRI data were analyzed by Statistical Parametric Mapping (SPM8, www.fil.ion.ucl.ac.uk/spm). First, the data of all participants were preprocessed. Images were realigned using a six-parameter rigid body transformation to correct for head movement. The functional images were coregistered to the structural image and normalized to the MNI template. Spatial smoothing was performed with a 7-mm full width at half maximum isotropic Gaussian kernel (two times the voxel size).
The general linear model was applied to estimate the effects of the Appraisal conditions vs Baseline for each participant. A block design was specified and convolved with a canonical hemodynamic response function. The condition blocks with a length of 20 s, the rest periods of 10 s and the 6 s during which the instruction was presented, were modeled separately. High-pass filtering was used to remove low-frequency noise. Motion parameters were included as regressors of no interest. The resulting contrast images were entered into second-level analyses.
All four groups were included and simple effects were calculated to reveal average brain activation during the different Appraisal conditions (vs Baseline). A threshold of P < 0.05, family wise error (FWE) rate corrected, was used. Across all groups, the conditions Other and Reflective were compared with the condition Self using T-tests (P < 0.05, FWE corrected). The conditions Other and Reflective were not compared with each other as there were no specific hypotheses about this contrast.
Next, an ANOVA was conducted with within-subject factor Appraisal condition (Self, Other and Reflective) and between-subject factors Sex (female, male) and Age (late adolescent, young adult). The effects of Sex and Age on Appraisal conditions were assessed in F-tests for the three-way interaction, two-way interactions and main effects. In the presence of an interaction effect, a main effect cannot be meaningfully interpreted. Therefore, in the case of significant interaction effects, these contrasts were used as an explicit mask when testing for main effects. All analyses were thresholded at P < 0.005, uncorrected for height and corrected for magnitude with a cluster size k = 50 derived from a Monte-Carlo simulation (3dClustSim in AFNI; http://afni.nimh.nih.gov/afni), equivalent to a threshold of P < 0.05, FWE corrected. For clusters equal or larger than 50 voxels significant at P < 0.005, mean parameter estimates were extracted using the SPM toolbox MarsBaR (http://marsbar.sourceforge.net). These were subjected to post hoc tests (P < 0.05, Bonferroni corrected for multiple comparisons) to interrogate the effects.
RESULTS
Behavioral results
For number of errors in the Baseline condition (mean = 3.78, s.d. = 3.49), there was no main effect of Sex (F = 1.25, P = 0.27), no main effect of Age (F = 0.16, P = 0.69) and no interaction effect of Sex and Age (F = 0.03, P = 0.86). This suggests that all four groups performed the task equally well.
For the number of different answers between Self and Other (mean = 10.06, s.d. = 4.56), no effect of Sex (F = 1.03, P = 0.31), Age (F = 0.007, P = 0.94) or interaction effect between Sex and Age (F = 1.63, P = 0.21) was observed. In addition, no effect of Sex (F = 0.82, P = 0.37), Age (F = 0.001, P = 0.97) or interaction effect between Sex and Age (F = 1.16, P = 0.29) was found for the number of different answers between Self and Reflective (mean = 6.81, P = 3.49).
A repeated measures ANOVA on the reaction times showed no interaction effect between Condition, Sex and Age (F = 0.13, P = 0.94), no interaction between Condition and Sex (F = 1.78, P = 0.15) and no interaction between Condition and Age (F = 0.11, P = 0.96). There was a main effect of the within-subject factor Condition (F = 3.00, P < 0.05) but no effects of between-subject factors Sex (F = 0.68, P = 0.41), Age (F = 0.02, P = 0.88) or the interaction Sex and Age (F = 1.65, P = 0.20). Post hoc tests for the factor Condition indicated that reaction times were longer for Reflective than Self (t = 2.79, P < 0.01). All other pairwise comparisons were not significant (See Supplementary Table S1 for descriptives).
fMRI results
Appraisal conditions
To replicate previous findings on brain areas involved in social understanding, the Appraisal conditions Self, Other and Reflective were contrasted with the Baseline condition. These simple effect analyses were performed on the entire sample of 73 participants, thus collapsing across sex and age. The results are displayed in Table 1 (See Supplementary Table S2 for post hoc tests on these regions in each group separately).
Table 1.
MNI coordinates for the simple effects of Appraisal conditions compared with Baseline (threshold P < 0.05, FWE corrected)
| Peak of activation | x | y | z | T-value | Z-value | Cluster size |
|---|---|---|---|---|---|---|
| Self | ||||||
| Medial prefrontal cortex | 0 | 60 | 0 | 8.64 | 7.97 | 626 |
| Medial posterior parietal cortex | −11 | −60 | 12 | 8.45 | 7.81 | 665 |
| Left dorsolateral prefrontal cortex | −21 | 32 | 45 | 6.52 | 6.21 | 114 |
| Left posterior parietal cortex | −39 | −84 | 30 | 6.25 | 5.79 | 39 |
| Right dorsolateral prefrontal cortex | 21 | 39 | 42 | 5.58 | 5.38 | 45 |
| Ventral striatum | 4 | 14 | −3 | 4.73 | 4.60 | 4 |
| Left fusiform gyrus | −28 | −42 | −15 | 4.65 | 4.53 | 3 |
| Dorsal striatum | 14 | 18 | 15 | 4.63 | 4.51 | 3 |
| Other | ||||||
| Medial posterior parietal cortex | 0 | −63 | 24 | 7.82 | 7.31 | 617 |
| Medial prefrontal cortex | 4 | 56 | −3 | 5.66 | 5.45 | 18 |
| Left posterior parietal cortex | −39 | −81 | 33 | 5.30 | 5.12 | 24 |
| Right dorsolateral prefrontal cortex | 21 | 28 | 48 | 5.06 | 4.90 | 17 |
| Left dorsolateral prefrontal cortex | −21 | 32 | 45 | 4.94 | 4.79 | 11 |
| Right temporoparietal junction | 49 | −63 | 24 | 4.55 | 4.42 | 2 |
| Reflective | ||||||
| Medial posterior parietal cortex | −7 | −56 | 33 | 10.78 | 9.59 | 1035 |
| Left dorsolateral prefrontal cortex | −25 | 35 | 45 | 8.51 | 7.87 | 1017 |
| Medial prefrontal cortex | −4 | 60 | 0 | 8.32 | 7.71 | Same cluster |
| Left temporoparietal junction | −46 | −60 | 24 | 8.45 | 7.81 | 409 |
| Left posterior parietal cortex | −42 | −77 | 33 | 8.40 | 7.77 | Same cluster |
| Right temporoparietal junction | 53 | −60 | 24 | 6.25 | 5.79 | 98 |
| Right dorsolateral prefrontal cortex | 25 | 39 | 45 | 5.58 | 5.38 | 82 |
| Left supplementary motor area | −4 | 18 | 66 | 4.99 | 4.84 | 22 |
| Ventral striatum | 4 | 18 | −3 | 4.58 | 4.46 | 2 |
During Self, activation was observed in the medial prefrontal cortex and the medial posterior parietal cortex. The left dorsolateral prefrontal cortex, left posterior parietal cortex and right dorsolateral prefrontal cortex were also activated. Small clusters (k < 10) were found in the ventral striatum, left fusiform gyrus and dorsal striatum.
For Other, activation was demonstrated in the medial posterior parietal cortex. In addition, the medial prefrontal cortex, the left posterior parietal cortex, right dorsolateral prefrontal cortex, left dorsolateral prefrontal cortex and a small cluster (k < 10) in right temporoparietal junction were activated.
During Reflective, there was activation in the medial posterior parietal cortex and the left dorsolateral prefrontal cortex extending into medial prefrontal cortex. The left temporoparietal junction extending into left posterior parietal cortex, the right temporoparietal junction and right dorsolateral prefrontal cortex were also engaged. Additional activation was observed in the left supplementary motor area and a small cluster (k < 10) in ventral striatum.
Contrasting the condition Self with Other revealed activation in the medial prefrontal cortex and left ventrolateral prefrontal cortex. Small clusters were also found in dorsal striatum, bilateral occipital regions and left supplementary motor area. No significant clusters were shown in the reverse contrast. A small cluster in right cuneus was activated more during Reflective compared with Self, whereas the left precuneus and left temporoparietal junction were activated more during Self vs Reflective (Table 2).
Table 2.
MNI coordinates for the direct comparisons of Appraisal conditions (threshold P < 0.05, FWE corrected)
| Peak of activation | x | y | z | T-value | Z-value | Cluster size |
|---|---|---|---|---|---|---|
| Self vs. Other | ||||||
| Medial prefrontal cortex | −4 | 56 | 6 | 5.71 | 5.50 | 216 |
| Left ventrolateral prefrontal cortex | −42 | 28 | 0 | 5.55 | 5.35 | 57 |
| Left calcarine cortex | −7 | −102 | 0 | 5.40 | 5.22 | 13 |
| Dorsal striatum | −7 | 7 | 6 | 4.89 | 4.75 | 11 |
| Right calcarine cortex | 14 | −98 | 6 | 4.87 | 4.73 | 3 |
| Right middle occipital cortex | −28 | −91 | 3 | 4.81 | 4.68 | 5 |
| Dorsomedial prefrontal cortex | −7 | 28 | 54 | 4.76 | 4.63 | 4 |
| Left supplementary motor area | −11 | 14 | 69 | 4.71 | 4.59 | 3 |
| Other vs. Self | ||||||
| No suprathreshold clusters | ||||||
| Self vs. Reflective | ||||||
| Right cuneus | 11 | −95 | 9 | 4.96 | 4.82 | 7 |
| Reflective vs. Self | ||||||
| Medial posterior parietal cortex | −7 | −67 | 42 | 6.24 | 5.96 | 92 |
| Left temporoparietal junction | −53 | −60 | 24 | 5.98 | 5.73 | 79 |
Sex differences
The effects of Sex and Age on brain activation during the conditions Self, Other and Reflective (vs Baseline) were assessed in an ANOVA. No main effect of Age or interaction effects involving the factor Age was found.
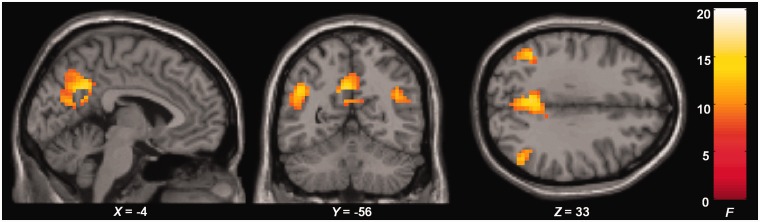
To investigate differences between females and males across Appraisal conditions, the main effect of Sex was calculated, masked by the interaction effect between Sex and Appraisal condition (thresholded at P < 0.005). An effect was demonstrated in the right temporoparietal junction (MNI = 49 − 63 27, Z = 4.22), the left temporoparietal junction (MNI = − 46 − 60 27, Z = 3.72) and the medial posterior parietal cortex (MNI = − 4 −56 33, Z = 3.77; Figure 1). Post hoc tests revealed that activation of these three regions was stronger for males than females (t = 3.83, P < 0.005; t = 3.36, P < 0.005; t = 3.60, P < 0.005, respectively).
Fig. 1.
Sex-related differences across Appraisal conditions. Activation of the medial posterior parietal cortex and bilateral temporoparietal junction was stronger for males compared with females during Self, Other and Reflective.
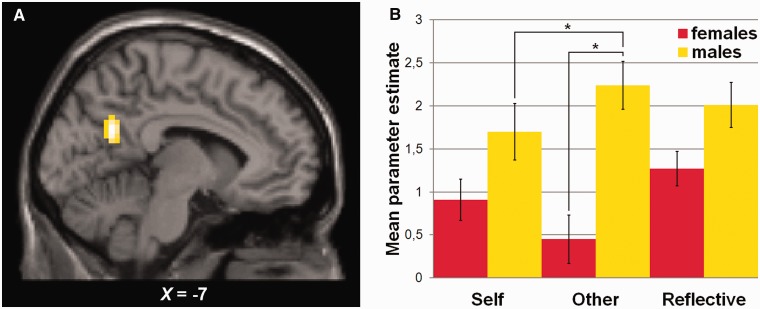
A significant interaction between Sex and Appraisal condition was observed in the precuneus (MNI = −7 −60 24, Z = 3.41, k = 73). Mean parameter estimates of this region were extracted and are shown in Figure 2. Post hoc tests were performed to investigate differences in mean parameter estimates between females and males for each Appraisal condition. In addition, as the precuneus is known to be especially involved in understanding others (Pfeifer et al., 2007), the conditions Other and Reflective were compared with the condition Self for females and males separately. Males showed stronger activation than females during all Appraisal conditions but this effect only reached significance in the condition Other (t = 4.62, P < 0.005). For males, the activation during the condition Other was larger than during the condition Self (t = 2.73, P < 0.005). There was no significant difference between the mean parameter estimates of the conditions Reflective and Self. For females, there was no effect of Appraisal condition.
Fig. 2.
(A) The precuneus showed an interaction effect between Sex and Appraisal condition. (B) Mean parameter estimates of the precuneus. Activation of this area was larger in males than females during Other and for males, activation during Other was larger than during Self.
DISCUSSION
The current fMRI study investigated self- and other-appraisals as well as reflected self-appraisals in male and female late adolescents (18–19 years old) and young adults (23–25 years old). Across all four groups, it was found that the medial prefrontal cortex, medial posterior parietal cortex, left and right dorsolateral prefrontal cortex as well as left posterior parietal cortex were engaged during all appraisal conditions. These results confirm previous research which showed that cortical midline structures play a role in social understanding (Uddin et al., 2007). The medial as well as lateral prefrontal and parietal regions have been implicated in controlled social cognition, as opposed to more automatic processes (Satpute and Lieberman, 2006). The medial prefrontal cortex was activated more during self-appraisals than other-appraisals. This is consistent with the notion that anterior midline brain areas are particularly important for self-related processing (Northoff et al., 2011). The left ventrolateral prefrontal cortex was also preferentially involved in self-appraisals compared with other-appraisals. Stronger activation of the medial posterior parietal cortex and left temporoparietal junction was observed for reflected self-appraisals compared with self-appraisals, as demonstrated previously on a similar paradigm (Pfeifer et al., 2009).
Over all appraisal conditions, males activated bilateral temporoparietal junction more than females. This region has been related to thinking about the content of mental states (Saxe, 2006) and distinguishing between self and other (Decety and Sommerville, 2003). Our finding of increased activation in this area for males compared with females is in line with studies on emotions. When focusing on emotions of oneself, stronger recruitment of the left temporoparietal junction was observed in males (Schulte-Ruther et al., 2008). Increased activation in the right temporoparietal junction for males compared with females was demonstrated during understanding of others’ emotions (Derntl et al., 2010). The current results suggest that similar sex differences are present on a more cognitive task. Thus, males rely to a greater extent on a neural mechanism for self and other processing when making social appraisals.
More activation for males than females was also found in the medial posterior parietal cortex. A small cluster of the medial posterior parietal cortex, specifically the precuneus, showed an interaction effect between sex and appraisal condition. Stronger activation of this region for males compared with females occurred only during other-appraisals. Males recruited the precuneus more for other-appraisals than self-appraisals while there was no difference in females. A recent meta-analysis proposes that the medial posterior parietal cortex preferentially responds to stimuli related to personally familiar people in contrast to self-specific stimuli (Qin et al., 2012). Our results indicate that this effect might be more profound in males.
No differences between males and females were observed in the medial prefrontal cortex. Krach et al. (2009) demonstrated stronger activation of this area in males compared with females when taking the perspective of a game partner. However, they employed a mentalizing task requiring online reasoning while our study targets knowledge about relatively stable attributes and preferences. Sex-related differences in brain activation might depend on the type of social paradigm used.
The current findings suggest that the neural bases of self-appraisals, other-appraisals and reflected self-appraisals differ between males and females. This adds to research showing that females usually outperform males on social tasks (Baron-Cohen, 2002). Our results demonstrate that sex differences can also exist at the neural level, even in the absence of evident behavioral differences. Unfortunately, it is difficult to make inferences about behavior in our study. A limitation of this paradigm is that accuracy measures are not available, thus it is unclear how the participants carried out the task. There were no sex differences in reaction times, pointing to equal performance between groups. In addition, males and females did not differ in the extent to which they judged other as similar to self or in how far they thought the other judged them similar as they judged themselves. This indicates that similarity does not play a role in the reported sex differences in brain activation.
We compared late adolescents 18–19 years old with young adults 23–25 years old. Between these ages, significant life changes occur (Arnett, 2000) and the cortex reaches maturity (Giedd and Rapoport, 2010). Nonetheless, we did not observe an effect of age on brain activation when making appraisals. This may suggest that understanding of self and others is already fully developed at age 18. Differences between late adolescents and young adults might be more evident when affective processes play a role (Steinberg, 2005; Casey et al., 2011). In contrast, our task involved social knowledge, without a clear emotional component. Adolescents aged 11–14 did show stronger activation of cortical midline regions and temporoparietal junction during self-appraisals compared with adults (Pfeifer et al., 2009). Future research might benefit from using a wider age range than in this study, by including for example adolescents 16–17 years old.
An alternative interpretation for not finding age differences may be that the 23- to 25-year old participants and the 18- to 19-year olds have not reached their full potential in social skills. All participants are students in Medical College so one could argue that even participants aged 23–25 have not assumed a stable adult role yet and therefore resemble late adolescents. However, these students are in the final years of the curriculum which involve clinical training according to full-time working schedules. This makes their life styles more similar to those of adults than to those of students aged 18–19, who mainly follow courses and do practical work in a class-room setting.
A homogeneous sample of students from Medical College was recruited to exclude possible confounding factors, such as intelligence, thereby increasing the possibility of finding sex differences. A drawback of this approach is that the results cannot be easily generalized. The observed differences between males and females may not be present in other age groups or in people with a different educational background. Regardless, our study provides a crucial first step towards gaining more insight into sex differences during appraisals of self and others.
To conclude, we demonstrated that there are important differences between males and females in the neural bases of social understanding during the transition from late adolescence to young adulthood. These findings should be taken into account when designing future studies in the field of social cognitive neuroscience. Some researchers have already begun to do this by focusing on either males or females. However, it is even more important to include both sexes within a single study to further investigate differences.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
We would like to thank Jennifer Pfeifer for providing suggestions related to the Appraisal task and Martina Knežević, Imke Jansen, Dirk Heslenfeld and Ton Schweigmann for help with data collection.
This work was supported by The Netherlands Organization for Scientific Research (VICI grant 453 11 005 to L.K.).
REFERENCES
- Arnett JJ. Emerging adulthood: a theory of development from the late teens through the twenties. American Psychologist. 2000;55(5):469–80. [PubMed] [Google Scholar]
- Baron-Cohen S. The extreme male brain theory of autism. Trends in Cognitive Sciences. 2002;6(6):248–54. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders. 2004;34(2):163–75. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Social Cognitive and Affective Neuroscience. 2007;2(2):130–9. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosacki SL. Theory of mind and self-concept in preadolescents: links with gender and language. Journal of Educational Psychology. 2000;92(4):709–17. [Google Scholar]
- Casey BJ, Duhoux S, Malter Cohen M. Adolescence: what do transmission, transition, and translation have to do with it? Neuron. 2010;67(5):749–60. doi: 10.1016/j.neuron.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Somerville LH. Braking and accelerating of the adolescent brain. Journal of Research on Adolescence. 2011;21(1):21–33. doi: 10.1111/j.1532-7795.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–50. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Collette F, Van der Linden M, et al. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. 2005;25(2):616–24. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Decety J, Sommerville JA. Shared representations between self and others: a social cognitive neuroscience view. Trends in Cognitive Sciences. 2003;7(12):527–533. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Eickhoff S, et al. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology. 2010;35(1):67–82. doi: 10.1016/j.psyneuen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Lennon R. Sex differences in empathy and related capacities. Psychological Bulletin. 1983;94(1):100–31. [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London: B Biological Sciences. 2003;358(1431):459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67(5):728–34. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience. 2006;1(1):18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickes W, Gesn PR, Graham T. Gender differences in empathic accuracy: differential ability or differential motivation? Personal Relationships. 2000;7(1):95–109. [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(8):1808–14. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(5):785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Keulers EH, Evers EA, Stiers P, Jolles J. Age, sex, and pubertal phase influence mentalizing about emotions and actions in adolescents. Developmental Neuropsychology. 2010;35(5):555–69. doi: 10.1080/87565641.2010.494920. [DOI] [PubMed] [Google Scholar]
- Klein KJK, Hodges SD. Gender differences, motivation, and empathic accuracy: when it pays to understand. Personality and Social Psychology Bulletin. 2001;27(6):720–30. [Google Scholar]
- Krach S, Blumel I, Marjoram D, et al. Are women better mindreaders? Sex differences in neural correlates of mentalizing detected with functional MRI. BMC Neuroscience. 2009;10:9. doi: 10.1186/1471-2202-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kret ME, De Gelder B. Sex differences in processing emotional signals of others. Neuropsychologia. 2012;50(7):1211–21. doi: 10.1016/j.neuropsychologia.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Kret ME, Pichon S, Grèzes J, De Gelder B. Men fear other men most: gender specific brain activations in perceiving threat from dynamic faces and bodies—an fMRI study. Frontiers in Psychology. 2011;2:3. doi: 10.3389/fpsyg.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annual Review of Psychology. 2007;58:259–89. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, et al. Shared neural circuits for mentalizing about the self and others. Journal of Cognitive Neuroscience. 2010;22(7):1623–35. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, et al. A developmental examination of gender differences in brain engagement during evaluation of threat. Biological Psychiatry. 2004;55(11):1047–55. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Northoff G, Qin P, Feinberg TE. Brain imaging of the self—conceptual, anatomical and methodological issues. Consciousness and Cognition. 2011;20(1):52–63. doi: 10.1016/j.concog.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28(4):797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. "I know you are but what am I?!": neural bases of self- and social knowledge retrieval in children and adults. Journal of Cognitive Neuroscience. 2007;19(8):1323–37. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, Lieberman MD. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Development. 2009;80(4):1016–38. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Liu Y, Shi J, et al. Dissociation between anterior and posterior cortical regions during self-specificity and familiarity: a combined fMRI-meta-analytic study. Human Brain Mapping. 2012;33(1):154–64. doi: 10.1002/hbm.21201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. Journal of Cognitive Neuroscience. 2004;16(6):988–99. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Satpute AB, Lieberman MD. Integrating automatic and controlled processes into neurocognitive models of social cognition. Brain Research. 2006;1079(1):86–97. doi: 10.1016/j.brainres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006;16(2):235–9. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in "theory of mind". Neuroimage. 2003;19(4):1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schlichting L. Peabody Picture Vocabulary Test-III-NL. Amsterdam: Harcourt Test Publisher; 2005. [Google Scholar]
- Schulte-Ruther M, Markowitsch HJ, Shah NJ, Fink GR, Piefke M. Gender differences in brain networks supporting empathy. Neuroimage. 2008;42(1):393–403. doi: 10.1016/j.neuroimage.2008.04.180. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Fontaine NM, Bird G, et al. Neural processing associated with cognitive and affective Theory of Mind in adolescents and adults. Social Cognitive and Affective Neuroscience. 2012;7(1):53–63. doi: 10.1093/scan/nsr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439(7075):466–9. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences. 2007;11(4):153–7. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J. The Concept Shifting Test: adult normative data. Psychological Assessment. 2006a;18(4):424–32. doi: 10.1037/1040-3590.18.4.424. [DOI] [PubMed] [Google Scholar]
- Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J. The Letter Digit Substitution Test: Normative data for 1,858 healthy participants aged 24-81 from the Maastricht Aging Study (MAAS): influence of age, education, and sex. Journal of Clinical and Experimental Neuropsychology. 2006b;28(6):998–1009. doi: 10.1080/13803390591004428. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2009;30(3):829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veroude K, Keulers EH, Evers EA, Stiers P, Krabbendam L, Jolles J. The effect of perspective and content on brain activation during mentalizing in young females. Journal of Clinical and Experimental Neuropsychology. 2012;34(3):227–34. doi: 10.1080/13803395.2011.630650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.